Abstract
Background
Resection of colorectal liver metastases(CRLM) is associated with improved survival; however, the impact of time to resection on survival is unknown. The current multi-institutional study sought to evaluate the influence of time from diagnosis to resection(Dx-Rx) on survival outcomes among patients with resectable, metachronous CRLM and to compare practice patterns across hospitals.
Study Design
Medical records of patients with ≤ 4 metachronous CRLM treated with surgery were reviewed and analyzed retrospectively. Time from Dx-Rx, was analyzed as a continuous variable and also dichotomized into two groups [Dx-Rx<3 months (Group 1), Dx-Rx≥ 3months (Group 2)] for further analysis. Survival time distributions after resection were estimated using the Kaplan-Meier method. Between group univariate comparisons were based on the log-rank test and multivariable analysis was done using Cox-proportional hazards model.
Results
From 2000-2010, 626 patients were identified. Type of initial referral(p<0.0001) and use of neoadjuvant(p=0.04) and/or adjuvant (p<0.0001)chemotherapy were significantly different among hospitals. Patients treated with neoadjuvant chemotherapy(n=108) and those with unresectable disease at laparotomy(n=5) were excluded from final evaluation. Median overall(OS) and recurrence free(RFS) survival [median(min-max)] were 74(63.8-84.2)months and 29(23.9-34.1)months, respectively. For the entire cohort, longer time from Dx-Rx was independently associated with shorter OS[Hazard ratio(HR), 95% Confidence Interval(CI)];(HR 1.12, 95% CI 1.06-1.18, p<0.0001) but not RFS. Median OS for Group 1 was 76(62.0-89.2)months vs. 58(34.3-81.7) months in Group 2, p=0.10. Among patients with available data pertaining to adjuvant chemotherapy (n=457; 318 treated, 139 untreated), OS[87(71.2-102.8) vs. 48(25.3-70.7) months, p<0.0001] and RFS[33(25.3-40.7) vs. 22(14.5-29.5) months, p=0.05]were significantly improved.
Conclusions
In select patients undergoing initial resection for CRLM, longer time from Dx-Rx is independently associated with worse OS. Furthermore, despite uniform disease characteristics, practice patterns related to definitely resectable CRLM vary significantly across hospitals.
Introduction
Complete resection of colorectal cancer liver metastases (CRLM) is the only treatment consistently associated with long term survival and potential cure in up to 16% of patients[1]. Unfortunately, resection is only feasible in 10-20% of patients at presentation[2]and despite improved overall survival(OS) following hepatectomy, disease recurrence is common[3]. As a consequence, the potential utility of chemotherapy amongst patients with clearly resectable CRLM has been of clinical interest. Initial results of the European Organization for Research and Treatment of Cancer (EORTC) prospective randomized trial of surgery +/− perioperative chemotherapy for resectable CRLM suggested a small but significant benefit in progression free survival(PFS) at 3 years in the chemotherapy treatment arm [4], however, this benefit disappeared with longer follow-up[5]. Additionally, retrospective series have shown that outcomes in patients with minimal liver only metastatic disease treated with surgery first are superior to those observed in patients treated with neoadjuvant chemotherapy [6, 7]. These findings suggest that patients with clearly resectable, metachronous, liver only CRLM do not benefit from chemotherapy prior to hepatectomy and primary treatment should be directed at definitive oncologic resection.
Nevertheless, determining resectability is challenging and differing notions of resectable disease may give rise to uncertainty as to when, and in whom, surgical referral is appropriate. The number and location of metastases, length of disease free interval and presence of extrahepatic disease are the most common clinical factors used to ascertain resectability [8, 9]; however, the relative importance of each is debatable and subject to individual and institutional variability. Given the lack of uniformity in defining resectability and differing attitudes regarding chemotherapy, significant heterogeneity in referral and treatment of metachronous CRLM amenable to surgical resection exists. Lack of consensus regarding management in this setting may lead to unnecessary time delays to definitive treatment and potentially impact clinical and oncologic outcomes.
Time delay to definitive treatment has been evaluated for a number of primary cancers, with mixed results[10-19]. However, no study has specifically evaluated the influence of time to surgery on clinical and oncologic outcomes in patients with resectable CRLM. The primary objective of this multi-institutional study was to evaluate the potential association between time from diagnosis to resection on survival outcomes in patients with resectable, metachronous CRLM and to characterize/compare practice patterns across study centers. To minimize variability related to institutional differences in the treatment of patients with very advanced disease, the current study specifically focused on patients with minimal, liver only, metastatic disease with the most favorable risk.
Methods
Study Design
Four academic referral hospitals participated in this multi-institutional study. Ethics approval was obtained through institutional review at each site. Patients with metachronous, resectable CRLM diagnosed from 2000 to 2010 were identified from institutional databases and retrospectively reviewed. CRLM were considered metachronous if disease free interval was >6 months, and resectable if there were ≤ 4 tumors amenable to complete resection in a single stage operation. Patients were excluded based on the following: presence of extrahepatic disease, use of ablative therapy to achieve R0 resection, recurrent CRLM, concomitant malignancy and/or use of neoadjuvant chemotherapy.
Data Collection
Demographic and clinicopathologic characteristics were obtained from institutional databases and supplemented with information from the medical record where necessary. Date of diagnosis was defined as the first radiographic evidence of CRLM and date of referral as the initial clinic visit addressing CRLM. Clinically relevant time intervals included: time from diagnosis to initial referral (Dx-Rf), initial referral to resection (Rf-Rx) and diagnosis to resection (Dx-Rx). Clinical risk scores (CRS) were calculated as previously described by Fong et al [20]. Modified CRS were tabulated for patients with missing data pertaining to 1 or 2 variables (n=116), no score was assigned if >2 variables were missing (n= 5). Patients were then stratified into either low (CRS 0-2), or high (CRS 3-5) risk categories prior to analysis. Operative reports were reviewed and patients with unresectable disease at laparotomy were excluded from final analysis. Resection margin positivity was defined by the presence of tumor cells at the inked margin. Postoperative complications were graded on a scale from 1 to 5 [21], with complications graded ≥ 3 considered major.
Follow-up time for OS analyses was from the date of surgery to the date of death or date last clinical contact, at which time data were censored. Post-operative disease surveillance was not standardized across centers. In general, however, follow-up was based on guidelines available at the time of resection [22]. Typically this included clinical assessment +/− serial CEA every 3-6 months for 2 years, then every 6 months for 3 years. Radiographic imaging of the chest, abdomen and pelvis was also obtained every 6-12 months for a minimum of 5 years following resection. Disease recurrence was determined radiographically. Review of medical records, public access obituaries and social security death index were used to determine survival status. These sources were last searched and vital statistics updated in January 2015.
Data Analysis
Demographic, clinicopathologic, operative and time to surgery data are presented as frequencies (%) or mean +/− standard deviation. All baseline measures were obtained on the date of surgery. Length of stay, follow up time, recurrence and survival data are expressed as median (min-max). Continuous data were compared using Mann-Whitney U or t-tests where appropriate and categorical data compared using Chi-square tests. Survival times were evaluated from the date of surgery to the date of censoring. The endpoint for RFS was disease recurrence and/or death and for OS it was death from any cause. The Kaplan-Meier method was used to estimate survival times. OS and RFS are reported as median (95% CI). Actuarial 1-, 2-, and 5-year survival rates are also reported. To identify variables independently associated with survival outcomes univariate analysis was completed and variables with p<0.10, were subsequently included in a multivariable model using Cox proportional hazards regression. A stepwise backward Wald method using p>0.05 as criteria for exclusion was employed to determine the final model. Of note, variables with more than 10% missing data were excluded from multivariable analysis.
A maximal chi square test was used to determine if a single cutpoint in time from Dx-Rx existed whereby OS and/or RFS began to differ significantly [23]. Using this test no statistically significant cutpoint was identified. Consequently a clinically relevant cutpoint of 3 months was determined based on the mean time from Dx-Rx from the current study and previously published data regarding optimal timing of resection for hepatobiliary malignancies [24]. Patients were stratified into two groups: Group 1- Dx-Rx <3 months and Group 2- Dx-Rx ≥3 months, and outcomes were compared. OS and RFS were assessed using the Kaplan-Meier method and comparison between groups made using the log rank test. Similar subgroup analyses based on use of adjuvant chemotherapy and treating hospital were also performed. All statistical analysis was completed using SPSS software version 21 (Chicago IL, USA), all tests were two-tailed and significance was set at p<0.05.
Results
Patient Characteristics and Operative Outcomes
From January 2000 -December 2010, 626 patients with metachronous, resectable CRLM were identified; of these, 108 patients treated with neoadjuvant chemotherapy and 5 patients unresectable at laparotomy were excluded. The remaining 513 patients underwent surgery as the first mode of treatment and were included in primary analysis. Baseline demographics and clinicopathologic characteristics are outlined in Table 1. Following diagnosis of CRLM the majority of patients were initially referred to a surgeon (88.1%, n=452) rather than a medical oncologist. Mean time from Dx-Rf was 0.65 +/−1.43 months, from Rf-Rx 0.68 +/−1.04 months and from Dx-Rx 1.92 +/−2.5 months. Operative characteristics and outcomes are outlined in Table 2. Postoperatively, 146(28.5%) patients experienced a complication, 63(12.3%) were considered major. Overall, 30 and 60-day mortality were 0.2%(n=1) and 1.2%(n=6), respectively. Positive resection margins were documented in 10.3% (n=53) of patients and 62%(n=318) of patients received adjuvant chemotherapy.
Table 1.
Baseline Demographic and Clinicopathologic Characteristics Stratified by Time from Diagnosis to Resection < 3 months vs ≥ 3 months
| Variable | Study cohort | Time from diagnosis to surgery < 3 mo |
Time from diagnosis to surgery ≥ 3 mo |
p Value |
|---|---|---|---|---|
| n | 513 | 394 (76.8) | 119 (23.20 | |
| Sex | 0.52 | |||
| Female | 194(37.8) | 152(38.6) | 42(35.3) | |
| Male | 319(62.2) | 242(61.4) | 77(64.7) | |
| Age, y | 64.1(11.5) | 63.6(11.4) | 65.7(11.7) | 0.08 |
| BMI, kg/m2 | 28.3(5.5) | 28.2(5.4) | 28.3(5.6) | 0.95 |
| Type of initial referral | 0.55 | |||
| Medical | 61(11.9) | 45(11.4) | 16(13.4) | |
| Surgical | 452(88.1) | 349(88.6) | 103(86.6) | |
| Year of surgery | 0.06 | |||
| 2000-05 | 259(50.5) | 208(52.8) | 51(42.9) | |
| 2006-10 | 254(49.5) | 186(47.2) | 68(57.1) | |
| Preoperative PVE | 18(3.5) | 6(1.5) | 12(10.1) | <0.0001 |
| Lymph node + primary | 227(44.2) | 174(44.2) | 53(44.5) | 0.68 |
| Preoperative CEA, ng/mL | 0.72 | |||
| CEA ≤ 200 | 424(82.7) | 336(85.3) | 88(73.9) | |
| CEA >200 | 12(2.3) | 9(2.3) | 3(2.5) | |
| Diameter of largest metastasis, cm | 0.94 | |||
| ≤5 | 374(72.9) | 287(72.8) | 87(73.1) | |
| > 5 | 135(26.3) | 104(26.4) | 31(26.1) | |
| No. of metastases | 0.07 | |||
| 1 | 316(61.6) | 252(64.0) | 64(53.8) | |
| >1 | 194(37.8) | 141(35.8) | 53(44.5) | |
| Disease-free interval, mo | 26.7(21.5) | 0.56 | ||
| <12 | 91(17.7) | 68(17.3) | 23(19.3) | |
| ≥ 12 | 414(80.7) | 321(81.5) | 93(78.2) | |
| Clinical Risk Score | 0.82 | |||
| 0 | 88(17.2) | 71(18.0) | 17(14.3) | |
| 1 | 232(45.2) | 179(45.4) | 53(44.5) | |
| 2 | 136(26.5) | 102(25.9) | 34(28.6) | |
| 3 | 47(9.2) | 34(8.6) | 13(10.9) | |
| 4 | 5(1.0) | 4(1.0) | 1(0.8) | |
| 5 | - | - | - | |
| Low risk (0-2) | 456(88.9) | 352(89.3) | 104(87.4) | 0.51 |
| High risk (3-5) | 52(10.1) | 38(9.6) | 14(11.8) | |
| Time from diagnosis to referral, mo |
0.65 +/− 1.43 | 0.21+/−0.43 | 2.13+/−2.30 | <0.0001 |
| Time from referral to resection, mo |
0.68 +/− 1.04 | 0.41+/−0.60 | 1.56+/−1.56 | <0.0001 |
| Time from diagnosis to resection, mo |
1.92 +/− 2.5 | 0.98+/−0.70 | 5.06+/−3.46 | <0.0001 |
Percentages for individual variables are tabulated based on total number of patients in each group (n= 394, diagnosis to resection <3 months and n=119, diagnosis to resection ≥ 3 months). Categorical data are presented as frequency (%) and continuous data mean +/− standard deviation. p<0.05 was considered significant.
PVE, portal vein embolization; CEA, carcinoembryonic antigen.
Table 2.
Operative Characteristics and Postoperative Outcomes of Patients with Resectable Colorectal Liver Metastases Stratified by Time from Diagnosis to Resection < 3 months vs ≥ 3 months
| Variable | Study cohort |
Time from
diagnosis to resection < 3 mo |
Time from
diagnosis to resection ≥ 3 mo |
p Value |
|---|---|---|---|---|
| n (%) | 513 | 394(76.8) | 119(23.2) | |
| Operative procedure, n (%) |
0.05 | |||
| Wedge | 26(5.1) | 22(5.6) | 4(3.4) | |
| Right lobectomy | 144(28.1) | 107(27.2) | 37(31.1) | |
| Left lobectomy | 54(10.5) | 44(11.2) | 10(8.4) | |
| Right trisegmentectomy |
43(8.4) | 25(6.3) | 18(15.1) | |
| Left trisegmentectomy |
16(3.1) | 12(3.0) | 4(3.4) | |
| Central hepatectomy |
11(2.1) | 10(2.5) | 1(0.8) | |
| Anatomic segmentectomy or sectorectomy |
219(42.7) | 174(44.2) | 45(37.8) | |
| Procedure time, 30 minute blocks (%) |
7.3(2.6) | 7.2(2.5) | 7.6(2.7) | 0.17 |
| EBL, per 100 mL, (%) | 5.6(6.6) | 5.6(7.2) | 5.6(4.4) | 0.97 |
| LOS, d, mean±SD | 7.0+/− | 7.8+/−4.9 | 8.8+/−9.2 | 0.10 |
| 30-d Complication, n (%) |
146(28.5) | 112(28.4) | 34(28.6) | 0.98 |
| Major complication, n (%) |
63(12.3) | 46(11.7) | 17(14.3) | 0.45 |
| 30-d Mortality , n (%) | 1(0.2) | 1(0.3) | 0(0.0) | 0.59 |
| 60-d Mortality, n (%) | 6(1.2) | 5(1.2) | 1(0.8) | 0.72 |
| Margin status , n (%) | 0.68 | |||
| Positive | 53(10.3) | 42(10.7) | 11(9.2) | |
| Negative | 459(89.5) | 352(89.3) | 107(89.9) | |
| Adjuvant chemotherapy, n (%) |
0.004 | |||
| Yes | 318(62) | 260(66.0) | 58(48.7) | |
| No | 139(27) | 97(24.6) | 42(35.3) | |
| Disease recurrence, n (%) |
0.25 | |||
| Yes | 243(47.4) | 192(48.7) | 51(42.9) | |
| No | 269(52.4) | 201(51.0) | 68(57.1) | |
| Time to recurrence, mo (minimum to maximum) |
13.0(1-80) | 13(1-80) | 13(3-73) | 0.72 |
| Site of first recurrence, n (%) |
0.96 | |||
| Lung | 103(20.1) | 79(20.1) | 24(20.2) | |
| Nodal | 17(3.3) | 14(3.6) | 3(2.5) | |
| Liver | 77(15.0) | 60(15.2) | 17(14.3) | |
| Local/pelvic | 12(2.3) | 10(2.5) | 2(1.7) | |
| Peritoneal | 15(2.9) | 13(3.3) | 2(1.7) | |
| Anastomotic | 3(0.6) | 2(0.5) | 1(0.8) | |
| Adrenal | 1(0.2) | 1(0.3) | 0(0) | |
| Other | 14(2.7) | 12(3.0) | 2(1.7) | |
| Status at last follow up, n (%) |
0.86 | |||
| NED | 291(56.7) | 221(56.1) | 70(58.8) | |
| AWD | 198(38.6) | 152(38.6) | 43(36.1) | |
| DWD | 24(4.7) | 18(4.6) | 6(5.0) | |
| Follow-up time, mo (minimum to maximum) |
37.0(0-163) | 40(0-163) | 32(0-146) | 0.02 |
Percentages for individual variables are tabulated based on total number of patients in each group (n= 394, Dx-Rx <3 months and n=119, Dx-Rx ≥ 3 months). Categorical data are presented as frequency (%), continuous data as mean +/− standard deviation and follow-up time/time to recurrence are presented as median (min-max). p<0.05 was considered significant.
EBL, estimated blood loss; LOS, length of stay; NED, no evidence of disease; AWD, alive with disease; DWD, dead with disease.
Recurrence/Survival Analysis
Median follow-up time was 37 (0-163) months for the entire cohort and 54 (0-163) months for survivors. Disease recurrence occurred in 243 (47.4%) patients, median RFS was 29 (23.9-34.1) months an estimated 1, 2, and 5 year RFS rates were 74.3%, 53.6%, and 34.1%, respectively (Figure 1A). Baseline demographic, clinicopathologic and operative characteristics outlined in Table 1 and 2 were included in univariate RFS analysis. Age, treating hospital, EBL, Pringle time, CRS, diameter of largest metastases, number of metastases, use of adjuvant chemotherapy and resection margin status were significantly associated with RFS (Table 3). However, time from Dx-Rf [HR (95% CI); 1.02 (0.95-1.11), p=0.54], Rf-Rx [0.98 (0.88-1.09), p=0.70] and Dx-Rx [1.02 (0.97-1.06), p=0.49] were not significantly associated with RFS. Covariates included in multivariable RFS analysis are outlined in Table 3. Adjuvant chemotherapy (11% missing data) and Pringle time (51% missing data) were not included. Furthermore, CRS correlated with tumor size (Spearman r=0.17, p<0.0001) and was also excluded from the model. Age, treating hospital and diameter of largest metastases > 5cm retained significance and were independently associated with RFS.
Figure 1.
Kaplan-Meier (A) recurrence-free and (B) overall survival curves for patients with metachronous, resectable colorectal liver metastases. Time zero = Date of surgery. Patients were censored at the time of event occurrence (death/recurrence) or date of last contact at the treating hospital.
Table 3.
Univariate and Multivariable Recurrence-Free Survival Analysis for Patients with Low Volume, Metachronous, Colorectal Liver Metastases
| Variable | Univariate HR |
Univariate 95% CI |
p Value | Multivariable HR |
Multivariable 95% CI |
p Value |
|---|---|---|---|---|---|---|
| Age, y | 1.02 | (1.01-1.03) | 0.004 | 1.01 | 1.00-1.03 | 0.01 |
| Hospital | 0.02 | 0.03 | ||||
| 1 | 1.0 (ref) | - | 1.0 (ref) | - | ||
| 2 | 1.22 | (0.86-1.71) | 1.09 | 0.76-1.57 | ||
| 3 | 1.49 | (1.15-1.93) | 1.52 | 1.15-2.03 | ||
| 4 | 0.95 | (0.54-1.67) | 0.83 | 0.42-1.62 | ||
| EBL, per 100 mL |
1.02 | (1.00-1.03) | 0.02 | 1.01 | 1.00-1.02 | 0.13 |
| Pringle time, min |
1.02 | (1.01-1.03) | 0.004 | - | - | - |
| No. of metastases |
0.06 | 0.31 | ||||
| 1 | 1.0 (ref) | - | 1.0 (ref) | - | ||
| >1 | 1.24 | (0.99-1.55) | 1.14 | 0.89-1.46 | ||
| Diameter of largest metastases, cm |
0.02 | 0.01 | ||||
| ≤ 5 | 1.0 (ref) | - | 1.0 (ref) | - | ||
| >5 | 1.34 | (1.05-1.70) | 1.40 | 1.08-1.82 | ||
| Clinical risk score |
<0.0001 | |||||
| Low risk(0- 2) |
1.0 (ref) | - | - | - | - | |
| High risk(3- 5) |
1.86 | (1.35-2.57) | ||||
| Positive resection margin |
1.43 | (1.03-1.99) | 0.03 | 1.38 | 0.97-1.97 | 0.07 |
| 30-d Complication |
1.23 | (0.97-1.56) | 0.09 | 0.98 | 0.75-1.28 | 0.88 |
| Adjuvant chemotherapy |
0.78 | (0.60-1.00) | 0.05 | - | - | - |
53 (11%) patients and 262 (51%) of patients were missing data pertaining to adjuvant chemotherapy use and Pringle time, respectively, as such these were not included in the final multivariable model. Clinical risk score was correlated with tumor size (Spearman r=0.17, p<0.0001) and was excluded from the final multivariable model.
HR, hazard ratio; 95% CI, 95% confidence interval; (ref), reference category; EBL, estimated blood loss.
At the time of analysis, 234(45.6%) patients had died. Median OS was 74(63.8-84.2) months an estimated 1, 2, and 5-year OS rates were 94.5%, 86.5% and 55.7%, respectively (Figure 1B). Among variables assessed in univariate OS analysis, age, BMI, treating hospital, operative procedure, EBL (per 100mL), CRS, diameter of largest metastasis, major complication, use of adjuvant chemotherapy and disease recurrence were significantly associated with OS. Time from Dx-Rf and from Dx-Rx were also significantly associated with OS (Table 4). Since time from Dx-Rx was correlated with time from Dx-Rf (Spearman r=0.60, p<0.0001) only time from Dx-Rx was included in further analysis. Results from multivariable OS analysis are shown in Table 4. Age, BMI, treating hospital, EBL (per 100mL), diameter of largest metastases, disease recurrence and time from Dx-Rx maintained significance and were independently associated with OS.
Table 4.
Univariate and Multivariable Overall Survival Analysis for Patients with Low Volume, Metachronous, Colorectal Liver Metastases
| Variable | Univariate HR |
Univariate 95% CI |
P-value | Multivariabl e HR |
Multivariabl e 95% CI |
p Value |
|---|---|---|---|---|---|---|
| Age | 1.02 | (1.01-1.03) | 0.001 | 1.02 | 1.00-1.03 | 0.005 |
| BMI | 0.97 | (0.94-0.99) | 0.02 | 0.97 | 0.94-1.00 | 0.04 |
| Hospital | <0.0001 | <0.0001 | ||||
| 1 | 1.0 (ref) | - | 1.0 (ref) | - | ||
| 2 | 1.07 | (0.69-1.66) | 0.56 | 0.33-0.95 | ||
| 3 | 1.92 | (1.43-2.57) | 1.8 | 1.28-2.53 | ||
| 4 | 0.95 | (0.48-1.87) | 0.79 | 0.34-1.81 | ||
| Primary procedure | 0.02 | 0.12 | ||||
| Wedge | 1.0 (ref) | - | 1.0 (ref) | - | ||
| Right lobectomy | 0.80 | (0.45-1.42) | 0.77 | 0.37-1.58 | ||
| Left lobectomy | 0.65 | (0.33-1.28) | 0.56 | 0.24-1.29 | ||
| Right trisegmentectomy |
0.93 | (0.48-1.82) | 0.98 | 0.43-2.23 | ||
| Left trisegmentectomy |
1.01 | (0.45-2.28) | 0.86 | 0.33-2.26 | ||
| Central hepatectomy |
2.12 | (0.89-5.07) | 2.21 | 0.79-6.20 | ||
| Anatomic segmentectomy |
0.63 | (0.36-1.11) | 0.71 | 0.36-1.39 | ||
| EBL, per 100 mL | 1.02 | (1.01-1.04) | 0.006 | 1.02 | 1.0-1.04 | 0.05 |
| No. of metastases | 0.06 | 0.64 | ||||
| 1 | 1.0 (ref) | - | 1.0 (ref) | - | ||
| >1 | 1.29 | (0.99-1.67) | 1.08 | 0.79-1.47 | ||
| Diameter of largest metastases, cm |
0.003 | 0.02 | ||||
| ≤5 | 1.0 (ref) | - | 1.0 (ref) | - | ||
| >5 | 1.51 | (1.15-1.99) | 1.45 | 1.07-2.00 | ||
| Clinical risk score | 0.001 | |||||
| Low risk (0-2) | 1 (ref) | - | - | - | - | |
| High risk (3-5) | 1.84 | (1.28-2.65) | - | - | - | |
| Time from Dx-Rf | 1.08 | (1.01-1.17) | 0.03 | - | - | - |
| Time from Dx-Rx | 1.06 | (1.01-1.12) | 0.01 | 1.12 | 1.06-1.18 | <0.0001 |
| 30-d Complication | 1.29 | (0.97-1.70) | 0.08 | 0.81 | 0.54-1.22 | 0.31 |
| Major complication | 1.47 | (1.03-2.10) | 0.04 | 1.15 | 0.75-1.75 | 0.53 |
| Positive resection margin |
1.44 | (0.98-2.10) | 0.06 | 1.07 | 0.69-1.65 | 0.77 |
| Adjuvant chemotherapy |
0.58 | (0.43-0.78) | <0.0001 | - | - | - |
| Recurrence | 2.69 | (2.03-3.57) | <0.0001 | 2.68 | 1.95-3.67 | <0.0001 |
53 (11%) patients were missing data pertaining to adjuvant chemotherapy use and were not included in the final multivariable model. Clinical risk score correlated with tumor size (Spearman r=0.17, p<0.0001), and time from Dx-Rf correlated with time from Dx-Rx (Spearman r=0.6) and were excluded from multivariable model.
HR, hazard ratio; 95% CI, 95% confidence interval; (ref), reference category; BMI, basal metabolic index; EBL, estimated blood loss; Dx-Rf, diagnosis to referral; Dx-Rx, diagnosis to resection.
Time from Diagnosis to Resection: <3 months versus ≥3 months
No definitive cutpoint in time from Dx-Rx was found. Thus, using current literature [10] and the distribution of time from Dx-Rx in the current study (1.92 +/− 2.5 months), a cutpoint of 3 months was chosen for stratification and comparison of survival outcomes; Group 1(Dx-Rx < 3 months, n=394) and Group 2(Dx-Rx ≥ 3 months, n=119). In terms of baseline demographic and clinicopathologic characteristics the groups were comparable (Table 1). The mean time +/− SD from Dx-Rx was 1.0+/− 0.7 months for Group 1 and 5.1+/−3.5 months for Group 2 (p<0.0001), respectively. Time from Dx-Rf for Group 1 was 0.2+/−0.4 months versus 2.1+/−2.3 months for Group 2 (p<0.0001). Similarly, time from Rf-Rx was significantly shorter in Group 1 (0.4+/−0.6 months) compared to Group 2 (1.6+/−1.6 months, p<0.0001). Table 2 outlines operative characteristics and clinical outcomes of the groups. No differences were observed in postoperative complications, margin positivity, or disease recurrence; however, use of adjuvant chemotherapy was more common in Group 1 compared to Group 2 (66% vs. 49%, p=0.004).
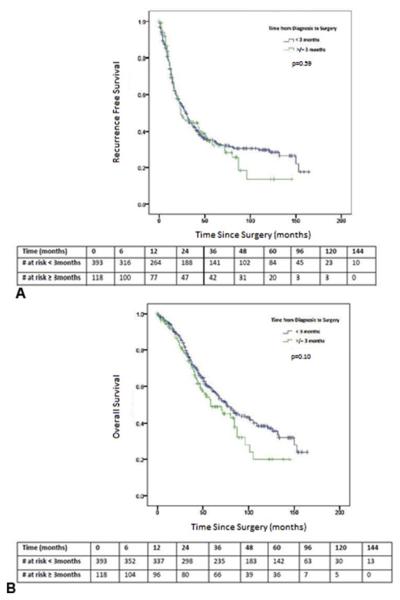
Median follow-up time was significantly different between Groups [40 (0-163) months in Group 1 versus 32 (0-146) months in Group 2, p=0.02]. Median RFS was 29 (24.0-34.0) months in Group 1 and was not different compared to Group 2 [24(10.1-37.9) months, p=0.59,Figure 2A]. Median OS for Group 1 was 76 (62.8-89.2) months versus 58 (34.3-81.7) months for Group 2 (p=0.10). Differences in OS did not achieve statistical significance; however, a clinically relevant tendency towards improved OS in Group 1 compared to Group 2 was observed (Figure 2B).
Figure 2.
Comparison of Kaplan-Meier (A) recurrence-free and (B) overall survival curves based on time from diagnosis to resection < 3 months vs ≥ 3 months. Time zero = Date of surgery. Patients were censored at the time of event occurrence (death/recurrence) or date of last contact at the treating hospital.
Subgroup Analysis
Adjuvant Chemotherapy
Of the 513 study patients, data pertaining to adjuvant chemotherapy administration was available for 457 (89.1%) patients. Of these patient, 318 (69.6%) received adjuvant chemotherapy and 139 (30.4%) did not. Of the treated patients, 224 (70.4%) received systemic chemotherapy alone, 91 (28.6%) received combination hepatic artery infusion (HAI) + systemic chemotherapy and 1(0.02%) received HAI alone. Demographic, operative, and outcome variables were compared between groups (Table 5). No differences between treated and untreated patients were observed in CRS, operative characteristics, or margin positivity rate. Patients receiving adjuvant therapy had shorter time from Dx-Rx (1.58 +/−1.71 months) compared to those who did not (2.18+/−2.68), p=0.005.
Table 5.
Demographic, Clinicopathologic, Operative and Outcomes Characteristics of Patients with Resectable Metachronous Colorectal Liver Metastases Stratified by Use of Adjuvant Chemotherapy
| Variable | No adjuvant chemotherapy |
Adjuvant chemotherapy |
p Value |
|---|---|---|---|
| n=457 (%) | 139(30.4) | 318(69.6) | |
| Sex | |||
| Female | 61(44.0) | 117(37) | 0.15 |
| Male | 78(56.0) | 201(63) | |
| Age, y | 66.8+/−11.6 | 62.3+/−11.3 | <0.0001 |
| BMI, kg/m2 | 28.1+/−6.0 | 28.3+/−5.1 | 0.79 |
| Year of surgery | |||
| 2000-05 | 54(38.8) | 174(54.7) | 0.002 |
| 2006-10 | 85(61.2) | 144(45.3) | |
| Hospital site | <0.0001 | ||
| 1 | 50(36.0) | 212(66.7) | |
| 2 | 34(24.5) | 35(11.0) | |
| 3 | 47(33.8) | 60(18.9) | |
| 4 | 8(5.8) | 11(3.5) | |
| Country | <0.0001 | ||
| USA | 97(69.8) | 272(85.5) | |
| Canada | 42(30.2) | 46(14.5) | |
| Type of initial referral | 0.23 | ||
| Medical | 14(10.1) | 45(14.2) | |
| Surgical | 125(89.9) | 273(85.8) | |
| Type of procedure | 0.64 | ||
| Wedge | 10(7.2) | 15(4.7) | |
| Right hepatectomy | 43(30.9) | 84(26.4) | |
| Left hepatectomy | 13(9.4) | 35(11.0) | |
| Extended right hepatectomy |
8(5.8) | 30(9.4) | |
| Extended left hepatectomy |
4(2.9) | 11(3.5) | |
| Central hepatectomy | 4(2.9) | 6(1.9) | |
| Anatomic Segmentectomy (including left lateral) |
57(41.0) | 137(43.1) | |
| Preoperative PVE | 5(3.6) | 12(3.8) | 0.98 |
| HAI | |||
| No | - | 224(70.4) | - |
| Yes | - | 91(28.6) | |
| 30-d Mortality | 1(0.7) | 0(0) | 0.12 |
| 30-d Complication | 50(36.0) | 83(26.1) | 0.03 |
| Major complication | 21(15.1) | 36(11.3) | 0.26 |
| Lymph node + primary | 72(51.8) | 140(44.0) | 0.003 |
| Preoperative CEA, ng/mL | 0.44 | ||
| ≤200 | 112(80.6) | 275(86.5) | |
| >200 | 2(1.4) | 9(2.8) | |
| Diameter of largest metastases, cm |
0.93 | ||
| ≤5 | 102(73.4) | 234(73.6) | |
| > 5 | 35(25.2) | 82(25.8) | |
| No. of tumors | 0.15 | ||
| 1 | 79(56.8) | 202(63.5) | |
| >1 | 60(43.2) | 114(35.8) | |
| Disease-free interval, mo | 0.99 | ||
| <12 | 23(16.5) | 53(16.7) | |
| ≥12 | 114(82.0) | 262(82.4) | |
| Clinical risk score | 0.07 | ||
| 0 | 23(16.5) | 52(16.4) | |
| 1 | 53(38.1) | 157(49.4) | |
| 2 | 48(34.5) | 73(23.0) | |
| 3 | 11(7.9) | 32(10.1) | |
| 4 | 2(1.4) | 2(0.6) | |
| 5 | - | - | |
| Clinical risk score dichotomized |
0.68 | ||
| Low risk (CRS 0-2) | 124(89.2) | 284(88.7) | |
| High risk (CRS 3-5) | 13(9.4) | 34(10.7) | |
| Margin status | 0.06 | ||
| Positive | 9(6.5) | 39(12.3) | |
| Negative | 130(93.5) | 278(87.4) | |
| Recurrence | 0.06 | ||
| Yes | 59(42.4) | 166(52.2) | |
| No | 80(57.6) | 152(47.8) | |
| Site of first recurrence | 0.73 | ||
| Lung | 27(19.4) | 71(22.3) | |
| Nodal | 3(2.2) | 13(4.1) | |
| Liver | 18(12.9) | 52(16.4) | |
| Local/Pelvic | 2(1.4) | 10(3.1) | |
| Peritoneal | 5(3.6) | 10(3.1) | |
| Anastomotic | 2(1.4) | 1(0.3) | |
| Adrenal | 0(0) | 1(0.3) | |
| Other | 3(2.2) | 7(2.2) | |
| Status at last follow-up | 0.59 | ||
| NED | 82(59.0) | 172(54.1) | |
| AWD | 50(36.0) | 129(40.6) | |
| DOD | 6(4.3) | 16(5.0) | |
| Survival status | 0.60 | ||
| Dead | 64(46.0) | 180(56.6) | |
| Alive | 75(54.0) | 138(43.4) | |
| LOS, d | 8.3+/−6.7 | 8.0+/−6.4 | 0.72 |
| Time from Dx-Rf, mo | 0.72+/−1.4 | 0.55+/−1.30 | 0.23 |
| Time from Rf-Rx, mo | 0.78+/−1.10 | 0.63+/−0.96 | 0.14 |
| Time from Dx-Rx, mo | 2.18+/−2.68 | 1.58+/−1.71 | 0.005 |
| Procedure time, per 30 min | 6.9+/−2.3 | 7.6+/−2.7 | 0.007 |
| Pringle time, min | 33.1+/−13.9 | 34.9+/−14.0 | 0.44 |
| EBL, per 100mL | 6.2+/−6.7 | 5.2+/−4.8 | 0.08 |
| Follow-up time from surgery, mo |
24(0-150) | 49(0-163) | <0.0001 |
Percentages for individual variables are tabulated based on total number of patients in each group (n=139, no adjuvant chemotherapy group and n=318, adjuvant chemotherapy). Categorical data are presented as frequency (%), continuous data as mean +/− standard deviation and follow-up time median (min-max). P<0.05 was considered significant.
BMI, basal metabolic index; PVE, portal vein embolization; EBL, estimated blood loss; CEA, carcinoembryonic antigen; HAI, hepatic artery infusion; NED, no evidence of disease; AWD, alive with disease; DOD, dead of disease; LOS, length of stay; Dx-Rf, diagnosis to referral; Rf-Rx, referral to resection; Dx-Rx, diagnosis to resection.
Disease recurrence rate was not different between treated (166/318, 52.2%) and untreated (59/139, 42.4%) patients, p=0.06. However, median RFS was significantly longer in the adjuvant chemotherapy group [33 (25.3-40.7) months] compared to the no adjuvant chemotherapy group [22(14.5-29.5) months, p=0.05]. Similarly, median OS was significantly longer in treated patients [87(71.2-102.8) months vs. 48 (25.3-70.7) months, p<0.0001 (Figure 3). When patients receiving adjuvant HAI chemotherapy were excluded (n=92), OS remained significantly greater among treated [84(67-100) months] versus untreated [48(25-70) months, p=0.005] patients, while RFS did not (p=0.15). As a consequence of missing data (11%), adjuvant chemotherapy was not included in primary multivariable analysis. However, we did perform a sensitivity analysis in which adjuvant chemotherapy was included. In this analysis adjuvant chemotherapy was independently associated with OS but not with RFS. Importantly, even with the addition of adjuvant chemotherapy to the multivariable OS model, the association between times from Dx-Rx remained significant.
Figure 3.
Comparison of Kaplan-Meier (A) recurrence-free and (B) overall survival curves based on the use of adjuvant chemotherapy. Time zero = Date of surgery. Patients were censored at the time of event occurrence (death/recurrence) or date of last contact at the treating hospital.
Treating Hospital
Demographic, operative, and outcome variables were compared between study centers. Time from Dx-Rf, Rf-Rx and Dx-Rx were significantly different across treating hospitals (p<0.0001). The proportion of initial referrals made to a surgeon varied from 73.9% to 99.3% (p<0.0001), use of neoadjuvant chemotherapy from 7.4-28%, (p=0.03) and adjuvant chemotherapy use from 44%-76%, (p<0.0001) across treating hospitals.
Disease recurrence rates were not different between centers (p=0.75); however, significant differences were observed in median RFS [32 (24.3-39.7) months, 18(7.5-28.5) months, 22(16.0-28.0) months and 47(9.2-84.8) months, at Hospitals 1-4, respectively, p=0.02]. Likewise, median OS was significantly different amongst study centers [86 (66.6-105.4) months, 80 (72.5-87.5) months, 45 (40.4-49.6) months and median OS not reached, at Hospitals 1-4, respectively, p<0.0001].
Discussion
The survival advantage for patients with CRLM amenable to resection is well established [1, 25-28]; however, the potential relationship between time to surgical resection and survival outcomes is unknown. In this study, a uniform group of patients with minimal (≤ 4 metastases), metachronous CRLM treated with surgery first were reviewed, practice patterns characterized, and the association of time from Dx-Rx on disease recurrence and survival was evaluated.
Based on clinical criteria, the current study reflects outcomes in patients with the most favorable risk (88% CRS 0-2). Observed OS (74 months) and RFS (29 months) are amongst the highest reported in modern series [29] and represent a benchmark for outcomes in this select population [30]. In conjunction with commonly cited clinicopathologic predictors of RFS and OS, the current study suggests that time from Dx-Rx is independently associated with OS (HR 1.13 95% CI 1.07-1.20, p<0.0001); however, it was unrelated to RFS. To date, time to definitive intervention has been shown to impact OS in a variety of other malignancies [10-13, 17, 19], however, this has not previously been reported in the setting of resectable CRLM. In 2010, Croome et al. [24] evaluated the impact of time from clinical presentation to surgical referral on resectability rates amongst 350 patients with a variety of hepatobiliary malignancies (76, 22% CRLM). Impact on OS was assessed as a secondary endpoint. It was found that time delay from Dx-Rf of >30 days was a significant independent predictor of worse OS (p=0.04). Unfortunately, the heterogeneity of cancer diagnoses included in the analysis makes interpretation difficult and application to resectable CRLM specifically is likely not justified.
The relationship between time to surgery and survival in the current study appears to be continuous in nature, with a clinically relevant tendency towards improved OS observed when time from Dx-Rx was < 3 months. This finding is similar to that observed in patients with primary hepatocellular cancer where delay to surgery of > 3 months independently predicted OS (Relative risk; RR 3.67, p= 0.002)[10]. In our study cohort, patients waiting ≥ 3 months vs. < 3 months were not different in terms of age, sex, BMI, or CRS suggesting that longer time to surgery was unlikely related to differences in time required for surgical optimization and/or burden of disease; however, details regarding specific comorbid conditions were not available for analysis. Furthermore, longer time from Dx-Rx was composed of both longer time from Dx- Rf and from Rf-Rx. Prolongation of both intervals suggests that delays are not a consequence of a single variable but are multifactorial and related to the combination of patient factors, physician biases and resource availability.
To date, randomized trials have failed to show an OS benefit for adjuvant chemotherapy amongst patients with resectable CRLM [5, 31]. Despite this, 69.6% of 457 patients with available data received adjuvant chemotherapy, this rate varied significantly (44.3% to 80.9%) across study centers (p<0.0001). Patients receiving adjuvant chemotherapy were not different in terms disease burden, operative characteristics or margin positivity, suggesting variability in chemotherapy use may be related to institutional bias as opposed to patient or tumor characteristics. Adjuvant chemotherapy was more common amongst patients with shorter time from Dx-Rx and was strongly associated with OS and RFS on univariate analysis but due to missing data was not included in formal multivariable analysis. Notably, inclusion of adjuvant chemotherapy in a multivariable sensitivity analysis did not negate the independent association between time from Dx-Rx and OS, indicating that the improved OS observed with shorter time to surgery was not merely a surrogate for adjuvant chemotherapy.
In conjunction with adjuvant chemotherapy use, initial referral type and neoadjuvant chemotherapy use varied significantly across individual hospitals and likely contributed to differences in time delay to surgery. Importantly, these variations in practice patterns were observed despite uniform disease characteristics within the study cohort, and may contribute to differences in RFS and OS across study centers. At present, it is unlikely that any one treatment center’s approach to the management of this select group of patients is superior. However, the current study does highlight the fact that development and implementation of more uniform treatment strategies are requisite to reduce time delay to surgery and potentially improve survival outcomes.
This study is subject to the typical limitations associated with its retrospective design. Surveillance of patients following resection, although similar, was not standardized and may have impacted timing of documented recurrence events. Likewise, post-operative treatment and management of disease recurrence, was not consistent across study centers and limits direct comparisons. To date, studies of CRLM have evaluated time from presentation to definitive diagnosis [32], clinical characteristics and predictors of surgical referral [24, 33, 34] and time related to therapeutic decision making [6, 9, 16]. To our knowledge, ours is the first study to suggest that time from Dx-Rx is significantly associated with OS and propose that longer time intervals from Dx-Rx portend worse OS in patients with best risk resectable CRLM. These findings are critically important, in that, time from Dx-Rx is a potentially modifiable risk factor and they suggest that increased uniformity and efficiency in delivery of care may impact oncologic outcomes.
Conclusion
Time from Dx-Rx is independently associated with OS in patients with the best risk CRLM. Its impact appears to vary along a continuum with a clinically meaningful tendency towards worse OS when delay is ≥ 3 months. Furthermore, even amongst high volume academic centers, practice patterns surrounding clearly resectable CRLM varied significantly, raising considerable clinical concern. These findings are novel and suggest that vigilant attention to timely referral, assessment, therapeutic decision making and surgical intervention in patients with low volume (≤4) metachronous CRLM is essential to improving patient outcomes. Further evaluation of this complex relationship is needed.
Acknowledgments
Support: This study was supported in part by NIH/NCI P30 CA008748 (Cancer Center Support Grant).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
References
- 1.Tomlinson J, Jarnagin W, DeMatteo R, et al. Actual 10-year survival after resection of colorectal liver metastases. J Clin Oncol. 2007;25:4575–4582. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 2.Frankel TL, D'Angelica MI. Hepatic resection for colorectal metastases. J Surg Oncol. 2014;109:2–7. doi: 10.1002/jso.23371. [DOI] [PubMed] [Google Scholar]
- 3.Power DG, Kemeny NE. Role of adjuvant therapy after resection of colorectal cancer liver metastases. J Clin Oncol. 2010;28:2300–2309. doi: 10.1200/JCO.2009.26.9340. [DOI] [PubMed] [Google Scholar]
- 4.Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1208–1215. doi: 10.1016/S1470-2045(13)70447-9. [DOI] [PubMed] [Google Scholar]
- 6.Scartozzi M, Siquini W, Galizia E, et al. The timing of surgery for resectable metachronous liver metastases from colorectal cancer: Better sooner than later? A retrospective analysis. Dig Liver Dis. 2011;43:194–198. doi: 10.1016/j.dld.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Adam R, Bhangui P, Poston G, et al. Is perioperative chemotherapy useful for solitary, metachronous, colorectal liver metastases? Ann Surg. 2010;252:774–787. doi: 10.1097/SLA.0b013e3181fcf3e3. [DOI] [PubMed] [Google Scholar]
- 8.Sandhu L, Fox A, Nhan C, et al. Assessing the management of hepatic colorectal cancer metastases: is treatment consistent in Ontario? HPB (Oxford) 2012;14:409–413. doi: 10.1111/j.1477-2574.2012.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nathan H, Bridges J, Cosgrove D, et al. Treating patients with colon cancer liver metastasis: A nation wide analysis of therapeutic dicision making. Ann Surg Oncol. 2012;19:3668–3676. doi: 10.1245/s10434-012-2564-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huo TI, Huang YH, Chiang JH, et al. Survival impact of delayed treatment in patients with hepatocellular carcinoma undergoing locoregional therapy: is there a lead-time bias? Scand J Gastroenterol. 2007;42:485–492. doi: 10.1080/00365520600931402. [DOI] [PubMed] [Google Scholar]
- 11.Sloane D, Chen H, Howell C. Racial disparity in primary hepatocellular carcinoma: tumor stage at presentation, surgical treatment and survival. J Natl Med Assoc. 2006;98:1934–1939. [PMC free article] [PubMed] [Google Scholar]
- 12.Christensen ED, Harvald T, Jendresen M, et al. The impact of delayed diagnosis of lung cancer on the stage at the time of operation. Eur J Cardiothorac Surg. 1997;12:880–884. doi: 10.1016/s1010-7940(97)00275-3. [DOI] [PubMed] [Google Scholar]
- 13.Sainsbury R, Johnston C, Haward B. Effect on survival of delays in referral of patients with breast-cancer symptoms: a retrospective analysis. Lancet. 1999;353:1132–1135. doi: 10.1016/s0140-6736(99)02374-0. [DOI] [PubMed] [Google Scholar]
- 14.Coates AS. Breast cancer: delays, dilemmas, and delusions. Lancet. 1999;353:1112–1113. doi: 10.1016/S0140-6736(99)00082-3. [DOI] [PubMed] [Google Scholar]
- 15.Caplan LS, Helzlsouer KJ. Delay in breast cancer: a review of the literature. Public Health Rev. 1992;20:187–214. [PubMed] [Google Scholar]
- 16.Robertson R, Campbell NC, Smith S, et al. Factors influencing time from presentation to treatment of colorectal and breast cancer in urban and rural areas. Br J Cancer. 2004;90:1479–1485. doi: 10.1038/sj.bjc.6601753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roncoroni L, Pietra N, Violi V, et al. Delay in the diagnosis and outcome of colorectal cancer: a prospective study. Eur J Surg Oncol. 1999;25:173–178. doi: 10.1053/ejso.1998.0622. [DOI] [PubMed] [Google Scholar]
- 18.Holliday HW, Hardcastle JD. Delay in diagnosis and treatment of symptomatic colorectal cancer. Lancet. 1979;1:309–311. doi: 10.1016/s0140-6736(79)90718-9. [DOI] [PubMed] [Google Scholar]
- 19.Young CJ, Sweeney JL, Hunter A. Implications of delayed diagnosis in colorectal cancer. Aust N Z J Surg. 2000;70:635–638. doi: 10.1046/j.1440-1622.2000.01916.x. [DOI] [PubMed] [Google Scholar]
- 20.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309, 318. doi: 10.1097/00000658-199909000-00004. discussion 318-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Comprehensive Cancer Network NCCN clinical practice guidelines in oncology; version 3.2014. Available at http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed October 30, 2014.
- 23.Miller RS. D. Maximally selected chi square statistics. Biometrics. 1982;38:1011–1016. [Google Scholar]
- 24.Croome KP, Chudzinski R, Hanto DW. Increasing time delay from presentation until surgical referral for hepatobiliary malignancies. HPB (Oxford) 2010;12:644–648. doi: 10.1111/j.1477-2574.2010.00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biasco G, Derenzini E, Grazi G, et al. Treatment of hepatic metastases from colorectal cancer: many doubts, some certainties. Cancer Treat Rev. 2006;32:214–228. doi: 10.1016/j.ctrv.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 26.Cummings LC, Payes JD, Cooper GS. Survival after hepatic resection in metastatic colorectal cancer: a population-based study. Cancer. 2007;109:718–726. doi: 10.1002/cncr.22448. [DOI] [PubMed] [Google Scholar]
- 27.Stangl R, Altendorf-Hofmann A, Charnley RM, Scheele J. Factors influencing the natural history of colorectal liver metastases. Lancet. 1994;343:1405–1410. doi: 10.1016/s0140-6736(94)92529-1. [DOI] [PubMed] [Google Scholar]
- 28.Robertson DJ, Stukel TA, Gottlieb DJ, et al. Survival after hepatic resection of colorectal cancer metastases: a national experience. Cancer. 2009;115:752–759. doi: 10.1002/cncr.24081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinto Marques H, Barroso E, de Jong MC, et al. Peri-operative chemotherapy for resectable colorectal liver metastasis: does timing of systemic therapy matter? J Surg Oncol. 2012;105:511–519. doi: 10.1002/jso.22133. [DOI] [PubMed] [Google Scholar]
- 30.Kanas GP, Taylor A, Primrose JN, et al. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol. 2012;4:283–301. doi: 10.2147/CLEP.S34285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Primrose JN. Surgery for colorectal liver metastases. Br J Cancer. 2010;102:1313–1318. doi: 10.1038/sj.bjc.6605659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyratzopoulos G, Wardle J, Rubin G. Rethinking diagnostic delay in cancer: how difficult is the diagnosis? BMJ. 2014;349:g7400. doi: 10.1136/bmj.g7400. [DOI] [PubMed] [Google Scholar]
- 33.Al-Sahaf O, Al-Azawi D, Al-Khudairy A, et al. Referral patterns of patients with liver metastases due to colorectal cancer for resection. Int J Colorectal Dis. 2009;24:79–82. doi: 10.1007/s00384-008-0561-6. [DOI] [PubMed] [Google Scholar]
- 34.Ksienski D, Woods R, Speers C, Kennecke H. Patterns of referral and resection among patients with liver-only metastatic colorectal cancer (MCRC) Ann Surg Oncol. 2010;17:3085–3093. doi: 10.1245/s10434-010-1304-9. [DOI] [PubMed] [Google Scholar]