Abstract
INTRODUCTION
Treatment of recurrent platinum-resistant ovarian and peritoneal cancers represents a therapeutic challenge. The aim of this Phase III prospective study was to compare the survival benefits, objective response rate, and toxicities among patients treated by weekly paclitaxel with those who underwent three-weekly paclitaxel in recurrent platinum-resistant ovarian and peritoneal cancers.
METHOD
Patients with recurrent platinum-resistant ovarian and peritoneal cancer were allocated to receive either weekly paclitaxel (arm 1) at 80 m/m2 or three-weekly paclitaxel (arm 2) at 175 mg/m2.
RESULTS
Fifty-five patients were enrolled (30 arm 1, 25 arm 2). The mean age was 56.7 years, and the median performance status was 0 (Eastern Cooperative Oncology Group [ECOG]). For arms 1 and 2, the objective response rates were 27% and 16%, the median progression-free survival were 7 and 4.5 months, and the median overall survival were 15.5 and 12.5 months, respectively. Treatments also significantly improved the quality of life. Treatment was associated with mild toxicities, and while neuropathy was slightly higher for weekly paclitaxel over three-weekly paclitaxel, hematological toxicities were significantly lower for the former than the latter.
CONCLUSION
Paclitaxel rechallenge showed antitumor activity in recurrent platinum-resistant ovarian and peritoneal cancers. Weekly paclitaxel achieved better results than three-weekly paclitaxel in terms of survival benefits, quality of life, and toxicities.
Keywords: weekly, paclitaxel, platinum-resistant, ovary, ORR, PFS, QOL
Introduction
Ovarian cancer is the most common cause of death among women with gynecological malignancies. Nearly 70% of ovarian cancers recur by time, and approximately 13,850 women in the USA die each year from ovarian cancer.1
Patients with recurrent disease are categorized as platinum-sensitive, partly sensitive, or platinum-resistant. Platinum-resistant disease defines patients who have progressed within six months of completing the initial platinum-based chemotherapy.2
Treatment of recurrent platinum-resistant ovarian and peritoneal cancers represents a therapeutic challenge. Patients with resistant disease have a worse prognosis when compared with those who have a treatment-free interval of more than six months.3
Commonly used chemotherapy agents in the treatment of recurrent platinum-resistant ovarian and peritoneal cancers are listed in Table 1.
Table 1.
Chemotherapy regimens in recurrent platinum-resistant ovarian and peritoneal cancers.
| STUDY | CHEMOTHERAPY | RESPONSE RATE | PROGRESSION FREE SURVIVAL (PFS) | TOXICITIES GRADE 3, 4 |
|---|---|---|---|---|
| Abushahin et al., 2008 (19) | Topotecan weekly, every 4 weeks | 15–20% | 5.7 months | 7% hematological |
| Gordon et al., 2000 (20) | Pegylated liposomal doxorubicin (PLD) every 4 wk | 20–26% | 7 months | Hand-foot syndrome 10% |
| Ferrandina et al., 2008 (21) | Gemcitabine on d 1, 8 every 3 weeks OR d 1, 8, 15 every 4 weeks | 25% | 7.2 months | 5% haematological |
| GOG et al., 2006 (15) | Paclitaxel weekly on d 1, 8, 15 every 4 weeks | 20.9% | 7 months | 8% fatigue, 6% hematological 4% neuropathy |
| Rose et al., 2003 (22) | Docetaxel every 3 weeks | 22.4% | 4 months | 25% hematological |
| Piccart et al., 2000 (16) | 3 Weekly paclitaxel | 17% | 4 months | 22% hematological |
Recently, the AURELIA trial by Pujade-Lauraine et al. showed that addition of bevacizumab to chemotherapy (paclitaxel, Pegylated liposomal doxorubicin (PLD), or topotecan) in recurrent platinum-resistant ovarian and peritoneal cancers resulted in an improvement in progression-free survival (PFS) by 3.3 months and in overall survival (OS) by 3.3 months. In addition, the objective response rate (ORR) was improved from 11.8% to 27.3% with the addition of bevacizumab.4
Study Objectives
The objective of this study was to compare the survival benefits, ORR, and toxicities among patients treated with weekly paclitaxel with those who underwent three-weekly paclitaxel in patients with recurrent platinum-resistant ovarian and peritoneal cancers. The primary end points were ORR and PFS. The secondary end points were OS, quality of life (QOL), and toxicities.
Patients and Methods
Inclusion criteria
Patients were eligible for inclusion in the current Phase III prospective study if they met the following criteria:
Histologically proven high-grade epithelial ovarian cancer or primary peritoneal cancer, which progressed or relapsed after first-line chemotherapy. Most recent carboplatin-based regimen should be less than six months earlier. Additionally, patients should have received only one line of chemotherapy, which should include paclitaxel as part of their first-line treatment. Furthermore, the progression or relapse must have been radiologically documented. Finally, patients should be between the ages of 18 and 70 years and had a performance status of ≤2 (ECOG). Patients should have adequate hematological, liver, and renal functions with baseline laboratory criteria that included neutrophils ≥1.5 × 103/mL; platelet count ≥15 × 103/mL; creatinine ≤ 1.6 mg/dL; total bilirubin level ≤ 1.25, the upper limit of normal; and ALT, AST ≤ 3, the upper limit of normal (<5 in the case of liver metastasis). Histologies included different subtypes of epithelial ovarian and peritoneal cancers, including serous, endometrioid, clear cell, mucinous, Brenner, undifferentiated, and others.
Patients were excluded from the trial if they did not meet the above criteria. Additionally, patients who received more than one line of chemotherapy and patients with significant comorbidities, including peripheral neuropathy Grade 4, were further excluded. The current research complied with the principles of the Declaration of Helsinki. The research was approved by the Ethical Committee of Ain Shams University, and patients gave their written, informed consent to participate.
Settings
Ain Shams University Hospital, Ain Shams University Specialized Hospital, and Ismailia Oncology Teaching Hospital, Egypt.
Study design
Arm 1 patients were enrolled to receive weekly paclitaxel at 80 mg/m2 on days 1, 8, and 15, every 28 days for a total of 6 cycles. Arm 2 patients were treated with paclitaxel at 175 mg/m2 on day 1, every 21 days for a total of 6 cycles. Prior to chemotherapy administration, CBC, renal function, liver function tests, and bilirubin were checked at days 1, 8, and 15 of each cycle. Dose modification was based on Table 2.5,6
Table 2.
| NEUTROPHILS (103/ml) | PLATELETS (103/ml) | DOSE | |
|---|---|---|---|
| 1-Hematology | |||
| For day 1 of each cycle: (for both weekly, 3 weekly paclitaxel) | |||
| ≥1 | and | >100 | 100% |
| <1 | or | <100 | For 3 weekly, Delay until recovery, and resume in the same dose level. For weekly, if the first occurrence: As 3 weeky. If second occurrence: Delay, reduce to 60 mg/m2 |
| Febrile neutropenia | For 3 weekly, Reduce dose to 155 mg/m2 after first occurrence. In case of second occurrence, use G-CSF together with the same dose of paclitaxel. For weekly: Reduce dose by 1 dose level* after first occurrence. If second occurrence, as for 3 weekly. |
||
| For day 8, 15 weekly paclitaxel | |||
| ≥0.5 | and | ≥50 | 100% |
| <0.5 | or | <50 | Omit & reduce subsequent treatments by 1 dose level* |
| ALT | TOTAL BILIRUBIN (mg/dl) | PACLITAXEL DOSE (mg/m2) WEEKLY | |
| 2-Hepatic function tests: weekly paclitaxel | |||
| <2 × ULN | ≤1.4 | 80 | |
| >2 × ULN Or>5 × ULN If liver metastases | ≤1.4 | 65 | |
| <10 × ULN | >1.4–2.9 | 40 | |
| ≥10 × ULN | >2.9 | 25 | |
| ALT | TOTAL BILIRUBIN | PACLITAXEL DOSE (mg/m2) 3 WEEKLY | |
| 3-Hepatic function tests: 3 weekly paclitaxel | |||
| <10 × ULN | ≤1.25 × ULN | 175 | |
| <10 × ULN | 1.26–2 × ULN | 135 | |
| <10 × ULN | 2.01–5 × ULN | 90 | |
| ≥10 × ULN | >5 × ULN | Not recommended | |
| Dose modification for paclitaxel by other toxicites | |||
| Grade | Paclitaxel dose | ||
| Grade 2 motor or sensory neuropathy | Decrease paclitaxel dose by 1 dose level*,** | ||
| All other Grade 2 non-hematologic toxicities | Hold treatment until toxicity resolved to less than or equal to Grade 1 and Decrease subsequent paclitaxel doses by 1 dose level*,** | ||
| Greater than or equal to Grade 3 non-hematologic toxicities | Hold treatment. Re-evaluate treatment plan. Consider discontinuing treatment with this protocol | ||
Notes:
Dose levels for weekly paclitaxel: 70 then 60 then 50 mg/m2.
Dose levels for 3 weekly paclitaxel: 175 then 135 then 90 mg/m2.
Evaluation
Baseline CT thoracic–abdominal–pelvic (TAP) was performed before treatment protocol and serum level of CA 125 was checked at baseline. CA 125 was repeated every other cycle from Cycle 3. After completion of chemotherapy, patients underwent CT TAP and CA 125 to evaluate chemotherapy response. CT TAP was requested at any point where disease progression (DP) was suspected.
Response definitions were based on “racist 1.0,”, where complete response (CR) was defined as complete disappearance of the tumors, partial response (PR) included at least a 30% decrease in the sum of the longest diameters, disease progression (DP) meant at least a 20% increase in the sum of the longest diameters, and stable disease (SD) was defined as all other situations.7
Follow-up
After the treatment protocol, patients were followed up according to the NCCN guideline – every three months in the first two years, every six months for the following three years, and then annually thereafter.
At each clinic interview, patients underwent history, physical examination, and laboratory investigations in the form of CA 125. CT TAP was undertaken as clinically indicated.8
QOL evaluation
We applied The Functional Assessment of Cancer Therapy-General (FACT-G) questionnaires (Arabic translation version) to assess the “quality of life.” The questionnaires were put to our patients on the following four occasions: at the start of treatment (baseline), after the end of the treatment protocol, eight weeks after treatment, and eight weeks thereafter.9
Toxicity
Toxic effects were graded according to the National Cancer Institute Common Toxicity Criteria – version 2.0.10
Early toxicity was defined as that occurring during chemotherapy up to eight weeks “post chemotherapy.” Late toxicity referred to that occurring more than eight weeks after treatment.
Statistical analysis
All calculations were carried out using Prism 6 software. Analyses were carried with the intention to treat. The mean and median were used for the description of data. t-test and P value were used to compare between the two group characters. OS and PFS for each arm were analyzed using Kaplan–Meier method. PFS were checked from the time of randomization until relapse or last follow-up visit, while the OS was defined from the time of randomization until death or the last follow-up visit. P value was statistically significant at ≤0.05.
Results
Between September 2010 and September 2014, 55 patients were enrolled: 30 patients were assigned to treatment arm 1, and 25 patients were assigned to arm 2. All our patients fulfilled the eligibility criteria of the current study. The mean age was 56.7 years (Table 3).
Table 3.
Patient characteristics of each treatment arm.
| CHARACTERISTICS | ARM 1 | ARM 2 | P VALUE | ||
|---|---|---|---|---|---|
| NUMBER | % | NUMBER | % | ||
| Age | |||||
| Mean | 56.5 | – | 57 | – | 0.4 |
| Median | 56 | – | 57 | – | |
| Range | 17–70 | – | 17–70 | – | |
| Performance status: (ECOG) | |||||
| 0 | 20 | 67% | 18 | 72% | – |
| 1 | 6 | 20% | 4 | 16% | – |
| 2 | 4 | 13% | 3 | 12% | – |
| Performance status (median) | 0 | – | 0 | – | 0.5 |
| Pathological classification | |||||
| Serous endometrioid clear cell | 25 | 83% | 22 | 88% | 0.3 |
| 2 | 7% | 1 | 4% | 0.4 | |
| 3 | 10% | 2 | 8% | 0.4 | |
| Origin | |||||
| Ovarian | 28 | 93% | 23 | 92% | 0.1 |
| peritoneal | 2 | 7% | 2 | 8% | 0.3 |
| Time interval from last chemotherapy | |||||
| 0–2 months | 4 | 13% | 3 | 12% | 0.3 |
| >2–4 months | 16 | 54% | 12 | 48% | 0.2 |
| >4–6 months | 10 | 33% | 10 | 40% | 0.5 |
| Recurrance | |||||
| Locoregional | 14 | 46% | 10 | 40% | 0.4 |
| Distant | 8 | 27% | 7 | 28% | 0.2 |
| Both | 8 | 27% | 8 | 32% | 0.7 |
Treatment protocol
All the 55 included patients had been treated according to our protocol. The median number of therapy was 6 cycles in both arms (range 4–8). Six patients from arm 1, and four patients from arm 2 received beyond six cycles. A total of 327 chemotherapy cycles were given to both arms (182 arm 1, 145 arm 2).
Response data
All patients underwent response assessment: 2 patients achieved CR (arm 1), 10 patients achieved PR (6 arm 1, 4 arm 2), and 17 patients had SD (10 arm 1, 7 arm 2). The remaining 26 patients had DP (12 arm1, 14 arm 2). The ORRs in the current study were 27% and 16%, for arms 1 and 2, respectively (Tables 4 and 5).
Table 4.
Response evaluation for the treatment arms.
| ARM 1 (WEEKLY) | ARM 2 (THRICE WEEKLY) | |||
|---|---|---|---|---|
| NUMBER | % | NUMBER | % | |
| CR | 2 | 7% | 0 | 0% |
| PR | 6 | 20% | 4 | 16% |
| SD | 10 | 33% | 7 | 28% |
| DP | 12 | 40% | 14 | 56% |
Table 5.
Characteristics of the 12 patients who achieved objective responses.
| PATIENT NUMBER | RESPONSE | AGE | PS | PATHOLOGY | ORIGIN | TIME INTERVAL* MONTHS | RECURRENCE |
|---|---|---|---|---|---|---|---|
| 1 arm 1 (Weekly) | CR | 48 | 0 | Serous | Ovarian | 5 | Both |
| 2 arm 1 (Weekly) | CR | 36 | 1 | Endometrioid | Ovarian | 5.5 | Locoregional |
| 3 arm 1 (Weekly) | PR | 50 | 0 | Serous | Peritoneal | 5 | Both |
| 4 arm 1 (Weekly) | PR | 19 | 0 | Serous | Ovarian | 3 | Locoregional |
| 5 arm 1 (Weekly) | PR | 51 | 0 | Endometrioid | Ovarian | 4.5 | Distant |
| 6 arm 1 (Weekly) | PR | 55 | 0 | Serous | Ovarian | 4 | Distant |
| 7 arm 1 (Weekly) | PR | 57 | 1 | Serous | Ovarian | 5 | Locoregional |
| 8 arm 1 (Weekly) | PR | 58 | 1 | Serous | Peritoneal | 5.5 | Distant |
| 9 arm 2 (Thrice weekly) | PR | 59 | 0 | Serous | Ovarian | 5.5 | Distant |
| 10 arm 2 (Thrice weekly) | PR | 53 | 0 | Endometrioid | Ovarian | 5 | Locoregional |
| 11 arm 2 (Thrice weekly) | PR | 56 | 0 | Serous | Ovarian | 5 | Locoregional |
| 12 arm 2 (Thrice weekly) | PR | 54 | 1 | Serous | Ovarian | 4.5 | Distant |
Note:
Time interval: The time from adjuvant chemotherapy completion to the first relapse.
Patients who had DP were reassessed for their fitness to further treatment. Of those, 8 were lost follow-up, 5 received further treatment (third line; 3 from arm 1 and 2 from arm 2), and the remaining 13 did not receive additional therapy.
Survival data
After a follow-up period of 24 months, the median PFS for arms 1 and 2 were 7 and 4.5 months, respectively, and the mean PFS for arms 1 and 2 were 6.9 and 5 months, respectively. The median OS for arms 1 and 2 were 15.5 and 12.5 months, respectively, and the mean OS for arms 1 and 2 were 16 and 11.9 months, respectively. The six-month PFS were 60% and 40%, while the six-month OS were 82% and 65%, respectively (Figs. 1 and 2).
Figure 1.
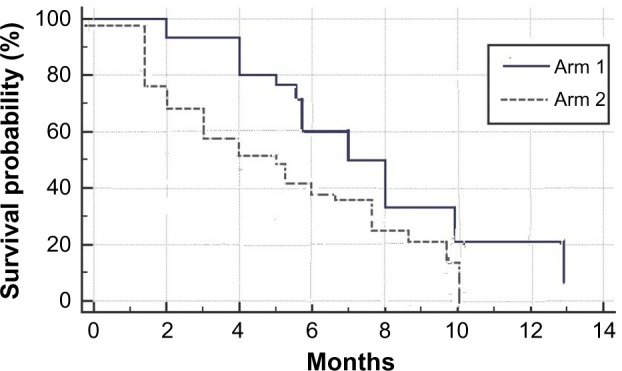
PFS for the study groups – P value: 0.02.
Figure 2.
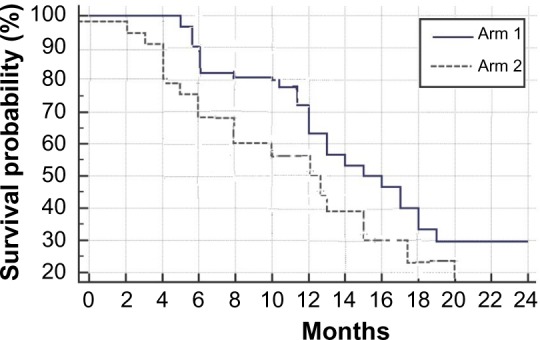
OS for the study groups – P value: 0.03.
QOL assessment
At baseline, 44 patients (80% of total) underwent QOL assessment (24 patients from arm 1 and 20 patients from arm 2). At baseline, the mean score was 40/108 (range 34/108 to 48/108). Just after treatment, 37 patients underwent assessment (those who achieved ORR, SD, and a further 8 patients who had DP), the mean score was 42/108 (range 30/108 to 50/108). At 8 weeks after treatment, 35 patients underwent assessment. These were the same patients who underwent previous assessment with the exception of two DP patients who refused participation. The mean score was 48/108 (range 36/108 to 54/108).
At eight weeks thereafter, 30 patients underwent assessment. These were the same patients who underwent assessment at 4 weeks posttreatment, with the exception of 2 DP and 3 SD patients who refused participation. The mean score was 50/108 (range 42/108 to 62/108; Fig. 3).
Figure 3.
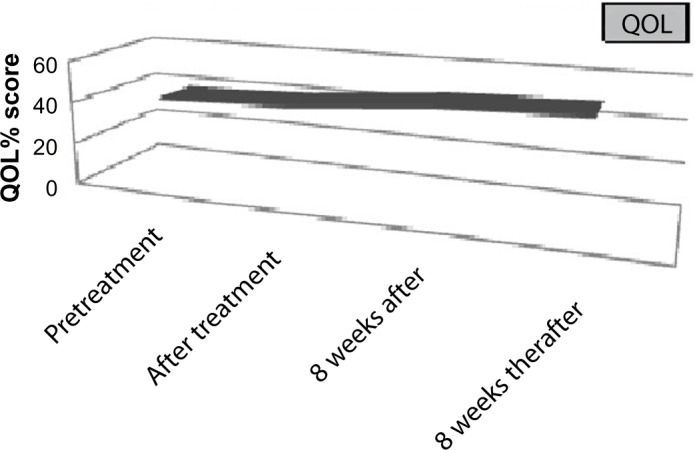
QOl assessment for the study group.
Toxicities
Early toxicities
A total of 327 chemotherapy cycles were given to both arms (182 to arm 1 and 145 to arm 2). Generally, for both arms, treatment protocols were tolerated well. No deaths have been reported in relation to treatment protocols. Dose reductions happened in 25% and 34% of cycles in arms 1 and 2, respectively. Treatment was discontinued in 4 patients from arm 1 (at cycle 6, 2 patients for grade 3 and 4 neuropathy and 2 for grade 4 neutropenia) and in 5 patients from arm 2 (4 at cycle 6, 1 at cycle 4). Of those, 4 patients for grade 3 and 4 neutropenia, and 1 for grade 4 neuropathy. Grades >2 toxicities for both arms are summarized in Table 6.
Table 6.
Grade >2 early toxicities for arms 1 and 2.
| TOXICITY | ARM 1 (WEEKLY) 172 CYCLES |
ARM 2 (THRICE WEEKLY) 138 CYCLES |
||
|---|---|---|---|---|
| GRADE 3 (%) | GRADE 4 (%) | GRADE 3 (%) | GRADE 4 (%) | |
| Leuconeutropenia | 18 | 1 | 20 | 2 |
| Anemia | 0 | 0 | 1 | 1 |
| Thrombocytopenia | 0 | 0 | 1 | 0 |
| Febrile neutropneia | 3 | 0 | 5 | 0 |
| Nausea, Vomiting, GIT Upset | 1 | 0 | 2 | 0 |
| Mucositis | 0 | 0 | 3 | 0 |
| Neuropathy | 11 | 1 | 11 | 1 |
| Fatigue | 3 | 0 | 5 | 0 |
Late toxicities
There was no grade 3 or 4 late morbidity related to treatment in the follow-up period.
Discussion
Despite several advances in the understanding of its pathobiology, ovarian cancer remains an incurable disease with frequent relapses. The survival rate of ovarian cancer is still disappointingly low when compared with that of breast or prostate cancer. One of the factors contributing to the poor survival rate in ovarian cancer is the development of chemotherapy resistance following several rounds of chemotherapy. Furthermore, there is not yet any standard chemotherapy for platinum-resistant recurrence.11,12
The aim of chemotherapy in recurrent platinum-resistant ovarian cancer is palliative with improvement of QOL being the major goal.13
The primary objectives of the current Phase III prospective study were to compare between weekly paclitaxel and three-weekly paclitaxel as salvage treatment for recurrent platinum-resistant ovarian and perotoneal cancers in terms of ORR and PFS. Both of these represented the main objective for treatment of such an incurable disease. Toxicities were the other important factor taken into account, especially as paclitaxel was previously administered to all patients.13,14
Confirmatory data for paclitaxel rechallenge came from metastatic breast cancer studies, where several trials confirmed its effectiveness and mild toxicity profile. Additionally, in recurrent platinum-resistant ovarian cancer, there is not yet any standard treatment protocol, although previous Phase II studies confirmed the effectiveness and low toxicity profile of paclitaxel in recurrent ovarian cancer.12,15–18
We applied simple randomization to select patients of each treatment arm13 with an initial randomization plan of 1:1. However, this was addended to be as follows. For the first 24 patients, the selection criterion was 2:1 in favor of arm 1, and for the remaining 31 patients, the selection criterion was 1:1. This was because the study was run in three institutes with different patient loads and the investigators’ preferences of initially recruiting more arm 1 patients based on preliminary results. The power of the current study was 70%. Furthermore, the two groups were matched with no statistically significant differences in their characteristics. Additionally, our patient groups showed no ethnic or geographical variations.
Limited data are available to compare between chemotherapy agents in recurrent platinum-resistant ovarian and peritoneal cancers. The majority of studies in recurrent platinum-resistant disease were Phase II rather than Phase III studies.
The current trial aims to answer important research questions in recurrent platinum-resistant ovarian and peritoneal cancers, including the possibility for a first-line regimen upon recurrence and the feasibility of paclitaxel rechallenge. We selected to run a Phase III trial to compare different dose schedules of paclitaxel, looking for the best dose schedule with the fewest and least impacting side effects. Most likely, our trial is one of the few that compared chemotheraputic agents in such a disease. Furthermore, to the best of our knowledge up to the date of publication, there was no Phase III trial to compare taxene agents and taxene schedules.
In the current study, weekly paclitaxel achieved better ORR and PFS results than three-weekly paclitaxel. The difference in ORR between the two regimens may be attributed to relative cancer resistance to three-weekly paclitaxel that was given as first-line therapy. Confirmatory evidence of the effectiveness of weekly pactiaxel over three-weekly regimen came from the trial of Katsumata et al. They observed a better OS and PFS with dose-dense paclitaxel regimen in comparison with the conventional dose in the adjuvant setting.18
The authors did not perform further multivariate analysis for confounders because of the small number of patients, the aim of the current study being to look for a new hope treatment and the nature of the disease with frequent and short interval relapses.
Furthermore, although both regimens were associated with a mild toxicity profile, the toxicity profile for weekly paclitaxel was significantly lower than three-weekly paclitaxel. Hematological toxicities and neuropathy were the most commonly encountered side effects, specifically 19% for grade 3 and 12% for grade 4.
When comparing our results with those of Gynecologic Oncology Group (GOG) et al.15, the current study achieved better ORR with more grade 3 and 4 neuropathy. This difference may be explained in part by the relatively small sample size of both trials and in part to our guidelines for treatment delay. Our guideline stated that treatment delay applies only for grade 3 and 4 neuropathy. This might explain the relatively higher neuropathy numbers in our trial.
In the current study, the QOL data were analyzed as they were considered key indictators as to the response to treatment. A subjective response is considered an important factor for response assessment in such palliative disease. There was no previous guideline to follow in evaluating QOL in participating patients. Therefore, the authors selected to evaluate QOL mainly on patients who achieved ORR and/or SD rather than those who achieved DP. This was to show the changes in QOL just in responders rather than through the whole group, although some data were also included from those. Including more patients with DP would flaw the QOL results. Furthermore, willingness of patients was another important factor to consider in such assessment, and the authors observed that patients with DP participated less in QOL assessment. The authors calculated the QOL changes for the group as a whole, due to the small number of patients in addition to the authors planned to check the subjective changes in patients treated with paclitaxel rechallenge either weekly or thrice weekly.
Paclitaxel significantly improved the QOL in patients with recurrent platinum-resistant ovarian and peritoneal cancers. Importantly, when we assessed the QOL at the end of the treatment, there were no significant changes or treatment-related side effects.
Further studies are required to confirm our results, ideally with a relatively larger number of patients and more research expenditures.
Conclusion
Paclitaxel rechallenge still showed antitumor activity in recurrent platinum-resistant ovarian and peritoneal cancers. Weekly paclitaxel achieved significant better survival results and lower toxicities than three-weekly paclitaxel. Weekly paclitaxel represented a good treatment hope for such patients.
Footnotes
ACADEMIC EDITOR: William Chi-shing Cho, Editor in Chief
PEER REVIEW: Six peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1149 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE). Provenance the authors were invited to submit this paper.
Author contributions
Conceived and designed the experiments: MO, ME, KN. Analyzed the data: MO, ME, KN. Wrote the first draft of the manuscript: MO, ME, KN. Contributed to the writing of the manuscript: MO, ME, KN. Agree with manuscript results and conclusions: MO, ME, KN. Jointly developed the structure and arguments for the paper: MO, ME, KN. Made critical revisions and approved final version: MO, ME, KN. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, Ward E, Cancer WE. statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Jelovac D, Armstrong DK. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J Clin. 2011;61:183–203. doi: 10.3322/caac.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pujade-Lauraine E, Paraiso D, Cure H, et al. Predicting the effectiveness of chemotherapy (Cx) in patients with recurrent ovarian cancer (ROC): a GINECO study. Proc Am Soc Clin Oncol. 2002:21. Abstract829. [Google Scholar]
- 4.Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32(13):1302–8. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 5.BCCA Protocol Summary. 2010. Available at: http://www.bccancer.bc.ca/HPI/Drug-Database/DrugIndexPro/Paclitaxel.htm.
- 6.Bristol-Myers Squibb TAXOL® product monograph. Feb 22, 2010. www.bms.com.
- 7.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 8.NCCN Clinical Practice Guidelines in Oncology, Ovarian Cancer, Version 1. 2013. Available at http://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf.
- 9.Cella D, Tulsky D, Gray G, et al. The functional assessment of cancer therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–9. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 10.Common Toxicity Criteria (CTC) Version 2.0. Available at http://www.eortc.be/services/doc/ctc/ctcv204-30-992.pdf Published April 30, 1999.
- 11.Chien J, Kuang R, Landen C, Shridhar V. Platinum-sensitive recurrence in ovarian cancer: the role of tumor microenvironment. Front Oncol. 2013;3:251. doi: 10.3389/fonc.2013.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez EA, Vogel CL, Irwin DH, Kirshner JJ, Patel R. Multicenter phase II trial of weekly paclitaxel in women with metastatic breast cancer. J Clin Oncol. 2001;19:4216–23. doi: 10.1200/JCO.2001.19.22.4216. [DOI] [PubMed] [Google Scholar]
- 13.Taguchi T, Aihara T, Takatsuka Y, et al. Phase II study of weekly paclitaxel for docetaxel-resistant metastatic breast cancer in Japan. Breast J. 2004;10:509–13. doi: 10.1111/j.1075-122X.2004.21555.x. [DOI] [PubMed] [Google Scholar]
- 14.Yonemori K, Katsumata N, Uno H, et al. Efficacy of weekly paclitaxel for docetaxel-resistant metastatic breast cancer. Breast Cancer Res Treat. 2005;89:237–41. doi: 10.1007/s10549-004-2184-0. [DOI] [PubMed] [Google Scholar]
- 15.Gynecologic Oncology Group (GOG) Markman M, Blessing J, et al. Phase II trial of weekly paclitaxel (80 mg/m2) in platinum and paclitaxel-resistant ovarian and primary peritoneal cancers: a Gynecologic Oncology Group study. Gynecol Oncol. 2006;101:436–40. doi: 10.1016/j.ygyno.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 16.Piccart MJ, Green JA, Lacave AJ, et al. Oxaliplatin or paclitaxel. in patients with platinum-pretreated advanced ovarian cancer: a randomized phase II study of the European Organization for Research and Treatment of Cancer Gynecology Group. J Clin Oncol. 2000;18:1193–202. doi: 10.1200/JCO.2000.18.6.1193. [DOI] [PubMed] [Google Scholar]
- 17.Suresh KP. An overview of randomization techniques: an unbiased assessment of outcome in clinical research. J Hum Reprod Sci. 2011;4(1):8–11. doi: 10.4103/0974-1208.82352. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Katsumata N, Yasuda M, Takahashi F, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374(9698):1331–8. doi: 10.1016/S0140-6736(09)61157-0. [DOI] [PubMed] [Google Scholar]
- 19.Abushahin F, Singh DK, Lurain JR, Grendys EC, Rademaker AW, Schink JC. Weekly topotecan for recurrent platinum resistant ovarian cancer. Gynecol Oncol. 2008;108:53–7. doi: 10.1016/j.ygyno.2007.08.062. [DOI] [PubMed] [Google Scholar]
- 20.Gordon AN, Granai CO, Rose PG, et al. Phase II study of liposomal doxorubicin in platinum- and paclitaxel-refractory epithelial ovarian cancer. J Clin Oncol. 2000;18:3093–100. doi: 10.1200/JCO.2000.18.17.3093. [DOI] [PubMed] [Google Scholar]
- 21.Ferrandina G, Ludovisi M, Lorusso D, et al. Phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in progressive or recurrent ovarian cancer. J Clin Oncol. 2008;26:890–6. doi: 10.1200/JCO.2007.13.6606. [DOI] [PubMed] [Google Scholar]
- 22.Rose PG, Blessing JA, Ball HG, et al. A phase II study of docetaxel in paclitaxelresistant ovarian and peritoneal carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2003;88:130–135. doi: 10.1016/s0090-8258(02)00091-4. [DOI] [PubMed] [Google Scholar]; J Clin Oncol. 1997 Jun;15(6):2183–93. doi: 10.1200/JCO.1997.15.6.2183. [DOI] [PubMed] [Google Scholar]


