Abstract
The discovery of the myofibroblast has allowed definition of the cell responsible for wound contraction and for the development of fibrotic changes. This review summarizes the main features of the myofibroblast and the mechanisms of myofibroblast generation. Myofibroblasts originate from a variety of cells according to the organ and the type of lesion. The mechanisms of myofibroblast contraction, which appear clearly different to those of smooth muscle cell contraction, are described. Finally, we summarize the possible strategies in order to reduce myofibroblast activities and thus influence several pathologies, such as hypertrophic scars and organ fibrosis.
Keywords: Myofibroblast, mechanotransduction, myofibroblast generation, myofibroblast contraction, hypertrophic scars, organ fibroses
Introduction
Wound healing has interested the medical praxis since the beginning of human history, but for many centuries the effort of physicians has concentrated more on empirical therapeutic strategies rather than on the understanding of its biological mechanisms. During the last few centuries, however, a gradual progress has been achieved in defining and understanding several physiological aspects of wound healing. In particular, the formation and evolution of granulation tissue has been described in the second half of the 18th century, mainly thanks to the British surgeon John Hunter, and in the last century it has been shown that wound contraction is due to an active contraction of granulation tissue, mainly thanks to the work of the French surgeon Alexis Carrel 1. The discovery of the myofibroblast more than forty years ago allowed the identification of the cell responsible for this phenomenon 2. This coincided with the early establishment of the cytoskeleton concept 3. The myofibroblast was then considered to be a contractile non-muscle cell 4. Since the first description, our knowledge of myofibroblast structure and activity has progressed enormously. The purpose of this article is to briefly summarize the biological features of the myofibroblast and to discuss some of the promising strategies to suppress this cell’s activity in order to achieve the possibility of influencing important pathological situations, such as fibrotic lesions, that presently cannot be cured successfully.
Evolution of the myofibroblast concept
Initially, the myofibroblast was described by means of electron microscopy revealing the presence of prominent cytoplasmic microfilament bundles and peripheral focal adhesions in the fibroblastic cells of granulation tissue 2. Electron microscopy further showed the existence of gap junctions connecting myofibroblasts, thus reinforcing the suggestion of similarity between myofibroblasts and smooth muscle (SM) cells 5. The production of a specific antibody against α-SM actin, the actin isoform typical of vascular SM cells, allowed the demonstration that myofibroblasts express α-SM actin and are hence equipped with a typical SM protein 6.
In the early phases of granulation tissue formation after the production of a wound, local fibroblasts begin moving from the unaffected dermis and subcutaneous tissue toward the wound center and acquire bundles of microfilaments, similar to in vitro stress fibers, containing only β- and γ-cytoplasmic actins; these cells have been named proto-myofibroblasts and evolve generally into α-SM actin containing differentiated myofibroblasts that are responsible for wound contraction 7. When the wound closes, myofibroblasts disappear through apoptosis 8, and a scar persists in the affected area. When myofibroblasts persist in a closed wound, they indicate the development of a hypertrophic scar, an important pathological evolution of wound healing, particularly frequent after burn injury 7, 9. Myofibroblasts are also present in all fibrotic diseases, such as scleroderma, as well as liver, kidney, and lung fibrosis and are prominent in heart failure and repair after myocardial infarction. Finally, myofibroblasts are the main components of the stromal reaction to several epithelial tumors 7, 10. It should be noted that both proto-myofibroblasts and differentiated myofibroblasts can be found in normal tissues, for example in lung alveolar septa and at the periphery of intestinal crypts, respectively 7, where they probably exert physiological mechanical functions.
Much work has been performed in order to find specific markers of the myofibroblastic phenotype. As stated above, α-SM actin discriminates myofibroblasts from fibroblasts and has become the most used marker for this cell. Several other markers have been proposed, but no specific marker has been identified until now. However, several SM cell markers are not expressed in myofibroblasts, such as SM myosin heavy chains, h-caldesmon, and smoothelin 11; this underlines the functional differences between the two cells, as we shall discuss below.
Mechanisms of myofibroblast formation and evolution
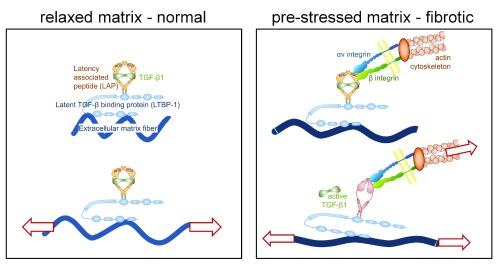
After a wounding insult, blood extravasation and clot formation occur followed by an inflammatory phase that allows an accumulation of blood-borne cells, liberating many cytokines and growth factors essential for the onset of the following phase of granulation tissue formation 12. In early granulation tissue, motile proto-myofibroblasts appear and start to synthesize extracellular matrix (ECM) components, such as collagen type I and III 7. Another relevant new component of ECM is cellular fibronectin, which contains the alternatively spliced segments EDA (or EIIIA) and EDB (or EIIIB) and is present in connective tissue during development but reappears in pathological situations such as granulation tissue and fibrotic lesions 13, 14. EDA fibronectin has been shown to be essential for the differentiation of myofibroblasts 15. The early transformation of fibroblasts into proto-myofibroblasts appears to depend on the mechanical changes taking place in the wound compared to the normal skin, in particular increased stiffness 7, 16; moreover, platelet-derived growth factor has been shown to stimulate proto-myofibroblast motility 17. The development of α-SM actin synthesizing differentiated myofibroblasts is essentially due to the action of transforming growth factor (TGF)-β1 in the presence of EDA fibronectin 15, 18. TGF-β1 is present in the ECM as a large latent complex including latency-associated peptide and latent TGF-β1-binding protein 9. It can be liberated by proteolytic enzymes as well as by integrin-dependent mechanically induced mechanisms 9. The force exerted by stress fibers through transmembrane integrins is enough to free TGF-β1 from the large latent complex, and the strained ECM is capable of maintaining a feedback mechanism, assuring a persistent fibrotic activity by the myofibroblast 19, 20; moreover, straining and/or stiffening of the ECM can increase the availability of TGF-β1 21, 22 ( Figure 1). Straining and stiffening are consequences of fibroblast and myofibroblast remodeling activities. Matrix stiffening is additionally promoted by fibroblast and inflammatory cell-derived collagen crosslinking enzymes including lysyl oxidases and lysyl oxidase-like enzymes, as reviewed in 20, 23. The incorporation of α-SM actin into stress fibers has been shown to significantly increase the contractile activity of fibroblasts 24; the force generated by myofibroblast stress fibers is transmitted to the ECM through focal adhesions that contain specialized transmembrane integrins 25. As stated above, myofibroblasts disappear when a wound closes, mainly through apoptosis 8. The mechanisms of apoptosis induction, or conversely of myofibroblast persistence, in hypertrophic scars are not clarified; however, the importance of a focal adhesion complex component, Hic-5, a paxillin homologue, in maintaining the myofibroblast phenotype has been demonstrated 26. Moreover, myofibroblasts can disappear by means of accelerated senescence 27 and even, at least in some instances, revert to the normal phenotype 28.
Figure 1. Mechanical activation of TGF-β1.
In normal connective tissue, loosely arranged collagen protects resident fibroblasts and latent transforming growth factor (TGF)-β1 complexes from being strained with the extracellular matrix (ECM). Fibroblasts in normal tissue do not express or present the integrin receptors that bind and activate latent TGF-β1. During tissue repair and in organ fibrosis, activated myofibroblasts express αv integrins that connect the contractile actin/myosin cytoskeleton to latent TGF-β1. The accumulation of collagen and its excessive remodeling (crosslinking) by these myofibroblasts result in denser and straighter ECM fibers, which leads to overall higher tissue stiffness. Because ECM fibers are straighter, even smaller strains applied to the fibrotic ECM externally, or by residing myofibroblasts, will be sufficient for the release of active TGF-β1 (modified from Hinz B and Suki B [2016] Does breathing amplify fibrosis? Editorial on 21).
Mechanism of myofibroblast contraction and mechanotransduction
The initial studies suggesting a similarity between myofibroblast and SM cell contractile activities 4 were gradually reconsidered in light of the consideration of the different functional activities of the two cells: SM cell contraction is rapid and short in duration, whereas myofibroblast contraction is rather long lasting and results in a permanent tissue retraction, probably stabilized by ECM deposition 7. Evidence has gradually accumulated suggesting that, in addition to the classical calcium-calmodulin-myosin light chain kinase-dependent SM cell contraction mechanism 11, myofibroblast contractile activity can be regulated by the activation of the Rho/ROCK/myosin light chain phosphatase pathway 7, 29– 31. This long-duration type of contraction underlines an essential difference between the SM cell and the myofibroblast and could explain the characteristic tissue remodeling activity of this cell.
The forces generated by the contractile activity of myofibroblasts are transmitted to the surrounding ECM through specialized focal adhesions containing transmembrane integrins. As a result, strained and more compacted ECM develops. Interestingly, the mechanical conditions generated by the myofibroblast feedback leads to their sustained pro-fibrotic activity 10, 19. More recently, it has been shown that megakaryoblastic leukemia factor 1 (MKL1), also named myocardin-related transcription factor (MRTF), is crucial for myofibroblast differentiation and mechanotransduction. In various myofibroblast precursor cells, it links mechanical stress to the transcriptional activity of muscle-cell genes via the polymerization state of actin 32– 36. Inhibition of MRTF reduces experimentally induced skin fibrosis in rodents 37, as well as differentiation of human colonic myofibroblasts 38. Similarly, YAP/TAZ transcription factors, known to mediate mechano-responses 39, positively regulate myofibroblast activation 40– 44.
Myofibroblast origin
One of the intriguing features of the myofibroblast is that it can derive from a large variety of cell types. As stated above, mesenchymal cells with myofibroblastic features are present in normal tissues, including the uterine submucosa, follicles of lymph nodes and spleen, intestinal villous cores and crypts, theca externa of the ovary, periodontal ligament, adrenal capsule, lung septa, and bone marrow stroma 7. It appears more and more evident that the term fibroblast comprises a heterogeneous cell population 10, 45, thus it is possible that only some specialized fibroblastic cells generate myofibroblasts in normal and pathological situations, as recently supported by studies on skin and heart fibrosis 46– 48. During pathological situations, local fibroblasts allegedly represent the major source of myofibroblastic cells 10; however, in particular cases, other local cells become the main precursors, such as SM cells in coronary atheromatous plaque 49, keratocytes in the eye 50, 51, perisinusoidal cells in the liver 52, and pericytes in many organs 53– 55. In addition, myofibroblasts may develop through the process of epithelial-mesenchymal transition 56, 57 or endothelial-mesenchymal transition 58. Finally, myofibroblasts may derive from circulating bone marrow-derived specialized inflammatory cells called fibrocytes and participate in fibrotic lesions in several organs 59, 60. Circulating and/or resident mesenchymal stromal/stem cells (MSCs) are prominent precursors of myofibroblasts in a variety of organs and injury situations 61, 62. Because delivery of MSCs is an attractive approach to regenerate organs that are beyond repair within the body’s own capacity 63, 64, understanding MSC-to-myofibroblast activation (fibrogenesis) will be of particular importance for the success of MSC therapies 40, 65.
There is considerable variability and dispute in the literature concerning the proportions of different precursor cells contributing to the myofibroblast pool. However, different research groups seem to generally agree that myofibroblast sources can differ between different individuals, organs, animals, or particular injury models. For example, in the corneal fibrosis model, 30 to 70% of myofibroblasts are derived from bone marrow-derived precursors depending on the type of wound and the individual that is wounded (reviewed in 51). Thus, using drugs that modulate myofibroblast activation from specific precursor cells can be an effective strategy to inhibit fibrosis in an organ-specific manner.
Perspectives
As we have seen, the myofibroblast represents an eclectic cell whose major function appears to be the remodeling of connective tissue. If we consider the variety of its possible origins, the myofibroblast could be defined as a phenotypic variant of many cell types, developing upon the appearance of appropriate stimuli. Myofibroblast activity can be physiological, e.g., regulation of ventilation/perfusion ratio in pulmonary alveoli, and useful for wound healing but noxious in many pathological situations, e.g., fibrotic lesions 7.
Despite many attempts and despite the clinical importance of fibrotic lesions 9, 12, there is not at present any clinically accepted pharmacological tool capable of influencing myofibroblast activity and thus the evolution of these diseases. We shall discuss some strategies that could possibly lead to the development of efficient tools. Despite the heterogeneity of origin, all differentiated myofibroblasts perform the same functions, i.e. tissue remodeling and synthesis of ECM. Hence, the processes regulating these functions appear to represent promising targets of therapeutic strategies. TGF-β1 would appear as an ideal target in order to control myofibroblast activity. Unfortunately, until now no relevant results have been obtained by using direct inhibitors; however, several pathways of TGF-β1 action remain to be explored and a number of clinical trials that target TGF-β1 are pending 66. EDA fibronectin is necessary for myofibroblast differentiation 15 and its absence results in wound healing or pulmonary fibrosis reduction 67, suggesting that EDA fibronectin could be addressed as a therapeutic target. The observation that α-SM actin is essential for the remodeling activity of the myofibroblast and the finding that its N-terminal peptide Ac-EEED is essential for α-SM actin incorporation into stress fibers 68 have suggested that this peptide could represent a tool for decreasing myofibroblast activity. This possibility has been demonstrated in vitro and in rat wound healing 69, suggesting that this peptide or, possibly more efficiently, a mimetic compound could be used therapeutically. The recent observation that tropomyosin 1.6/7 isoforms play an essential role in the stable incorporation of α-SM actin into fibroblast stress fibers 70 points to a new target for the reduction of α-SM actin expression in myofibroblasts with the consequent reduction of their remodeling activity 71. Another way to regulate myofibroblast remodeling activity could be the control of the Rho/ROCK/myosin light chain phosphatase pathway. In this respect, it has been shown that the ROCK inhibitor Y-27632 decreases granulation tissue contraction 31. Closely related to reducing cell contraction is the idea of blocking myofibroblast adhesion to the ECM via integrins. This will have two potential beneficial outcomes: reduced force transmission to the ECM and, provided the correct integrins are targeted, reduction of TGF-β1 activation 72, 73.
Further work is needed in order to develop an efficient therapeutic approach to excessive wound healing and fibrotic diseases. Importantly, myofibroblast research will need to cross organ boundaries to exploit the full potential of drugs that are effective in one system but not studied in others. For instance, mitomycin C was shown to block fibrosis development after eye surgery 74, but its action in other organs is unknown. We feel confident that during the next few years the above-discussed strategies will allow the discovery of new, efficient tools to control these devastating diseases.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Steven E. Wilson, Cole Eye Institute, Cleveland Clinic, Cleveland, OH, USA
Jack Gauldie, Department of Pathology and Molecular Medicine, McMaster University, Hamilton, Ontario, Canada
Funding Statement
The research of BH is supported by Canadian Institutes of Health Research (CIHR) (grants #210820, #286920, #286720, and #497202), the Collaborative Health Research Programme (CIHR/NSERC) (grants #1004005 and #413783), and the Canada Foundation for Innovation and Ontario Research Fund (CFI/ORF) (grant #26653). The research of MLBP is supported by the Swiss National Science Foundation (grant #146790/1).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved]
References
- 1. Whipple AO: The story of wound healing and wound repair.Springfield,1963. Reference Source [Google Scholar]
- 2. Gabbiani G, Ryan GB, Majno G: Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27(5):549–50. 10.1007/BF02147594 [DOI] [PubMed] [Google Scholar]
- 3. Zampieri F, Coen M, Gabbiani G: The prehistory of the cytoskeleton concept. Cytoskeleton (Hoboken). 2014;71(8):464–71. 10.1002/cm.21177 [DOI] [PubMed] [Google Scholar]
- 4. Gabbiani G, Hirschel BJ, Ryan GB, et al. : Granulation tissue as a contractile organ. A study of structure and function. J Exp Med. 1972;135(4):719–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gabbiani G, Chaponnier C, Hüttner I: Cytoplasmic filaments and gap junctions in epithelial cells and myofibroblasts during wound healing. J Cell Biol. 1978;76(3):561–8. 10.1083/jcb.76.3.561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Skalli O, Ropraz P, Trzeciak A, et al. : A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986;103(6 Pt 2):2787–96. 10.1083/jcb.103.6.2787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tomasek JJ, Gabbiani G, Hinz B, et al. : Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3(5):349–63. 10.1038/nrm809 [DOI] [PubMed] [Google Scholar]
- 8. Desmoulière A, Redard M, Darby I, et al. : Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995;146(1):56–66. [PMC free article] [PubMed] [Google Scholar]
- 9. Van De Water L, Varney S, Tomasek JJ: Mechanoregulation of the Myofibroblast in Wound Contraction, Scarring, and Fibrosis: Opportunities for New Therapeutic Intervention. Adv Wound Care (New Rochelle). 2013;2(4):122–41. 10.1089/wound.2012.0393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hinz B, Phan SH, Thannickal VJ, et al. : Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180(4):1340–55. 10.1016/j.ajpath.2012.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arnoldi R, Chaponnier C, Gabbiani G, et al. : Heterogeneity of Smooth Muscle.In: Muscle: Elsevier,2012;1183–1195. 10.1016/B978-0-12-381510-1.00088-0 [DOI] [Google Scholar]
- 12. Wynn TA, Ramalingam TR: Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18(7):1028–40. 10.1038/nm.2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brown LF, Dubin D, Lavigne L, et al. : Macrophages and fibroblasts express embryonic fibronectins during cutaneous wound healing. Am J Pathol. 1993;142(3):793–801. [PMC free article] [PubMed] [Google Scholar]
- 14. Singh P, Reimer CL, Peters JH, et al. : The spatial and temporal expression patterns of integrin alpha9beta1 and one of its ligands, the EIIIA segment of fibronectin, in cutaneous wound healing. J Invest Dermatol. 2004;123(6):1176–81. 10.1111/j.0022-202X.2004.23485.x [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 15. Serini G, Bochaton-Piallat ML, Ropraz P, et al. : The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol. 1998;142(3):873–81. 10.1083/jcb.142.3.873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goffin JM, Pittet P, Csucs G, et al. : Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers. J Cell Biol. 2006;172(2):259–68. 10.1083/jcb.200506179 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 17. Grinnell F, Petroll WM: Cell motility and mechanics in three-dimensional collagen matrices. Annu Rev Cell Dev Biol. 2010;26:335–61. 10.1146/annurev.cellbio.042308.113318 [DOI] [PubMed] [Google Scholar]
- 18. Desmoulière A, Geinoz A, Gabbiani F, et al. : Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122(1):103–11. 10.1083/jcb.122.1.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duscher D, Maan ZN, Wong VW, et al. : Mechanotransduction and fibrosis. J Biomech. 2014;47(9):1997–2005. 10.1016/j.jbiomech.2014.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hinz B: The extracellular matrix and transforming growth factor-β1: Tale of a strained relationship. Matrix Biol. 2015;47:54–65. 10.1016/j.matbio.2015.05.006 [DOI] [PubMed] [Google Scholar]
- 21. Froese AR, Shimbori C, Bellaye PS, et al. : Stretch Induced Activation of TGF-β1 in Pulmonary Fibrosis. Am J Respir Crit Care Med. 2016. 10.1164/rccm.201508-1638OC [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 22. Klingberg F, Chow ML, Koehler A, et al. : Prestress in the extracellular matrix sensitizes latent TGF-β1 for activation. J Cell Biol. 2014;207(2):283–97. 10.1083/jcb.201402006 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 23. Klingberg F, Hinz B, White ES: The myofibroblast matrix: implications for tissue repair and fibrosis. J Pathol. 2013;229(2):298–309. 10.1002/path.4104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hinz B, Celetta G, Tomasek JJ, et al. : Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12(9):2730–41. 10.1091/mbc.12.9.2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dugina V, Fontao L, Chaponnier C, et al. : Focal adhesion features during myofibroblastic differentiation are controlled by intracellular and extracellular factors. J Cell Sci. 2001;114(Pt 18):3285–96. [DOI] [PubMed] [Google Scholar]
- 26. Varney SD, Betts CB, Zheng R, et al. : Hic-5 is required for myofibroblast differentiation by regulating mechanically dependent MRTF-A nuclear accumulation. J Cell Sci. 2016;129(4):774–87. 10.1242/jcs.170589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jun JI, Lau LF: The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol. 2010;12(7):676–85. 10.1038/ncb2070 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 28. Kisseleva T, Cong M, Paik Y, et al. : Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci U S A. 2012;109(24):9448–53. 10.1073/pnas.1201840109 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Parizi M, Howard EW, Tomasek JJ: Regulation of LPA-promoted myofibroblast contraction: role of Rho, myosin light chain kinase, and myosin light chain phosphatase. Exp Cell Res. 2000;254(2):210–20. 10.1006/excr.1999.4754 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Anderson S, DiCesare L, Tan I, et al. : Rho-mediated assembly of stress fibers is differentially regulated in corneal fibroblasts and myofibroblasts. Exp Cell Res. 2004;298(2):574–83. 10.1016/j.yexcr.2004.05.005 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. Tomasek JJ, Vaughan MB, Kropp BP, et al. : Contraction of myofibroblasts in granulation tissue is dependent on Rho/Rho kinase/myosin light chain phosphatase activity. Wound Repair Regen. 2006;14(3):313–20. 10.1111/j.1743-6109.2006.00126.x [DOI] [PubMed] [Google Scholar]
- 32. Crider BJ, Risinger GM, Jr, Haaksma CJ, et al. : Myocardin-related transcription factors A and B are key regulators of TGF-β1-induced fibroblast to myofibroblast differentiation. J Invest Dermatol. 2011;131(12):2378–85. 10.1038/jid.2011.219 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. Scharenberg MA, Pippenger BE, Sack R, et al. : TGF-β-induced differentiation into myofibroblasts involves specific regulation of two MKL1 isoforms. J Cell Sci. 2014;127(Pt 5):1079–91. 10.1242/jcs.142075 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Zhou Y, Huang X, Hecker L, et al. : Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Invest. 2013;123(3):1096–108. 10.1172/JCI66700 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Small EM, Thatcher JE, Sutherland LB, et al. : Myocardin-related transcription factor-a controls myofibroblast activation and fibrosis in response to myocardial infarction. Circ Res. 2010;107(2):294–304. 10.1161/CIRCRESAHA.110.223172 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Luchsinger LL, Patenaude CA, Smith BD, et al. : Myocardin-related transcription factor-A complexes activate type I collagen expression in lung fibroblasts. J Biol Chem. 2011;286(51):44116–25. 10.1074/jbc.M111.276931 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 37. Haak AJ, Tsou PS, Amin MA, et al. : Targeting the myofibroblast genetic switch: inhibitors of myocardin-related transcription factor/serum response factor-regulated gene transcription prevent fibrosis in a murine model of skin injury. J Pharmacol Exp Ther. 2014;349(3):480–6. 10.1124/jpet.114.213520 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Johnson LA, Rodansky ES, Haak AJ, et al. : Novel Rho/MRTF/SRF inhibitors block matrix-stiffness and TGF-β-induced fibrogenesis in human colonic myofibroblasts. Inflamm Bowel Dis. 2014;20(1):154–65. 10.1097/01.MIB.0000437615.98881.31 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. Halder G, Dupont S, Piccolo S: Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol. 2012;13(9):591–600. 10.1038/nrm3416 [DOI] [PubMed] [Google Scholar]
- 40. Talele NP, Fradette J, Davies JE, et al. : Expression of α-Smooth Muscle Actin Determines the Fate of Mesenchymal Stromal Cells. Stem Cell Reports. 2015;4(6):1016–30. 10.1016/j.stemcr.2015.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Calvo F, Ege N, Grande-Garcia A, et al. : Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol. 2013;15(6):637–46. 10.1038/ncb2756 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Liu F, Lagares D, Choi KM, et al. : Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol Lung Cell Mol Physiol. 2015;308(4):L344–57. 10.1152/ajplung.00300.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Speight P, Nakano H, Kelley TJ, et al. : Differential topical susceptibility to TGFβ in intact and injured regions of the epithelium: key role in myofibroblast transition. Mol Biol Cell. 2013;24(21):3326–36. 10.1091/mbc.E13-04-0220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Piersma B, de Rond S, Werker PM, et al. : YAP1 Is a Driver of Myofibroblast Differentiation in Normal and Diseased Fibroblasts. Am J Pathol. 2015;185(12):3326–37. 10.1016/j.ajpath.2015.08.011 [DOI] [PubMed] [Google Scholar]
- 45. Driskell RR, Lichtenberger BM, Hoste E, et al. : Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504(7479):277–81. 10.1038/nature12783 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Rinkevich Y, Walmsley GG, Hu MS, et al. : Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science. 2015;348(6232):aaa2151. 10.1126/science.aaa2151 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Moore-Morris T, Guimarães-Camboa N, Banerjee I, et al. : Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J Clin Invest. 2014;124(7):2921–34. 10.1172/JCI74783 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Marangoni RG, Korman BD, Wei J, et al. : Myofibroblasts in murine cutaneous fibrosis originate from adiponectin-positive intradermal progenitors. Arthritis Rheumatol. 2015;67(4):1062–73. 10.1002/art.38990 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Hao H, Gabbiani G, Camenzind E, et al. : Phenotypic modulation of intima and media smooth muscle cells in fatal cases of coronary artery lesion. Arterioscler Thromb Vasc Biol. 2006;26(2):326–32. 10.1161/01.ATV.0000199393.74656.4c [DOI] [PubMed] [Google Scholar]
- 50. Hinz B: Myofibroblasts. Exp Eye Res. 2016;142:56–70. 10.1016/j.exer.2015.07.009 [DOI] [PubMed] [Google Scholar]
- 51. Torricelli AA, Santhanam A, Wu J, et al. : The corneal fibrosis response to epithelial-stromal injury. Exp Eye Res. 2016;142:110–8. 10.1016/j.exer.2014.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Friedman SL: Evolving challenges in hepatic fibrosis. Nat Rev Gastroenterol Hepatol. 2010;7(8):425–36. 10.1038/nrgastro.2010.97 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. Kramann R, Schneider RK, DiRocco DP, et al. : Perivascular Gli1 + progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell. 2015;16(1):51–66. 10.1016/j.stem.2014.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 54. Greenhalgh SN, Iredale JP, Henderson NC: Origins of fibrosis: pericytes take centre stage. F1000Prime Rep. 2013;5:37. 10.12703/P5-37 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 55. Duffield JS: Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest. 2014;124(6):2299–306. 10.1172/JCI72267 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 56. Zeisberg M, Kalluri R: The role of epithelial-to-mesenchymal transition in renal fibrosis. J Mol Med (Berl). 2004;82(3):175–81. 10.1007/s00109-003-0517-9 [DOI] [PubMed] [Google Scholar]
- 57. Kim KK, Kugler MC, Wolters PJ, et al. : Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A. 2006;103(35):13180–5. 10.1073/pnas.0605669103 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. Ho WT, Chang JS, Su CC, et al. : Inhibition of matrix metalloproteinase activity reverses corneal endothelial-mesenchymal transition. Am J Pathol. 2015;185(8):2158–67. 10.1016/j.ajpath.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 59. Galligan CL, Fish EN: The role of circulating fibrocytes in inflammation and autoimmunity. J Leukoc Biol. 2013;93(1):45–50. 10.1189/jlb.0712365 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 60. Reilkoff RA, Bucala R, Herzog EL: Fibrocytes: emerging effector cells in chronic inflammation. Nat Rev Immunol. 2011;11(6):427–35. 10.1038/nri2990 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 61. Barbosa FL, Chaurasia SS, Cutler A, et al. : Corneal myofibroblast generation from bone marrow-derived cells. Exp Eye Res. 2010;91(1):92–6. 10.1016/j.exer.2010.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 62. Direkze NC, Hodivala-Dilke K, Jeffery R, et al. : Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res. 2004;64(23):8492–5. 10.1158/0008-5472.CAN-04-1708 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 63. Behfar A, Crespo-Diaz R, Terzic A, et al. : Cell therapy for cardiac repair--lessons from clinical trials. Nat Rev Cardiol. 2014;11(4):232–46. 10.1038/nrcardio.2014.9 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 64. Bianco P, Cao X, Frenette PS, et al. : The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19(1):35–42. 10.1038/nm.3028 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 65. Hinz B: The myofibroblast in connective tissue repair and regeneration. In: Regenerative Medicine and Biomaterials for the Repair of Connective Tissues: Elsevier;2010;39–80. 10.1533/9781845697792.39 [DOI] [Google Scholar]
- 66. Friedman SL, Sheppard D, Duffield JS, et al. : Therapy for fibrotic diseases: nearing the starting line. Sci Transl Med. 2013;5(167):167sr1. 10.1126/scitranslmed.3004700 [DOI] [PubMed] [Google Scholar]
- 67. Muro AF, Moretti FA, Moore BB, et al. : An essential role for fibronectin extra type III domain A in pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177(6):638–45. 10.1164/rccm.200708-1291OC [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 68. Chaponnier C, Goethals M, Janmey PA, et al. : The specific NH2-terminal sequence Ac-EEED of alpha-smooth muscle actin plays a role in polymerization in vitro and in vivo. J Cell Biol. 1995;130(4):887–95. 10.1083/jcb.130.4.887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hinz B, Gabbiani G, Chaponnier C: The NH2-terminal peptide of alpha-smooth muscle actin inhibits force generation by the myofibroblast in vitro and in vivo. J Cell Biol. 2002;157(4):657–63. 10.1083/jcb.200201049 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 70. Prunotto M, Bruschi M, Gunning P, et al. : Stable incorporation of α-smooth muscle actin into stress fibers is dependent on specific tropomyosin isoforms. Cytoskeleton (Hoboken). 2015;72(6):257–67. 10.1002/cm.21230 [DOI] [PubMed] [Google Scholar]
- 71. Gunning PW, Hardeman EC, Lappalainen P, et al. : Tropomyosin - master regulator of actin filament function in the cytoskeleton. J Cell Sci. 2015;128(16):2965–74. 10.1242/jcs.172502 [DOI] [PubMed] [Google Scholar]
- 72. Reed NI, Jo H, Chen C, et al. : The α vβ 1 integrin plays a critical in vivo role in tissue fibrosis. Sci Transl Med. 2015;7(288):288ra79. 10.1126/scitranslmed.aaa5094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Henderson NC, Arnold TD, Katamura Y, et al. : Targeting of α v integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med. 2013;19(12):1617–24. 10.1038/nm.3282 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 74. Santhiago MR, Netto MV, Wilson SE: Mitomycin C: biological effects and use in refractive surgery. Cornea. 2012;31(3):311–21. 10.1097/ICO.0b013e31821e429d [DOI] [PubMed] [Google Scholar]; F1000 Recommendation