Abstract
Background
The nicotinamide adenine dinucleotide phosphate-dependent membrane protein 5α-reductase catalyses the conversion of testosterone to the most potent androgen – 5α-dihydrotestosterone. Two 5α-reductase isoenzymes are expressed in humans: type I and type II. The latter is found primarily in prostate tissue. Saw palmetto extract (SPE) has been used extensively in the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia (BPH). The pharmacological effects of SPE include the inhibition of 5α-reductase, as well as anti-inflammatory and antiproliferative effects. Clinical studies of SPE have been inconclusive – some have shown significant results, and others have not – possibly the result of varying bioactivities of the SPEs used in the studies.
Purpose
To determine the in vitro potency in a cell-free test system of a novel SP supercritical CO2 extract (SPSE), an inhibitor of the 5α-reductase isoenzyme type II.
Materials and methods
The inhibitory potency of SPSE was compared to that of finasteride, an approved 5α-reductase inhibitor, on the basis of the enzymatic conversion of the substrate androstenedione to the 5α-reduced product 5α-androstanedione.
Results
By concentration-dependent inhibition of 5α-reductase type II in vitro (half-maximal inhibitory concentration 3.58±0.05 μg/mL), SPSE demonstrated competitive binding toward the active site of the enzyme. Finasteride, the approved 5α-reductase inhibitor tested as positive control, led to 63%–75% inhibition of 5α-reductase type II.
Conclusion
SPSE effectively inhibits the enzyme that has been linked to BPH, and the amount of extract required for activity is comparatively low. It can be confirmed from the results of this study that SPSE has bioactivity that promotes prostate health at a level that is superior to that of many other phytotherapeutic extracts. The bioactivity of SPSE corresponds favorably to that reported for the hexane extract used in a large number of positive BPH clinical trials, as well as to finasteride, the established standard of therapy among prescription drugs. Future in vitro and clinical trials involving SPEs would be useful for elucidating their comparative differences, as well as appropriate patient selection for their use.
Keywords: benign prostatic hyperplasia, prostate, saw palmetto supercritical CO2 extract, SPSE, 5α-reductase
Introduction
5α-reductase and androgen biosynthesis
A nicotinamide adenine dinucleotide phosphate (NADPH)-dependent membrane protein, 5α-reductase catalyzes the reduction of 4-en-3-oxosteroids irreversibly, resulting in the corresponding 5α-3-oxosteroids. The most important reaction is the conversion of testosterone to the most potent androgen, 5α-dihydrotestosterone (DHT), which displays the highest affinity toward the androgen receptor. The final step in androgen biosynthesis is the irreversible reduction of testosterone to DHT.
Two 5α-reductase isoenzymes are expressed in humans: type I and type II. Distinct biochemical and pharmacological properties, such as pH optimum and Km,1–3 characterize the isoenzymes. Their differing patterns of distribution are still being elucidated. The type I isoenzyme is expressed primarily in skin, scalp, and follicles, and is the predominant form in oil and sweat glands. Found primarily in prostate tissue, type II is expressed in stroma and basal cells of the prostate; type I is also expressed in epithelial cells of the prostate.1
Boudon et al performed specific enzyme amplification after reverse transcription to determine whether the two known 5α-reductase isoenzymes were expressed in prostate tissue. Their study reported the presence of 274 and 383 base-pair fragments that were specific for 5α-reductase type I and type II, respectively. These results extended the evidence that 5α-reductase type II activity is largely responsible for the production of DHT by human prostatic cells through demonstrating the expression of messenger RNA in enlarged prostate tissue for both isoenzymes.2,3 Shirakawa et al subsequently identified specific enzyme activity for 5α-reductase type I in the prostate, supporting the hypothesis that this isoenzyme may also play an important role in maintaining prostate enlargement.1
Clinical applications of 5α-reductase inhibition
BPH
The most common benign proliferative disorder of unknown etiology found in men, benign prostatic hyperplasia (BPH) affects over 50% of males older than 70 years. BPH confers its morbidity through potentially bothersome lower urinary tract symptoms.4 Urinary hesitancy, nocturia, incontinence, weak stream, and recurrent urinary tract infections are typical presenting symptoms.
The sole function of the prostate is to secrete fluid containing substances that are essential for reproduction, requiring an extremely high tissue concentration of androgens.5 Dysregulation of the conversion of testosterone to DHT by 5α-reductase is generally considered to be a key step in the development of BPH. Moreover, elevated DHT levels have been shown to correlate with the pathogenesis and progression of androgen-dependent diseases (eg, prostate cancer and BPH).
5α-reductase inhibitors lower the level of DHT available to prostate tissue and hair follicles, and have been used extensively to treat BPH. These agents block the action of the 5α-reductase that converts testosterone into DHT. Other treatments for BPH symptoms include α-adrenergic receptor blockers (the pharmacological agents of choice for the primary and secondary symptoms of BPH), phosphodiesterase type 5 (PDE5) inhibitors,6,7 simple nonsteroidal anti-inflammatory drugs,8–10 minimally invasive therapies that use heat to damage or destroy prostate tissue, and surgery, including transurethral resection of the prostate. In the US, dietary supplements for promoting prostate health have gained popularity over the last decade.4,11 The herbal agents saw palmetto extract (SPE), rye grass-pollen extract, and pygeum have also been shown to relieve symptoms of BPH.
Currently available treatment options in the field of phytotherapy were recently reviewed by Allkanjari and Vitalone, who found that randomized clinical trials indicated significant efficacy in improving urinary symptoms with mild adverse effects for some phytotherapeutic agents, but that further clinical evidence was needed for others (eg, Epilobium spp., Secale cereale, Roystonea regia).12
Saw palmetto extracts and BPH
SPE has been extensively utilized for the management of lower urinary tract symptoms secondary to BPH. In addition to the inhibition of 5α-reductase, the pharmacological effects of SPE include antiandrogenic effects, antiproliferative effects, and anti-inflammatory effects.13
The numerous SPEs available today feature several different extraction processes, resulting in varying levels of BPH-related bioactivity. SPE is the phytotherapeutic agent that is used most frequently for the treatment of the urological symptoms caused by BPH.
In comparing the in vitro potency of various brands of SPE, Scaglione et al found that all extracts tested were able to inhibit both isoforms of 5α-reductase to one degree or another. The potency of the extracts, as well as the potencies of two different batches of the same extract, appeared to be very different; qualitative and quantitative differences in the active ingredients may explain these results. The investigators recommended that the extract product of each company be tested to evaluate bioactivity and clinical efficacy.14
In a more recent study, Scaglione et al compared the activity of different SPEs that are widely available on the world market. Although differences in activity were observed between the tested extracts, all were able to inhibit 5α-reductase type I and II isoenzymes as well as fibroblast proliferation. The investigators concluded that extract potency differs between products, as does proliferation-inhibition potency, again citing quantitative and qualitative variations in the active ingredient as likely reasons for these differences.15
Due in part to varying bioactivities of the SPEs used, clinical studies of SPE in BPH have been inconsistent, with some showing significant results and others not.4,16–19 For example, Bent et al randomly assigned 225 men over the age of 49 years who had moderate-to-severe symptoms of BPH to 1 year of treatment with SPE (160 mg twice a day) or placebo. The investigators found no significant difference between the SPE and placebo groups in change in American Urological Association Symptom Index scores, maximal urinary flow rate, prostate size, residual volume after voiding, quality of life, or serum prostate-specific antigen levels during the study.16
An earlier publication by our group reported the results of an in vitro study of an SPE made using ethanol as the solvent.20 The current manuscript reports the results of an in vitro study involving an improved, supercritical SPE, a different product in that a supercritical CO2 solvent extracts chemistries that differ from those of the ethanol-extracted product. In order to make a comparison to the earlier-generation product that was as valid as possible, we felt it was essential that the second trial evaluate the new product – SP supercritical CO2 extract (SPSE), Prosterol® (Euromed) – following in vitro methods and conditions that were identical to those used in the earlier trial.
Objective
The objective of the present study was to determine in a cell-free test system the in vitro potency of a novel SPSE, Prosterol®, an inhibitor of the 5α-reductase isoenzyme type II.
Materials and methods
Homogenates containing 5α-reductase isoenzyme II were isolated from transfected human embryonic kidney cells (HEK293), stably expressing the 5α-reductase isoenzyme type II (HEK293-5α2 = HEK II).21,22 The inhibitory potency of SPSE was measured on the basis of the enzymatic conversion of the substrate androstenedione to the 5α-reduced product 5α-androstanedione, and compared to that of finasteride, an approved 5α-reductase inhibitor.2,3 The HEK cells are a commercially available cell line, and ethical approval was neither required nor available for the HEK cell lines used in this study.
Cell culture
HEK II cells were cultivated in Dulbecco’s Modified Eagle’s Medium (pH 7.4) with 10% fetal bovine serum, penicillin–streptomycin (100 U/mL and 100 μg/mL), and 0.5 mg/mL of Geneticin 418 sulfate in a humidified 5% CO2 atmosphere at 37°C.
Preparation of 5α-reductase type I-containing cell fractions
For the cell-free in vitro assays, HEK II cells were harvested. They were then freed from culture medium by centrifugation (500 ×g), and resuspended in a homogenate buffer consisting of 50 mM Tris-HCl (pH 7.4), 300 mM sucrose, 0.1 mM ethylenediaminetetraacetic acid, 10 mM dithiothreitol, and 100 μM phenylmethylsulfonyl fluoride. The cells were solubilized by freezing at −196°C and thawing on ice.
Following incubation for 30 minutes at 4°C with 1 mg/mL DNase in 50 mM MgCl2 and vigorous shaking in between, the obtained homogenate was centrifuged at 20,200 ×g for 50 minutes at 4°C in a refrigerated centrifuge. The resulting pellet was resuspended in homogenate buffer and again centrifuged at 20,200 ×g for 30 minutes at 4°C. This procedure was repeated twice. Using homogenate buffer, the homogenate pellet was detached from the tube bottom and resuspended by Ultra-Turrax® mixing at the highest speed. With the commercially available RotiQuant® reagent, protein content was quantified according to the Bradford method; the fractionated cell suspension was then aliquoted and stored at −80°C.
Assays of cell-free in vitro inhibition
Provided as oily extract, SPSE was diluted to working solutions of fivefold-higher strength than the intended test dilutions: SPSE extract was dissolved in dimethyl sulfoxide (DMSO) at a concentration of 0.5 g/mL and was diluted further 1:2 (v/v) in ethanol to obtain a 0.25 g/mL stock solution containing 50% ethanol and 25% DMSO (v/v).
Since the stock solution was insoluble in sodium citrate assay buffer, dilutions of the extract were prepared as follows. The stock solution was diluted in phosphate-buffered saline (PBS), and NaOH was then added until the test item was dissolved (pH in the range, 10.7 to 12.2). With a concentration of 5 mg/mL and 0.5 mg/mL for concentration finding- and half-maximal inhibitory concentration (IC50)-determination assays, respectively, the predilution was used to prepare the respective fivefold-concentrated test-item starting solutions.
Prior to application, aliquots of test item starting solutions were diluted with sodium citrate buffer (at a pH of 5.5) to the intended final test concentration for the purpose of checking again the final solubility as well as the pH values. The final solvent concentrations were 0.2% ethanol and 0.1% DMSO for concentration finding or 0.02% ethanol and 0.01% DMSO for IC50 determination.
Incubations were performed in a sodium citrate buffer incubation mixture at 37°C, with a final volume of 250 μL containing 0.24 mM NADPH, 250 nM androstenedione, and 10 μg cell homogenate and inhibitor dilutions. Serving as an internal control, finasteride was dissolved in ethanol, further diluted in PBS, and tested at a final concentration of 6 nM.
Solvent-treated controls were processed similarly, and contained 0.2% ethanol and 0.1% DMSO for concentration finding or 0.02% ethanol and 0.01% DMSO for IC50 determination, respectively. The addition of cellular homogenates started the enzyme reactions. After 15 minutes, incubations were brought to a stop by the addition of 50 μL 10 N NaOH, followed by shaking at 800 rpm.
For the extraction of product and unconverted substrate, 600 μL ethyl acetate containing internal-standard griseofulvin (0.03 μM) were added to each sample. Samples were centrifuged for 5 minutes at approximately 4,800 ×g following 10 minutes of shaking, and the ethyl acetate supernatant was pipetted into fresh cups. The solvent was then evaporated, and the dried residues reconstituted in 35 μL methanol and subsequently subjected to liquid chromatography/mass spectrometry. The injection volume per sample was 12 μL.
Liquid chromatography/mass spectrometry method
The high-performance liquid chromatography system consisted of an MS Plus pump (Surveyor, Thermo Fisher Scientific, Waltham, MA, USA) and an AS Plus autosampler (Surveyor). Mass spectrometry was performed on a TSQ Quantum Discovery Max triple-quadrupole mass spectrometer equipped with an atmospheric pressure chemical ionization interface (Thermo Fisher Scientific, Waltham, MA, USA) connected to a PC running the standard software Xcalibur 2.0.7.
Full-scan mass spectra were acquired in the positive mode using syringe-pump infusion to identify the protonated quasimolecular ions [M+H]+. Autotuning was carried out for maximizing ion abundance, followed by the identification of characteristic fragment ions using a generic parameter set (parameters will be listed). Ions with the highest signal-to-noise ratios were used to quantify the item in the selected reaction-monitoring mode and as qualifier, respectively.
The pump flow rate was set to 300 μL/min and the compounds separated on a Gemini C6-phenyl, 3 μm, 50×2 mm analytical column with a Gemini C6-phenyl, 3 μm, 4×2 mm precolumn (Phenomenex, Torrance, CA, USA). The gradients presented in Table 1 were used to detect the test items. The organic and aqueous components consisted of acetonitrile/0.1% (v/v) formic acid and 10 mM ammonium formate/0.5% formic acid, respectively. Mass spectrometry and chromatographic parameters are presented in Table 2.
Table 1.
HPLC gradients (test and reference items)
| Androstenedione, 5α-androstanedione (minutes) | Mobile phase
|
|
|---|---|---|
| A (%) | B (%) | |
| 0 | 5 | 95 |
| 0.2 | 5 | 95 |
| 1.0 | 97 | 3 |
| 2.5 | 97 | 3 |
| 2.6 | 5 | 95 |
| 4.0 | 5 | 95 |
Notes: A, acetonitrile/0.1% (v/v) formic acid; B, 10 mM ammonium formate/0.5% formic acid.
Abbreviation: HPLC, high-performance liquid chromatography.
Table 2.
Mass spectrometry and chromatographic parameters (test and reference items)
| Analyte
|
|||
|---|---|---|---|
| Androstenedione | 5α-androstanedione | ISTD (griseofulvin) | |
| Molecular weight (g/mol) | 286.4 | 288.4 | 352.8 |
| [M+H]+ (m/z) | 287.1 | 289.1 | 353.2 |
| Monitoring ion (m/z) | 109 | 213 | 215 |
| Scan time (seconds) | 0.15 | 0.15 | 0.05 |
| Collision energy (V) | 25 | 25 | 20 |
| Retention time (minutes) | 2.6 | 2.7 | 2.5 |
Abbreviations: ISTD, internal standard; m/z, mass-to-charge ratio.
Data analysis
Results are displayed as peak area ratio, in which the area of the analyte peak is divided by the area of the internal standard peak. Conversion rates were calculated according to the following formula:
| (1) |
Expressed as percentage-inhibition values relative to untreated controls, inhibition rates were calculated out of the mean conversion rates with (n=2) and without inhibitor (n=2). IC50 values were calculated by linear interpolation of the concentrations of test compounds and the corresponding percentage of inhibition that bracketed 50%:
| (2) |
Quality control
Assay acceptability
The assay setup included a positive-control inhibitor. The in vitro assays were deemed to be acceptable if they met ≥60% inhibition of 5α-reductase type II by finasteride at a concentration of 6 nM for the cell-free test system.
Results
Assay
A cell-free assay using cell homogenates isolated from stably transfected HEK293 cells was used to test SPSE in vitro for its inhibitory potential toward 5α-reductase type II. The following final concentrations of SPSE were tested: 10, 5, 2.5, 1.25, 1, and 0.5 μg/mL. The test concentrations were selected according to the results of the concentration-finding experiments (Tables 3 and 4).
Table 3.
Inhibition of 5α-reductase isotype II by saw palmetto supercritical CO2 extract in vitro (5α-reductase isotype II-containing cell fractions, concentration finding 1)
| Concentration (μg/mL) | 200 | 100 | 50 | 6 nM finasteride |
| Mean inhibition (%), n=2 | 99 | 99 | 99 | 61 |
Table 4.
Inhibition of 5α-reductase isotype II by saw palmetto supercritical CO2 extract in vitro (5α-reductase isotype II-containing cell fractions, concentration finding 2)
| Concentration (μg/mL) | 100 | 10 | 1 | 6 nM finasteride |
| Mean inhibition (%), n=2 | 99 | 93 | 22 | 63 |
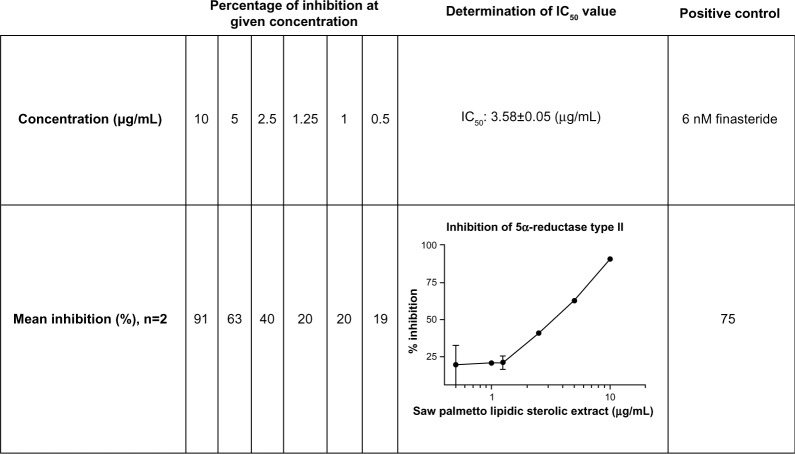
SPSE concentration-dependently inhibited 5α-reductase isoform type II. The IC50 value was calculated to be 3.58±0.05 μg/mL. Competitive binding of the test items toward the active site of the enzyme can be assumed, based on the resulting concentration–response curve. Figure 1 displays the results obtained from the cell-free IC50-determination experiment.
Figure 1.
Inhibition of 5α-reductase isotype II by saw palmetto supercritical CO2 extract in vitro (5α-reductase isotype II-containing cell fractions, determination of IC50 value, mean ± SEM).
Abbreviations: IC50, half-maximal inhibitory concentration; SEM, standard error of mean.
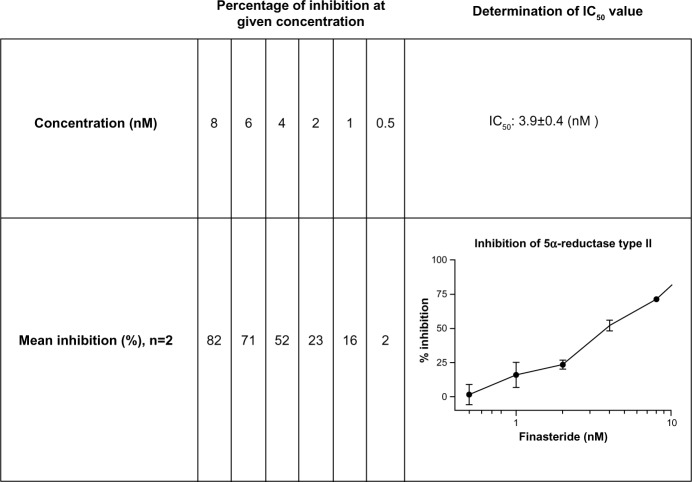
Finasteride, the established 5α-reductase inhibitor, was tested as positive control for the cell-free experiments at 6 nM, and led to 63%–75% inhibition. Figure 2 presents the dose–response curve for finasteride obtained with the cell homogenate that was used for the assays.
Figure 2.
Inhibition of 5α-reductase isotype II by finasteride extract in vitro (5α-reductase isotype II-containing cell fractions, determination of the IC50 value, mean ± SEM).
Abbreviations: IC50, half-maximal inhibitory concentration; SEM, standard error of mean.
Discussion
Summary of results of this trial
The novel SPSE was tested in vitro for its inhibitory potential toward 5α-reductase type II. Testing took place in a cell-free assay, and used cell homogenates that had been isolated from stably transfected HEK293 cells.
5α-reductase type II was inhibited in vitro by SPSE in a concentration-dependent fashion. The IC50 for this extract was determined to be 3.58±0.05 μg/mL, demonstrating competitive binding toward the active site of the enzyme. These BPH-bioassay results for SPSE compared very favorably with those for the standard of drug therapy – finasteride.
Mechanism of action of saw palmetto
Substantial research has shown SP to be an effective inhibitor of 5α-reductase activity in prostate-gland tissue.23,24 Unlike other inhibitors, SPE does not interfere with cellular capacity to secrete prostate specific antigen. SP, moreover, exerts anti-inflammatory effects that may also have salubrious effects on prostate health.5
Nonclinical trials with saw palmetto
The bioactive compounds contained in hexane and diethyl ether extracts of SP were isolated and characterized by Abe et al. With female rat liver microsomes as protein source and testosterone as substrate, the components (oleic acid and lauric acid) were tested for their in vitro potency toward 5α-reductase. IC50 values in the range of 54–68 μg/mL were determined using this methodology.13 5α-reductase type II inhibition by SPE was assessed in a cell-based system by Habib et al. The investigators found that SP at 10 μg/mL substantially inhibited the activity of 5α-reductase type II by 76%.23
Several brands of SPE were tested for their inhibitory potential toward 5α-reductase type I and II in another trial,14 using prostatic fibroblast and epithelial cells as an enzyme source. Although all extracts inhibited 5α-reductase, the potency of the extracts appeared to be very different (IC50 values ranging from 3.8 to 908 mg/L). With IC50 values of 3.841 and 4.313 mg/L for different batches, a hexane extract of SP was determined to be the most potent product in this study.14
Clinical trials with saw palmetto monotherapy
SP relieves urinary symptoms as effectively as the pharmaceutical 5α-reductase inhibitor finasteride in patients with BPH.5 The efficacy of 160 mg of SP taken twice daily for 2 years was assessed by Gerber et al. Patients’ quality of life and maximum urinary flow had improved and both prostate size and symptoms had decreased at each 6-month evaluation. As reported by study participants, sexual function remained stable for the first year of treatment and significantly improved during the second year.25
After receiving placebo for 1 month, men aged 45 years or older with moderate-to-severe symptoms of BPH were randomly assigned in another study to receive either SP or placebo for an additional 6 months.25 In addition to a significant decrease in prostate-symptom scores, quality-of-life scores increased to a greater degree in the SP group compared to men taking placebo. The investigators concluded that SP significantly improved urinary symptoms compared to placebo.25
Suter et al conducted an open clinical pilot trial to investigate whether an SP berry preparation influenced BPH symptoms and sexual dysfunction. Investigators’ and patients’ assessments confirmed good efficacy and tolerability. Correlation analyses confirmed the relationship between improved BPH symptoms and reduced sexual dysfunction. This was the first trial with SP to show improvement in BPH symptoms as well as sexual function.26
Giulianelli et al evaluated the efficacy of the hexane extract of SP in the treatment of lower urinary tract symptoms in 591 patients with chronic benign prostate diseases with associated inflammation, also taking into consideration the influence of treatment on sexual function and thus on patients’ quality of life. The investigators concluded that treatment for 6 months with the hexane extract of SP in routine clinical practice gave rise to a statistically significant improvement in peak flow rate and the International Prostate Symptom Score, National Institutes of Health Chronic Prostatitis Symptom Index, and International Index of Erectile Function 5 questionnaire scores, reflecting not only an improvement in urinary symptoms but also an overall improvement in patients’ quality of life.27
Avins et al randomized 369 patients in the CAMUS trial to 320 mg, 640 mg, and 960 mg daily of an ethanolic SPE or an identical-appearing placebo in an escalating manner at 6-month intervals, for a total of 18 months’ follow-up. The investigators found that SPE showed no evidence of toxicity at doses up to three times the usual clinical dose over a period of 18 months.28
The strongest evidence for the competitive efficacy and tolerability of SP in the treatment of BPH comes from randomized studies. Either alone or in combination with other plant extracts, SPE appears to be a viable option for improving lower urinary tract symptoms and flow measures. Although the evidence is less strong for Hypoxis rooperi and Secale cereale, these extracts also appear to improve symptoms of BPH.24
Combination therapy with saw palmetto extracts
In combination with an α-blocker, the hexane extract of SP is associated with benefits in terms of onset of action, reduction of inflammation, and improving sexual function when compared with use of α-blocker monotherapy in patients with BPH. Prescribing this extract in combination with α-blockers can provide benefits beyond those achieved with either therapy alone.29
In a recent review of the literature, Minutoli et al concluded that SP may have greater potential for the management of BPH when combined with selenium and lycopene.30 Comparative efficacy in BPH was assessed for the following three treatment regimens in another prospective trial: SPE 320 mg per day (n=20), tamsulosin 0.4 mg per day (n=20), and SPE + tamsulosin (n=20). The investigators in that study reported SPE and tamsulosin to be effective alone, but that no treatment demonstrated superiority, and combined therapy provided no further benefit. The investigators concluded that SPE is well tolerated and can be effective in the treatment of urinary tract symptoms secondary to BPH.31
As described, the results of clinical studies involving SPE for BPH have been equivocal: some have shown significant results, and others have not. Such inconsistent results may partly be the result of the varying bioactivities of the extracts used in those trials.4,16–19 Fully half of German urologists surveyed in a recent European study expressed a preference for SPE to pharmaceutical agents for the treatment of BPH.32 A Cochrane review, moreover, concluded that SPE produces improvements in urinary symptoms and flow measures that are comparable to those of finasteride.33 Investigators in another well-designed randomized controlled trial, however, reported no benefit in symptom relief or urinary flow measures following 1 year of therapy with SPE in patients with BPH.16
Further evaluations of standardized preparations of phytotherapeutic agents in randomized controlled trials with longer study durations will help in determining the long-term effectiveness of these products in the treatment of BPH. The strengths of this study lie in its well-validated scientific methods and the robust data generated, particularly the striking IC50 found for SPSE and its ability to reduce DHT, the main factor leading to prostate growth. Areas for future investigation, however, could include direct comparisons to competitor products, such as other CO2 or ethanolic extracts. Focusing on 5α-reductase inhibition is extremely important, but in order to address the quality and activity of a multicompound mixture like SPE, it might also be useful to look at other parameters, such as anti-inflammatory activity and PDE5 inhibition. The comparative differences among these extracts might also be further elucidated through analytical comparisons, such as those conducted by Booker et al, who characterized 57 different SP products using two distinct analytical approaches and found a high level of heterogeneity among the nine fatty acids contained in the various products.34
Conclusion
In this study a SPSE, Prosterol®, concentration-dependently inhibited 5α-reductase type II in vitro. Not only does SPSE effectively inhibit the enzyme that has been linked to BPH, but the amount of extract required for activity is also very low compared to data for other extracts that have been reported in similar tests. Data from the medical literature support the conclusion that SPSE is as effective as the hexane extract of SP that demonstrated the highest levels of in vitro bioactivity, and that SPSE is also more effective than other SPEs tested.
It can be confirmed from the results of this study that the SPSE Prosterol® has bioactivity that promotes prostate health at a level that is superior to that of many other phytotherapeutic extracts. The bioactivity of Prosterol® corresponds favorably to that reported for the hexane extract used in a large number of positive BPH clinical trials, as well as to finasteride, the established standard of therapy among prescription drugs. By establishing the bioactivity of SPSE, the present study reinforces the importance of conducting such trials prior to attempting clinical studies. Confirming bioactivity increases the likelihood of showing positive effects clinically, while reducing the chances of negative studies, such as those reported in the literature. Future in vitro and clinical trials involving SPEs would be useful for elucidating their comparative differences as well as appropriate patient selection for their use.
Acknowledgments
This study was sponsored by Euromed, Barcelona, Spain. The authors thank Pharmacelsus, Saarbrücken, Germany for their contributions to the design and conduct of this study, as well as for data analyses and data reporting. The authors also thank Aesculapius Consulting, Inc., for editorial assistance in developing this manuscript. Support for this assistance was provided by Euromed.
Footnotes
Disclosure
This study was funded by Euromed, Barcelona, Spain. PP, AV, and SR are employed by Euromed. The authors report no other conflicts of interest in this work.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
References
- 1.Shirakawa T, Okada H, Acharya B, et al. Messenger RNA levels and enzyme activities of 5 alpha-reductase types 1 and 2 in human benign prostatic hyperplasia (BPH) tissue. Prostate. 2004;58:33–40. doi: 10.1002/pros.10313. [DOI] [PubMed] [Google Scholar]
- 2.Délos S, Iehlé C, Martin PM, Raynaud JP. Inhibition of the activity of ‘basic’ 5α-reductase (type 1) detected in DU 145 cells and expressed in insect cells. J Steroid Biochem Mol Biol. 1994;48:347–352. doi: 10.1016/0960-0760(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 3.Boudon C, Lobaccaro JM, Lumbroso S, et al. 5 alpha-reductase activity in cultured epithelial and stromal cells from normal and hyperplastic human prostates – effect of finasteride (Proscar), a 5 alpha-reductase inhibitor. Cell Mol Biol (Noisy-le-Grand) 1995;41:1007–1015. [PubMed] [Google Scholar]
- 4.Lee J, Andriole G, Avins A, et al. Redesigning a large-scale clinical trial in response to negative external trial results: the CAMUS study of phytotherapy for benign prostatic hyperplasia. Clin Trials. 2009;6:628–636. doi: 10.1177/1740774509352199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Comhaire F, Mahmoud A. Preventing diseases of the prostate in the elderly using hormones and nutriceuticals. Aging Male. 2004;7:155–169. doi: 10.1080/13685530412331284722. [DOI] [PubMed] [Google Scholar]
- 6.Laydner HK, Oliveira P, Oliveira CR, et al. Phosphodiesterase 5 inhibitors for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a systematic review. BJU Int. 2011;107:1104–1109. doi: 10.1111/j.1464-410X.2010.09698.x. [DOI] [PubMed] [Google Scholar]
- 7.Bechara A, Casabe A, Rodriguez Baigorri G, Cobreros C. Effectiveness of tadalafil 5 mg once daily in the treatment of men with lower urinary tract symptoms suggestive to benign prostatic hyperplasia with or without erectile dysfunction: results from naturalistic observational TadaLutsEd study. J Sex Med. 2014;11:498–505. doi: 10.1111/jsm.12386. [DOI] [PubMed] [Google Scholar]
- 8.Falahatkar S, Mokhtari G, Pourreza F, Asgari SA, Kamran AN. Celecoxib for treatment of nocturia caused by benign prostatic hyperplasia: a prospective, randomized, double-blind, placebo-controlled study. Urology. 2008;72:813–816. doi: 10.1016/j.urology.2008.04.069. [DOI] [PubMed] [Google Scholar]
- 9.Araki T, Yokoyama T, Kumon H. Effectiveness of a nonsteroidal anti-inflammatory drug for nocturia on patients with benign prostatic hyperplasia: a prospective non-randomized study of loxoprofen sodium 60 mg once daily before sleeping. Acta Med Okayama. 2004;58:45–49. doi: 10.18926/AMO/32115. [DOI] [PubMed] [Google Scholar]
- 10.Kahokehr A, Vather R, Nixon A, Hill AG. Non-steroidal anti-inflammatory drugs for lower urinary tract symptoms in benign prostatic hyperplasia: systematic review and meta-analysis of randomized controlled trials. BJU Int. 2013;111:304–311. doi: 10.1111/j.1464-410X.2012.11559.x. [DOI] [PubMed] [Google Scholar]
- 11.Edwards JL. Diagnosis and management of benign prostatic hyperplasia. Am Fam Physician. 2008;77:1403–1410. [PubMed] [Google Scholar]
- 12.Allkanjari O, Vitalone A. What do we know about phytotherapy of benign prostatic hyperplasia? Life Sci. 2015;126:42–56. doi: 10.1016/j.lfs.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 13.Abe M, Ito Y, Suzuki A, Onoue S, Noguchi H, Yamada S. Isolation and pharmacologic characterization of fatty acids from saw palmetto extract. Anal Sci. 2009;25:553–557. doi: 10.2116/analsci.25.553. [DOI] [PubMed] [Google Scholar]
- 14.Scaglione F, Lucini V, Pannacci M, Caronno A, Leone C. Comparison of the potency of different brands of Serenoa repens extract on 5α-reductase types I and II in prostatic co-cultured epithelial and fibroblast cells. Pharmacology. 2008;82:270–275. doi: 10.1159/000161128. [DOI] [PubMed] [Google Scholar]
- 15.Scaglione F, Lucini V, Pannacci M, Dugnani S, Leone C. Comparison of the potency of 10 different brands of Serenoa repens extracts. Eur Rev Med Pharmacol Sci. 2012;16:569–574. [PubMed] [Google Scholar]
- 16.Bent S, Kane C, Shinohara K, et al. Saw palmetto for benign prostatic hyperplasia. N Engl J Med. 2006;354:557–566. doi: 10.1056/NEJMoa053085. [DOI] [PubMed] [Google Scholar]
- 17.Log T. Serenoa repens in benign prostatic hyperplasia. Tidsskr Nor Laegeforen. 2008;128:1293–1294. Norwegian. [PubMed] [Google Scholar]
- 18.MacDonald R, Tacklind JW, Rutks I, Wilt TJ. Serenoa repens monotherapy for benign prostatic hyperplasia (BPH): an updated Cochrane systematic review. BJU Int. 2012;109:1756–1761. doi: 10.1111/j.1464-410X.2012.11172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barry MJ, Meleth S, Lee JY, et al. Effect of increasing doses of saw palmetto extract on lower urinary tract symptoms: a randomized trial. JAMA. 2011;306:1344–1351. doi: 10.1001/jama.2011.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pais P. Potency of a novel saw palmetto ethanol extract, SPET-085, for inhibition of 5α-reductase II. Adv Ther. 2010;27:555–563. doi: 10.1007/s12325-010-0041-6. [DOI] [PubMed] [Google Scholar]
- 21.Reichert W, Harmann RW, Jose J. Stable expression of the human 5α-reductase isoenzymes type I and type II in HEK293 cells to identify dual and selective inhibitors. J Enzyme Inhib. 2001;16:47–53. doi: 10.1080/14756360109162354. [DOI] [PubMed] [Google Scholar]
- 22.Andersson S, Russell DW. Structural and biochemical properties of cloned and expressed human and rat steroid 5α-reductases. Proc Natl Acad Sci U S A. 1990;87:3640–3644. doi: 10.1073/pnas.87.10.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Habib FK, Ross M, Ho CK, Lyons V, Chapman K. Serenoa repens (Permixon) inhibits the 5α-reductase activity of human prostate cancer cell lines without interfering with PSA expression. Int J Cancer. 2005;114:190–194. doi: 10.1002/ijc.20701. [DOI] [PubMed] [Google Scholar]
- 24.Wilt TJ, Ishani A, Rutks I, MacDonald R. Phytotherapy for benign prostatic hyperplasia. Public Health Nutr. 2000;3:459–472. doi: 10.1017/s1368980000000549. [DOI] [PubMed] [Google Scholar]
- 25.Gerber GS, Kuznetsov D, Johnson BC, Burstein JD. Randomized, double-blind, placebo-controlled trial of saw palmetto in men with lower urinary tract infections. Urology. 2001;58:960–964. doi: 10.1016/s0090-4295(01)01442-x. [DOI] [PubMed] [Google Scholar]
- 26.Suter A, Saller R, Riedi E, Heinrich M. Improving BPH symptoms and sexual dysfunctions with a saw palmetto preparation? Results from a pilot trial. Phytother Res. 2013;27:218–226. doi: 10.1002/ptr.4696. [DOI] [PubMed] [Google Scholar]
- 27.Giulianelli R, Pecoraro S, Sepe G, et al. Multicentre study on the efficacy and tolerability of an extract of Serenoa repens in patients with chronic benign prostate conditions associated with inflammation. Arch Ital Urol Androl. 2012;84:94–98. [PubMed] [Google Scholar]
- 28.Avins AL, Lee JY, Meyers CM, Barry MJ, CAMUS Study Group Safety and toxicity of saw palmetto in the CAMUS trial. J Urol. 2013;189:1415–1420. doi: 10.1016/j.juro.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perry R, Milligan G, Anderson P, Gillon A, White M. Real-world use of Permixon in benign prostatic hyperplasia–determining appropriate monotherapy and combination treatment. Adv Ther. 2012;29:538–550. doi: 10.1007/s12325-012-0024-x. [DOI] [PubMed] [Google Scholar]
- 30.Minutoli L, Bitto A, Squadrito F, et al. Serenoa repens, lycopene and selenium: a triple therapeutic approach to manage benign prostatic hyperplasia. Curr Med Chem. 2013;20:1306–1312. doi: 10.2174/0929867311320100007. [DOI] [PubMed] [Google Scholar]
- 31.Hizli F, Uygur MC. A prospective study of the efficacy of Serenoa repens, tamsulosin, and Serenoa repens plus tamsulosin treatment for patients with benign prostate hyperplasia. Int Urol Nephrol. 2007;39:879–886. doi: 10.1007/s11255-006-9106-5. [DOI] [PubMed] [Google Scholar]
- 32.Lowe FC, Ku JC. Phytotherapy in treatment of benign prostatic hyperplasia: a critical review. Urology. 1996;48:12–20. doi: 10.1016/s0090-4295(96)00077-5. [DOI] [PubMed] [Google Scholar]
- 33.Wilt T, Ishani A, MacDonald R. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2002;3:CD001423. doi: 10.1002/14651858.CD001423. [DOI] [PubMed] [Google Scholar]
- 34.Booker A, Suter A, Krnjic A, et al. A phytochemical comparison of saw palmetto products using gas chromatography and 1H nuclear magnetic resonance spectroscopy metabolomic profiling. J Pharm Pharmacol. 2014;66:811–822. doi: 10.1111/jphp.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]