Abstract
STUDY HYPOTHESIS
Are active ovarian germ stem cells present in postnatal mouse ovaries under physiological conditions?
STUDY FINDING
Active ovarian germ stem cells exist and function in adult mouse ovaries under physiological conditions.
WHAT IS KNOWN ALREADY
In vitro studies suggested the existence of germ stem cells in postnatal ovaries of mouse, pig and human. However, in vivo studies provided evidence against the existence of active germ stem cells in postnatal mouse ovaries. Thus, it remains controversial whether such germ stem cells really exist and function in vivo in postnatal mammalian ovaries.
STUDY DESIGN, SAMPLES/MATERIALS, METHODS
Octamer-binding transcription factor 4 (Oct4)-MerCreMer transgenic mice were crossed with R26R-enhanced yellow fluorescent protein (EYFP) mice to establish a tamoxifen-inducible tracing system so that Oct4-expressing potential ovarian germ stem cells in young adult mice (5–6 weeks old) can be labeled with EYFP. The germ cell activities of DNA replication, mitotic division, entry into meiosis and progression to primordial follicle stage were investigated by means of immunofluorescent staining of ovarian tissues collected at different time points post-tamoxifen injection (1 day, 3 days, 2 months and 4 months). Meiosis entry and primordial follicle formation were also measured by EYFP-labeled single-cell RT–PCR. Germ cell proliferation and mitotic division were examined through 5-bromodeoxyuridine triphosphate incorporation assay. At each time point, ovaries from two to three animals were used for each set of experiment.
MAIN RESULTS AND THE ROLE OF CHANCE
By labeling the Oct4-expressing small germ cells and tracing their fates for up to 4 months, we observed persistent meiosis entry and primordial follicle replenishment. Furthermore, we captured the transient processes of mitotic DNA replication as well as mitotic division of the marked germ cells at various time periods after tracing. These lines of evidence unambiguously support the presence of active germ stem cells in postnatal ovaries and their function in replenishing primordial follicle pool under physiological conditions. Moreover, we pointed out that Oct4+ deleted in azoospermia-like (Dazl)− but not Oct4+Dazl+ or Oct4+ DEAD (Asp–Glu–Ala–Asp) Box Polypeptide 4 (Ddx4)+ cells contain a population of germ stem cells in mouse ovary.
LIMITATIONS, REASONS FOR CAUTION
This study was conducted in mice. Whether or not the results are applicable to human remain unclear. The future work should aim at identifying the specific ovarian germ stem cell marker and evaluating the significance of these stem cells to normal ovarian function.
WIDER IMPLICATIONS OF THE FINDINGS
Clarifying the existence of active germ stem cells and their functional significance in postnatal mammalian ovaries could provide new insights in understanding the mechanism of ovarian aging and failure.
LARGE SCALE DATA
Not applicable.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported by the National Key Basic Research Program of China (grant number 2012CBA01300) and the National Natural Science Foundation of China to P.Z. (31571484). No competing interests are reported.
Keywords: genetic tracing, primordial follicle replenishment, ovarian germ stem cells, postnatal mouse ovary, meiosis, neo-oogenesis
Introduction
Some invertebrate (e.g. Drosophila) and lower vertebrate females (e.g. fish) continuously produce gametes from germ stem cells in ovaries after birth (Draper et al., 2007; Xie et al., 2008; Nakamura et al., 2010). In mammals, the prevailing dogma in the field declares that neo-oogenesis ceases at birth as oocytes enter the prophase of the first meiotic division and arrest at the dictyate. Under this rubric, newborn females possess their life time complement of germ cells stored as primordial follicles that are not replenished after birth. Recently, this model has been challenged by several important findings, which reported the observation of meiosis entry in postnatal mouse ovary (Johnson et al., 2004), or isolating rare cells in adult mouse and human ovarian tissue that can proliferate in dishes and form developmentally competent oocytes following transplantation (Zou et al., 2009; White et al., 2012). Although these pioneer works and other follow-up studies (Pacchiarotti et al., 2010; Zou et al., 2011; Bai et al., 2013; Bui et al., 2014; Zhou et al., 2014) suggest the presence of germ stem cells in postnatal ovaries of several mammalian species, they do not address whether such stem cells actually exist and function in vivo under physiological conditions. Instead, they reignited debate regarding if these stem cells were an artifact of purification or culture methods (Telfer and Albertini, 2012; Vogel, 2012; Evron and Blumenfeld, 2013; Hernandez et al., 2015). For instance, by reconstructing female cell lineage trees, Shapiro et al. offered indirect evidence supporting the postnatal oocyte renewal from germ-line stem cells under physiological conditions (Reizel et al., 2012; Woods et al., 2012). On the other hand, several recent studies presented evidence indicating no germ stem cell activity in postnatal mouse ovaries under physiological and injury conditions (Lei and Spradling, 2013a; Zhang et al., 2014, 2015). Thus, whether there are active germ stem cells and primordial follicle replenishment in postnatal ovaries in mammals remains controversial.
In vivo genetic cell fate tracing using Cre-loxP system is a definitive method for investigating the contribution of stem cells to tissue development and homeostasis (Kretzschmar and Watt, 2012). It provides information on how cells behave in the context of intact tissue, as opposed to what can be learned about the cells following their isolation, in vitro culture and transplantation. The key to successful genetic cell fate tracing is the selection of the gene driving Cre recombinase expression. Ideally, stem-cell-specific gene is used to drive the tracing system. However, other genes that are not restrictively expressed in stem cells of the investigated tissue can also be utilized in case of lacking stem cell marker (Morris et al., 2004; Kretzschmar and Watt, 2012). To investigate whether the putative germ stem cells function in vivo in adult mouse ovaries, one recent work employed CAG (chicken β-actin) promoter to drive the Cre-loxP system in a tamoxifen-inducible manner. They observed a stable primordial follicle pool, but did not detect the presence of DEAD (Asp–Glu–Ala–Asp) box polypeptide 4 (Ddx4)-expressing germ cell clusters, which they considered as an indicator of the existence of active germ stem cells. Therefore, they concluded that adult female mice lacked germ stem cells but sustained oogenesis using stable primordial follicles (Lei and Spradling, 2013a). Germ-cell-specific gene Ddx4 (also known as Vasa) is considered to be expressed and localized in plasma membrane of putative germ stem cells of postnatal ovaries. Its extracellular epitope was utilized to isolate a germ stem cell population in previous studies (Zou et al., 2009; White et al., 2012; Imudia et al., 2013). However, Ddx4 expression is not detected in undifferentiated primitive germ cells of fetal ovaries (Toyooka et al., 2000; Anderson et al., 2007). Utilizing Ddx4-driving Cre-loxp system to label the Ddx4-expressing cells also failed to detect mitotic cell division of such cells or de novo folliculogenesis in mice (Zhang et al., 2012). Moreover, recent two studies challenged the idea that Ddx4 extracellular epitope can be used to isolate putative ovarian germ stem cells (Hernandez et al., 2015; Zhang et al., 2015). Thus, whether putative ovarian germ stem cells express Ddx4 or if the Ddx4-driven Cre-loxp system can mark ovarian germ stem cells are questioned (Bhartiya et al., 2013). Freshly purified putative germ stem cells from postnatal mouse ovary and undifferentiated primitive germ cells in fetal ovary express octamer-binding transcription factor 4 (Oct4) (Bowles and Koopman, 2007; Imudia et al., 2013). Therefore, we used Oct4 promoter to drive a tamoxifen-inducible Cre-loxP tracing system to investigate whether there are active germ stem cells in postnatal mouse ovaries under physiological conditions.
Materials and Methods
Mice
Normal CD1 female mice (3–4 weeks old) were used for these investigations. Mouse lines Oct4-MerCreMer, R26R-enhanced yellow fluorescent protein (EYFP) and OG2 were obtained from the Jackson Laboratory (Bar Harbor, ME, USA; stock numbers 016829, 006148, and 004654, respectively, C57BL/6 background). Mice were maintained in specific-pathogen-free condition. Oct4-MerCreMer mice were crossed with R26R-EYFP mice. Oct4-MerCreMer+/Tm; R26R-EYFPTm/Tm female and male mice (5–6 weeks old) were used for tamoxifen injection.
Ethics
Animal care and experimental procedures were approved by the Animal Care and Use Committee of the Kunming Institute of Zoology, Chinese Academy of Sciences. The experiments were carried out in accordance with the approved guidelines.
Tamoxifen preparation and injection
Mice were weighed and the amount of tamoxifen (Sigma-Aldrich, St. Louis, MO, USA; T5648) required for administration was calculated according to the working concentration (e.g. 0.25 mg/g body weight). Tamoxifen, which was sufficient for a single injection, was dissolved in 300 μl 100% ethanol. Corn oil (300 μl) (Sigma-Aldrich, St. Louis, MO, USA) was then added into the tamoxifen ethanol solution to make a working solution. The mixture of tamoxifen-containing ethanol and corn oil was vortexed and pipetted up and down to eliminate precipitation. Residual ethanol was removed by vacuum centrifuge. The whole of the corn-oil solution tamoxifen was then administered to a mouse by intraperitoneal injection (i.p. injection).
Frozen tissue section preparation and immunofluorescence staining
Ovarian and testis tissues were fixed in formalin (10%, v/v, buffered neutral) at room temperature for 14 h, followed by incubation in 15 (w/v) and 30% (w/v) sucrose overnight and embedded in O.C.T. (Tissue Freezing Medium, Leica, Wetzlar, Germany; 020108926). The specimens were stored at −80°C before cutting 8-μm sections. For immunofluorescence staining, antigen retrieval was performed by placing slides in a pressure cooker in citrate antigen retrieval buffer (pH 6.0) (Fuzhou Maixin Biotech, Fuzhou, China; MVS-0100) for 2 min. Slides were then washed in phosphate buffered saline (PBS) and permeabilized in 0.1% (v/v) Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA) for 15 min. Sections were blocked with 5% (w/v) bovine serum albumin (BSA) (Sangon, Shanghai, China; A0332) followed by sequential incubations with primary and secondary antibodies (Table I). The specificity of all primary antibodies was examined by omitting primary antibodies in immunostaining. DNA was counterstained with 4′,6-diamidino-2-phenylindole (Sigma-Aldrich, St. Louis, MO, USA). Mounted slides were imaged using a laser scanning confocal microscope (Olympus, Tokyo, Japan). Images were analyzed using the FV10-ASW 2.1 viewer software. For each experiment, testis or ovaries from two to three animals were used for investigation.
Table I.
Information of primary and secondary antibodies.
| Antibody | Company | Catalog number | Dilution |
|---|---|---|---|
| Primary antibodies | |||
| Oct4 | Abcam | AB19857 | 1:300 |
| Oct4 | Santa Cruz | SC8628 | 1:200 |
| Sycp3 | Abcam | AB97672 | 1:300 |
| Stra8 | Abcam | AB49602 | 1:300 |
| Ddx4 | Abcam | AB13840 | 1:300 |
| GFP | Abcam | AB13970 | 1:2000 |
| GFP | Cell Signaling Technology | 2956 | 1:200 |
| BrdU | Novus Biologicals | NB500-169 | 1:300 |
| Dazl | Novus Biologicals | NB100-2437 | 1:300 |
| Stella | Abcam | AB19878 | 1:300 |
| Foxl2 | Abcam | AB5096 | 1:200 |
| Secondary antibodies | |||
| Alexa Fluor® 488 Donkey anti Rabbit IgG | Invitrogen | A-21206 | 1:500 |
| Alexa Fluor® 555 Donkey anti Rabbit IgG | Invitrogen | A-31572 | 1:500 |
| Alexa Fluor® 488 Goat anti Rabbit IgG | Invitrogen | A-11008 | 1:500 |
| Alexa Fluor® 647 Goat anti Rabbit IgG | Invitrogen | A-21244 | 1:500 |
| Alexa Fluor® 555 Donkey anti Goat IgG | Invitrogen | A-21432 | 1:500 |
| Alexa Fluor® 488 Goat anti Mouse IgG | Invitrogen | A-11029 | 1:500 |
| Alexa Fluor® 555 Donkey anti Mouse IgG | Invitrogen | A-31570 | 1:500 |
| Alexa Fluor® 488 Goat anti Chicken IgG | Invitrogen | A-11039 | 1:500 |
| Alexa Fluor® 488 Donkey anti Rat IgG | Invitrogen | A-21208 | 1:500 |
| Alexa Fluor® 647 Goat anti Rat IgG | Invitrogen | A-21247 | 1:500 |
5-Bromo-2′-deoxyuridine triphosphate incorporation assay
BrdU (5-Bromo-2′-deoxyuridine; Sigma-Aldrich, St. Louis, MO, USA) was re-suspended in PBS before administration. BrdU solution was injected i.p. into 3- to 4-week-old CD1 females (50 mg/kg body weight) and tamoxifen-treated double-mutant mice. Ovarian tissues were collected 2 (for CD1 mice) or 12 h (for tamoxinfen treated mice) after BrdU injection and processed for frozen sections as described above. To probe with BrdU primary antibody, sections were sequentially treated with 2N HCl for 30 min and 0.1 M sodium borate for 10 min prior to permeabilization and immunofluorescence staining.
Isolation of EYFP-expressing ovarian germ cells
Tamoxifen was injected into 5- to 6-week-old double-targeted mutation mice (Oct4-MerCreMer+/Tm; R26R-EYFPTm/Tm). Ovarian tissues were collected at 1 day, 3 days, 2 weeks and 1 month after tamoxifen injection. Ovarian single-cell suspension was prepared by two-step enzymatic digestion described previously (Zou et al., 2009). In brief, ovaries were placed in PBS solution without calcium or magnesium but containing 1 mg/ml type IV collagenase (Life Technologies, Carlsbad, CA, USA) and incubated at 37°C for 15 min. After washing two times in PBS, ovarian tissue was placed in PBS containing 0.05% (w/v) trypsin (Life Technologies, Carlsbad, CA, USA.) and incubated at 37°C for 5–10 min. Trypsin was neutralized by adding 10% (v/v) fetal bovine serum. The clumps of residual tissue were removed by passing the suspension through a 70-μm nylon cell strainer. The cell suspension was centrifuged at 300g for 5 min. The pellet was re-suspended in 0.3% (w/v) PBS-BSA. EYFP-expressing germ cells were then manually picked by using a mouth pipette under the fluorescent microscope (Nikon Eclipse Ti-S, Tokyo, Japan).
Single-cell cDNAs preparation and amplification
Single-cell cDNAs preparation and amplification were performed according to the published protocol (Tang et al., 2010). Briefly, cDNAs were prepared from single cell and amplified by 20 cycles of PCR. cDNA samples were then stored at −80°C.
Reverse transcription and polymerase chain reaction
PCR primers to detect gene expression are described in Table II. Upper and lower primers of all genes except for EYFP were designed to span across introns. The PCR condition consists of 1 cycle at 94°C for 5 min; 35 cycles at 94°C for 30 s, 57°C for 40 s, and 72°C for 40 s; and an extension cycle at 72°C for 10 min. When amplifying synaptonemal complex protein 3 (Sycp3), stimulated by retinoic acid gene 8 (Stra8), spermatogenesis- and oogenesis-specific basic helix–loop–helix 1 (Sohlh1) and EYFP, the annealing temperature was set at 54°C.
Table II.
Details for PCR primers used to analyze gene expression in cDNA samples prepared from mouse cells and tissues.
| Gene | Accession number | Primer sequences (5′ to 3′; F, forward; R, reverse) | Size (bp) |
|---|---|---|---|
| Oct4 | NM_013633 | F: GGAGGAAGCCGACAACAA | 322 |
| R: GGGCAGAGGAAAGGATACAG | |||
| Stella | NM_139218 | F: ATGAAGAGGACGCTTTGGA | 382 |
| R: CCGATTTTCGCATTCTCAG | |||
| Ddx4 | NM_010029 | F: CATTCCCATTGTATTAGCAGG | 481 |
| R: AAGGGTTTGGCGTTGTTC | |||
| Dazl | NM_010021 | F: CTCCTCCACCACAGTTCCA | 365 |
| R: CACTGTCTGTATGCTTCGGTC | |||
| Sycp3 | NM_011517 | F: AGCCACCTTTGGTTGATC | 497 |
| R: CTGCTCGTGTATCTGTTTGA | |||
| Stra8 | NM_009292 | F: ACAACCTAAGGAAGGCAGTTTAC | 173 |
| R: GACCTCCTCTAAGCTGTTGGG | |||
| EYFP | KP171163 | F: CACAAGTTCAGCGTGTCCG | 421 |
| R: AGTTCACCTTGATGCCGTTC | |||
| Gapdh | NM_008084 | F: TTGAGGTCAATGAAGGGGTC | 132 |
| R: TCGTCCCGTAGACAAAATGG | |||
| Sohlh1 | NM_001001714.1 | F: GATGTCTGTGTACTTCCTCC | 320 |
| R: CTGGCTCACTGAATGACAAC |
The following controls were set up in performing PCR: cDNA templates from mouse testis were used as positive controls in detecting Sycp3, Stra8 and Sohlh1 mRNA expression; cDNAs from single germinal vesicle stage oocyte were used as positive controls for detection of Oct4, developmental pluripotency-associated protein 3 (Stella), deleted in azoospermia-like (Dazl), and Ddx4 expression; genomic DNAs prepared from R26R-EYFPTm/Tm mouse tail tip were used as positive control for detection of EYFP. cDNA samples from somatic cells were used as negative control in detection of germ-cell-specific gene expression.
Results
Oct4 is expressed in primitive germ cells of postnatal mouse ovaries and can be used for marking putative germ stem cells
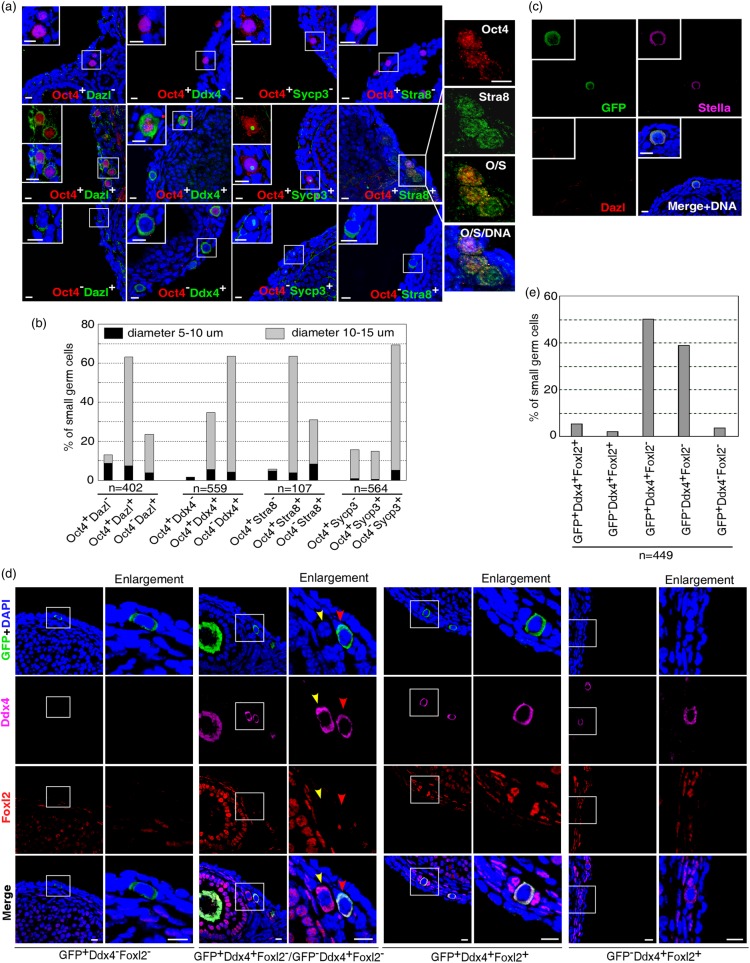
Initiation of female germ cell differentiation and entrance into meiosis occur prior to follicle formation. Previous studies reported the detection of small germ cells not intimately associated with a layer of squamous epithelial cells in the surface epithelium, resembling germ cells in fetal ovaries (Johnson et al., 2004; Niikura et al., 2009). Importantly, some of these small germ cells were capable of DNA replication or expressing Sycp3 indicative of the meiosis entry (Johnson et al., 2004; Zou et al., 2009). We validated these observations by immunostaining ovaries of juvenile mice (3–4 weeks old, 2–3 animals for each group of immunostaining examination) with germ cell differentiation markers Dazl, Ddx4, Stra8 and Sycp3. The specificity of the commercial antibodies to Stra8 and Sycp3 was verified in testicular samples (Supplementary Fig. S1). It should be mentioned that the protein distribution pattern of Sycp3 in ovary is distinct from that in testis. As previously reported (Yang et al., 2006; Morelli et al., 2008; Tsutsumi et al., 2011), Sycp3 aligns on chromosomes in testis (Supplementary Fig. S1a). However, it is distributed as punctuates in ovary (Supplementary Fig. S1e) (Liu et al., 2007; Zou et al., 2009; Lei and Spradling, 2013b). Consistently, a population of small germ cells not morphologically in any type of follicular structure were detected at the ovarian surface (refer to as small germ cells thereafter, 98% of them have diameter <15 μm, n = 1476) (Fig. 1a). Co-staining these cells with antibody to Oct4, a pluripotency and germ-line-specific marker expressed in primitive germ cells and freshly isolated putative ovarian germ stem cells (Scholer et al., 1989; Bowles and Koopman, 2007; Imudia et al., 2013), documented their heterogeneity in developmental stage (Fig. 1a and b). For instance, co-staining of Oct4 with Stra8 revealed three populations. Among them, Oct4+Stra8− (5.61%, n = 107) germ cells were more primitive than Oct4+Stra8+ (63.55%, n = 107) followed by Oct4−stra8+ (30.84%, n = 107) germ cells. Importantly, a minority of the cells were small (5–10 μm in diameter), expressed Oct4, but did not initiate the early germ cell differentiation marker Dazl or Ddx4 (Oct4+Dazl− or Oct4+Ddx4−) (Fig. 1a), and thus represented the most primitive germ cells in postnatal ovaries. To confirm the germ cell identity of these Oct4+Dazl− or Oct4+Ddx4− cells, we examined whether these Oct4-expressing cells express another primitive germ cell marker Stella (Saitou et al., 2002; Payer et al., 2003). To do this, we used the OG2-enhanced green fluorescent protein (EGFP) transgenic mice (3–4 weeks old) in which EGFP expression is driven by germ-line-specific Oct4 promoter (Yeom et al., 1996). Triple staining with EGFP, Stella and Dazl revealed that all EGFP/Oct4-expressing cells are germ cell lineage as evidenced by the expression of germ cell marker Dazl and/or Stella (Fig. 1c, 288 EGFP+ small germ cells were examined in total). Moreover, co-staining of OG2-EGFP mouse ovaries with EGFP (Oct4), Ddx4, and forkhead box L2 (Foxl2), a marker of the granulosa cells (Schmidt et al., 2004; Matson et al., 2011; Zhang et al., 2014), showed that the majority (92.4%, n = 449) of the small germ cells (not morphologically in any type of follicular structure) was not surrounded by Foxl2-expressing granulosa cells (Fig. 1d and e). These results validated that >90% of small germ cells were indeed at pre-follicular stage. In particular, all EGFP(Oct4)+Ddx4− small germ cells did not form a follicle (Fig. 1d and e). Taken together, these observations suggested the presence of primitive germ cells and proposed the use of Oct4 promoter to establish a Cre-loxP genetic tracing system for labeling putative germ stem cells and investigating their physiological activities in postnatal mouse ovary.
Figure 1.
Small germ cells at differential developmental stages in juvenile (3–4 weeks old) mouse ovaries. (a) Co-staining Oct4 with Dazl, Ddx4, Sycp3 or Stra8 documented different types of small germ cells. Insets show the higher magnification of germ cells indicated by squares. (b) Proportions of each type of small germ cells. (c) In OG2 transgenic mouse ovaries, EGFP+Dazl− (n = 288) cells express germ cell marker Stella, indicating that Oct4+Dazl− cells are germ cells. (d) Co-staining of OG2-EGFP mouse ovaries with EGFP, Ddx4 and Foxl2 documented five types of small germ cells. Enlargement columns show the higher magnification of germ cells in squares. Red arrowhead indicates the EGFP+Ddx4+ germ cell not surrounded by Foxl2-expressing granulosa cells. Yellow arrowhead, the EGFP−Ddx4+ small germ cell not surrounded by Foxl2-expressing granulosa cells. (e) Proportions of each type of small germ cells in OG2 transgenic mouse ovaries according to the expression of EGFP, Ddx4 and Foxl2. Ovaries from two to three animals were used for each group of immunostaining. Scale bar, 10 μm.
Cre-loxP genetic tracing of Oct4-expressing cells in postnatal mouse ovaries reveals persistent meiosis entry and formation of new primordial follicles
Oct4-MerCreMer mice were crossed with R26R-EYFP mice to establish a tamoxifen-inducible tracing system in which cells expressing Oct4 at the time of tamoxifen administration are permanently marked with EYFP (Supplementary Fig. S2). Because Oct4 is expressed in mouse spermatogonial stem cells (SSCs) (Ohbo et al., 2003), we first tested whether this cell tracing system functioned in males. No germ cells in testis expressed EYFP without tamoxifen induction, indicating the absence of constitutive expression or of system leak (Supplementary Fig. S3a). At 3 days post-injection (dpi) of tamoxifen (0.25 mg/g body weight), some EYFP+ germ cells were specifically detected near the basal membrane of seminiferous tubule (Supplementary Fig. S3b and c). Given that the completion of spermatogenesis takes about 1 month in mice, we examined the fates of EYFP+ cells at 1 and 4 months post-injection (mpi). EYFP+ germ cells at differential spermatogenic stages were observed at both time points (Supplementary Fig. S3d–g), indicating continuous spermatogenesis from the EYFP-labeled SSCs and the soundness of the tracing system.
In postnatal ovaries, Oct4 is expressed not only in a fraction of non-follicular germ cells (Fig. 1), but also in oocytes as they progress through folliculogenesis. Thus, Oct4-expressing germ cells prior to follicle formation and within follicles can be marked with EYFP. Nevertheless, the initiation and entry into meiosis, which are defined by induction of Stra8 and Sycp3 expression, occurs exclusively in non-follicular germ cells. We therefore focused on the EYFP+ small germ cells. It should be noted that meiosis markers Stra8 and Sycp3 are temporarily expressed during prophase I and progressively degraded upon oocytes entering into dictyate arrest (Johnson et al., 2004; Prieto et al., 2004; Krentz et al., 2011). As a result, Stra8 and Sycp3 cannot be carried over in germ cells for long time and only germ cells that newly enter into meiosis are responsive for Stra8 or Sycp3 immunostaining. We first examined the influence of tamoxifen dosages on labeling efficiency in order to define a suitable dose that could specifically label Oct4+Sycp3− small germ cells. Double-mutant female mice (5–6 weeks old) were injected with tamoxifen at the following dosages: 0.25, 0.125, 0.06, 0.03, and 0.01 mg/g body weight. Consistently, no cells in ovaries expressed EYFP without tamoxifen induction. All dosages generated similar proportion of EYFP+Sycp3+ or EYFP+Stra8+ cells within the entire EYFP+ small germ cells at 1 dpi (Supplementary Fig. S4a and b), although the total number of EYFP+ small germ cells decreased with the reduction of injection dose. Specifically, at the dose of 0.25 mg/g body weight, 100% (n = 157) of Oct4-expressing small germ cells were labeled with EYFP. We therefore utilized this dosage in all subsequent cell tracing studies. Furthermore, the germ cell identity of the EYFP-labeled cells was once again verified by triple staining of ovarian sections collected at 3 dpi of tamoxifen with antibodies against EYFP, Dazl and Stella. All EYFP+ cells (n = 768) were positive for Dazl or/and Stella, indicative of germ cell identity (Supplementary Fig. S4c).
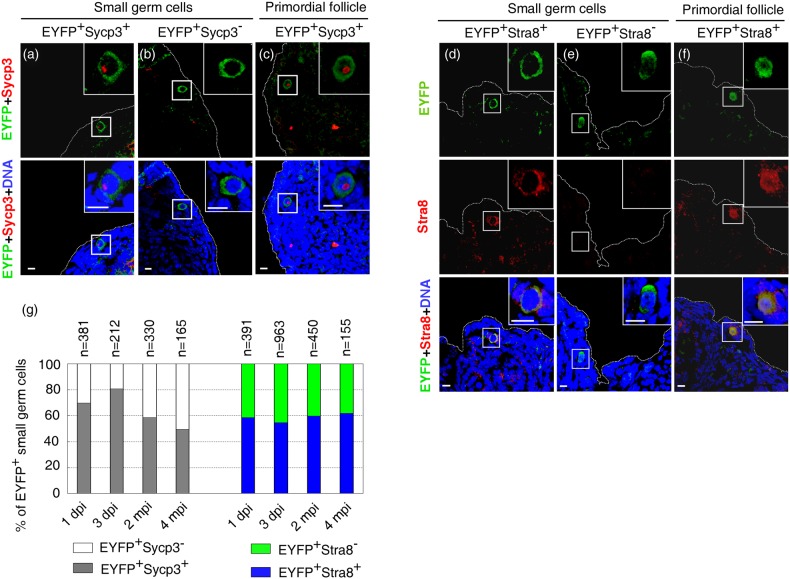
At 1 dpi, the majority of EYFP-labeled small germ cells still retained Oct4 staining (77.53%, n = 832), ∼70% of EYFP+ small germ cells (n = 381) expressed Sycp3 (EYFP+Sycp3+) (Fig. 2a and g). This population displayed a slight increase to 80% at 3 dpi (n = 212), suggesting that additional small germ cells moved from EYFP+Sycp3− to EYFP+Sycp3+ status (Fig. 2b and g). Sycp3 protein was also occasionally detected in some EYFP+ germ cells of primordial follicles, indicating that these primordial follicles were newly formed (Fig. 2c). Due to the temporary expression property of Sycp3 and Stra8, after a long time period of tracing, these EYFP+Sycp3+ germ cells would shift to EYFP+Sycp3− due to the degradation of Sycp3. Genetic tracing was then extended for 2 months, which is long enough for Sycp3 to undergo degradation. Notably, we persistently observed EYFP+Sycp3+ small germ cells, which accounted for 58% of total EYFP+ small germ cells (n = 330) (Fig. 2a and g). Moreover, a portion of EYFP+ germ cells within primordial follicle retained an obvious Sycp3 signal, indicating that these EYFP-marked primordial follicles are newly formed (Fig. 2c). These data suggested the new meiosis entry and primordial follicle replenishment in postnatal ovaries. To confirm the meiosis entry and follicle replenishment, we prolonged genetic tracing to 4 months and obtained similar results (Fig. 2a–c and g). We repeated the tracing experiments by examining the expression of another temporary meiosis marker Stra8. Consistently, the EYFP+Stra8+ small germ cells and primordial follicles containing EYFP+Stra8+ germ cells were persistent after 2 and 4 months of genetic tracing (Fig. 2d–g). Of note, the percentages of EYFP+Sycp3+ or EYFP+Stra8+ small germ cells remained relatively stable at all examined time points (Fig. 2g). This suggested that entry into meiosis might take place in a steady state manner in young adult females. Similarly, the proportions of newly formed EYFP+ primordial follicles among the total EYFP-labeled primordial follicles (ratio of primordial follicles containing EYFP+Stra8+ or EYFP+Sycp3+ germ cells to those containing EYFP-labeled germ cells) were constant at 2 and 4 mpi (∼45%, n > 100). At all time points, the EYFP+ oocytes in various follicle stages were observed (Supplementary Fig. S4d–h). No granulosa cells expressed EYFP (Supplementary Fig. S4d–h), confirming that Oct4-expressing stem cells contribute to germ cell lineage in folliculogenesis.
Figure 2.
Genetic tracing combined with immunostaining documents continuous meiosis entry and primordial follicle replenishment in postnatal mouse ovaries. Meiosis entry was persistently detected in postnatal mouse ovaries (5–6 weeks old) at 1 and 3 dpi, and 2 and 4 mpi of tamoxifen induction. Displays are representative data collected at 4 mpi. (a, b, d and e) Representative micrographs of EYFP+ small germ cells expressing Sycp3 or Stra8. (c and f) Newly formed primordial follicles retaining Sycp3 or Stra8. (g) Percentages of EYFP+Sycp3+ or EYFP+Stra8+ small germ cells at each time point following tamoxifen injection. Insets show the higher magnification of germ cells indicated by squares. At each time point, ovaries from two to three animals were used for investigation. Scale bar, 10 μm.
To further validate the persistent existence of EYFP+Sycp3+ and EYFP+Stra8+ germ cells and continuous primordial follicle replenishment in ovaries in our tracing experiment, we isolated EYFP-expressing single germ cells with diameters of 5–15 μm at 1 day, 3 days, 2 weeks and 1 month post-tamoxifen injection (Fig. 3a). cDNA was prepared and amplified from single cells according to the published protocol (Tang et al., 2010). The mRNA expression of glyceraldehyde 3-phosphate dehydrogenase (Gapdh), EYFP, Oct4, Stella, Dazl, Ddx4, Stra8, Sycp3 and Sohlh1 were examined by PCR. As shown (Fig. 3b), isolated EYFP-positive germ cells expressed Dazl mRNAs, indicating that they initiated differentiation process. Note that some EYFP+ germ cells (S5, S6, S7 and S8 in Fig. 3b) showed no or very little Oct4 mRNAs, indicating that they lost Oct4 expression along with differentiation (Pesce et al., 1998; Kehler et al., 2004; Anderson et al., 2007; Kretzschmar and Watt, 2012). Interestingly, these germ cells displayed four types of mRNA expression pattern for Stra8 and Sycp3, including Stra8+Sycp3− (S3 in Fig. 3b), Stra8−Sycp3+ (S2, S4, S6 and S10 in Fig. 3b), Stra8+Sycp3+ (S1, S5 and S9 in Fig. 3b), and Stra8−Scyp3− (S7 and S8 in Fig. 3b). Furthermore, some EYFP+ germ cells not only expressed a marker of primordial follicle Sohlh1 (Pangas et al., 2006; Zhang et al., 2014), but also retained Sycp3 and Stra8 expression (S5 and S6 in Fig. 3b). These expression patterns were persistently observed in individual EYFP-expressing germ cells at all time points examined. Thus, these data altogether support the notion that there are persistent meiosis entry and primordial follicle replenishment in postnatal mouse ovary under physiological conditions.
Figure 3.
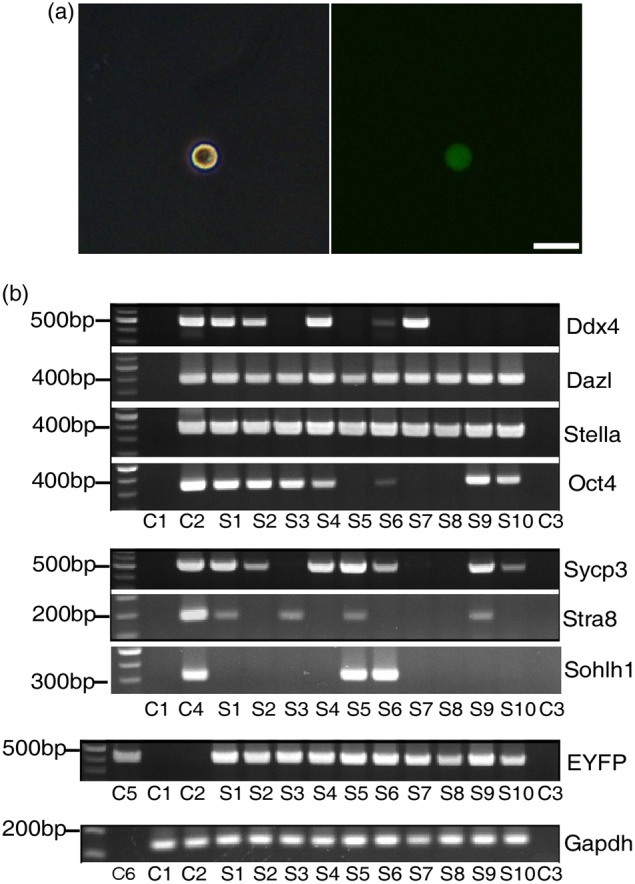
Genetic tracing combined with RT–PCR confirms continuous meiosis entry in postnatal mouse ovaries. Single-cell suspension was prepared from ovaries, and EYFP-expressing single cells were manually picked at 1 day, 3 days, 2 weeks, and 1 month post-injection of tamoxifen. (a) Representative EYFP-expressing single cell for cDNA amplification. The mRNAs expression of Gapdh, EYFP, Stra8, Sycp3, Sohlh1, Oct4, Stella, Dazl and Ddx4 was examined by RT–PCR at all time points. The representative data collected at 1 month post-injection of tamoxifen were shown in (b). C1, EYFP-negative single cell from single-cell suspension; C2, single germinal vesicle stage oocyte from wild-type C57BL/6 mouse; C3, double-distilled H2O; C4, mouse testicular tissues; C5, genome DNA from R26R-EYFPTm/Tm mouse; C6, single-cell suspension medium. Samples 1–10 (S1–S10), EYFP-expressing single cells. Scale bar, 20 μm.
Genetic tracing of Oct4-expressing cells suggests the presence of putative germ stem cells capable of proliferation and mitotic division
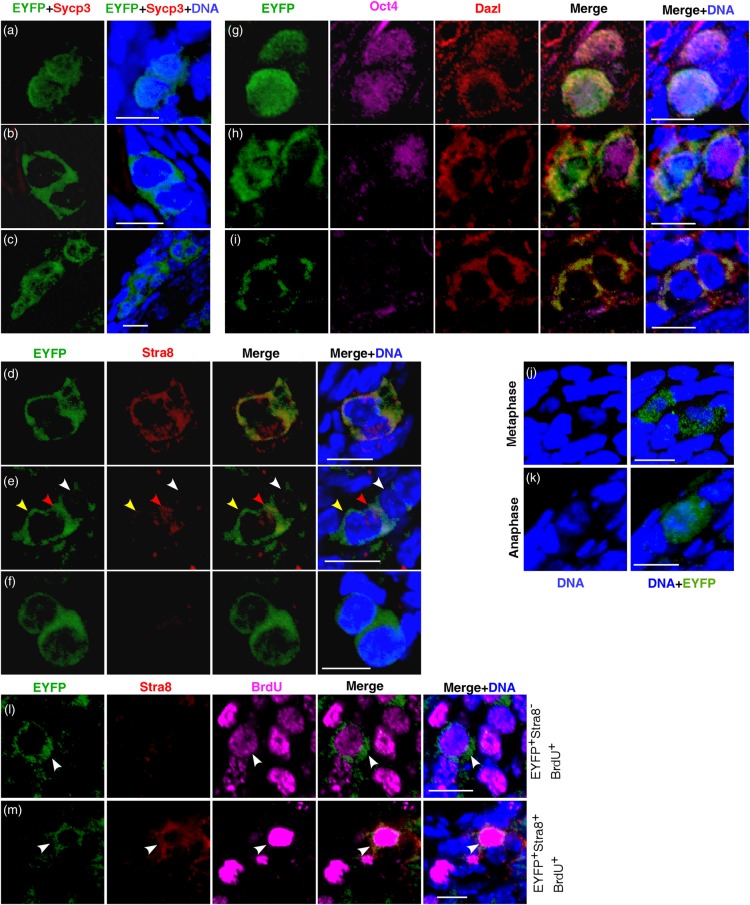
To understand whether the neo-oogenesis is based on the activities of putative germ stem cells, we examined if a portion of EYFP+ germ cells possess the stem cell properties of long-term proliferation and mitotic division. The formation of germ cell cluster in which cells are in the process of mitotic division or had just divided and remained in close proximity to one another is often considered as a critical evidence of germ cell mitotic division. Indeed, two or more clustered EYFP+ small germ cells were detected at each time point after tamoxifen induction (Fig. 4a–i), despite that the frequency was extremely low (<1%). Moreover, rare EYFP+ cells were captured at metaphase or anaphase as judged by the chromosome morphology (Fig. 4j and k) at all time points examined. These observations indicate that EYFP-labeled putative germ stem cells undergo mitotic division to generate descendent daughter cells. Co-staining EYFP with Stra8, Sycp3, Dazl or Oct4 documented different modes of germ stem cell division. For instance, co-staining EYFP with Stra8 revealed three types of division in which both (Fig. 4d), one (Fig. 4e) or neither (Fig. 4f) daughter cells initiated Stra8 expression. Similarly, triple staining of EYFP, Oct4 and Dazl showed different types of germ cell mitotic division (Fig. 4g–i).
Figure 4.
Genetic cell tracing reveals the continuous proliferation and mitotic division of germ cells. Representative micrographs were obtained at 4 mpi. (a–c) Co-staining EYFP+ cells with antibodies to Sycp3 documented a cluster of two or more germ cells that had just divided. None of the daughter cells initiated Sycp3 expression. (d–f) Co-staining EYFP+ cells with antibody to Stra8 revealed three modes of germ cell division. Arrowheads in (e) indicated three clustered EYFP+ germ cells. Red arrowhead, daughter cell initiating Stra8 expression; yellow arrowhead, daughter cell negative for Stra8; white arrowhead, germ cell negative for Stra8. (g–i) Different modes of germ cell division revealed by immunostaining EYFP+ germ cells with antibodies to Oct4 and Dazl. EYFP+ germ cells at metaphase (j) and anaphase (k). (l) After tracing for 4 months, EYFP+ germ cell (arrowhead) undergoes bona fide mitotic proliferation as indicated by the active BrdU incorporation and the absence of Stra8 expression. (m) After tracing for 4 months, EYFP+ germ cell (arrowhead) undergoes pre-meiotic DNA replication as evidenced by the active BrdU incorporation and the Stra8 expression. At each time point, ovaries from two to three animals were used for investigation. Scale bar, 10 μm.
We then examined if a fragment of EYFP+ small germ cells were capable of proliferation after long-term tracing. Adult female mice were injected with BrdU at 4 months post-tamoxifen administration, and ovaries were collected 12 h later for BrdU incorporation assay. Consistent with the observation of persistent germ cell mitotic division, rare EYFP+ germ cell nuclei were labeled with BrdU (EYFP+BrdU+) in comparable intensity to that observed in replicating somatic cells, indicative of the active genome DNA replication rather than DNA repair or mitochondria DNA replication (Fig. 4l and m, arrowhead). Some EYFP+BrdU+ germ cells simultaneously expressed Stra8 protein, suggesting that these cells were undergoing pre-meiotic DNA replication (Fig. 4m, arrowhead). In contrast, other EYFP+BrdU+ germ cells were negative for Stra8 expression, indicating that these germ cells underwent bona fide mitotic proliferation (Fig. 4l, arrowhead). These lines of evidence support the existence of putative ovarian germ stem cells capable of proliferation and mitotic division.
Oct4+Dazl− small germ cells contain a population of germ stem cells in postnatal mouse ovary
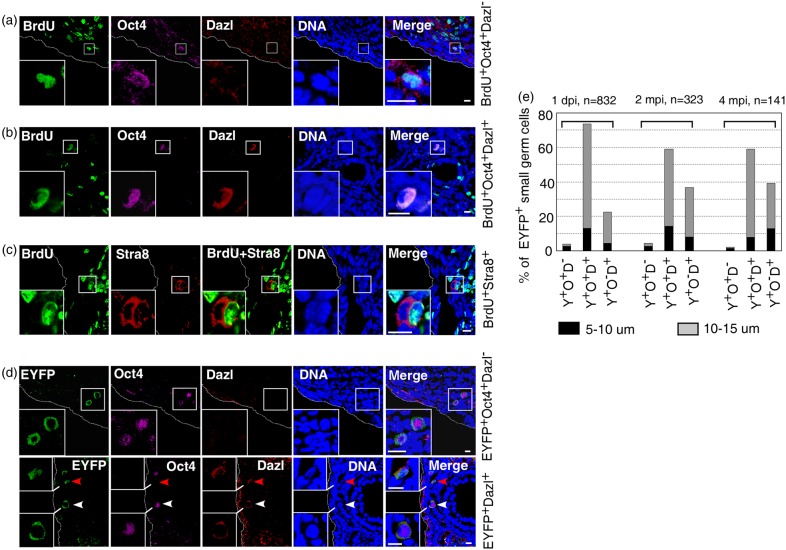
Although the specific marker of ovarian germ stem cells is unclear, we postulated that they express Oct4 but not early germ cell differentiation marker Dazl or Ddx4. To test this hypothesis, we examined the proliferative activity of the Oct4+Dazl− small germ cells in juvenile female mice (3–4 weeks old) by BrdU incorporation assay as described above. Rare Oct4+Dazl− small germ cell nucleus was specifically labeled with intensive BrdU signal indicative of the active genome DNA replication (Fig. 5a). A small number of Oct4+Dazl+ small germ cells were also positive for intensive nuclear BrdU labeling (Fig. 5b), which agreed with previous observation of BrdU+Ddx4+ cells in postnatal ovaries (Johnson et al., 2004; Zou et al., 2009). Given that Dazl expression is required for Stra8 initiation (Baltus et al., 2006; Lin et al., 2008), we concluded that BrdU labeling in Oct4+Dazl− germ cells reflected their bona fide mitotic proliferation, whereas BrdU incorporation in Oct4+Dazl+ cells might reflect pre-meiotic DNA replication. Indeed, some Stra8+ germ cells were captured at pre-meiosis DNA replication (Fig. 5c). In concordance with their proliferation competence, genetic tracing revealed that EYFP+Oct4+Dazl− germ cells were small (diameters of 5–10 μm) and persisted in ovaries for 2 (4.33%, n = 323) and 4 months (2.13%, n = 141), although their number was extremely low (Fig. 5d and e). These investigations suggested that Oct4+Dazl− but not Oct4+Dazl+ small germ cells contained a rare population of germ stem cells responsible for primordial follicle replenishment in postnatal mouse ovaries.
Figure 5.
Oct4+Dazl− cells contain a population of ovarian germ stem cells. (a) Rare Oct4+Dazl− cells were labeled with BrdU, indicative of active genome DNA replication. (b) Rare Oct4+Dazl+ cells were also labeled with BrdU. (c) Germ cell undergoing meiotic DNA replication. (d) Genetic cell fate tracing documented the persistence of EYFP+Oct4+Dazl− germ cells. EYFP+Oct4+Dazl+ (white arrowhead, Y+O+D+) and EYFP+Oct4−Dazl+ (red arrowhead, Y+O−D+) germ cells were also detected, respectively. (e) Percentages of EYFP+Oct4+Dazl− (Y+O+D−) small germ cells at each time point following tamoxifen injection. Insets show the higher magnification of germ cells indicated by squares. At each time point, ovaries from two to three animals were used for investigation. Scale bar, 10 μm.
Dazl and Ddx4 are early differentiation markers of germ cells. In mouse and human fetal germ cell development, Dazl expression is initiated earlier than Ddx4 (Toyooka et al., 2000; Anderson et al., 2007; Bowles and Koopman, 2007; Guo et al., 2015). In juvenile mice (3–4 weeks old), we co-immunostained ovarian sections with Dazl and Ddx4 antibodies and observed that 86.7% (n = 136) of Dazl+Ddx4− small germ cells were at 5–10 μm in diameter, whereas 81.2% (n = 171) of Dazl−Ddx4+ small germ cells had diameter of 10–15 μm. Thus, Ddx4 is expressed in larger germ cells, suggesting that Ddx4 expression represents more advanced stage of differentiation during the germ stem cell-based oogenesis in postnatal ovaries.
Discussion
Three recent studies utilized in vivo approaches to investigate the possible existence of putative ovarian germ stem cells but found no mitotically active germ stem/progenitor cells in postnatal mouse ovaries (Lei and Spradling, 2013a; Zhang et al., 2012, 2014). One study used CAG promoter to drive Cre-loxp system and marked all types of cells in ovaries with YFP upon tamoxifen induction. By examining the numbers of YFP-marked primordial follicles and total primordial follicles at different time points following tamoxifen administration, they found that YFP-labeled primordial follicles and the total primordial follicle pool were stable as a whole. Moreover, they failed to detect the YFP+Ddx4+ germ cell cluster, which they considered as an unambiguous indicator of the germ stem cell mitotic activity. Based on these observations, this study concluded that there was no active germ stem cells in postnatal ovaries (Lei and Spradling, 2013a). However, the observed stability of YFP-marked and total primordial follicles as a whole can be alternatively explained by the replenishment of new primordial follicles from YFP-marked and -unmarked putative germ stem cells. Furthermore, Ddx4 was not expressed in putative ovarian germ stem cells, as suggested in this study and others. Rather, it is expressed in differentiated germ cells that are unable to undergo mitotic division or form germ cell clusters. The other work by Zhang et al. also utilized Ddx4 to mark the putative germ stem cells and failed to detect germ stem cell activities in vivo (Zhang et al., 2012). Thus, utilizing an inappropriate molecule, for instance Ddx4, to label putative germ stem cells could lead to failure in detecting ovarian germ stem cell activities as well as the wrong conclusion. The most recent work by Zhang et al. employed other strategies to address this question and found no neo-oogenesis in vivo (Zhang et al., 2014). In this study, all germ cells expressing Gdf9 were eliminated upon administration of the diphtheria toxin into the Gdf9-Cre;iDTR-targeted mutant mice. No new germ cell was regenerated after germ cell depletion. However, it is not clear if elimination of all existing germ cells in ovaries impairs the somatic cell functions and disrupts the stem cell niche necessary for stem cell regeneration. Moreover, Sohlh1-CreERT2;R26R genetic tracing was employed to label a portion of primordial follicles. The ratios of the labeled to unlabeled oocytes were compared at 2 and 8 months following tamoxifen injection at postnatal day 28. They observed similar ratios at the two time points and assumed no neo-oogenesis. However, animal age can compromise putative germ stem cell functions (Niikura et al., 2009). It remains unknown if functional decline of putative ovarian germ stem cells already occurs in 9-month-old mice. More time points, e.g. 4 and 6 months post-tamoxifen injection, should be considered at this study. Furthermore, it was not clear if the unlabeled immature oocytes within primordial follicles were all counted in this study.
To find out an appropriate gene promoter to drive Cre-loxP system and to study the putative ovarian germ stem cells, we reviewed the gene expression dynamics of primordial germ cells (PGCs) in mouse and human fetal ovaries. Undifferentiated mouse PGCs abundantly express pluripotency marker Oct4. Between embryonic day 10.5 (E 10.5) and E 11.5, PGCs initiate the expression of meiotic competence marker Dazl (Ruggiu et al., 1997; Seligman and Page, 1998), which in turn induces the expression of Stra8 required for pre-meiotic DNA replication and subsequent events of prophase I (Baltus et al., 2006; Lin et al., 2008). Shortly after Stra8 induction, Sycp3, required for meiotic synaptonemal complex assembly, is strongly expressed (Feng et al., 2014). Like Dazl, Ddx4 expression is initiated in differentiated PGCs at E 11.5 and increases at E 12.5 (Toyooka et al., 2000). Coinciding with the progress of germ cell differentiation, Oct4 expression in PGCs undergoes down-regulation (Pesce et al., 1998; Bullejos and Koopman, 2004; Bowles and Koopman, 2007). Thus, gene expression shifts from Oct4 to differentiation markers (Dazl, Ddx4, Stra8 and Sycp3) along with the commitment of PGCs to meiosis. Similar gene expression dynamics was reported in the development of human fetal PGCs (Guo et al., 2015). Concordantly, we detected several types of small germ cells in the ovarian surface of juvenile mice. Those expressing Oct4 but not differentiation marker Dazl or Ddx4 have diameter of 5–10 μm and are not in follicular structure. However, the majority of small germ cells initiating the expression of differentiation markers have a diameter of 10–15 μm. These observations suggest that Oct4 is expressed in the primitive germ cells and can be used for tracing the putative germ stem cells in postnatal mouse ovaries.
After marking the Oct4-expressing small germ cells with EYFP and tracing their fates for up to 4 months, we obtained the following findings: first, by two independent approaches of immunostaining and single-cell RT–PCR, we consistently observed the persistent mRNA and protein expression of meiosis markers Stra8 and Sycp3 in some EYFP-labeled small germ cells. Second, primordial follicles expressing Sycp3 or Stra8 were continuously detected by both immunostaining and single-cell RT–PCR. Because Stra8 and Sycp3 expression is temporary, these mRNAs or proteins cannot be carried over from the initially labeled germ cells. These results do not reconcile with the prevailing dogma. Rather, they support that meiosis entry and primordial follicle replenishment continuously take place in EYFP-labeled germ cells in postnatal ovaries. Third, we captured the transient processes of mitotic DNA replication as well as mitotic division of the marked germ cells at various time periods after marking, thereby providing the most direct evidence to support the neo-oogenesis in postnatal mouse ovaries under physiological conditions.
Due to the ubiquitous expression of Oct4 in germ cells during folliculogenesis, it is not feasible to distinguish the regenerated EYFP+ oocytes from pre-existing EYFP+ oocytes and to verify the competence of regenerated germ cells by producing the next-generation mice. It is also difficult to assess the developmental wave of labeled germ stem cells and the contribution of germ stem cell activity to follicle pool. Nevertheless, the key features of genetic cell tracing are that it does not change the properties of the marked cell, its progeny, and its neighbors (Kretzschmar and Watt, 2012). This makes the functional test of the progeny of the marked cells not obligatory in many genetic cell lineage tracing studies (Bonaguidi et al., 2011; Schepers et al., 2012). In addition, our work proposed that Oct4+Dazl− but not Oct4+Dazl+ or Oct4+Ddx4+ cells display bona fide mitotic proliferation and contain a population of germ stem cells in postnatal mouse ovary. This does not reconcile with the previous studies that reported the isolation of ovarian germ stem cells with antibody to Ddx4 (Zou et al., 2009; White et al., 2012). Whether germ cells purified via Ddx4 antibody are dedifferentiated during in vitro culture requires further investigation (Vogel, 2012). The future work should aim at identifying the specific ovarian germ stem cell marker and improving the lineage tracing system. Refined lineage tracing driven by stem-cell-specific marker gene is necessary to evaluate the ovarian germ stem cell activities under physiological conditions and their contributions to normal as well as pathological ovarian functions.
Supplementary data
Supplementary data are available at http://molehr.oxfordjournals.org/.
Authors' roles
P.Z. organized and designed the study. K.G., C.L., X.W. and D.H. performed the experiments. P.Z. and K.G. wrote the manuscript. All authors gave final approval for publication.
Funding
This work is supported by the National Key Basic Research Program of China (grant number 2012CBA01300) and the National Natural Science Foundation of China to P.Z. (31571484).
Conflict of interest
None declared.
Supplementary Material
Acknowledgements
We thank Dr Jurrien Dean in National Institutes of Health, USA, and Dr Kui Liu in University of Gothenburg, Sweden, for critical discussion and editorial comments.
References
- Anderson RA, Fulton N, Cowan G, Coutts S, Saunders PT. Conserved and divergent patterns of expression of DAZL, VASA and OCT4 in the germ cells of the human fetal ovary and testis. BMC Dev Biol 2007;7:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, Yu M, Hu Y, Qiu P, Liu W, Zheng W, Peng S, Hua J. Location and characterization of female germline stem cells (FGSCs) in juvenile porcine ovary. Cell Prolif 2013;5:516–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltus AE, Menke DB, Hu YC, Goodheart ML, Carpenter AE, de Rooij DG, Page DC. In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet 2006;12:1430–1434. [DOI] [PubMed] [Google Scholar]
- Bhartiya D, Sriraman K, Parte S, Patel H. Ovarian stem cells: absence of evidence is not evidence of absence. J Ovarian Res 2013;1:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, Song H. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell 2011;7:1142–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles J, Koopman P. Retinoic acid, meiosis and germ cell fate in mammals. Development 2007;19:3401–3411. [DOI] [PubMed] [Google Scholar]
- Bui HT, Van Thuan N, Kwon DN, Choi YJ, Kang MH, Han JW, Kim T, Kim JH. Identification and characterization of putative stem cells in the adult pig ovary. Development 2014;11:2235–2244. [DOI] [PubMed] [Google Scholar]
- Bullejos M, Koopman P. Germ cells enter meiosis in a rostro-caudal wave during development of the mouse ovary. Mol Reprod Dev 2004;4:422–428. [DOI] [PubMed] [Google Scholar]
- Draper BW, McCallum CM, Moens CB. Nanos1 is required to maintain oocyte production in adult zebrafish. Dev Biol 2007;2:589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evron A, Blumenfeld Z. Ovarian stem cells—the pros and cons. Clin Med Insights Reprod Health 2013;7:43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng CW, Bowles J, Koopman P. Control of mammalian germ cell entry into meiosis. Mol Cell Endocrinol 2014;1:488–497. [DOI] [PubMed] [Google Scholar]
- Guo F, Yan L, Guo H, Li L, Hu B, Zhao Y, Yong J, Hu Y, Wang X, Wei Y, et al. The transcriptome and DNA methylome landscapes of human primordial germ cells. Cell 2015;6:1437–1452. [DOI] [PubMed] [Google Scholar]
- Hernandez SF, Vahidi NA, Park S, Weitzel RP, Tisdale J, Rueda BR, Wolff EF. Characterization of extracellular DDX4- or Ddx4-positive ovarian cells. Nat Med 2015;10:1114–1116. [DOI] [PubMed] [Google Scholar]
- Imudia AN, Wang N, Tanaka Y, White YA, Woods DC, Tilly JL. Comparative gene expression profiling of adult mouse ovary-derived oogonial stem cells supports a distinct cellular identity. Fertil Steril 2013;5:1451–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature 2004;6979:145–150. [DOI] [PubMed] [Google Scholar]
- Kehler J, Tolkunova E, Koschorz B, Pesce M, Gentile L, Boiani M, Lomeli H, Nagy A, McLaughlin KJ, Scholer HR, et al. Oct4 is required for primordial germ cell survival. EMBO Rep 2004;11:1078–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krentz AD, Murphy MW, Sarver AL, Griswold MD, Bardwell VJ, Zarkower D. DMRT1 promotes oogenesis by transcriptional activation of Stra8 in the mammalian fetal ovary. Dev Biol 2011;1:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar K, Watt FM. Lineage tracing. Cell 2012;1–2:33–45. [DOI] [PubMed] [Google Scholar]
- Lei L, Spradling AC. Female mice lack adult germ-line stem cells but sustain oogenesis using stable primordial follicles. Proc Natl Acad Sci USA 2013a;21:8585–8590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei L, Spradling AC. Mouse primordial germ cells produce cysts that partially fragment prior to meiosis. Development 2013b;10:2075–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Gill ME, Koubova J, Page DC. Germ cell-intrinsic and -extrinsic factors govern meiotic initiation in mouse embryos. Science 2008;5908:1685–1687. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wu C, Lyu Q, Yang D, Albertini DF, Keefe DL, Liu L. Germline stem cells and neo-oogenesis in the adult human ovary. Dev Biol 2007;1:112–120. [DOI] [PubMed] [Google Scholar]
- Matson CK, Murphy MW, Sarver AL, Griswold MD, Bardwell VJ, Zarkower D. DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature 2011;7358:101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli MA, Werling U, Edelmann W, Roberson MS, Cohen PE. Analysis of meiotic prophase I in live mouse spermatocytes. Chromosome Res 2008;5:743–760. [DOI] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol 2004;4:411–417. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Kobayashi K, Nishimura T, Higashijima S, Tanaka M. Identification of germline stem cells in the ovary of the teleost medaka. Science 2010;5985:1561–1563. [DOI] [PubMed] [Google Scholar]
- Niikura Y, Niikura T, Tilly JL. Aged mouse ovaries possess rare premeiotic germ cells that can generate oocytes following transplantation into a young host environment. Aging 2009;12:971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohbo K, Yoshida S, Ohmura M, Ohneda O, Ogawa T, Tsuchiya H, Kuwana T, Kehler J, Abe K, Scholer HR, et al. Identification and characterization of stem cells in prepubertal spermatogenesis in mice. Dev Biol 2003;1:209–225. [DOI] [PubMed] [Google Scholar]
- Pacchiarotti J, Maki C, Ramos T, Marh J, Howerton K, Wong J, Pham J, Anorve S, Chow YC, Izadyar F. Differentiation potential of germ line stem cells derived from the postnatal mouse ovary. Differentiation 2010;3:159–170. [DOI] [PubMed] [Google Scholar]
- Pangas SA, Choi Y, Ballow DJ, Zhao Y, Westphal H, Matzuk MM, Rajkovic A. Oogenesis requires germ cell-specific transcriptional regulators Sohlh1 and Lhx8. Proc Natl Acad Sci USA 2006;21:8090–8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payer B, Saitou M, Barton SC, Thresher R, Dixon JP, Zahn D, Colledge WH, Carlton MB, Nakano T, Surani MA. Stella is a maternal effect gene required for normal early development in mice. Curr Biol 2003;23:2110–2117. [DOI] [PubMed] [Google Scholar]
- Pesce M, Wang X, Wolgemuth DJ, Scholer H. Differential expression of the Oct-4 transcription factor during mouse germ cell differentiation. Mech Dev 1998;1–2:89–98. [DOI] [PubMed] [Google Scholar]
- Prieto I, Tease C, Pezzi N, Buesa JM, Ortega S, Kremer L, Martinez A, Martinez AC, Hulten MA, Barbero JL. Cohesin component dynamics during meiotic prophase I in mammalian oocytes. Chromosome Res 2004;3:197–213. [DOI] [PubMed] [Google Scholar]
- Reizel Y, Itzkovitz S, Adar R, Elbaz J, Jinich A, Chapal-Ilani N, Maruvka YE, Nevo N, Marx Z, Horovitz I, et al. Cell lineage analysis of the mammalian female germline. PLoS Genet 2012;2:e1002477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiu M, Speed R, Taggart M, McKay SJ, Kilanowski F, Saunders P, Dorin J, Cooke HJ. The mouse Dazla gene encodes a cytoplasmic protein essential for gametogenesis. Nature 1997;6646:73–77. [DOI] [PubMed] [Google Scholar]
- Saitou M, Barton SC, Surani MA. A molecular programme for the specification of germ cell fate in mice. Nature 2002;6895:293–300. [DOI] [PubMed] [Google Scholar]
- Schepers AG, Snippert HJ, Stange DE, van den Born M, van Es JH, van de Wetering M, Clevers H. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science 2012;6095:730–735. [DOI] [PubMed] [Google Scholar]
- Schmidt D, Ovitt CE, Anlag K, Fehsenfeld S, Gredsted L, Treier AC, Treier M. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development 2004;4:933–942. [DOI] [PubMed] [Google Scholar]
- Scholer HR, Balling R, Hatzopoulos AK, Suzuki N, Gruss P. Octamer binding proteins confer transcriptional activity in early mouse embryogenesis. EMBO J 1989;9:2551–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman J, Page DC. The Dazh gene is expressed in male and female embryonic gonads before germ cell sex differentiation. Biochem Biophys Res Commun 1998;3:878–882. [DOI] [PubMed] [Google Scholar]
- Tang F, Barbacioru C, Nordman E, Li B, Xu N, Bashkirov VI, Lao K, Surani MA. RNA-Seq analysis to capture the transcriptome landscape of a single cell. Nat Protoc 2010;3:516–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telfer EE, Albertini DF. The quest for human ovarian stem cells. Nat Med 2012;3:353–354. [DOI] [PubMed] [Google Scholar]
- Toyooka Y, Tsunekawa N, Takahashi Y, Matsui Y, Satoh M, Noce T. Expression and intracellular localization of mouse Vasa-homologue protein during germ cell development. Mech Dev 2000;1–2:139–149. [DOI] [PubMed] [Google Scholar]
- Tsutsumi M, Kogo H, Kowa-Sugiyama H, Inagaki H, Ohye T, Kurahashi H. Characterization of a novel mouse gene encoding an SYCP3-like protein that relocalizes from the XY body to the nucleolus during prophase of male meiosis I. Biol Reprod 2011;1:165–171. [DOI] [PubMed] [Google Scholar]
- Vogel G. Reproductive biology. Potential egg stem cells reignite debate. Science 2012;6072:1029–1030. [DOI] [PubMed] [Google Scholar]
- White YA, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med 2012;3:413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods DC, Telfer EE, Tilly JL. Oocyte family trees: old branches or new stems? PLoS Genet 2012;7:e1002848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T, Song X, Jin Z, Pan L, Weng C, Chen S, Zhang N. Interactions between stem cells and their niche in the Drosophila ovary. Cold Spring Harb Symp Quant Biol 2008;73:39–47. [DOI] [PubMed] [Google Scholar]
- Yang F, De La Fuente R, Leu NA, Baumann C, McLaughlin KJ, Wang PJ. Mouse SYCP2 is required for synaptonemal complex assembly and chromosomal synapsis during male meiosis. J Cell Biol 2006;4:497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeom YI, Fuhrmann G, Ovitt CE, Brehm A, Ohbo K, Gross M, Hubner K, Scholer HR. Germline regulatory element of Oct-4 specific for the totipotent cycle of embryonal cells. Development 1996;3:881–894. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zheng W, Shen Y, Adhikari D, Ueno H, Liu K. Experimental evidence showing that no mitotically active female germline progenitors exist in postnatal mouse ovaries. Proc Natl Acad Sci USA 2012;31:12580–12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Liu L, Li X, Busayavalasa K, Shen Y, Hovatta O, Gustafsson JA, Liu K. Life-long in vivo cell-lineage tracing shows that no oogenesis originates from putative germline stem cells in adult mice. Proc Natl Acad Sci USA 2014;50:17983–17988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Panula S, Petropoulos S, Edsgard D, Busayavalasa K, Liu L, Li X, Risal S, Shen Y, Shao J, et al. Adult human and mouse ovaries lack DDX4-expressing functional oogonial stem cells. Nat Med 2015;10:1116–1118. [DOI] [PubMed] [Google Scholar]
- Zhou L, Wang L, Kang JX, Xie W, Li X, Wu C, Xu B, Wu J. Production of fat-1 transgenic rats using a post-natal female germline stem cell line. Mol Hum Reprod 2014;3:271–281. [DOI] [PubMed] [Google Scholar]
- Zou K, Yuan Z, Yang Z, Luo H, Sun K, Zhou L, Xiang J, Shi L, Yu Q, Zhang Y, et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol 2009;5:631–636. [DOI] [PubMed] [Google Scholar]
- Zou K, Hou L, Sun K, Xie W, Wu J. Improved efficiency of female germline stem cell purification using fragilis-based magnetic bead sorting. Stem Cells Dev 2011;12:2197–2204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.