Abstract
As a transplant surgeon, my interest in glycobiology began through my research into ABO-incompatible allotransplantation, and grew when my goal became overcoming the shortage of organs from deceased human donors by the transplantation of pig organs into patients with terminal organ failure (xenotransplantation/cross-species transplantation). The major target for human “natural” (preformed) anti-pig antibodies is galactose-α(1,3)-galactose (the “Gal” epitope), which is expressed on many pig cells, including the vascular endothelium. The binding of human IgM and IgG antibodies to Gal antigens initiates the process of hyperacute rejection, resulting in destruction of the pig graft within minutes or hours. This major barrier has been overcome by the production of pigs in which the gene for the enzyme α(1,3)-galactosyltransferase (GT) has been deleted by genetic engineering, resulting in GT knockout (GTKO) pigs. The two other known carbohydrate antigenic targets on pig cells for human anti-pig antibodies are (i) the product of the cytidine monophosphate-N-acetylneuraminic acid hydroxylase (CMAH) gene, i.e., N-glycolylneuraminic acid, and (ii) the product of the β1,4 N-acetylgalactosaminyltransferase gene, i.e., the Sd(a) antigen. Expression of these two has also been deleted in pigs. These genetic manipulations, together with others directed to overcoming primate complement and coagulation activation (the latter of which also relates to glycobiology) have contributed to the prolongation of pig graft survival in nonhuman primate recipients to many months rather than a few minutes. Clinical trials of the transplantation of pig cells are already underway and transplantation of pig organs may be expected within the relatively near future.
Keywords: ABO-incompatibility; β(1,4)N-acetylgalactosaminyltransferase; galactose-α(1,3)-galactose; glycobiology; N-glycolylneuraminic acid; pig; xenotransplantation
I am sometimes reminded of the differences between a rhinoceros and a cardiac surgeon. One has a thick skin, a small brain, and charges a lot. The other is a large animal that lives in Africa.
David Wheatley (British cardiac surgeon)
Introduction
A cardiac surgeon does not generally expect to find him/herself involved in the field of glycobiology. However, if the surgeon's main research interest becomes heart transplantation and, ultimately, heart xenotransplantation (cross-species transplantation), then glycobiology plays a significant role.
As a medical student at Guy's Hospital in London in the late 1950s and early 1960s I had little interest in—or aptitude for—biochemistry. My intention was to become a cardiac surgeon and so the anatomy and physiology of the cardiovascular system proved much more interesting to me. After graduation and completion of house appointments (internships) in London, and a year spent teaching anatomy at Harvard Medical School in Boston, I began the prolonged surgical residency program in the UK. I interrupted this program to spend 3 years obtaining a PhD degree at the University of London in which I studied how best to preserve the donor heart for purposes of heart transplantation.
I was fortunate to be present at the first human heart transplant carried out in the UK in 1968, and subsequently participated in the first truly successful heart transplant program in the UK in 1979. In 1980, I obtained an appointment with the heart transplant pioneer, Christiaan Barnard, at the University of Cape Town, where I had responsibility for the day-to-day care of heart transplant patients and also participated in a very active experimental research program.
ABO-incompatible organ allotransplantation
Baboons were readily available for research in South Africa. As they have the oligosaccharide AB blood groups (A, B and AB, but not O) similar to humans, I used the baboon heart transplantation model as a surrogate for ABO-compatible or incompatible organ transplantation in humans (Cooper et al. 1988b). I found that approximately one-third of AB-incompatible heart transplants in baboons were hyperacutely rejected (within 24 h) in comparison with approximately two-thirds when heart transplantation was carried out across this barrier in humans (Cooper 1990). These results confirmed previous studies by several other researchers that it would be risky to transplant an ABO-incompatible heart. ABO-incompatibility also played a small role in failure of grafts between closely related species (Cooper et al. 1989).
I moved from Cape Town to Oklahoma City in 1987, where I shared responsibility for the development of a new clinical heart transplant program. I was contacted by members of a small company, Chembiomed (Edmonton, Canada; established through the work of a carbohydrate chemist, Ray Lemieux), who were aware of my work on ABO-incompatibility. These researchers had evidence to suggest that the intravenous (i.v.) infusion of synthetic A or B oligosaccharides would be bound by the respective anti-A or -B antibodies in the blood and that this antibody–antigen complex would be cleared, thus reducing the antibody level in the blood and enabling an ABO-incompatible organ graft to be transplanted without fear of hyperacute rejection. In view of the model I had established of AB-incompatible heart transplantation in baboons, they invited me to collaborate with them to test the infusion of the relevant oligosaccharides.
I was somewhat doubtful that their hypothesis would be corroborated, but I readily agreed to carry out a series of heart transplants in baboons. With the collaboration of one of their consultant scientists, Egidio Romano, who (after testing the safety of i.v. infusion of these oligosaccharides on himself) had carried out preliminary clinical studies in hemolytic disease of the newborn (Romano et al. 1987a, b, c), we began the study. To my relative surprise and satisfaction, the continuous i.v. infusion of the A or B oligosaccharides into baboons allowed AB-incompatible heart grafts to function until cellular rejection (unrelated to AB-incompatibility) developed, as it would in an AB-compatible heart transplant (Cooper et al. 1993c; Ye et al. 1994b).
One of the major problems in translating this research to the clinic at that time was the cost of the oligosaccharides. I estimated that, if I had purchased the oligosaccharides we had infused, each baboon would have received almost $1 million of synthetic oligosaccharide every day—but, of course, the company provided these free of charge.
Xenotransplantation using pig organs
Previously, in the UK and South Africa, I had realized that one of the major problems with heart transplantation was obtaining sufficient hearts from deceased human donors to satisfy the needs of those in terminal heart failure. Indeed, this is still a major limiting factor today. In Cape Town, I had begun to explore different sources of hearts. I initially considered the possibility of using baboons as organ donors for humans (Neethling et al. 1990; Cooper 1992a; Luo et al. 1996). To set up a relevant experimental model, I selected cynomolgus (vervet) monkeys as donors with baboons as recipients (to simulate the baboon-to-human species immunological discrepancy) (Cooper et al. 1989; Reichenspurner et al. 1990; Cooper 1991).
Rejection of a heart from a closely related primate species was rather more rapid than after allotransplantation, but could be delayed by standard immunosuppressive therapy. I soon realized, however, that there were many reasons why pigs would be preferable sources of organs and cells than nonhuman primates (Table I). However, a pig organ transplanted into a baboon was rejected within minutes or hours rather than days or weeks (Lexer et al. 1986; Cooper et al. 1988a).
Table I.
The advantages and disadvantages of the pig as a potential source of organs and cells for humans, in contrast with those of the baboon in this role
| Pig | Baboon | |
|---|---|---|
| Availability | Unlimited | Limited |
| Breeding potential | Good | Poor |
| Period to reproductive maturity | 4–8 months | 3–5 years |
| Length of pregnancy | 114 ± 2 days | 173–193 days |
| Number of offspring | 5–12 | 1–2 |
| Growth | Rapid (adult human size within 6 months)a | Slow (9 years to reach maximum size) |
| Size of adult organs | Adequate | Inadequateb |
| Cost of maintenance | Relatively low | High |
| Anatomical similarity to humans | Moderately close | Close |
| Physiological similarity to humans | Moderately close | Close |
| Relationship of immune system to humans | Distant | Close |
| Knowledge of tissue typing | Considerable (in selected herds) | Limited |
| Necessity for blood type compatibility with humans | Probably unimportant | Important |
| Experience with genetic engineering | Considerable | None |
| Risk of transfer of infection (xenozoonosis) | Low | High |
| Availability of specific pathogen-free animals | Yes | No |
| Public opinion | Generally in favor | Mixed |
aBreeds of miniature swine are ∼50% of the weight of domestic pigs at birth and sexual maturity, and reach a maximum weight of ∼30% of standard breeds.
bThe size of certain organs, e.g., the heart, would be inadequate (too small) for transplantation into adult humans.
Reproduced with permission from Cooper and Bottino (2015).
On the basis of my experience with ABO-incompatible organ transplants, I gave thought to whether the hyperacute rejection that occurred uniformly after the transplantation of a pig organ into a baboon was associated with recognition by the recipient of a carbohydrate on the surface of the pig organ. I had seen nothing to support this idea in the literature but the more I thought about it, the more it appeared to be likely. My rather naive thinking at the time was that, if we could overcome this single problem, we would be able to use pigs as sources of organs for transplantation into humans (just as we could use ABO-incompatible allografts when steps to overcome hyperacute rejection were undertaken). The barriers to successful xenotransplantation, however, proved much more complex.
By this time, I was collaborating on a daily basis with Eugene Koren, my scientific colleague at the Oklahoma Medical Research Foundation. He had suggested ways by which we could identify the potential carbohydrate targets on pig organs against which humans have anti-pig antibodies. The key proposal was to perfuse human plasma through isolated pig kidneys and hearts ex vivo, then to elute the antibodies that had bound to the vascular endothelium of the organ, and send these antibodies to our colleagues at Chembiomed to identify their carbohydrate specificities using a “glycan array” approach—a large library of synthetic oligosaccharides that the company had accumulated.
The role of Gal in xenotransplantation
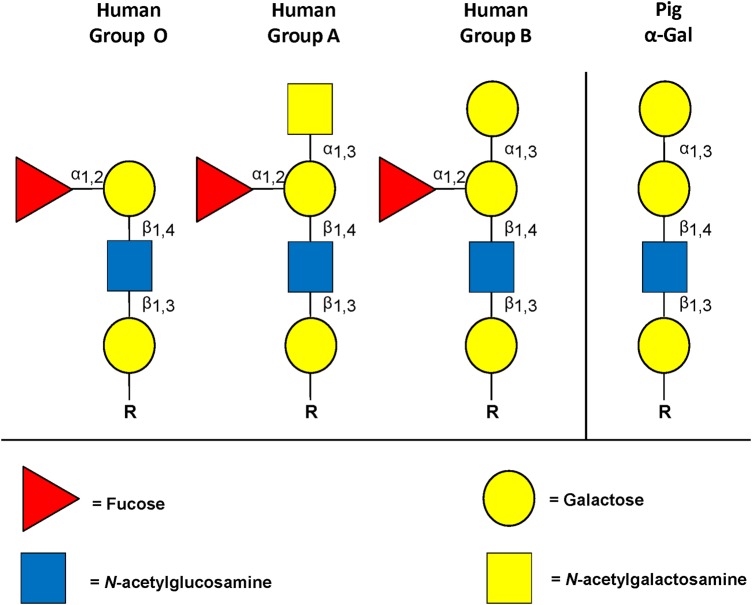
Based on this approach, I was greatly excited when one of the Chembiomed scientists, Heather Good, informed me that a major target for human anti-pig antibodies was the Gal epitope (galactose-α(1,3)-galactose) (Cooper 1992b, 1994; Good et al. 1992; Cooper et al. 1993a; ; Kobayashi and Cooper 1999). There were a few other oligosaccharide targets, but Gal was by far the most important. I was encouraged by the observation that there are similarities between the structure of Gal and those of the ABO blood group antigens (Stussi et al. 2006) (Figure 1), particularly between Gal and blood group B (Galili et al. 1987; Galili 2006), suggesting that the human immune response would be similar to both.
Fig. 1.
Carbohydrate structure on human and pig RBCs. Pig RBCs express Gal epitopes on oligosaccharides that are similar in structure to the human blood type B oligosaccharide (which has a fucose side arm).
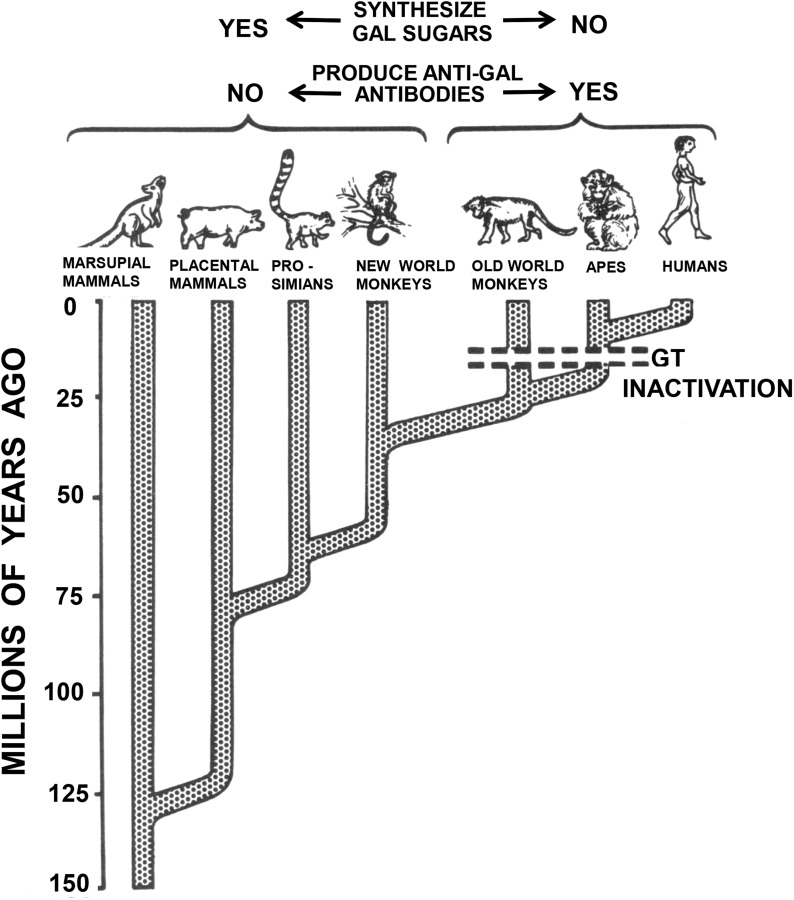
I had never heard of Gal previously, but the Chembiomed team informed me that they had prior knowledge of it as they had carried out some work for Uri Galili, who had identified that all mammals below Old World monkeys express Gal whereas Old World nonhuman primates and humans lack the requisite enzyme α(1,3)-galactosyltransferase (GT), and thus lack Gal in their tissues and produce anti-Gal antibodies (Figure 2) (Galili et al. 1984; 1988b). If they already knew this information, I asked them, why had they not mentioned this to me when we were planning the study, but they were at a loss to explain why.
Fig. 2.
Evolutionary time scale of mammals during the past 125 million years. All mammals originally synthesized the Gal sugar, which was made by the enzyme GT. Evidence suggests that ∼10–25 million years ago the Old World higher primates were afflicted by a lethal infection caused by a microorganism that also expressed the Gal determinant. Only those primates that could produce anti-Gal antibodies survived. This necessitated suppression of the synthesis of the Gal sugar by mutation of the gene for the enzyme GT. (Modified from Galili 1998.)
I then avidly read all I could about Gal antigens and anti-Gal antibodies, largely from the writings of Galili and his colleagues (Galili 1999). Galili had put forward the hypothesis that humans, apes, and Old World monkeys develop anti-Gal antibodies (so-called “natural” or “preformed” antibodies) during infancy as a “defensive” response to the colonization of their gastrointestinal tracts by microorganisms and viruses that express Gal (Galili et al. 1988a). My colleagues and I later confirmed that infant humans and baboons do not make anti-pig (or anti-Gal) antibodies (or anti-A or B antibodies) for the first 3 months or so of life (Neethling et al. 1995; Minanov et al. 1997; Rood et al. 2007; Dons et al. 2012), at which time they begin to develop these antibodies, presumably after colonization of the GI tracts.
The more I read, the more I was convinced that this antibody–antigen reaction was key to xenotransplantation. In vitro studies by our group showed this to be the case (Table II) (Koren et al. 1993; Oriol et al. 1993, 1994, 1999; Kujundzic et al. 1994; Neethling et al. 1994, 1996; Neethling and Cooper 1999). The identification of Gal as the prime target for anti-pig antibodies was presented at the First International Congress on Xenotransplantation held in Minneapolis in 1991 (Cooper 1992b; Good et al. 1992), the first definitive identification of the role of Gal in xenotransplantation (Cooper et al. 1996b; Cooper 2009b). Others soon confirmed our conclusion (Sandrin et al. 1993). Although Galili had carried out several studies on the nature of anti-Gal antibodies, he had not directed his attention towards xenotransplantation before this time (Galili 1993).
Table II.
Micromolal concentration of each oligosaccharide needed to obtain 50% inhibition of cytotoxicity of unmodified human or baboon serum on pig kidney (PK15) cells
| Inhibitor oligosaccharide | Serum |
|
|---|---|---|
| Human | Baboon | |
| Fucα1–2Galβ1-R | >10,000 | >10,000 |
| Galβ1-R | >10,000 | >10,000 |
| Galα1–2Galβ1-R′ | 7000 | >10,000 |
| Galα1–3Gal | 386 ± 149a | 301 ± 44e |
| Galα1–3Galβ1–4Gal | 163 ± 73b | 141 ± 60f |
| Galα1–3Galβ1–4Galα1–3Gal | 54 ± 31c | 119 ± 30g |
| Galα1–3Galβ1–4GlcNAc | 27 ± 11d | 31 ± 4h |
Bold type indicates structural differences of the oligosaccharide with the major pig vascular endothelium glycolipid Galα1–3Galβ1–4GlcNAcβ1–3Galβ1–4Glcβ1-Cer. R represents 1–3 or 1–4 linkages to Gal or to GlcNAc; R′ is –O(CH2)3NHCOCF3. The results of the strong inhibitors (a–h) are expressed as mean ± SD (n = 3). Statistical significance: a vs. c (t = 3.0); a vs. d (t = 3.9); b vs. d (t = 3.1); e vs. h (t = 9.7); f vs. h (t = 3.1); g vs. h (t = 5.4), all with P < 0.02. The other comparisons did not reach the P = 0.05 level of significance, but both human and baboon serum inhibition tests follow a similar trend. Reproduced with permission from Neethling et al. (1996).
We reasoned that if we could obtain sufficient synthetic Gal oligosaccharide, or alternatively immunoaffinity columns of synthetic Gal, we could test whether removal of anti-Gal antibodies in nonhuman primates would allow prolonged survival of a pig organ graft. Initially, we were unable to obtain sufficient synthetic Gal to carry out this study, but through my reading I realized that melibiose had sufficient similarity to Gal that it might have some effect in delaying hyperacute rejection. We therefore perfused melibiose continuously at high concentrations into baboons, and indeed there was some delay in rejection of a pig heart graft (Ye et al. 1994a).
The difficulty in obtaining synthetic Gal led us to seek natural sources of Gal. In Oklahoma City at that time, we were fortunate to have the advice and collaboration of the well-known glycobiologist, Richard Cummings, with whom we explored other options for obtaining Gal sugars and of reducing antibody binding (Li et al. 1995, 1996; Luo et al. 1999). We even briefly explored the possibility of using organs from non-mammal species, e.g., ratites (ostriches, emus) that do not express Gal (Taniguchi et al. 1996a). Eventually, we were able to obtain sufficient specific synthetic Gal from several different sources (including Nicolai Bovin in Moscow) to test our hypothesis in vivo in baboons (Rieben et al. 1995; Cooper et al. 1996a; Taniguchi et al. 1996b), and indeed found that antibody-mediated rejection of a pig heart was delayed (Simon et al. 1998; Romano et al. 1999).
Later, after I moved to the Transplantation Biology Research Center (TBRC) at the Massachusetts General Hospital/Harvard Medical School in 1996, we studied Gal expression in different tissues and cell types in pigs (Chae et al. 1999; Gojo et al. 2002; Dor et al. 2004a), and anti-Gal antibody in various species under differing circumstances (Gojo et al. 2000; Teranishi et al. 2002b). We also carried out >300 extracorporeal immunoadsorptions of anti-Gal antibodies (using immunoaffinity columns of synthetic Gal) in baboons, some of whom subsequently underwent organ or bone marrow transplantation (Kozlowski et al. 1998a, b; Lambrigts et al. 1998; Xu et al. 1998; Watts et al. 2000; Buhler et al. 2001; Kuwaki et al. 2004). We also intravenously infused Gal conjugates (Teranishi et al. 2002a, 2003; Gollackner et al. 2003; Kuwaki et al. 2004). In these studies, for a short period of time, we were able to consult with Robert Sackstein who, although then new to the field of glycobiology, was invaluable to us for his expertise and advice in bone marrow transplantation as a means to induce immune tolerance (Tseng et al. 2004, 2005b). These data demonstrated that, although extracorporeal immunoadsorption certainly delayed antibody-mediated rejection of a pig graft, the continuing production of anti-Gal antibodies resulted in the graft being lost from a delayed form of antibody-mediated rejection known as acute humoral xenograft rejection.
The generation of GTKO pigs
At the time of the Minneapolis meeting, in discussion with my two invaluable colleagues, Eugene Koren and Rafael Oriol (an immunogeneticist from Paris with a special interest in ABO blood group antigens), we determined that, when the technology was available, it should be possible to knockout the gene for the enzyme that attaches Gal to underlying carbohydrate structures on the surface of the pig vascular endothelium (Cooper et al. 1993b). This approach would involve producing GTKO pigs.
It was soon possible to produce a GTKO mouse (Thall et al. 1995; Tearle et al. 1996), but knockout of the GT gene in pigs only became possible after the development of the technology that produced the world's first cloned mammal, the sheep known as “Dolly” (Campbell et al. 1996) and, subsequently, the first cloned pig (Polejaeva et al. 2000). This would clearly be a more successful approach than i.v. oligosaccharide infusion or extracorporeal immunoadsorption as it would permanently delete Gal as a target for primate anti-pig antibodies.
The first GTKO pigs did not become available until 2003 (Phelps et al. 2003; Kolber-Simonds et al. 2004), and my colleagues and I at the TBRC were the first to test the transplantation of organs from these pigs in immunosuppressed baboons (Kuwaki et al. 2005; Tseng et al. 2005a; Yamada et al. 2005; Hisashi et al. 2008; Shimizu et al. 2008). The transplantation of a GTKO pig heart or kidney was associated with markedly prolonged survival of the grafts, particularly of the heart grafts (one of which functioned for almost 6 months), a major advance in the field of xenotransplantation research.
I moved to the University of Pittsburgh in 2004 and, around that time and subsequently, several studies were carried out on GTKO pigs (Dor et al. 2004b; Knosalla et al. 2004; Ekser et al. 2012; Fang et al. 2012) and their cells were used for in vitro assays (Ezzelarab et al. 2006; Hara et al. 2006; Rood et al. 2006; Wong et al. 2006). Although not anticipated, the absence of Gal expression on pig tissues was associated with a reduction in the primate cellular response (as well as the expected humoral response) to the graft, which has proved beneficial in overcoming the immunological barriers to successful xenotransplantation (Wilhite et al. 2012). Subsequently, GTKO pigs were genetically engineered to express a human complement-regulatory protein, which has further enhanced graft survival (Azimzadeh et al. 2015).
Nevertheless, in part due to low-grade activation of the vascular endothelium of the transplanted pig organ by remaining anti-pig antibodies (i.e., anti-non-Gal antibodies, the nature of which remained unknown at the time), a thrombotic microangiopathy developed that ultimately led to graft failure (Buhler et al. 2000; Houser et al. 2004). If the thrombotic microangiopathy became advanced (by aggregation of platelets and fibrin in the graft), a consumptive coagulopathy could develop that could be life-threatening to the recipient baboon (Ierino et al. 1998; Kozlowski et al. 1999; Buhler et al. 2000; Ezzelarab et al. 2009). Emergent excision of the pig graft would reverse the situation, confirming it was the presence of the graft that was the major factor in the development of this complication.
Coagulation dysregulation
These observations (and important previous studies by others (reviewed in Robson et al. 2000), generated investigation into the nature of the coagulation/anticoagulation discrepancies between pigs and primates and eventually to the genetic modification of GTKO pigs to express human coagulation-regulatory proteins, e.g., thrombomodulin, endothelial protein C-receptor, tissue factor pathway inhibitor, and/or CD39, which, individually or in combination, to some extent corrected this dysregulation. Efforts are currently underway to replace porcine von Willebrand factor with human von Willebrand factor. Our mutual interest in preventing this thrombotic microangiopathy has resulted in an ongoing collaboration with Umesh Desai and his glycobiology colleagues at Virginia Commonwealth University to create potent analogs of heparin (A Azimzadeh et al. in preparation).
The role of non-Gal oligosaccharides in xenotransplantation
Soon after we identified Gal as the major target for human anti-pig antibodies, others reported that it was already known that pigs expressed N-glycolylneuraminic acid (NeuGc) on the vascular endothelium (Bouhours et al. 1996). NeuGc is not expressed in humans (but was thought to be present in all other mammals) due to the absence of the enzyme creating this modified form of the monosaccharide neuraminic acid, cytidine monophosphate-N-acetylneuraminic acid hydroxylase (CMAH). The development of anti-NeuGc antibodies in humans would therefore be detrimental to survival of a pig organ graft transplanted into a patient (though not into a baboon). Just as I had read all of Uri Galili's papers on Gal, I now followed the studies by Ajit Varki's group on NeuGc (reviewed in Padler-Karavani and Varki 2011).
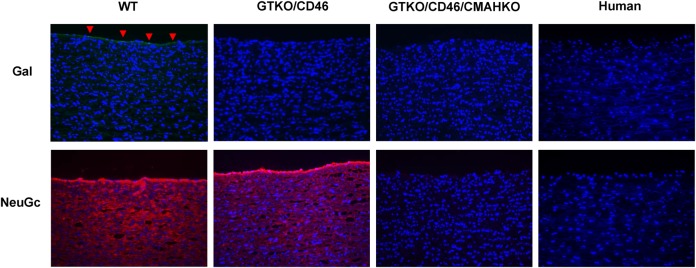
The relevance of anti-NeuGc antibodies has been tested in vitro using human serum and pig cells, but it has only been relatively recently that pigs in which expression of both Gal and NeuGc has been deleted have become available (Figure 3) (Lutz et al. 2013; Lee et al. 2016a). The effect of CMAH gene-knockout has not yet been explored in vivo because it was thought there was no relevant animal model, but it has been recently demonstrated that NeuGc is not expressed in New World monkeys (Springer et al. 2014), which therefore can develop anti-NeuGc antibodies. Plans are afoot to carry out organ transplants from NeuGcKO pigs in New World monkeys, which should provide evidence of the role NeuGc may play when clinical xenotransplantation is introduced, and how well the grafts are tolerated.
Fig. 3.
(Left to right) Expression of Gal and NeuGc on aortas from wild-type, GTKO/CD46 and GTKO/CD46/CMAHKO pigs, and also on a human aorta. Expression of Gal was determined by staining with the isolectin B4 from Bandeiraea simplicifolia, and expression of NeuGc by staining with a chicken-derived anti-NeuGc immunohistochemistry set. Therefore, it is not possible to make a direct quantitative comparison of the level of expression between the two oligosaccharides. However, Gal (green) is expressed mainly on the vascular endothelium (indicated by red arrowheads), whereas NeuGc (red) is much more widely expressed in all layers, including the vascular endothelium. (Cell nuclei—blue; Gal—green; NeuGc—red. Magnification ×200.) (Figure kindly provided by W. Lee, MD).
Despite considerable investigation (Cooper 1998; Ezzelarab et al. 2005; Yeh et al. 2010), only one further oligosaccharide antigen has been definitively identified as being of relevance to xenotransplantation, which is a product of the gene, β(1,4)N-acetylgalactosaminyltransferase (β4GalNT2) (Byrne et al. 2014). Like Gal, the oligosaccharide created by this enzyme is not expressed in humans and Old World monkeys and so it will be possible to test the effect of primate anti-β4GalNT2 antibodies on pig grafts in the pig-to-nonhuman primate transplant model. Furthermore, β4GalNT2-knockout (β4GalNT2KO) pigs that do not express the Sd(a) antigen are also now available (Estrada et al. 2015), and transplants using organs from GTKO/β4GalNT2KO pigs are being carried out in monkeys by a group of investigators at the University of Indiana and Emory University, though in vivo data have not yet been reported.
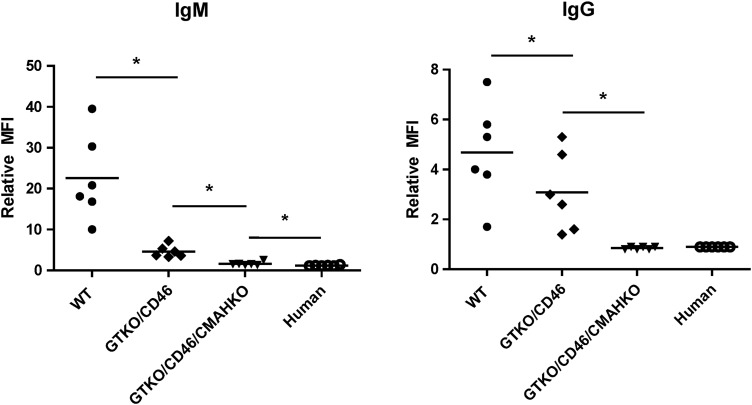
Recent studies using human serum and cells from pigs in which the enzymes GT+/− CMAH+/− β4GalNT2 have been knocked out provide great encouragement for the future of clinical transplantation as human antibody binding to these cells is often minimal (Figure 4). Tector and his colleagues have demonstrated that ∼25% of humans do not appear to have natural antibodies to any other targets on these “triple-knockout” pig cells (Estrada et al. 2015).
Fig. 4.
Human IgM (A) and IgG (B) antibody binding to pig and human aortic endothelial cells by flow cytometry (n = 6). Human IgM and IgG binding to GTKO/CD46 pAECs was significantly decreased compared with wild-type (WT, i.e., genetically unmodified) pAECs (*P < 0.05), and was further decreased to GTKO/CD46/CMAHKO pAECs (*P < 0.05). There was significantly greater IgM binding to GTKO/CD46/CMAHKO pAECs than to human AECs, but there was no statistical significance in the extent of IgG binding between them. (Figure kindly provided by H. Hara, MD, PhD)
The future of pig organ and cell xenotransplantation
When pigs in which these three carbohydrate antigens have been deleted and which also express one or more human complement-regulatory proteins and one or more human coagulation-regulatory proteins, a clinical trial of kidney or heart xenotransplantation will likely be justified (Cooper 2015; Cooper and Bottino 2015; Cooper et al. 2016). However, there are numerous other genetic manipulations that may benefit the outcome of a xenotransplant, and these include the expression of anti-inflammatory genes and manipulations that reduce or suppress the adaptive immune response (Table III). Currently, there are an estimated 40 different genetically modified pigs worldwide, with up to 7 modifications combined in a single pig.
Table III.
Selected genetically modified pigs currently available for xenotransplantation research
| Complement regulation by human complement-regulatory gene expression |
| CD46 (membrane cofactor protein) |
| CD55 (decay-accelerating factor) |
| CD59 (protectin or membrane inhibitor of reactive lysis) |
| Gal or non-Gal antigen “masking” or deletion |
| Human H-transferase gene expression (expression of blood type O antigen) |
| Endo-β-galactosidase C (reduction of Gal antigen expression) |
| α(1,3)-galactosyltransferase gene-knockout (GTKO) |
| Cytidine monophosphate-N-acetylneuraminic acid hydroxylase (CMAH) gene-knockout (CMAHKO) |
| β4GalNT2 (β(1,4)-N-acetylgalactosaminyltransferase) gene-knockout (β4GalNT2KO) |
| Suppression of cellular immune response by gene expression or downregulation |
| CIITA-DN (MHC class II transactivator knockdown, resulting in swine leukocyte antigen class II knockdown) |
| Class I MHC-knockout (MHC-IKO) |
| HLA-E/human β2-microglobulin (inhibits human natural killer cell cytotoxicity) |
| Human FAS ligand (CD95L) |
| Human N-acetylglucosaminyltransferase III gene |
| Porcine CTLA4-Ig (cytotoxic T-lymphocyte antigen 4 or CD152) |
| Human tumor necrosis factor-α-related apoptosis-inducing ligand |
| Anticoagulation and anti-inflammatory gene expression or deletion |
| von Willebrand factor-deficient (natural mutant) |
| Human tissue factor pathway inhibitor |
| Human thrombomodulin |
| Human endothelial protein C receptor |
| Human CD39 (ectonucleoside triphosphate diphosphohydrolase-1) |
| Anticoagulation, anti-inflammatory, and anti-apoptotic gene expression |
| Human A20 (tumor necrosis factor-α-induced protein 3) |
| Human heme oxygenase-1 |
| Human CD47 (species-specific interaction with SIRP-α inhibits phagocytosis) |
| Porcine asialoglycoprotein receptor 1 gene-knockout (decreases platelet phagocytosis) |
| Human signal regulatory protein α (decreases platelet phagocytosis by “self”-recognition) |
| Prevention of porcine endogenous retrovirus (PERV) activation |
| PERV siRNA |
Modified from Cooper et al. (2016).
We and other groups have extended our interest in the glycobiology of xenotransplantation to include the transplantation of the pig liver, lung, pancreatic islets, corneas, neuronal cells, and red blood cells. For example, the transplantation of pig pancreatic islets could provide a cure for the millions of patients worldwide with diabetes (Cooper and Bottino 2015). Neuronal cells from genetically engineered pigs may cure neurodegenerative diseases, such as Parkinson's disease (Leveque et al. 2011). The absence of Gal and NeuGc expression on erythrocytes takes us one step closer to being able to use pig red blood cells for transfusion in humans (Rouhani et al. 2004; Cooper et al. 2010; Wang et al. 2014). Genetically engineered pigs could also be a source of corneas for the hundreds of thousands of patients worldwide with corneal blindness (Hara and Cooper 2011; Lamm et al. 2014; Lee et al. 2016b). Bioprosthetic heart valves from pigs (that are implanted in their thousands each year into patients with cardiac valve disease) will almost certainly function for longer periods if obtained from GTKO/CMAHKO/β4GalNT2KO pigs (Cooper 2009a; Manji et al. 2014).
Conclusions
In summary, therefore, my career has taken an unexpected turn towards glycobiology, a field that, as I have shared here, has played center-stage in my research activities. My firm belief is that the transplantation of pig organs and cells into humans will be achieved successfully within the foreseeable future and that its success will be due in no small part to the developments that have taken place in glycobiology during the past 30 years.
History tells us that procedures that were inconceivable yesterday, and barely achievable today, often become routine tomorrow.
Thomas Starzl (American transplant pioneer)
Conflict of interest statement
None declared.
Abbreviations
β4GalNT2, pig antigen encoded by β(1,4)N-acetylgalactosaminyltransferase; CMAH, cytidine monophosphate-N-acetylneuraminic acid hydroxylase; Gal, galactose-α(1,3)-galactose; GTKO, α(1,3)-galactosyltransferance gene-knockout; NeuGc, N-glycolylneuraminic acid; NeuGcKO, cytidine monophosphate-N-acetylneuraminic acid hydroxylase gene-knockout.
Acknowledgements
I thank my many former and current colleagues with whom I have worked on the studies summarized here. Work on xenotransplantation in the Thomas E. Starzl Transplantation Institute at the University of Pittsburgh has been supported in part by NIH grants U01 AI068642, R21 AI074844 and U19 AI090959, and by Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Inc., Blacksburg, VA.
References
- Azimzadeh A, Kelishadi S, Ezzelarab MB, Singh A, Stoddard T, Zhang T, Burdorf L, Avon C, Laaris A, Cheng X et al. 2015. Early graft failure of GalTKO pig organs in baboons is reduced by expression of a human complement-regulatory protein. Xenotransplantation. 22:310–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhours D, Pourcel C, Bouhours JE. 1996. Simultaneous expression by porcine aorta endothelial cells of glycosphingolipids bearing the major epitope for human xenoreactive antibodies (Gal alpha 1-3Gal), blood group H determinant and N-glycolylneuraminic acid. Glycoconj J. 13:947–953. [DOI] [PubMed] [Google Scholar]
- Buhler L, Basker M, Alwayn IP, Goepfert C, Kitamura H, Kawai T, Gojo S, Kozlowski T, Ierino FL, Awwad M et al. 2000. Coagulation and thrombotic disorders associated with pig organ and hematopoietic cell transplantation in nonhuman primates. Transplantation. 70:1323–1331. [DOI] [PubMed] [Google Scholar]
- Buhler L, Yamada K, Kitamura H, Alwayn IP, Basker M, Appel JZ III, Colvin RB, White-Scharf ME, Sachs DH, Robson SC et al. 2001. Pig kidney transplantation in baboons: Anti-Gal(alpha)1-3Gal IgM alone is associated with acute humoral xenograft rejection and disseminated intravascular coagulation. Transplantation. 72:1743–1752. [DOI] [PubMed] [Google Scholar]
- Byrne GW, Du Z, Stalboerger P, Kogelberg H, McGregor CG. 2014. Cloning and expression of porcine beta1,4 N-acetylgalactosaminyl transferase encoding a new xenoreactive antigen. Xenotransplantation. 21:543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KH, McWhir J, Ritchie WA, Wilmut I. 1996. Sheep cloned by nuclear transfer from a cultured cell line. Nature. 380:64–66. [DOI] [PubMed] [Google Scholar]
- Chae SJ, Kramer AD, Zhao Y, Arn S, Cooper DK, Sachs DH. 1999. Lack of variation in alphaGal expression on lymphocytes in miniature swine of different genotypes. Xenotransplantation. 6:43–51. [DOI] [PubMed] [Google Scholar]
- Cooper DK. 1990. Clinical survey of heart transplantation between ABO blood group-incompatible recipients and donors. J Heart Transplant. 9:376–381. [PubMed] [Google Scholar]
- Cooper DKC. 1991. Allo- and xeno- transplantation in non-human primates. Minerva Chirurgica. 46(Suppl. 1 al N. 11):107–116. [Google Scholar]
- Cooper DK. 1992a. The Chacma baboon as an organ donor for man. Transplantation. 53:1380–1381. [DOI] [PubMed] [Google Scholar]
- Cooper DK. 1992b. Depletion of natural antibodies in non-human primates – A step towards successful discordant xenografting in humans. Clin Transplant. 6:178–183. [PubMed] [Google Scholar]
- Cooper DK. 1998. Xenoantigens and xenoantibodies. Xenotransplantation. 5:6–17. [DOI] [PubMed] [Google Scholar]
- Cooper DK. 2009a. How important is the anti-Gal antibody response following the implantation of a porcine bioprosthesis? J Heart Valve Dis. 18:671–672. [PubMed] [Google Scholar]
- Cooper DK. 2009b. Identification of alpha Gal as the major target for human anti-pig antibodies. Xenotransplantation. 16:47–49. [DOI] [PubMed] [Google Scholar]
- Cooper DK. 2015. The case for xenotransplantation. Clin Transplant. 29:288–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DK, Bottino R. 2015. Recent advances in understanding xenotransplantation: Implications for the clinic. Expert Rev Clin Immunol. 11:1379–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DK, Cairns TD, Taube DH. 1996a. Extracorporeal immunoadsorption of alphaGal antibodies. Xeno. 4:27–29. [Google Scholar]
- Cooper DK, Ekser B, Ramsoondar J, Phelps C, Ayares D. 2016. The role of genetically-engineered pigs in xenotransplantation research. J Pathol. 238:288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DK, Good AH, Koren E, Oriol R, Malcolm AJ, Ippolito RM, Neethling FA, Ye Y, Romano E, Zuhdi N. 1993a. Identification of alpha-galactosyl and other carbohydrate epitopes that are bound by human anti-pig antibodies: Relevance to discordant xenografting in man. Transpl Immunol. 1:198–205. [DOI] [PubMed] [Google Scholar]
- Cooper DK, Hara H, Yazer M. 2010. Genetically engineered pigs as a source for clinical red blood cell transfusion. Clin Lab Med. 30:365–380. [DOI] [PubMed] [Google Scholar]
- Cooper DK, Human PA, Lexer G, Rose AG, Rees J, Keraan M, Du Toit E. 1988a. Effects of cyclosporine and antibody adsorption on pig cardiac xenograft survival in the baboon. J Heart Transplant. 7:238–246. [PubMed] [Google Scholar]
- Cooper DK, Human PA, Rose AG, Rees J, Keraan M, Reichart B, Du Toit E, Oriol R. 1989. The role of ABO blood group compatibility in heart transplantation between closely related animal species. An experimental study using the vervet monkey to baboon cardiac xenograft model. J Thorac Cardiovasc Surg. 97:447–455. [PubMed] [Google Scholar]
- Cooper DK, Koren E, Oriol R. 1993b. Genetically engineered pigs. Lancet. 342:682–683. [DOI] [PubMed] [Google Scholar]
- Cooper DK, Koren E, Oriol R. 1994. Oligosaccharides and discordant xenotransplantation. Immunol Rev. 141:31–58. [DOI] [PubMed] [Google Scholar]
- Cooper DKC, Koren E, Oriol R. 1996b. Manipulation of the anti-αGal antibody – αGal epitope system in experimental discordant xenotransplantation. Xenotransplantation. 3:102–111. [Google Scholar]
- Cooper DK, Lexer G, Rose AG, Keraan M, Rees J, Du Toit E, Oriol R. 1988b. Cardiac allotransplantation across major blood group barriers in the baboon. J Med Primatol. 17:333–346. [PubMed] [Google Scholar]
- Cooper DK, Ye Y, Niekrasz M, Kehoe M, Martin M, Neethling FA, Kosanke S, DeBault LE, Worsley G, Zuhdi N et al. 1993c. Specific intravenous carbohydrate therapy. A new concept in inhibiting antibody-mediated rejection – Experience with ABO-incompatible cardiac allografting in the baboon. Transplantation. 56:769–777. [DOI] [PubMed] [Google Scholar]
- Dons EM, Montoya C, Long CE, Hara H, Echeverri GJ, Ekser B, Ezzelarab C, Medellin DR, van der Windt DJ, Murase N et al. 2012. T-cell-based immunosuppressive therapy inhibits the development of natural antibodies in infant baboons. Transplantation. 93:769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dor FJ, Cheng J, Alt A, Cooper DK, Schuurman HJ. 2004a. Gal alpha 1,3Gal expression on porcine pancreatic islets, testis, spleen, and thymus. Xenotransplantation. 11:101–106. [DOI] [PubMed] [Google Scholar]
- Dor FJ, Tseng YL, Cheng J, Moran K, Sanderson TM, Lancos CJ, Shimizu A, Yamada K, Awwad M, Sachs DH et al. 2004b. Alpha1,3-Galactosyltransferase gene-knockout miniature swine produce natural cytotoxic anti-Gal antibodies. Transplantation. 78:15–20. [DOI] [PubMed] [Google Scholar]
- Ekser B, Bianchi J, Ball S, Iwase H, Walters A, Ezzelarab M, Veroux M, Gridelli B, Wagner R, Ayares D et al. 2012. Comparison of hematologic, biochemical, and coagulation parameters in alpha1,3-galactosyltransferase gene-knockout pigs, wild-type pigs, and four primate species. Xenotransplantation. 19:342–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada JL, Martens G, Li P, Adams A, Newell KA, Ford ML, Butler JR, Sidner R, Tector M, Tector J. 2015. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/beta4GalNT2 genes. Xenotransplantation. 22:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzelarab M, Ayares D, Cooper DK. 2005. Carbohydrates in xenotransplantation. Immunol Cell Biol. 83:396–404. [DOI] [PubMed] [Google Scholar]
- Ezzelarab M, Garcia B, Azimzadeh A, Sun H, Lin CC, Hara H, Kelishadi S, Zhang T, Lin YJ, Tai HC et al. 2009. The innate immune response and activation of coagulation in alpha1,3-galactosyltransferase gene-knockout xenograft recipients. Transplantation. 87:805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzelarab M, Hara H, Busch J, Rood PP, Zhu X, Ibrahim Z, Ball S, Ayares D, Awwad M, Cooper DK. 2006. Antibodies directed to pig non-Gal antigens in naive and sensitized baboons. Xenotransplantation. 13:400–407. [DOI] [PubMed] [Google Scholar]
- Fang J, Walters A, Hara H, Long C, Yeh P, Ayares D, Cooper DK, Bianchi J. 2012. Anti-gal antibodies in alpha1,3-galactosyltransferase gene-knockout pigs. Xenotransplantation. 19:305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili U. 1993. Interaction of the natural anti-Gal antibody with alpha-galactosyl epitopes: A major obstacle for xenotransplantation in humans. Immunol Today. 14:480–482. [DOI] [PubMed] [Google Scholar]
- Galili U. 1998. Anti-gal antibody prevents xenotransplantation. Sci Med. 5:28–37. [Google Scholar]
- Galili U. 1999. Evolution of alpha 1,3galactosyltransferase and of the alpha-Gal epitope. Subcell Biochem. 32:1–23. [DOI] [PubMed] [Google Scholar]
- Galili U. 2006. Xenotransplantation and ABO incompatible transplantation: The similarities they share. Transfus Apher Sci. 35:45–58. [DOI] [PubMed] [Google Scholar]
- Galili U, Buehler J, Shohet SB, Macher BA. 1987. The human natural anti-Gal IgG. III. The subtlety of immune tolerance in man as demonstrated by crossreactivity between natural anti-Gal and anti-B antibodies. J Exp Med. 165:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili U, Mandrell RE, Hamadeh RM, Shohet SB, Griffiss JM. 1988a. Interaction between human natural anti-alpha-galactosyl immunoglobulin G and bacteria of the human flora. Infect Immun. 56:1730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili U, Rachmilewitz EA, Peleg A, Flechner I. 1984. A unique natural human IgG antibody with anti-alpha-galactosyl specificity. J Exp Med. 160:1519–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili U, Shohet SB, Kobrin E, Stults CL, Macher BA. 1988b. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem. 263:17755–17762. [PubMed] [Google Scholar]
- Gojo S, Bartholomew A, Xu Y, Neethling FA, Awwad M, Saidman S, Cosimi AB, Cooper DK. 2000. Anti-Galalpha1-3Gal antibody levels in organ transplant recipients receiving immunosuppressive therapy. Transplantation. 69:914–917. [DOI] [PubMed] [Google Scholar]
- Gojo S, Harper D, Down J, Awwad M, Cooper DK. 2002. Differential expression of Galalpha1,3Gal epitopes on fetal and adult porcine hematopoietic cells. Xenotransplantation. 9:297–300. [DOI] [PubMed] [Google Scholar]
- Gollackner B, Knosalla C, Houser S, Mauiyyedi S, Buhler L, Kawai T, Duggan M, Sachs DH, Awwad M, Cooper DK. 2003. Pig kidney transplantation in baboons treated intravenously with a bovine serum albumin-Galalpha1-3Gal conjugate. Xenotransplantation. 10:606–614. [DOI] [PubMed] [Google Scholar]
- Good AH, Cooper DK, Malcolm AJ, Ippolito RM, Koren E, Neethling FA, Ye Y, Zuhdi N, Lamontagne LR. 1992. Identification of carbohydrate structures that bind human antiporcine antibodies: Implications for discordant xenografting in humans. Transplant Proc. 24:559–562. [PubMed] [Google Scholar]
- Hara H, Cooper DK. 2011. Xenotransplantation – The future of corneal transplantation? Cornea. 30:371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara H, Ezzelarab M, Rood PP, Lin YJ, Busch J, Ibrahim Z, Zhu X, Ball S, Ayares D, Zeevi A et al. 2006. Allosensitized humans are at no greater risk of humoral rejection of GT-KO pig organs than other humans. Xenotransplantation. 13:357–365. [DOI] [PubMed] [Google Scholar]
- Hisashi Y, Yamada K, Kuwaki K, Tseng YL, Dor FJ, Houser SL, Robson SC, Schuurman HJ, Cooper DK, Sachs DH et al. 2008. Rejection of cardiac xenografts transplanted from alpha1,3-galactosyltransferase gene-knockout (GalT-KO) pigs to baboons. Am J Transplant. 8:2516–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser SL, Kuwaki K, Knosalla C, Dor FJ, Gollackner B, Cheng J, Shimizu A, Schuurman HJ, Cooper DK. 2004. Thrombotic microangiopathy and graft arteriopathy in pig hearts following transplantation into baboons. Xenotransplantation. 11:416–425. [DOI] [PubMed] [Google Scholar]
- Ierino FL, Kozlowski T, Siegel JB, Shimizu A, Colvin RB, Banerjee PT, Cooper DK, Cosimi AB, Bach FH, Sachs DH et al. 1998. Disseminated intravascular coagulation in association with the delayed rejection of pig-to-baboon renal xenografts. Transplantation. 66:1439–1450. [DOI] [PubMed] [Google Scholar]
- Knosalla C, Giovino MA, Harper D, Kaczmarek E, Gollackner B, Cooper DK, Robson SC. 2004. Relative effects of GAL+ and GALlow/- porcine hematopoietic cells on primate platelet aggregation and endothelial cell activation: Implications for the induction of mixed hematopoietic chimerism in the pig-to-primate model. Xenotransplantation. 11:72–77. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Cooper DK. 1999. Anti-Gal, alpha-Gal epitopes, and xenotransplantation. Subcell Biochem. 32:229–257. [DOI] [PubMed] [Google Scholar]
- Kolber-Simonds D, Lai L, Watt SR, Denaro M, Arn S, Augenstein ML, Betthauser J, Carter DB, Greenstein JL, Hao Y et al. 2004. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci USA. 101:7335–7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren E, Neethling FA, Richards S, Koscec M, Ye Y, Zuhdi N, Cooper DK. 1993. Binding and specificity of major immunoglobulin classes of preformed human anti-pig heart antibodies. Transpl Int. 6:351–353. [DOI] [PubMed] [Google Scholar]
- Kozlowski T, Ierino FL, Lambrigts D, Foley A, Andrews D, Awwad M, Monroy R, Cosimi AB, Cooper DK, Sachs DH. 1998a. Depletion of anti-Gal(alpha)1-3Gal antibody in baboons by specific alpha-Gal immunoaffinity columns. Xenotransplantation. 5:122–131. [DOI] [PubMed] [Google Scholar]
- Kozlowski T, Monroy R, Xu Y, Glaser R, Awwad M, Cooper DK, Sachs DH. 1998b. Anti-Gal(alpha)1-3Gal antibody response to porcine bone marrow in unmodified baboons and baboons conditioned for tolerance induction. Transplantation. 66:176–182. [DOI] [PubMed] [Google Scholar]
- Kozlowski T, Shimizu A, Lambrigts D, Yamada K, Fuchimoto Y, Glaser R, Monroy R, Xu Y, Awwad M, Colvin RB et al. 1999. Porcine kidney and heart transplantation in baboons undergoing a tolerance induction regimen and antibody adsorption. Transplantation. 67:18–30. [DOI] [PubMed] [Google Scholar]
- Kujundzic M, Koren E, Neethling FA, Milotic F, Koscec M, Kujundzic T, Martin M, Cooper DK. 1994. Variability of anti-alphaGal antibodies in human serum and their relation to serum cytotoxicity against pig cells. Xenotransplantation. 1:58–65. [Google Scholar]
- Kuwaki K, Knosalla C, Moran K, Alt A, Katopodis AG, Duthaler RO, Schuurman HJ, Awwad M, Cooper DK. 2004. Reduction of anti-Galalpha1,3Gal antibodies by infusion of types 2 and 6 gal trisaccharides conjugated to poly-L-lysine. Xenotransplantation. 11:210–215. [DOI] [PubMed] [Google Scholar]
- Kuwaki K, Tseng YL, Dor FJ, Shimizu A, Houser SL, Sanderson TM, Lancos CJ, Prabharasuth DD, Cheng J, Moran K et al. 2005. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: Initial experience. Nat Med. 11:29–31. [DOI] [PubMed] [Google Scholar]
- Lambrigts D, Van Calster P, Xu Y, Awwad M, Neethling FA, Kozlowski T, Foley A, Watts A, Chae SJ, Fishman J et al. 1998. Pharmacologic immunosuppressive therapy and extracorporeal immunoadsorption in the suppression of anti-alphaGal antibody in the baboon. Xenotransplantation. 5:274–283. [DOI] [PubMed] [Google Scholar]
- Lamm V, Hara H, Mammen A, Dhaliwal D, Cooper DK. 2014. Corneal blindness and xenotransplantation. Xenotransplantation. 21:99–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Hara H, Ezzelarab MB, Iwase H, Bottino R, Long C, Ramsoondar J, Ayares D, Cooper DKC. 2016a. Initial in vitro studies on tissues and cells from GTKO/hCD46/NeuGcKO pigs. Xenotransplantation. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Miyagawa Y, Long C, Ekser B, Walters E, Ramsoondar J, Ayares D, Tector AJ, Cooper DK, Hara H. 2016b. Expression of NeuGc on pig corneas and its potential significance in pig corneal xenotransplantation. Cornea. 35:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveque X, Cozzi E, Naveilhan P, Neveu I. 2011. Intracerebral xenotransplantation: Recent findings and perspectives for local immunosuppression. Curr Opin Organ Transplant. 16:190–194. [DOI] [PubMed] [Google Scholar]
- Lexer G, Cooper DK, Rose AG, Wicomb WN, Rees J, Keraan M, Du Toit E. 1986. Hyperacute rejection in a discordant (pig to baboon) cardiac xenograft model. J Heart Transplant. 5:411–418. [PubMed] [Google Scholar]
- Li S, Yeh J-C, Cooper DKC, Cummings RD. 1995. Inhibition of human anti-alphaGal IgG by oligosaccharides derived from porcine stomach mucin. Xenotransplantation. 2:279–288. [Google Scholar]
- Li SF, Neethling FA, Taniguchi S, Yeh JC, Kobayashi T, Ye Y, Koren E, Cummings RD, Cooper DK. 1996. Glycans derived from porcine stomach mucin are effective inhibitors of natural anti-alpha-galactosyl antibodies in vitro and after intravenous infusion in baboons. Transplantation. 62:1324–1331. [DOI] [PubMed] [Google Scholar]
- Luo Y, Taniguchi S, Kobayashi T, Niekrasz M, Cooper DK. 1996. Screening of baboons as potential liver donors for humans. Transplant Proc. 28:855. [PubMed] [Google Scholar]
- Luo Y, Wen J, Luo C, Cummings RD, Cooper DK. 1999. Pig xenogeneic antigen modification with green coffee bean alpha-galactosidase. Xenotransplantation. 6:238–248. [DOI] [PubMed] [Google Scholar]
- Lutz AJ, Li P, Estrada JL, Sidner RA, Chihara RK, Downey SM, Burlak C, Wang ZY, Reyes LM, Ivary B et al. 2013. Double knockout pigs deficient in N-glycolylneuraminic acid and galactose alpha-1,3-galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation. 20:27–35. [DOI] [PubMed] [Google Scholar]
- Manji RA, Ekser B, Menkis AH, Cooper DK. 2014. Bioprosthetic heart valves of the future. Xenotransplantation. 21:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minanov OP, Itescu S, Neethling FA, Morgenthau AS, Kwiatkowski P, Cooper DK, Michler RE. 1997. Anti-GaL IgG antibodies in sera of newborn humans and baboons and its significance in pig xenotransplantation. Transplantation. 63:182–186. [DOI] [PubMed] [Google Scholar]
- Neethling FA, Cooper DK. 1999. Serum cytotoxicity to pig cells and anti-alphaGal antibody level and specificity in humans and baboons. Transplantation. 67:658–665. [DOI] [PubMed] [Google Scholar]
- Neethling F, Cooper DK, Xu H, Michler RE. 1995. Newborn baboon serum anti-alpha galactosyl antibody levels and cytotoxicity to cultured pig kidney (PK15) cells. Transplantation. 60:520–521. [DOI] [PubMed] [Google Scholar]
- Neethling FA, Joziasse D, Bovin N, Cooper DK, Oriol R. 1996. The reducing end of alpha Gal oligosaccharides contributes to their efficiency in blocking natural antibodies of human and baboon sera. Transpl Int. 9:98–101. [DOI] [PubMed] [Google Scholar]
- Neethling FA, Koren E, Ye Y, Richards SV, Kujundzic M, Oriol R, Cooper DK. 1994. Protection of pig kidney (PK15) cells from the cytotoxic effect of anti-pig antibodies by alpha-galactosyl oligosaccharides. Transplantation. 57:959–963. [DOI] [PubMed] [Google Scholar]
- Neethling FA, Nortman PJ, Cooper DK. 1990. Histocompatibility matching between humans and baboons. Transplant Proc. 22:1067–1069. [PubMed] [Google Scholar]
- Oriol R, Barthod F, Bergemer AM, Ye Y, Koren E, Cooper DK. 1994. Monomorphic and polymorphic carbohydrate antigens on pig tissues: Implications for organ xenotransplantation in the pig-to-human model. Transpl Int. 7:405–413. [DOI] [PubMed] [Google Scholar]
- Oriol R, Candelier JJ, Taniguchi S, Balanzino L, Peters L, Niekrasz M, Hammer C, Cooper DK. 1999. Major carbohydrate epitopes in tissues of domestic and African wild animals of potential interest for xenotransplantation research. Xenotransplantation. 6:79–89. [DOI] [PubMed] [Google Scholar]
- Oriol R, Ye Y, Koren E, Cooper DK. 1993. Carbohydrate antigens of pig tissues reacting with human natural antibodies as potential targets for hyperacute vascular rejection in pig-to-man organ xenotransplantation. Transplantation. 56:1433–1442. [DOI] [PubMed] [Google Scholar]
- Padler-Karavani V, Varki A. 2011. Potential impact of the non-human sialic acid N-glycolylneuraminic acid on transplant rejection risk. Xenotransplantation. 18:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, Ball S, Specht SM, Polejaeva IA, Monahan JA et al. 2003. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 299:411–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polejaeva IA, Chen SH, Vaught TD, Page RL, Mullins J, Ball S, Dai Y, Boone J, Walker S, Ayares DL et al. 2000. Cloned pigs produced by nuclear transfer from adult somatic cells. Nature. 407:86–90. [DOI] [PubMed] [Google Scholar]
- Reichenspurner H, Human PA, Rose AG, Reichart B, Cooper DK. 1990. Effect of pharmacologic immunosuppression on donor heart survival in a closely related nonhuman primate xenograft model. Transplant Proc. 22:1086–1087. [PubMed] [Google Scholar]
- Rieben R, Von Allmen E, Korchagina EY, Nydegger UE, Neethling FA, Kujundzic M, Koren E, Bovin N, Cooper DK. 1995. Detection, immunoabsorption, and inhibition of cytotoxic activity of anti-alphaGal antibodies using newly developed substances with synthetic Gal alpha1-3Gal disaccharide epitopes. Xenotransplantation. 2:98–106. [Google Scholar]
- Robson SC, Cooper DK, d'Apice AJ. 2000. Disordered regulation of coagulation and platelet activation in xenotransplantation. Xenotransplantation. 7:166–176. [DOI] [PubMed] [Google Scholar]
- Romano EL, Bhardwaj J, Kelly S, Olson M, Lamontagne LR, Leigh JB, Lauzon GJ. 1987a. Interaction of IgG and IgM anti-A with synthetic oligosaccharides. Transplant Proc. 19:4479–4483. [PubMed] [Google Scholar]
- Romano E, Neethling FA, Nilsson K, Kosanke S, Shimizu A, Magnusson S, Svensson L, Samuelsson B, Cooper DK. 1999. Intravenous synthetic alphaGal saccharides delay hyperacute rejection following pig-to-baboon heart transplantation. Xenotransplantation. 6:36–42. [DOI] [PubMed] [Google Scholar]
- Romano EL, Soyano A, Linares J. 1987b. Preliminary human study of synthetic trisaccharide representing blood substance A. Transplant Proc. 19:4475–4478. [PubMed] [Google Scholar]
- Romano EL, Soyano A, Linares J, Lauzon GJ. 1987c. Neutralization of ABO blood group antibodies by specific oligosaccharides. Transplant Proc. 19:4426–4430. [PubMed] [Google Scholar]
- Rood PP, Hara H, Busch JL, Ezzelarab M, Zhu X, Ball S, Ayares D, Awwad M, Cooper DK. 2006. Incidence and cytotoxicity of antibodies in cynomolgus monkeys directed to nonGal antigens, and their relevance for experimental models. Transpl Int. 19:158–165. [DOI] [PubMed] [Google Scholar]
- Rood PP, Tai HC, Hara H, Long C, Ezzelarab M, Lin YJ, van der Windt DJ, Busch J, Ayares D, Ijzermans JN et al. 2007. Late onset of development of natural anti-nonGal antibodies in infant humans and baboons: Implications for xenotransplantation in infants. Transpl Int. 20:1050–1058. [DOI] [PubMed] [Google Scholar]
- Rouhani FJ, Dor FJ, Cooper DK. 2004. Investigation of red blood cells from alpha1,3-galactosyltransferase-knockout pigs for human blood transfusion. Transfusion. 44:1004–1012. [DOI] [PubMed] [Google Scholar]
- Sandrin MS, Vaughan HA, Dabkowski PL, McKenzie IF. 1993. Anti-pig IgM antibodies in human serum react predominantly with Gal(alpha 1-3)Gal epitopes. Proc Natl Acad Sci USA. 90:11391–11395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu A, Hisashi Y, Kuwaki K, Tseng YL, Dor FJ, Houser SL, Robson SC, Schuurman HJ, Cooper DK, Sachs DH et al. 2008. Thrombotic microangiopathy associated with humoral rejection of cardiac xenografts from alpha1,3-galactosyltransferase gene-knockout pigs in baboons. Am J Pathol. 172:1471–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon PM, Neethling FA, Taniguchi S, Goode PL, Zopf D, Hancock WW, Cooper DK. 1998. Intravenous infusion of Galalpha1-3Gal oligosaccharides in baboons delays hyperacute rejection of porcine heart xenografts. Transplantation. 65:346–353. [DOI] [PubMed] [Google Scholar]
- Springer SA, Diaz SL, Gagneux P. 2014. Parallel evolution of a self-signal: Humans and new world monkeys independently lost the cell surface sugar Neu5Gc. Immunogenetics. 66:671–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stussi G, West L, Cooper DK, Seebach JD. 2006. ABO-incompatible allotransplantation as a basis for clinical xenotransplantation. Xenotransplantation. 13:390–399. [DOI] [PubMed] [Google Scholar]
- Taniguchi S, Neethling FA, Kobayashi T, Ye Y, Niekrasz M, Peters L, Koren E, Oriol R, Cooper DK. 1996a. Ratites (ostrich, emu) as potential heart donors for humans: Immunologic considerations. Transplant Proc. 28:561. [PubMed] [Google Scholar]
- Taniguchi S, Neethling FA, Korchagina EY, Bovin N, Ye Y, Kobayashi T, Niekrasz M, Li S, Koren E, Oriol R et al. 1996b. In vivo immunoadsorption of antipig antibodies in baboons using a specific Gal(alpha)1-3Gal column. Transplantation. 62:1379–1384. [DOI] [PubMed] [Google Scholar]
- Tearle RG, Tange MJ, Zannettino ZL, Katerelos M, Shinkel TA, Van Denderen BJ, Lonie AJ, Lyons I, Nottle MB, Cox T et al. 1996. The alpha-1,3-galactosyltransferase knockout mouse. Implications for xenotransplantation. Transplantation. 61:13–19. [DOI] [PubMed] [Google Scholar]
- Teranishi K, Alwayn IP, Buhler L, Gollackner B, Knosalla C, Huck J, Duthaler R, Katopodis A, Sachs DH, Schuurman HJ et al. 2003. Depletion of anti-Gal antibodies by the intravenous infusion of Gal type 2 and 6 glycoconjugates in baboons. Xenotransplantation. 10:357–367. [DOI] [PubMed] [Google Scholar]
- Teranishi K, Gollackner B, Buhler L, Knosalla C, Correa L, Down JD, White-Scharf ME, Sachs DH, Awwad M, Cooper DK. 2002a. Depletion of anti-gal antibodies in baboons by intravenous therapy with bovine serum albumin conjugated to gal oligosaccharides. Transplantation. 73:129–139. [DOI] [PubMed] [Google Scholar]
- Teranishi K, Manez R, Awwad M, Cooper DK. 2002b. Anti-Gal alpha 1-3Gal IgM and IgG antibody levels in sera of humans and old world non-human primates. Xenotransplantation. 9:148–154. [DOI] [PubMed] [Google Scholar]
- Thall AD, Maly P, Lowe JB. 1995. Oocyte Gal alpha 1,3Gal epitopes implicated in sperm adhesion to the zona pellucida glycoprotein ZP3 are not required for fertilization in the mouse. J Biol Chem. 270:21437–21440. [DOI] [PubMed] [Google Scholar]
- Tseng YL, Dor FJ, Kuwaki K, Ryan D, Wood J, Denaro M, Giovino M, Yamada K, Hawley R, Patience C et al. 2004. Bone marrow transplantation from alpha1,3-galactosyltransferase gene-knockout pigs in baboons. Xenotransplantation. 11:361–370. [DOI] [PubMed] [Google Scholar]
- Tseng YL, Kuwaki K, Dor FJ, Shimizu A, Houser S, Hisashi Y, Yamada K, Robson SC, Awwad M, Schuurman HJ et al. 2005a. alpha1,3-Galactosyltransferase gene-knockout pig heart transplantation in baboons with survival approaching 6 months. Transplantation. 80:1493–1500. [DOI] [PubMed] [Google Scholar]
- Tseng YL, Sachs DH, Cooper DK. 2005b. Porcine hematopoietic progenitor cell transplantation in nonhuman primates: A review of progress. Transplantation. 79:1–9. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Burlak C, Estrada JL, Li P, Tector MF, Tector AJ. 2014. Erythrocytes from GGTA1/CMAH knockout pigs: Implications for xenotransfusion and testing in non-human primates. Xenotransplantation. 21:376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts A, Foley A, Awwad M, Treter S, Oravec G, Buhler L, Alwayn IP, Kozlowski T, Lambrigts D, Gojo S et al. 2000. Plasma perfusion by apheresis through a Gal immunoaffinity column successfully depletes anti-Gal antibody: Experience with 320 aphereses in baboons. Xenotransplantation. 7:181–185. [DOI] [PubMed] [Google Scholar]
- Wilhite T, Ezzelarab C, Hara H, Long C, Ayares D, Cooper DK, Ezzelarab M. 2012. The effect of Gal expression on pig cells on the human T-cell xenoresponse. Xenotransplantation. 19:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong BS, Yamada K, Okumi M, Weiner J, O'Malley PE, Tseng YL, Dor FJ, Cooper DK, Saidman SL, Griesemer A et al. 2006. Allosensitization does not increase the risk of xenoreactivity to alpha1,3-galactosyltransferase gene-knockout miniature swine in patients on transplantation waiting lists. Transplantation. 82:314–319. [DOI] [PubMed] [Google Scholar]
- Xu Y, Lorf T, Sablinski T, Gianello P, Bailin M, Monroy R, Kozlowski T, Awwad M, Cooper DK, Sachs DH. 1998. Removal of anti-porcine natural antibodies from human and nonhuman primate plasma in vitro and in vivo by a Galalpha1-3Galbeta1-4betaGlc-X immunoaffinity column. Transplantation. 65:172–179. [DOI] [PubMed] [Google Scholar]
- Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, Nuhn M, O'Malley P, Nobori S, Vagefi PA, Patience C et al. 2005. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 11:32–34. [DOI] [PubMed] [Google Scholar]
- Ye Y, Neethling FA, Niekrasz M, Koren E, Richards SV, Martin M, Kosanke S, Oriol R, Cooper DK. 1994a. Evidence that intravenously administered alpha-galactosyl carbohydrates reduce baboon serum cytotoxicity to pig kidney cells (PK15) and transplanted pig hearts. Transplantation. 58:330–337. [PubMed] [Google Scholar]
- Ye Y, Niekrasz M, Kehoe M, Rolf LL Jr, Martin M, Baker J, Kosanke S, Romano E, Zuhdi N, Cooper DK. 1994b. Cardiac allotransplantation across the ABO-blood group barrier by the neutralization of preformed antibodies: The baboon as a model for the human. Lab Anim Sci. 44:121–124. [PubMed] [Google Scholar]
- Yeh P, Ezzelarab M, Bovin N, Hara H, Long C, Tomiyama K, Sun F, Ayares D, Awwad M, Cooper DK. 2010. Investigation of potential carbohydrate antigen targets for human and baboon antibodies. Xenotransplantation. 17:197–206. [DOI] [PubMed] [Google Scholar]