Abstract
Dermatology is a field that strives not only to alleviate skin disease (therapeutics) but also to improve the perception of wellness (cosmetics). Thus, in this special issue of Glycobiology, it seems appropriate to discuss the biology of a glycosaminoglycan, called hyaluronic acid (hyaluronan, or HA), that has become the most popular agent today for intradermal injections to improve wrinkles and other cosmetic defects. HA is a simple linear polymer in which a simple disaccharide is repeated thousands of time, thereby creating a huge hydrophilic molecule that confers a large volume of hydration and contributes to the turgor and flexibility of healthy skin. Beyond cosmetic considerations, however, HA also has important biological and physiological functions that were largely under-appreciated until recently. New research has confirmed that HA is dynamically produced by most skin cells, not only fibroblasts (the cells that make most of the skin's extracellular matrix) but also by keratinocytes in the outer protective layer (epidermis). For both fibroblasts and keratinocytes, HA plays a regulatory role in controlling cell physiology through interaction of extracellular HA with a major cell-surface receptor, CD44. This interaction mediates intracellular signaling both directly and indirectly, through CD44 interactions with the cytoskeleton and with EGF and TGFβ receptors. Furthermore, degradation of HA by specific hyaluronidase enzymes produces HA fragments that can help to regulate inflammatory processes. In this review, current knowledge about the role of HA in skin inflammation and wound healing are reviewed and possible future applications of such knowledge discussed.
Keywords: fibrosis, glycosaminoglycan, inflammation, skin, wound healing
Introduction
While in Dermatology Residency training at the Massachusetts General Hospital in the late 1980s, my exposure to glycobiology was extremely limited. I knew that leg ulcers in diabetic patients were somehow caused by hyperglycemia, and that staining of skin biopsy specimens with alcian blue would reveal a mysterious “ground substance” containing glycoproteins and glycosaminoglycans. Two decades later, as an academic dermatologist, I learned that one of these glycosaminoglycans, hyaluronic acid (HA), or hyaluronan, had become the most popular material for soft tissue augmentation by cosmetic dermatologists (Cohen et al. 2013; Gilbert, Hui, Meehan, et al. 2012; Gilbert, Hui, Waldorf, et al. 2012). This was news to me, since for the preceding two decades bovine collagen had been the principal agent used for wrinkle revision. Unlike the highly allergenic collagens, HA is a non-immunogenic molecule, a “pristine” polysaccharide polymer completely devoid of protein epitopes (Gilbert, Hui, Meehan, et al. 2012). Another advantage of HA is that if problems arise, it can be easily removed by digestion with an enzyme, hyaluronidase (Hirsch et al. 2007). Interestingly, since HA is relatively invisible (both optically and immunologically speaking), it was very helpful earlier in its development as a clear biopolymer for use in ophthalmological procedures such as cataract replacement (Pape and Balazs 1980; Vatne and Syrdalen 1986). By the mid-2000s, when HA-based products such as Restylane, Perlane and Juvederm were enjoying new-found fame among dermatologists and plastic surgeons (Gilbert, Hui, Waldorf, et al. 2012), I was at least aware that HA was important. However, when I moved from Boston to the Midwest in 2000 to set up a new skin biology laboratory at the Cleveland Clinic, I had never even heard of hyaluronan. As it turned out, my new office was located right next door to Vincent Hascall, a world-renowned expert in HA. While at the NIH Dental Branch in the 1970s and 1980s, Vince had figured out the molecular structure of cartilage, and showed that its unique tissue properties were attributable to a tough, compressible molecular scaffold structure consisting of HA strands bound by a specialized HA-binding proteoglycan called aggrecan (Hascall and Heinegard 1974a,b; Heinegard and Hascall 1974). The protein core of aggrecan is one of a small group of proteins (called “hyaladherins”) now known to bind to the HA polysaccharide and to alter its function (Knudson and Knudson 1993). This pioneering work made Vince famous in the glycobiology world, and from that time onward he became a tireless crusader in the pursuit of knowledge about what he calls “that darn ubiquitous molecule, HA HA HA!”
My initial plan for the new laboratory was to study transcription factors that regulate epidermal differentiation, and thereby cure the world of psoriasis, ichthyosis, and other scaly skin diseases. However, whilst struggling to write my first R01 on this topic, I would often take a break and talk to Vince about HA and its role in physiology and disease. Apparently, very little was known about HA in the skin, especially in the epidermis (my tissue of interest). Intrigued, I teamed up with Alberto Passi, a talented glycoscientist on sabbatical from Italy. The experiments we did together (Passi et al. 2004) became preliminary data for an NIH R01 proposal on HA in the skin. That grant was funded, establishing a base for my future work. Parenthetically, I actually never got the R01 funding to work on transcription factors, so my fortuitous encounter with HA was both a career changer and, actually, a research-funding lifesaver.
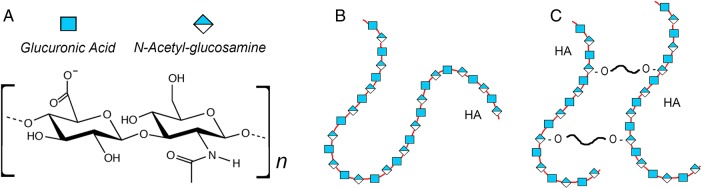
Even to a biochemically challenged dermatologist, it seems obvious that HA should make a good dermal filler. HA is extremely abundant in the dermis under normal circumstances. It is also a major ingredient in moisturizing creams, due to its tremendous hygroscopic (hydrating) properties, which also helps to explain why injected HA-based fillers excel at “plumping up” the dermis. HA consists of a simple non-sulfated 2-sugar subunit, glucuronic acid and N-acetylglucosamine (Figure 1A), which is repeated thousands of times (Figure 1B); for example, a large 2 MDa HA molecule contains 5000 disaccharides (Itano 2008). Though lacking sulfate substitutions, highly charged residues on the sugar moieties confer hydrophilic properties, but, more importantly, the very large molecular size provides a domain that retains large amounts of water. Modern HA-based fillers are created typically by cross-linking the HA chains by conjugation with butanediol diglycidyl ether (BDDE) (Figure 1C). This slows its degradation in tissue, and allows the manufacturer to control the stiffness of the gel (Gilbert, Hui, Waldorf, et al. 2012; Kablik et al. 2009).
Fig. 1.
Molecular structures of (A) the HA disaccharide subunit, (B) a native HA polysaccharide chain and (C) cross-linked HA used for tissue implantation.
HA boot camp: HA synthesis, degradation and binding to receptors
Although the molecular structure of HA seems simple, especially when compared with its cousins, the sulfated glycosaminoglycans (e.g., heparan sulfate or chondroitin sulfate), be not fooled: HA biology is anything but simple. The incredible abundance of HA (∼15 gm in a 70 kg man), and the fact that nearly one-third of it is removed and replaced each day (Fraser et al. 1997), suggests a highly regulated molecule with functional importance. The estimated half-life of HA in the epidermis is 1 day (Tammi et al. 1991). HA is synthesized by three different hyaluronan synthase (HAS) enzymes namely HAS1, HAS2 and HAS3, each of which is located on the plasma membrane (Itano and Kimata 2002). Of the six known hyaluronidase (HYAL) genes in mammals, HYAL1 and HYAL2 are expressed ubiquitously; HYAL2 on the cell membrane cleaves the HA, and the fragments are internalized into endosomes and then transported to lysosomes where HYAL1 degrades it further (Stern 2004). HA degradation has functional implications, since different-sized HA fragments have different effects. Although greatly oversimplified here, intact HA (high-molecular weight HA) tends to exert anti-inflammatory effects, whereas mid-sized and small fragments (oligosaccharides) have pro-inflammatory or variable effects, depending upon which cell-surface receptor binds HA (typically CD44 for large HA, and toll-like receptors for small HA; Petrey and de la Motte 2014).
The most well-studied HA receptor is CD44. Originally identified as the lymphocyte homing receptor (see review by Robert Sackstein in this Special Issue) and only later shown to bind to HA, CD44 generally upregulates pro-proliferative and migratory effects of cells in tissues that contain abundant HA (Jordan et al. 2015). A very significant concept is that CD44 can regulate cell behavior by interacting with other growth factor receptors in the cell membrane, such as EGFR/ErbB (Wang and Bourguignon 2011) and TGFβR (Webber, Jenkins, et al. 2009). The binding of HA in the matrix to CD44, which in turn interacts with the other receptors to modify their pathway activities, represents an important form of “Outside-In” signaling (see Sackstein 2011; Zoller 2011).
HA and skin wounding: no longer a passive “ground substance”
Regarding HA and wound healing, by the 1990s it was generally appreciated that large amounts of HA are secreted in acute wound fluids (Longaker et al. 1991; Dechert et al. 2006), and that relatively high levels of HA in fetal skin correlate with regenerative (scarless) healing, as opposed to scarring and fibrosis in adult skin (Longaker et al. 1991; Mack et al. 2003). Those observations were based on bulk measurements of HA, but more precise studies regarding individual cell types and microanatomical locations of HA were difficult because of high (saturating) amounts of HA in the dermis. In contrast, HA levels in epithelial tissues are lower, and changes are easier to quantify. Markku and Raija Tammi in Finland were able to document very interesting changes in levels of HA in epithelium of squamous skin cancers (Wang et al. 1996; Ropponen et al. 1998) and psoriatic skin (Tammi et al. 1994). The Tammi group went on to demonstrate, using skin organ cultures (Tammi et al. 1989) and a more-refined culture system (stratified keratinocytes growing on a collagen gel), that exposure to the growth factors and cytokines typically encountered in wounds and inflammatory diseases (e.g. EGF and TGFβ) induced robust HA synthesis via increased expression of HAS2 and HAS3 (Karvinen et al. 2003; Pasonen-Seppanen et al. 2003).
The Tammi's organotypic model comprised immortalized rat epidermal keratinocytes (REKs) grown on a collagen gel at the air–liquid interface to induce epidermal stratification. To improve this model, a pre-formed basement membrane (BM) was added to the collagen gel prior to seeding the keratinocytes; the BM helped to retain high-molecular weight HA within the epidermal compartment, to more accurately simulate the native epidermis (Tammi et al. 2000). Using this optimized model, my laboratory proceeded to ask functional questions about HA and the epidermis. First we found that normal levels of HA are important for proper proliferation and development of epidermal tissue (Passi et al. 2004). Further, we observed that normal levels of intact extracellular HA are required for normal keratinocyte differentiation, since in both the REK model (Passi et al. 2004) and in murine skin in vivo (Maytin et al. 2004), the addition of hyaluronidase led to acceleration of the epidermal differentiation program. We also explored mechanisms by which epidermal injury causes HA accumulation. For example, many skin diseases are characterized by a defective permeability barrier; we used acetone to disrupt the stratum corneum in the REK model and found that HA synthesis is vigorously induced (Ajani et al. 2007). Physically wounding the organotypic cultures by poking with a needle also led to a 3-fold increase in HA expression (Monslow et al. 2009). This HA accumulation was partly due to increased transcription of Has2 and Has3, but activation of pre-formed HAS enzymes was also involved because the initial increase in HA (first observed at 15 min post-injury) occurred too soon to be attributable to synthesis of new HAS enzymes. Another surprising finding was that HA induction requires a soluble factor. Media-transfer experiments, involving conditioned media from 3-D REK cultures, showed that EGF receptors must be specifically activated by cleaved heparin-binding EGF (HB-EGF) in order for injury-induced HA accumulation to occur (Monslow et al. 2009). Other research groups have shown (i) that CD44 interacts with EGF receptors (EGFR and other ErbB receptors), (ii) this interaction is mandatory for full EGFR activity and (iii) EGFR pathway activation induces the synthesis of HAS enzymes (Bourguignon 2008; Grass et al. 2013). Therefore, it seems entirely possible that the wounding-induced release of cleaved HB-EGF and the induction in HA synthesis observed in our organotypic cultures could be mediated via an HA-dependent CD44-EGFR interaction, perhaps as part of a feed-forward amplification loop. This made us realize that we should be paying more attention not only to growth factors and CD44 but also to paracrine effects and their role in regulating HA responses during wound healing.
Understanding the roles of HA in wound healing: going global
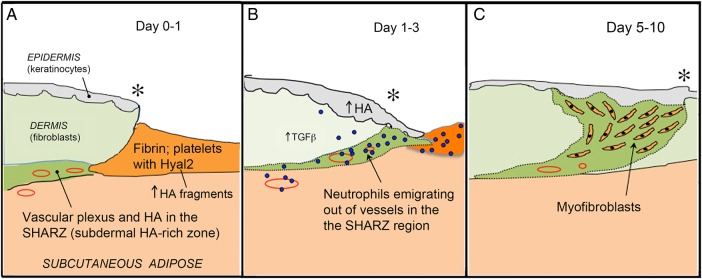
By now we were convinced that only limited insights would be gained by focusing exclusively on the epidermis, because wound healing involves multiple events at multiple locations in the skin. A more global perspective was needed. Wound healing, a complex and sequential process, can be divided into four distinct temporal phases (see reviews Singer and Clark 1999; Ebner and Maytin 2009; Ghatak et al. 2015). Briefly, the first stage (Hemostasis) occurs immediately, as platelets aggregate at the site of injury and trigger formation of a fibrin clot (Figure 2A). The clot represents a temporary, provisional matrix over and through which migrating cells can pass. In the second Inflammation phase, leukocytes are recruited from the bloodstream to the site of injury (Figure 2B). Neutrophils arrive within hours, to clear invading pathogens and cellular debris. After undergoing apoptosis, the dead neutrophils are phagocytosed by macrophages that begin arriving after 3 days. In the third phase, Proliferation and Migration, epithelial keratinocytes migrate from the wound edges toward the center (a process initiated within the first 24 h of injury), helping to seal the defect, prevent fluid loss and exclude infection. This keratinocyte migration coincides with a marked accumulation of HA in the epidermis at the wound edges (Monslow et al. 2009). Fibroblasts proliferate at the wound edge and then migrate into the provisional matrix, where after ∼1 week a subset of these transform into myofibroblasts (cells that produce large amounts of new collagen matrix and mediate wound contraction) (Figure 2C). Neoangiogenesis is also very important during this phase. The fourth stage is Remodeling. Extensive changes in the extracellular matrix (ECM) include assembly and maturation of fully developed collagen fibrils, in complex association with various proteoglycans and glycosaminoglycans. Remodeling takes several months but is not perfect, resulting in a scar.
Fig. 2.
Schematic cartoon depicting events at the wound edge during the first day (A), on the first to third day (B) and 5–10 days after wounding (C). Asterisk, wound edge.
To investigate wound healing in a skin model that has abnormal HA regulation, we decided to examine Has1/Has3 double knockout (Has1/3KO) mice, which lack two of the three known HA synthases. In these mice, only Has2 remains (Mack et al. 2012). Circular excisional wounds were made on the back skin, and closure rates and skin biopsies were examined at serial time points (Mack et al. 2012). Three major findings in the Has1/3KO mice, when compared with wild-type mice, were observed: (i) faster wound closure; (ii) accelerated influx of leukocytes; (iii) accelerated repair of the ECM. Some of these results (as described below) seemed counter-intuitive at the time. As usual, the enigmatic HA molecule was offering up additional puzzles.
What are the implications of accelerated wound closure?
Faster wound closure in the Has1/3KO mice was a surprise, because we were used to thinking of HA as a facilitator of keratinocyte migration. The relative absence of HA in the epidermis of Has1/3KO mice at the wound edge (Mack et al. 2012) might be expected to delay closure, if wound healing involved only the epidermis. In fact, no evidence for differences in epidermal migration rates was observed. One rather simplistic explanation for faster closure is that reduced amounts of HA in the wound bed make the dermal tissues less hydrated (drier), thereby promoting contraction. Another scenario might be that functional contractility of fibroblasts is increased in the Has1/3KO wounds, driven by high levels of TGFβ produced by leukocytes that are now present in greater numbers (which will be discussed further in the next section). Early wound closure was not due to a difference in myofibroblasts, since myofibroblasts do not appear until day 5, yet the accelerated closure rate was already observed by day 1. Since wounds in Has1/3KO and in wild-type mice are all fully closed by 11 days, faster closure in the first few days of wound healing may not be functionally very meaningful here, yet it remains an intriguing mechanistic puzzle.
Recruitment of leukocytes to the scene of the crime: does HA help direct traffic?
Our second finding was that neutrophil and macrophage recruitment happens faster in the Has1/3KO mice, relative to wild-type wounds; 2- to 3-fold more neutrophils are found in the dermis of Has1/3KO wounds at 24 h post-wounding, along with an increased influx of macrophages at day 3 (Mack et al. 2012). This phenomenon is most evident in one special region of the skin, a highly vascularized area located at the junction of the dermis and the subcutaneous fat, the site of the deep horizontal vascular plexus (Braverman 1997). We have nicknamed this area the subdermal HA-rich zone (“SHARZ”), because it contains the highest levels of HA observed anywhere in the skin (Figures 2 and 3). This HA appears to be organized in a linear, bundle-like structure that coincides with the location of blood vessels (Figure 3). We hypothesize that this structure is somehow involved in the regulation of leukocyte extravasation after wounding. Another interesting possibility, suggested by the discovery that platelets carry large amounts of HYAL2 on their surface (Albeiroti et al. 2015), is that platelets in the fibrin clot cleave dermal HA and generate bioactive HA fragments that might affect leukocyte recruitment. In Has1/3KO wounds, a local reduction in HA levels due to HAS enzyme deficiency could lead to increased leukocyte recruitment via several possible mechanisms. For example, if the HA coat on the lumenal surfaces of blood vessels normally acts to prevent leukoctyes from interacting with E-selectins and other adhesion molecules (VCAM-1, for example) on the endothelial wall, then a reduction in the HA coat might allow leukocytes greater access to those adhesion molecules and thereby facilitate leukocyte tethering, rolling and adhesion. If HA fragments released due to platelet-mediated hyaluronidase action were inhibitory for leukocyte recruitment (a still-open question), then release of fewer HA fragments due to an overall reduction of HA in the SHARZ, might release the inhibition and lead to a relative increase in leukocyte recruitment. Finally, there are good reasons to believe that modification of the cohort of proteins that are bound to HA (so-called hyaladherins or HA-binding proteins) may contribute to wounding-related phenomena by altering the ability of HA to interact with CD44 on leukocytes. This last hypothesis will now be expanded upon.
Fig. 3.
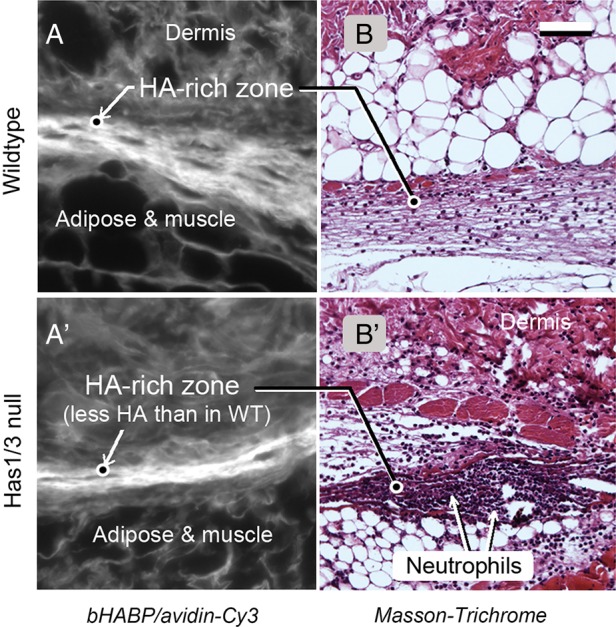
Image of the SHARZ region in skin, at a location adjacent to the wound edge in a wild-type mouse (top row), or a Has1/3KO mouse (bottom row). (A, A'), Histological staining with biotinylated HA-binding protein and an avidin-Cy3 probe. (B, B') Masson-Trichrome stain of an adjacent section. Scale bar, 50 µm. From Mack et al. (2012), used with permission.
The concept that injury can lead to changes in HA's physical 3-D structure and regulate binding of leukocytes to HA was pioneered by Carol de la Motte and colleagues, who showed that intestinal smooth muscle cells (SMCs), when exposed to ER stress inducing agents or infection with RNA viruses, respond by synthesizing an unusual ECM consisting of long, thick cables of HA (de La Motte et al. 1999, 2003; Majors et al. 2003). Other cell types have been shown to make these monocyte-adhesive cables as well, including renal mesangial cells (Wang and Hascall 2004) and skin keratinocytes (Jokela et al. 2008). Leukocytes do not usually bind to HA in the pericellular coat (glycocalyx) of SMCs, but they readily attach to inflammatory HA cables through interactions with CD44 on the leukocyte cell membrane (de La Motte et al. 1999). HA cables contain a protein called “heavy chain (HC)”; the latter is derived from inter-α-trypsin inhibitor (I-α-I), a widely circulating serum protein made in the liver (de la Motte et al. 2003). Evidence from a large body of work, including studies on expansion of the HA-rich cumulus oophorus in the ovary (another inflammation-like process), demonstrated that HA in inflammatory cables becomes cross-linked via covalent HC:HA attachments, and that an enzyme called TSG-6 (tumor necrosis factor-stimulated gene 6) is responsible for catalyzing the transfer of heavy chains from I-α-I onto the HA (reviewed in Day and de la Motte 2005). Theoretically, cross-linking of HA can be quite extensive, since a full-length 2 MDa strand of HA could accommodate ∼1000 HA-binding proteins (collectively known as “hyaladherins”) (Day and de la Motte 2005). However, the actual number of cross-links is probably much smaller, as synovial fluids from rheumatoid arthritis patients were shown to have between 3 and 5 HCs per 2 MDa HA molecule (Yingsung et al. 2003). One current idea is that monocytes are sequestered onto the inflammatory HA cables, preventing them from directly contacting and damaging the underlying tissues (Day and de la Motte 2005). In the SHARZ of the skin, the close proximity of HA to smooth muscle cells, pericytes and endothelial components of blood vessels, together with our unpublished findings that TSG-6 is induced in wounded skin, opens up the possibility that white blood cell sequestration onto leukocyte-adhesive HA structures represents a regulatory mechanism for leukocyte trafficking. We are now testing this hypothesis.
Fibrosis vs. regeneration: is faster necessarily better?
Normal wound healing in adult skin generates an imperfect ECM. The breaking strength of a fully-healed scar in adults is typically <70% the strength of uninjured skin. In contrast, fetal skin wounds regenerate nearly perfectly. We are studying the properties of the reconstructed ECM in Has1/3KO mice. We have observed that myofibroblasts appear earlier and that collagen is laid down and assembled more rapidly into mature fibrillar structures in the Has1/3KO wounds compared with normal wounds. High levels of TGFβ expression may be driving this myofibroblast conversion, because elevated TGFβ1 expression levels (probably leukocyte derived) are found in Has1/3KO wounds at days 1–4. Supporting a central role for TGFβ1 is the observation that primary fibroblast cultures from Has1/3KO mice respond to TGFβ1 by producing higher amounts of collagen, when compared with wild-type controls (Shakya et al. 2015). This suggests that Has1/3KO fibroblasts are intrinsically different, and/or that their physiological programming has been altered. For example, Has1/3KO fibroblasts are relatively resistant to apoptosis induced by serum starvation or UVB exposure (Wang et al. 2014). Their apoptosis resistance is related to Has2 overexpression, since the resistance disappears if Has2 expression is blocked with siRNA (Wang et al. 2014). Also, Has1/3KO fibroblasts produce a relatively enlarged pericellular HA coat, again due to compensatory overexpression of the Has2 gene (Wang et al. 2014). A functional link between HA in the glycocalyx and TGFβ signaling may explain why Has1/3KO fibroblasts exhibit exaggerated pro-fibrotic behavior, including increased collagen production. A highly active research group in Cardiff has elegantly shown that HA in the pericellular coat of skin fibroblasts acts to support TGFβ signaling, through a mechanism in which the TGFβ receptor interacts with the HA receptor, CD44 (Meran et al. 2007). HA binding to CD44 stimulates downstream TGFβ pathways and promotes fibroblast to myofibroblast conversion (Webber, Jenkins, et al. 2009; Webber Meran, et al. 2009). We are currently testing the hypothesis that a similar mechanism is operative in Has1/3KO skin. Whether or not an accelerated ECM maturation in fully healed Has1/3KO wounds is functionally better or worse than in wild-type wounds will require tensiometric experiments to measure wound-breaking strength, as previously performed in Hoxb13 null mice that express increased levels of HA in the skin (Mack et al. 2003).
What do wrinkle implants do for you, or to you?
Cross-linked HA materials for skin augmentation are quite effective, and are very safe because the implanted material can be subsequently removed with hyaluronidase. However, the question remains as to whether these tissue fillers are truly passive, or involve other mechanisms as well. In one study, after examining skin biopsies from human volunteers at 4 and 13 weeks after injection of cross-linked HA dermal fillers, researchers at University of Michigan observed activated fibroblasts at the edge of the implant along with increased procollagen synthesis; they postulated that fibroblasts had become activated via mechanical stretch receptors (Wang et al. 2007). However, another possibility to consider is that low-level inflammation was induced via binding of leukocytes to the cross-linked HA or by local production of cross-linked HA fragments. This is only one of the many questions that further, ongoing experiments on HA biology and physiology in the skin might hope to answer.
Taking a forward-looking view of skin research as it relates to translational glycobiology, a better understanding of HA biology in the skin might have far-reaching consequences not only for dermatologists but for other clinicians as well. One important therapeutic target for which manipulation of HA could potentially make a difference is the prevention of fibrosis and scarring. This might be done directly or indirectly. For a direct approach, the work from the group in Cardiff (Webber, Jenkins, et al. 2009) as well as our own laboratory (Wang et al. 2014) has made it clear that HA and CD44 on the outer surface of dermal fibroblasts act to regulate fibroblast physiology, by supporting and stimulating extracellular matrix molecule production. It might be possible to modify the amount of collagen produced in the skin by upregulating or downregulating HA synthesis, using specific HAS enyzme activators or inhibitors. Alternatively, the same thing could be accomplished by interrupting HA:CD44 interactions in the pericellular coat of dermal fibroblasts using an anti-CD44 antibody, or by injecting small fragments of HA into the skin. In this manner, the overproduction of collagen that leads to keloids and hypertrophic scars could be blocked. An indirect approach to preventing scar formation and fibrosis, especially during wound healing, would be to manipulate HA so as to reduce the extent and/or timing of inflammation that precedes fibrosis. As shown by our work with the Has1/3KO mice, loss of HAS enzyme activity in the dermis leads to accelerated neutrophil and monocyte influx, with a subsequent acceleration of collagen maturation and scar. By understanding the mechanisms that lead to rapid recruitment of leukocytes, one should be able to identify new targets for therapeutic blockade.
The ability to block neutrophil and monocyte influx in the skin, using a new understanding of HA, could have implications beyond wound healing. For example, pyoderma gangrenosum (PG) is a feared and little-understood disease of the skin, in which massive numbers of neutrophils are recruited to localized areas of the skin (often at sites of previous trauma), causing pustule formation, skin breakdown and the formation of gangrenous-appearing ulcers. The mechanism of this condition is completely unknown, but the sequence of the events (recruitment of neutrophils to injured areas of the skin) sounds very reminiscent of the normal events during wound healing, albeit highly exaggerated. Perhaps a clue to the dysregulated neutrophil recruitment in PG might lie within the realm of HA:HC:CD44 biology, whose mechanistic possibilities we continue to explore.
Finally, the fact that HA levels and/or the interactions of HA and CD44 are able to regulate cellular differentiation (e.g., the cornification of epidermal keratinocytes and the differentiation of fibroblasts into myofibroblasts) raises the possibility that the differentiation-modulatory ability of HA-regulated pathways might be employed therapeutically, especially in the field of oncology and cancer. The fact that HA-liganded CD44 can interact with EGFR, ErbB and c-met receptors in several systems (see “HA Boot Camp” section) is very intriguing; if HA and CD44 interactions are required to drive carcinogenesis and metastases, then it follows that manipulation of those interactions might be harnessed therapeutically. One concrete example of how manipulation of differentiation might be useful is provided by our use of “differentiation therapy” to enhance photodynamic therapy (PDT) of cancer (Anand et al. 2012). PDT uses a photosensitizing drug and visible light to target cancer cells. The most popular form of PDT in dermatology today uses a small precursor (5-aminolevulinic acid, ALA) that is selectively taken up by carcinoma cells and converted into mitochondrial porphyrins, and upon illumination with a strong visible light, the porphyrins become photoactivated, producing oxygen-dependent free radicals that destroy membranes and kill the carcinoma cells (Anand et al. 2012). To improve the effectiveness of ALA-PDT therapy, we have shown that three different small-molecule drugs (methotrexate; 5-fluorouracil; vitamin D), each of which can enhance the differentiation state of carcinoma cells, simultaneously enhances the ALA-mediated production of porphyrins within those cells, thereby enhancing the therapeutic response to light (Anand et al. 2009, 2011). It will be intriguing to see whether by modulating the differentiation state through manipulating HA and CD44 in the skin (e.g., by topical application of hyaluronidase or HA fragments, to a superficial skin cancer), one might also be able to improve the PDT response. One can fantasize that in the future, one might be able to treat a skin cancer simply by applying a sugar cream, an ALA cream, and some light… a bright day indeed for translational glycobiology!
Funding
This work was supported by the Program of Excellence in Glycosciences (PEG), Grant 1P01 HL107147 from the Heart, Lung and Blood Institute, National Institutes of Health, USA.
Conflict of interest statement
None declared.
Abbreviations
ALA, 5-aminolevulinic acid; BDDE, butanediol diglycidyl ether; BM, basement membrane; ECM, extracellular matrix; EGFR, EGF receptors; HA, hyaluronic acid; HAS, hyaluronan synthase; HB-EGF, heparin-binding EGF; HC, heavy chain; HYAL, hyaluronidase; PDT, photodynamic therapy; PG, pyoderma gangrenosum; REKs, rat epidermal keratinocytes.
References
- Ajani G, Sato N, Mack JA, Maytin EV. 2007. Cellular responses to disruption of the permeability barrier in a three-dimensional organotypic epidermal model. Exp Cell Res. 313:3005–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albeiroti S, Ayasoufi K, Hill DR, Shen B, de la Motte CA. 2015. Platelet hyaluronidase-2: An enzyme that translocates to the surface upon activation to function in extracellular matrix degradation. Blood. 125:1460–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand S, Honari G, Hasan T, Elson P, Maytin EV. 2009. Low-dose methotrexate enhances aminolevulinate-based photodynamic therapy in skin carcinoma cells in vitro and in vivo. Clin Cancer Res. 15:3333–3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand S, Ortel BJ, Pereira SP, Hasan T, Maytin EV. 2012. Biomodulatory approaches to photodynamic therapy for solid tumors. Cancer Lett. 326:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand S, Wilson C, Hasan T, Maytin EV. 2011. Vitamin D3 enhances the apoptotic response of epithelial tumors to aminolevulinate-based photodynamic therapy. Cancer Res. 71:6040–6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon LY. 2008. Hyaluronan-mediated CD44 activation of RhoGTPase signaling and cytoskeleton function promotes tumor progression. Semin Cancer Biol. 18:251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braverman IM. 1997. The cutaneous microcirculation: Ultrastructure and microanatomical organization. Microcirculation. 4:329–340. [DOI] [PubMed] [Google Scholar]
- Cohen JL, Dayan SH, Brandt FS, Nelson DB, Axford-Gatley RA, Theisen MJ, Narins RS. 2013. Systematic review of clinical trials of small- and large-gel-particle hyaluronic acid injectable fillers for aesthetic soft tissue augmentation. Dermatol Surg. 39:205–231. [DOI] [PubMed] [Google Scholar]
- Day AJ, de la Motte CA. 2005. Hyaluronan cross-linking: A protective mechanism in inflammation? Trends Immunol. 26:637–643. [DOI] [PubMed] [Google Scholar]
- de La Motte CA, Hascall VC, Calabro A, Yen-Lieberman B, Strong SA. 1999. Mononuclear leukocytes preferentially bind via CD44 to hyaluronan on human intestinal mucosal smooth muscle cells after virus infection or treatment with poly(I.C). J Biol Chem. 274:30747–30755. [DOI] [PubMed] [Google Scholar]
- de la Motte CA, Hascall VC, Drazba J, Bandyopadhyay SK, Strong SA. 2003. Mononuclear leukocytes bind to specific hyaluronan structures on colon mucosal smooth muscle cells treated with polyinosinic acid:polycytidylic acid: Inter-alpha-trypsin inhibitor is crucial to structure and function. Am J Pathol. 163:121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechert TA, Ducale AE, Ward SI, Yager DR. 2006. Hyaluronan in human acute and chronic dermal wounds. Wound Repair Regen. 14:252–258. [DOI] [PubMed] [Google Scholar]
- Ebner J, Maytin E. 2009. Cutaneous wound healing. In: Vidimos AT, Ammirati CT, Poblete-Lopez C, editors. Dermatogic Surgery. New York: Saunders/Elsevier; p. 81–100. [Google Scholar]
- Fraser JR, Laurent TC, Laurent UB. 1997. Hyaluronan: Its nature, distribution, functions and turnover. J Intern Med. 242:27–33. [DOI] [PubMed] [Google Scholar]
- Ghatak S, Maytin EV, Mack JA, Hascall VC, Atanelishvili I, Moreno Rodriguez R, Markwald RR, Misra S. 2015. Roles of proteoglycans and glycosaminoglycans in wound healing and fibrosis. Int J Cell Biol. 2015:834893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert E, Hui A, Meehan S, Waldorf HA. 2012. The basic science of dermal fillers: Past and present Part II: Adverse effects. J Drugs Dermatol. 11:1069–1077. [PubMed] [Google Scholar]
- Gilbert E, Hui A, Waldorf HA. 2012. The basic science of dermal fillers: Past and present Part I: Background and mechanisms of action. J Drugs Dermatol. 11:1059–1068. [PubMed] [Google Scholar]
- Grass GD, Tolliver LB, Bratoeva M, Toole BP. 2013. CD147, CD44, and the epidermal growth factor receptor (EGFR) signaling pathway cooperate to regulate breast epithelial cell invasiveness. J Biol Chem. 288:26089–26104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall VC, Heinegard D. 1974a. Aggregation of cartilage proteoglycans. I. The role of hyaluronic acid. J Biol Chem. 249:4232–4241. [PubMed] [Google Scholar]
- Hascall VC, Heinegard D. 1974b. Aggregation of cartilage proteoglycans. II. Oligosaccharide competitors of the proteoglycan-hyaluronic acid interaction. J Biol Chem. 249:4242–4249. [PubMed] [Google Scholar]
- Heinegard D, Hascall VC. 1974. Aggregation of cartilage proteoglycans. 3. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 249:4250–4256. [PubMed] [Google Scholar]
- Hirsch RJ, Brody HJ, Carruthers JD. 2007. Hyaluronidase in the office: A necessity for every dermasurgeon that injects hyaluronic acid. J Cosmet Laser Ther. 9:182–185. [DOI] [PubMed] [Google Scholar]
- Itano N. 2008. Simple primary structure, complex turnover regulation and multiple roles of hyaluronan. J Biochem. 144:131–137. [DOI] [PubMed] [Google Scholar]
- Itano N, Kimata K. 2002. Mammalian hyaluronan synthases. IUBMB Life. 54:195–199. [DOI] [PubMed] [Google Scholar]
- Jokela TA, Lindgren A, Rilla K, Maytin E, Hascall VC, Tammi RH, Tammi MI. 2008. Induction of hyaluronan cables and monocyte adherence in epidermal keratinocytes. Connect Tissue Res. 49:115–119. [DOI] [PubMed] [Google Scholar]
- Jordan AR, Racine RR, Hennig MJ, Lokeshwar VB. 2015. The role of CD44 in disease pathophysiology and targeted treatment. Front Immunol. 6:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kablik J, Monheit GD, Yu L, Chang G, Gershkovich J. 2009. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol Surg. 35(Suppl. 1):302–312. [DOI] [PubMed] [Google Scholar]
- Karvinen S, Pasonen-Seppanen S, Hyttinen JM, Pienimaki JP, Torronen K, Jokela TA, Tammi MI, Tammi R. 2003. Keratinocyte growth factor stimulates migration and hyaluronan synthesis in the epidermis by activation of keratinocyte hyaluronan synthases 2 and 3. J Biol Chem. 278:49495–49504. [DOI] [PubMed] [Google Scholar]
- Knudson CB, Knudson W. 1993. Hyaluronan-binding proteins in development, tissue homeostasis, and disease. FASEB J. 7:1233–1241. [PubMed] [Google Scholar]
- Longaker MT, Chiu ES, Adzick NS, Stern M, Harrison MR, Stern R. 1991. Studies in fetal wound healing. V. A prolonged presence of hyaluronic acid characterizes fetal wound fluid. Ann Surg. 213:292–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack JA, Abramson SR, Ben Y, Coffin JC, Rothrock JK, Maytin EV, Hascall VC, Largman C, Stelnicki EJ. 2003. Hoxb13 knockout adult skin exhibits high levels of hyaluronan and enhanced wound healing. FASEB J. 17:1352–1354. [DOI] [PubMed] [Google Scholar]
- Mack JA, Feldman RJ, Itano N, Kimata K, Lauer M, Hascall VC, Maytin EV. 2012. Enhanced inflammation and accelerated wound closure following tetraphorbol ester application or full-thickness wounding in mice lacking hyaluronan synthases Has1 and Has3. J Invest Dermatol. 132:198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majors AK, Austin RC, de la Motte CA, Pyeritz RE, Hascall VC, Kessler SP, Sen G, Strong SA. 2003. Endoplasmic reticulum stress induces hyaluronan deposition and leukocyte adhesion. J Biol Chem. 278:47223–47231. [DOI] [PubMed] [Google Scholar]
- Maytin EV, Chung HH, Seetharaman VM. 2004. Hyaluronan participates in the epidermal response to disruption of the permeability barrier in vivo. Am J Pathol. 165:1331–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meran S, Thomas D, Stephens P, Martin J, Bowen T, Phillips A, Steadman R. 2007. Involvement of hyaluronan in regulation of fibroblast phenotype. J Biol Chem. 282:25687–25697. [DOI] [PubMed] [Google Scholar]
- Monslow J, Sato N, Mack JA, Maytin EV. 2009. Wounding-induced synthesis of hyaluronic acid in organotypic epidermal cultures requires the release of heparin-binding EGF and activation of the EGFR. J Invest Dermatol. 129:2046–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape LG, Balazs EA. 1980. The use of sodium hyaluronate (Healon) in human anterior segment surgery. Ophthalmology. 87:699–705. [DOI] [PubMed] [Google Scholar]
- Pasonen-Seppanen S, Karvinen S, Torronen K, Hyttinen JM, Jokela T, Lammi MJ, Tammi MI, Tammi R. 2003. EGF upregulates, whereas TGF-beta downregulates, the hyaluronan synthases Has2 and Has3 in organotypic keratinocyte cultures: Correlations with epidermal proliferation and differentiation. J Invest Dermatol. 120:1038–1044. [DOI] [PubMed] [Google Scholar]
- Passi A, Sadeghi P, Kawamura H, Anand S, Sato N, White LE, Hascall VC, Maytin EV. 2004. Hyaluronan suppresses epidermal differentiation in organotypic cultures of rat keratinocytes. Exp Cell Res. 296:123–134. [DOI] [PubMed] [Google Scholar]
- Petrey AC, de la Motte CA. 2014. Hyaluronan, a crucial regulator of inflammation. Front Immunol. 5:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropponen K, Tammi M, Parkkinen J, Eskelinen M, Tammi R, Lipponen P, Agren U, Alhava E, Kosma VM. 1998. Tumor cell-associated hyaluronan as an unfavorable prognostic factor in colorectal cancer. Cancer Res. 58:342–347. [PubMed] [Google Scholar]
- Sackstein R. 2011. The biology of CD44 and HCELL in hematopoiesis: The ‘step 2-bypass pathway’ and other emerging perspectives. Curr Opin Hematol. 18:239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakya S, Wang Y, Mack JA, Maytin EV. 2015. Hyperglycemia-induced changes in hyaluronan contribute to impaired skin wound healing in diabetes: Review and perspective. Int J Cell Biol. 2015:701738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer AJ, Clark RA. 1999. Cutaneous wound healing. N Engl J Med. 341:738–746. [DOI] [PubMed] [Google Scholar]
- Stern R. 2004. Hyaluronan catabolism: A new metabolic pathway. Eur J Cell Biol. 83:317–325. [DOI] [PubMed] [Google Scholar]
- Tammi R, Paukkonen K, Wang C, Horsmanheimo M, Tammi M. 1994. Hyaluronan and CD44 in psoriatic skin. Intense staining for hyaluronan on dermal capillary loops and reduced expression of CD44 and hyaluronan in keratinocyte-leukocyte interfaces. Arch Dermatol Res. 286:21–29. [DOI] [PubMed] [Google Scholar]
- Tammi R, Ripellino JA, Margolis RU, Maibach HI, Tammi M. 1989. Hyaluronate accumulation in human epidermis treated with retinoic acid in skin organ culture. J Invest Dermatol. 92:326–332. [DOI] [PubMed] [Google Scholar]
- Tammi R, Saamanen AM, Maibach HI, Tammi M. 1991. Degradation of newly synthesized high molecular mass hyaluronan in the epidermal and dermal compartments of human skin in organ culture. J Invest Dermatol. 97:126–130. [DOI] [PubMed] [Google Scholar]
- Tammi RH, Tammi MI, Hascall VC, Hogg M, Pasonen S, MacCallum DK. 2000. A preformed basal lamina alters the metabolism and distribution of hyaluronan in epidermal keratinocyte “organotypic” cultures grown on collagen matrices. Histochem Cell Biol. 113:265–277. [DOI] [PubMed] [Google Scholar]
- Vatne HO, Syrdalen P. 1986. The use of sodium hyaluronate (Healon) in the treatment of complicated cases of retinal detachment. Acta Ophthalmol (Copenh). 64:169–172. [DOI] [PubMed] [Google Scholar]
- Wang A, Hascall VC. 2004. Hyaluronan structures synthesized by rat mesangial cells in response to hyperglycemia induce monocyte adhesion. J Biol Chem. 279:10279–10285. [DOI] [PubMed] [Google Scholar]
- Wang C, Tammi M, Guo H, Tammi R. 1996. Hyaluronan distribution in the normal epithelium of esophagus, stomach, and colon and their cancers. Am J Pathol. 148:1861–1869. [PMC free article] [PubMed] [Google Scholar]
- Wang F, Garza LA, Kang S, Varani J, Orringer JS, Fisher GJ, Voorhees JJ. 2007. In vivo stimulation of de novo collagen production caused by cross-linked hyaluronic acid dermal filler injections in photodamaged human skin. Arch Dermatol. 143:155–163. [DOI] [PubMed] [Google Scholar]
- Wang SJ, Bourguignon LY. 2011. Role of hyaluronan-mediated CD44 signaling in head and neck squamous cell carcinoma progression and chemoresistance. Am J Pathol. 178:956–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lauer ME, Anand S, Mack JA, Maytin EV. 2014. Hyaluronan synthase 2 protects skin fibroblasts against apoptosis induced by environmental stress. J Biol Chem. 289:32253–32265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber J, Jenkins RH, Meran S, Phillips A, Steadman R. 2009. Modulation of TGFbeta1-dependent myofibroblast differentiation by hyaluronan. Am J Pathol. 175:148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber J, Meran S, Steadman R, Phillips A. 2009. Hyaluronan orchestrates transforming growth factor-beta1-dependent maintenance of myofibroblast phenotype. J Biol Chem. 284:9083–9092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yingsung W, Zhuo L, Morgelin M, Yoneda M, Kida D, Watanabe H, Ishiguro N, Iwata H, Kimata K. 2003. Molecular heterogeneity of the SHAP-hyaluronan complex. Isolation and characterization of the complex in synovial fluid from patients with rheumatoid arthritis. J Biol Chem. 278:32710–32718. [DOI] [PubMed] [Google Scholar]
- Zoller M. 2011. CD44: Can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer. 11:254–267. [DOI] [PubMed] [Google Scholar]