Abstract
The apoC-III proteoform containing two sialic acid residues (apoC-III2) has different in vitro effects on lipid metabolism compared with asialylated (apoC-III0) or the most abundant monosialylated (apoC-III1) proteoforms. Cross-sectional and longitudinal associations between plasma apoC-III proteoforms (by mass spectrometric immunoassay) and plasma lipids were tested in two randomized clinical trials: ACT NOW, a study of pioglitazone in subjects with impaired glucose tolerance (n = 531), and RACED (n = 296), a study of intensive glycemic control and atherosclerosis in type 2 diabetes patients. At baseline, higher relative apoC-III2 and apoC-III2/apoC-III1 ratios were associated with lower triglycerides and total cholesterol in both cohorts, and with lower small dense LDL in the RACED. Longitudinally, changes in apoC-III2/apoC-III1 were inversely associated with changes in triglycerides in both cohorts, and with total and small dense LDL in the RACED. apoC-III2/apoC-III1 was also higher in patients treated with PPAR-γ agonists and was associated with reduced cardiovascular events in the RACED control group. Ex vivo studies of apoC-III complexes with higher apoC-III2/apoC-III1 showed attenuated inhibition of VLDL uptake by HepG2 cells and LPL-mediated lipolysis, providing possible functional explanations for the inverse association between a higher apoC-III2/apoC-III1 and hypertriglyceridemia, proatherogenic plasma lipid profiles, and cardiovascular risk.
Keywords: lipoproteins, mass spectrometry, proteomics, triglycerides, very low density lipoprotein
apoC-III is increasingly recognized as an important contributor to dyslipidemia and CVD risk in the setting of insulin resistance and type 2 diabetes (1). apoC-III is a major protein moiety of VLDL and chylomicron particles, and is also present in HDL and LDL (1, 2). In vitro, apoC-III inhibits LPL, reduces receptor-mediated uptake of triglyceride-rich lipoproteins, and facilitates VLDL production (3–5). In mice, reduced expression of the gene for apoC-III (APOC3) is associated with low plasma triglycerides, whereas APOC3 overexpression is associated with high triglyceride levels (6, 7). In humans, increased apoC-III on lipoproteins is associated with reduced hepatic uptake of apoB-containing lipoproteins and increased conversion of the light and more buoyant LDL to small dense LDL (2). Consequently, high plasma apoC-III levels in humans are associated with hypertriglyceridemia and a high prevalence of pro-atherogenic small dense LDL particles (8, 9).
In addition to promoting pro-atherogenic blood lipid profiles, apoC-III may facilitate atherosclerosis via actions on blood monocytes and vascular cells (10). It enhances binding of LDL particles to proteoglycans and, thereby, their retention in the vascular wall, and stimulates production of inflammatory cytokines and adhesion molecules in monocytes and endothelial cells (11–13). In humans, higher apoC-III concentrations on lipoprotein particles are associated with both atherosclerosis progression and incident coronary artery disease (14–17). Naturally occurring loss-of-function mutations in APOC3 are associated with decreased coronary artery calcification and a reduced risk of ischemic CVD (18, 19). Importantly, APOC3 expression is upregulated by glucose and downregulated by insulin (20, 21). The latter may be impaired in the setting of insulin resistance and diabetes, and together with hyperglycemia, may aggravate diabetes dyslipidemia, further increasing cardiovascular risk (20).
Mature apoC-III polypeptide undergoes glycosylation in the Golgi apparatus prior to incorporation in lipoproteins. The sugar moiety consists of galactose, N-acetyl-galactosamine, and either zero, one, or two molecules of sialic acid, resulting in three major proteoforms, apoC-III0, apoC-III1, and apoC-III2, respectively (22, 23). Sialylation of apoC-III is catalyzed by sialyltransferases, whereas desialylation of apoC-III is mediated by lysosomal neuraminidase (24). apoC-III proteoforms show substantial functional differences in regulating cellular VLDL uptake in vitro (25). The few small studies in humans also indicate distinct effects of major apoC-III proteoforms on metabolic phenotypes (12, 22, 26). The scarcity of available data characterizing relationships between apoC-III proteoforms and human lipid phenotypes is partly explained by the limitations of using the relatively labor intensive isoelectric focusing approach for studies in larger cohorts.
We recently developed a high throughput apoC mass spectrometric immunoassay (MSIA) (27) that can simultaneously measure both major and minor proteoforms of apoC-III, and applied it to a small group of obese youth participants without type 2 diabetes (28). Our results revealed a strong inverse association between the ratio of apoC-III2 to the most abundant apoC-III1 and fasting plasma triglycerides. In the present study, we validate and extend these findings by demonstrating the unique inverse association between the relative abundance of apoC-III2 and proatherogenic plasma lipids in individuals with impaired glucose tolerance and type 2 diabetes participating in two large clinical trials. We also evaluated the effect of a higher apoC-III2/apoC-III1 ratio on VLDL cellular uptake and LPL activity ex vivo, and investigated the relationships of higher relative apoC-III2 with pro-atherogenic lipoprotein subclasses and risk of developing cardiovascular events in patients with type 2 diabetes.
MATERIALS AND METHODS
Study populations
Clinical trial blood samples and data were obtained from participants of the Actos Now for Prevention of Diabetes (ACT NOW) study and the Risk Factors, Atherosclerosis, and Clinical Events in Diabetes (RACED) study (29, 30). Plasma samples for in vitro studies were obtained from a subset of participants in the University of Southern California Obesity Study (28). All studies were approved by the Institutional Review Boards of all study centers and all participants gave written informed consent.
ACT NOW was a prospective, randomized, double-blind, placebo-controlled trial to examine the effectiveness of pioglitazone in the prevention of type 2 diabetes (29). Participants were overweight adults with impaired glucose tolerance and at least one other risk factor for type 2 diabetes who were randomized to up to 45 mg pioglitazone or matching placebo per day. Participants returned every 2 months during the first year of the study and once every 3 months thereafter for up to 48 months, with a mean and median follow-up time of 2.3 years. The present analyses include plasma samples and matching data from the baseline visit and the latest available follow-up visit.
The RACED study included type 2 diabetes patients that were participating at seven study sites of the Veterans Affairs Diabetes Trial (VADT). Briefly, the VADT investigated the effect of tight glucose control versus standard therapy on cardiovascular events in veterans with uncontrolled diabetes, while the RACED substudy evaluated the effect of the VADT intervention on novel cardiovascular risk factors (30, 31). The primary VADT outcome was the time to the first occurrence of any one of a composite of macrovascular events including: myocardial infarction; stroke; death from cardiovascular causes; new or worsening congestive heart failure; surgical intervention for cardiac, cerebrovascular, or peripheral vascular disease; inoperable coronary artery disease; and amputation for ischemic gangrene. The current analysis includes plasma measurements at baseline and approximately 9 months after randomization.
MSIA
apoC-III proteoforms were measured by MSIA, as previously described (27). After rapid thawing on ice, samples were centrifuged for 5 min at 3,000 rpm and then aliquoted into 96-well microplates and stored at −80°C until measured. In brief, after sample processing and preparation of the polyclonal anti-apoC-III antibody (Academy Biomedical Co., Houston, TX) derivatized pipettes, apoC-III protein was captured from the analytical samples during repeated aspiration and dispensing cycles. Captured proteins were then eluted directly onto a 96-well formatted MALDI target using a sinapinic acid matrix. Linear mass spectra were then acquired from each sample spot using Bruker’s Ultraflex III MALDI-TOF instrument (Bruker, Billerica, MA) in positive ion mode. Mass spectra were internally calibrated using the protein calibration standard-I, and further processed with Flex Analysis 3.0 software (Bruker Daltonics). All peaks representing apolipoproteins and their proteoforms, along with a lysozyme control (internal reference standard) peak, were integrated baseline-to-baseline using Zebra 1.0 software (Intrinsic Bioprobes Inc.), and the obtained peak area values were tabulated. To distinguish between noise and low intensity signals, the peak areas were corrected individually with baseline noise-bin signals. The corrected apolipoprotein peak areas were then divided by the lysozyme peak area. Relative abundance of each apoC-III proteoform was expressed as relative peak area (RPA). To reduce the variation due to less precisely captured low-abundant proteoforms, major proteoforms were also expressed as ratios to the most abundant proteoform.
Biochemical assays
In the ACT NOW study, total plasma cholesterol and triglycerides were measured using the CHOD-DAOS method (WAKO, Richmond, VA) and an enzymatic assay (Stanbio Laboratory, Boerne, TX). HDL cholesterol was measured after precipitation of apoB-containing lipoproteins with dextran sulfate-Mg2+, using the CHOD-DAOS method (WAKO). LDL cholesterol was calculated by the Friedewald equation. In the RACED study samples, standard lipids and lipoprotein subclass concentrations were measured by the Vertical Auto Profile II method (VAP II; Atherotech, Inc., Birmingham, AL), as previously described (32). Total plasma apoC-III concentrations were measured in baseline samples from the RACED study by sandwich ELISA using an anti-apoC-III antibody (identical Academy Biomedical antibody as in the MSIA) and apoC-III protein standard obtained from Academy Biomedical, as previously described (33). The individual concentrations of apoC-III proteoforms were then computed for this subset by multiplying the total apoC-III concentration by the relative abundance of each proteoform.
Ex vivo measurement of VLDL uptake and LPL activity
Plasma samples from overweight and obese youth participants in the University of Southern California Obesity Study (28) with either high (upper quartile) or low (lower quartile) apoC-III2/apoC-III1 ratios were used to isolate VLDL and apoC-III complexes from the VLDL. These plasma samples for VLDL isolation were selected based on MSIAs performed on a total 72 available plasma samples.
VLDL isolation.
Plasma was overlaid with a density solution of 1.006 g/ml (11.40 g of NaCl plus 0.1 g of EDTA-2Na) in a 2:1 ratio. Samples were centrifuged at 100,000 rpm (8°C) for 2 h (Beckman Optima TLX 120.1). The top third layer (VLDL) was isolated and dialyzed for 2 h against PBS solution using Slide-A-Lyzer mini dialysis unit (Thermo Fisher Scientific, Waltham, MA).
Immunoprecipitation of apoC-III complexes from VLDL.
Dynabeads (Life Technologies, Grand Island, NY) were conjugated with an anti-apoC-III antibody per the manufacturer’s instructions. VLDL (150 μl) was added and gently pipetted to resuspend the Dynabeads-antibody complex and incubated for 45 min to allow VLDL binding. The supernatant was removed and the Dynabeads complex sample was washed three times using 200 μl phosphate buffered saline with Tween-20 (PBST) for each wash. After the third wash, the Dynabeads complex was resuspended in 100 μl PBST. After removal of the supernatant, apoC-III was eluted by adding 20 μl elution buffer [50 mM glycine (pH 2.8)] and gently pipetted and incubated with rotation for 2 min to dissociate the complex. The supernatant containing apoC-III was transferred to a clean tube, and this step was repeated until complete elution of all the protein. The protein was dialyzed against PBS using Slide-A-Lyzer MINI dialysis unit in the cold room.
HepG2 cell culture.
HepG2 cells were plated in 24-well (15 mm) culture plates in 1 ml of MEM containing 10% FBS and grown for 4 days. At 80% confluence, cells were incubated in MEM supplemented with 10% lipoprotein-deficient serum for 24 h.
VLDL-triglyceride delivery to HepG2 cells.
Isolated VLDL cholesterol (50 μg/ml) was added to the cells and incubated for 16 h at 37°C in 5% CO2 in the presence of 0.25 units/ml bovine milk LPL (Sigma-Aldrich, St. Louis, MO) (34). The cells were then washed twice with PBS and cell lipids were extracted in hexane:isopropanol. Total triglycerides in the cells were measured using a colorimetric assay and normalized to cell protein concentration.
Fluorescently labeled VLDL uptake.
Twenty micrograms of DiI-labeled VLDL cholesterol were added to HepG2 cells and incubated for 4 h at 37°C. The cells were preincubated for 30 min with 10 ng/ml recombinant human leptin (Life Technologies) for maximized expression of lipolysis-stimulated receptor (LSR) (25). Based on preliminary experiments (supplementary Fig. 1), the incubation was performed in the presence of 0.5 mM oleic acid (Sigma-Aldrich) (35), and isolated apoC-III complexes containing 5 μg/ml of apoC-III were used to inhibit DiI-VLDL uptake. The cell monolayers were washed twice with PBS (pH 7.4). DiI-fluorescence was measured at an excitation/emission of 513/550 nm. The cells were then fixed with 4% paraformaldehyde for 10 min. The DiI-values were normalized by staining the cells with 4′,6-diamidino-2-phenylindole (DAPI) (1:10,000, 10 min) followed by three washes with PBS. DAPI was measured at an excitation/emission 358/461 nm. VLDL uptake was defined as the ratio of DiI to DAPI fluorescence values.
LPL-mediated lipolysis.
A modification of a previously published protocol was used (36). Dialyzed VLDL was heated at 56°C for 1 h to inactivate any residual endogenous lipase activity. VLDL substrate (2 mM triglycerides) and 30 μg/ml apoC-III were added to the assay buffer [4% BSA, 180 mM Tris HCL, 100 mM NaCl, 1 mM EDTA, 0.01% NaN3, 66.7 μg/ml sodium heparin (pH 9)] with a final total volume 50 μl, which was then preincubated at 37°C on a shaker for 20 min. The dose of apoC-III was based on Wang et al. (37) and our initial studies with pure apoC-III (Academy Biomedical). The reaction was then initiated by adding 2 units/ml bovine milk LPL. At the beginning of the reaction, one tube was placed on ice and served as control, while the other tubes were incubated at 37°C on a shaker for 40 min. The reaction was stopped by placing the tubes on ice. NEFA release was assessed by a colorimetric assay at wavelength 550 nm and normalized to the reading from the control tube.
Statistical analyses
Depending on data distribution, differences in apoC-III measures were tested by Wilcoxon rank-sum test for two categories, and by Kruskal-Wallis test for three or more categories. Paired Student’s t-test or Wilcoxon signed rank test was used to determine differences from baseline within each treatment group. Relationships between continuous variables were evaluated using Spearman correlations. General linear models were used to test the relationships between apoC-III proteoforms and metabolic variables after adjustment for potential covariates, including age, gender, BMI, ethnicity, and use of lipid-lowering therapy. Data were log10 transformed if not normally distributed. All longitudinal analyses were adjusted for treatment assignment. Cox proportional hazards regression was used to determine the association between apoC-III proteoform measures and development of cardiovascular events in RACED in the models adjusted for treatment assignment. To control for cardiovascular risk, a United Kingdom Prospective Diabetes Study (UKPDS) risk engine coefficient representing a composite index of traditional cardiovascular risk factors [age, hemoglobin A1c (HbA1c), sex, blood pressure, race/ethnicity, smoking, and total and HDL cholesterol] was used. The interaction of apoC-III proteoform measures with the randomized treatment assignment was also tested in the Cox proportional hazard model along with the main effects, with P < 0.15 as threshold for stratified analyses. Statistical analyses were performed using SAS Institute software (version 9.2; Cary, NC). P < 0.05 was considered statistically significant.
RESULTS
Baseline plasma samples for measurements of apoC-III proteoforms were available for 531 participants with impaired glucose tolerance from the ACT NOW study and 296 participants with type 2 diabetes from the RACED study (Table 1). ACT NOW participants included more women and individuals of minority race, and were on average more obese than RACED participants. RACED participants were on average older predominantly white males with poor glucose control, and a high percentage were receiving lipid-lowering therapy and had a history of hypertension or CVD.
TABLE 1.
Characteristics of study cohorts
| Characteristic | ACT NOW (n = 531) | RACED (n = 296) |
| Age (yearrs) | 52 ± 12 | 61 ± 9 |
| Gender (males) | 40% | 95% |
| Race/ethnicity | ||
| White | 54% | 66% |
| African American | 15% | 13% |
| Hispanic | 27% | 17% |
| BMI (kg/m2) | 34.5 ± 6.8 | 31.5 ± 4.3 |
| History of CVD | 7% | 38% |
| Systolic BP (mmHg) | 128 ± 17 | 131 ± 17 |
| Diastolic BP (mmHg) | 73 ± 10 | 75 ± 10 |
| HbA1c (%) | 5.4 ± 0.4 | 9.3 ± 1.4 |
| HbA1c (mmol/mol) | 36 ± 4.8 | 78 ± 16 |
| Lipid-lowering therapy | 16% | 69% |
| Triglycerides (mg/dl) | 121 ± 57 | 201 ± 131 |
| Total cholesterol (mg/dl) | 170 ± 34 | 180 ± 38 |
| LDL cholesterol (mg/dl) | 106 ± 31 | 105 ± 31 |
| HDL cholesterol (mg/dl) | 40 ± 10 | 37 ± 10 |
Data are means ± SD or percentages. BP, blood pressure.
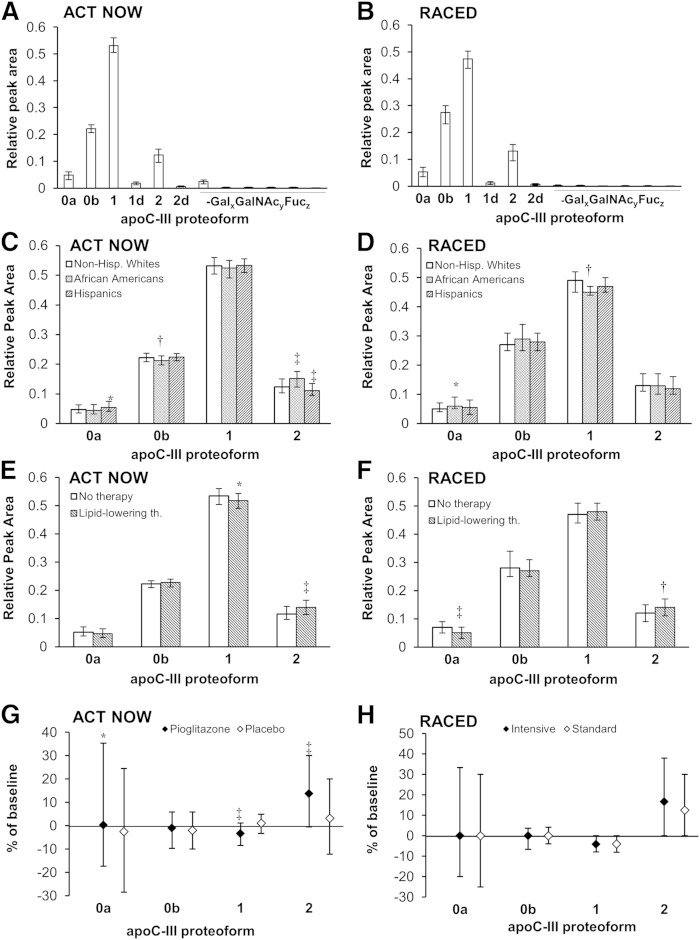
Consistent with our initial description of the assay (27, 28), 12 apoC-III proteoforms were detected by MSIA in both cohorts (supplementary Table 1). In both cohorts, the most abundant form (RPA) was apoC-III1, followed by glycosylated apoC-III0 (apoC-III0b), apoC-III2, and native apoC-III (apoC-III0a), while other forms were substantially less abundant compared with the four major proteoforms (Fig. 1A, B).
Fig. 1.
RPAs of all apoC-III proteoforms in the ACT NOW (n = 531) and RACED (n = 296) cohorts at baseline (A, B). 0a, native apoC-III; 0b, glycosylated nonsialylated apoC-III; 1, monosialylated apoC-III; 2, disialylated apoC-III; d, des-Alanine; Gal, galactose; GalNAc, N-acetylgalactosamine; Fuc, fucose. Major apoC-III proteoforms by race and ethnicity (C, D) and lipid-lowering therapy (Lipid-lowering th.) (E, F), and longitudinal changes in the major apoC-III proteoforms (G, H) (median follow-up 33 months ACT NOW and 9 months RACED). Data are medians ± quartiles; *P < 0.05, †P < 0.01, ‡P < 0.001 by Wilcoxon-rank-sum test or Kruskal-Wallis test [(E) vs. Non-Hispanic Whites].
apoC-III proteoforms’ RPAs and patients’ demographic and clinical characteristics at baseline
The apoC-III proteoforms’ distribution was related to several demographic and clinical characteristics. In the ACT NOW cohort, African Americans had lower apoC-III0b and higher apoC-III2, whereas Hispanics had higher apoC-III0b and lower apoC-III2 compared with non-Hispanic whites (Fig. 1C). In the RACED study, African Americans had higher apoC-III0a and lower apoC-III1 than non-Hispanic whites (Fig. 1D). In both cohorts, apoC-III2 was higher in those receiving lipid-lowering medications (Fig. 1E, F). In the ACT NOW study, lipid-lowering therapy was almost exclusively statins (one participant was on fibric acid). Most RACED patients were receiving statins (n = 149), but a number of patients were on fibric acid alone (n = 24) or in combination with statins (n = 27). The relative abundance of apoC-III2 was higher with use of statins, fibrates, or their combination (median RPAs were 0.14, 0.15, and 0.16, respectively, vs. 0.12 with no lipid-lowering drugs, P < 0.05 for all).
The effects of pioglitazone and intensive glucose lowering on apoC-III proteoforms
Follow-up plasma samples were available in 362 ACT NOW and 253 RACED participants. In the ACT NOW cohort, relative amounts of apoC-III0a and apoC-III2 were higher, and apoC-III1 was lower after pioglitazone (Fig. 1G). In the RACED cohort, apoC-III2 increased and apoC-III1 decreased similarly in both the standard and intensive glucose lowering groups (Fig. 1H). The increases in apoC-III2 after pioglitazone or after glucose lowering [both RACED treatment groups had significant declines in HbA1c from baseline at 9 months (32)] were the most pronounced (P < 0.05 for all) among the proteoforms (Fig. 1G, H).
Relationships between apoC-III proteoforms and metabolic variables
At baseline, higher relative apoC-III2 abundance was associated with lower triglycerides and total cholesterol, and higher HDL cholesterol concentrations in both cohorts, and with lower BMI in the RACED study (Table 2). In contrast, relative abundances of other major proteoforms showed mostly positive associations with BMI, triglycerides, and total cholesterol concentrations and negative associations with HDL cholesterol concentrations (Table 2). In the RACED study, apoC-III0a negatively correlated with fasting glucose and HbA1c concentrations (Table 2).
TABLE 2.
Association between relative abundance of major apoC-III proteoforms and metabolic characteristics in the ACT NOW and RACED cohorts at baseline
| apoC-III Proteoform | ||||||||
| apoC-III0a | apoC-III0b | apoC-III1 | apoC-III2 | |||||
| Variable | ACT NOW | RACED | ACT NOW | RACED | ACT NOW | RACED | ACT NOW | RACED |
| BMI | −0.06 | −0.01 | −0.01 | 0.03 | 0.19b | 0.24c | −0.08 | −0.16b |
| Fasting glucose | 0.05 | −0.16b | −0.03 | 0.04 | −0.04 | 0.06 | 0.02 | −0.01 |
| HbA1c | −0.02 | −0.17b | −0.08 | 0.09 | 0.01 | 0.06 | 0.05 | −0.08 |
| Triglycerides | 0.09 | 0.03 | 0.30c | 0.26c | 0.14a | 0.21c | −0.34c | −0.46c |
| Total cholesterol | 0.00 | 0.02 | 0.10a | 0.01 | 0.09a | 0.08 | −0.14c | −0.14a |
| LDL cholesterol | 0.00 | 0.02 | 0.06 | 0.04 | 0.04 | 0.12a | −0.07 | −0.16b |
| HDL cholesterol | −0.11a | 0.16b | −0.16c | −0.16b | 0.04 | −0.17b | 0.10a | 0.16b |
Data are Spearman correlation coefficients. Longitudinal correlations are partial for treatment group.
P < 0.05.
P < 0.01.
P < 0.001.
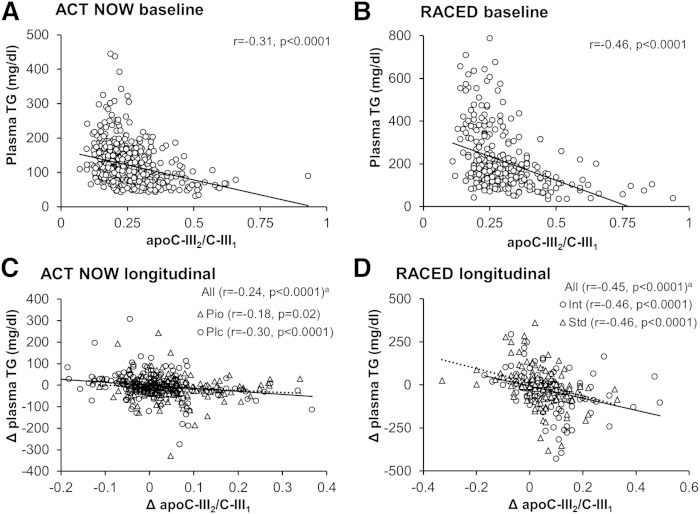
As apoC-III2 demonstrated different relationships with metabolic variables than the most abundant proteoform apoC-III1, we also examined relationships of the apoC-III2/apoC-III1 ratio in plasma. The apoC-III2/apoC-III1 showed a strong inverse association with plasma triglycerides in both cohorts (Fig. 2A, B). In the multivariate analyses, this association remained significant (P < 0.0001) after adjustment for age, gender, BMI, race, and use of lipid-lowering medications. Variation in apoC-III2/apoC-III1 in the ACT NOW cohort also accounted for approximately 23 and 30% of the difference in plasma triglyceride concentrations between African Americans and whites, and African Americans and Hispanics (African Americans, 91 ± 38 mg/dl; whites, 126 ± 59 mg/dl; Hispanic, 132 ± 57 mg/dl; P < 0.0001). Higher apoC-III2/apoC-III1 was also associated with lower total cholesterol and higher HDL cholesterol in both cohorts and with lower LDL cholesterol in the RACED cohort (Table 3).
Fig. 2.
Associations between the apoC-III2/apoC-III1 ratio and plasma triglycerides. Scatter plots (with regression lines) and Spearman correlation coefficients of baseline values of (A, B) and longitudinal changes in (C, D) plasma triglycerides with apoC-III2/apoC-III1 in the ACT NOW and RACED cohorts. a, partially adjusted for treatment. Pio, pioglitazone; Plc, placebo; Int, intensive glucose-lowering therapy; Std, standard glucose-lowering therapy.
TABLE 3.
Spearman correlations between baseline values and longitudinal changes in the ratios of major apoC-III proteoforms to the most abundant form (apoC-III1) and fasting plasma lipids in the ACT NOW and RACED cohorts
| C-III0a/C-III1 | C-III0b/C-III1 | C-III2/C-III1 | ||||
| Variable | ACT NOW | RACED | ACT NOW | RACED | ACT NOW | RACED |
| Triglycerides | 0.05 | −0.004 | 0.14b | 0.11 | −0.31c | −0.46c |
| Δ Triglycerides | 0.08 | 0.13a | 0.02 | −0.02 | −0.24c | −0.45c |
| Total cholesterol | −0.01 | 0.03 | 0.01 | −0.03 | −0.14b | −0.15b |
| Δ Total cholesterol | 0.19c | −0.04 | 0.02 | −0.10 | −0.30c | −0.19b |
| LDL cholesterol | −0.004 | −0.02 | 0.003 | −0.02 | −0.06 | −0.18b |
| Δ LDL cholesterol | 0.16b | −0.10 | 0.04 | −0.11 | −0.25c | −0.19b |
| HDL cholesterol | −0.10a | 0.19b | −0.12b | −0.06 | 0.07 | 0.18b |
| Δ HDL cholesterol | 0.09 | 0.10 | −0.02 | 0.01 | 0.02 | 0.16b |
Data are Spearman correlation coefficients. Δ, partially adjusted for treatment.
P < 0.05.
P < 0.01.
P < 0.001.
Longitudinal changes in apoC-III2/apoC-III1 also inversely correlated with changes in fasting triglycerides (Fig. 2C, D) and total and LDL cholesterol in both cohorts, and positively correlated with changes in HDL cholesterol in the RACED cohort (Table 3). The association between changes in apoC-III2/apoC-III1 and changes in plasma triglycerides was independent of changes in body weight and fasting glucose (P < 0.001 in both cohorts). Notably, adjusting for changes in apoC-III2/apoC-III1 appeared to account for 38% of the decline in plasma triglycerides after pioglitazone therapy in the ACT NOW cohort.
The ratios of other major apoC-III proteoforms to apoC-III1 in both a cross-sectional and a longitudinal setting tended to have positive associations with triglyceride levels and less favorable associations with other lipid values (Table 3).
Total plasma apoC-III concentrations were measured in the baseline plasma samples of the RACED cohort. Total apoC-III was not associated with apoC-III2/apoC-III1 (r = −0.06, P = 0.3). Plasma triglycerides positively correlated with total apoC-III (r = 0.19, P = 0.002), and total apoC-III-derived concentrations of apoC-III0a (r = 0.15, P = 0.01), apoC-III0b (r = 0.23, P = 0.0001), and apoC-III1 (r = 0.22, P = 0.0001). In contrast, triglycerides negatively correlated with apoC-III2 concentrations (r = −0.14, P = 0.02). Adjustment for total plasma apoC-III concentrations had no effect on the negative associations between apoC-III2 RPA or apoC-III2/apoC-III1 and plasma triglycerides (P < 0.0001 for both).
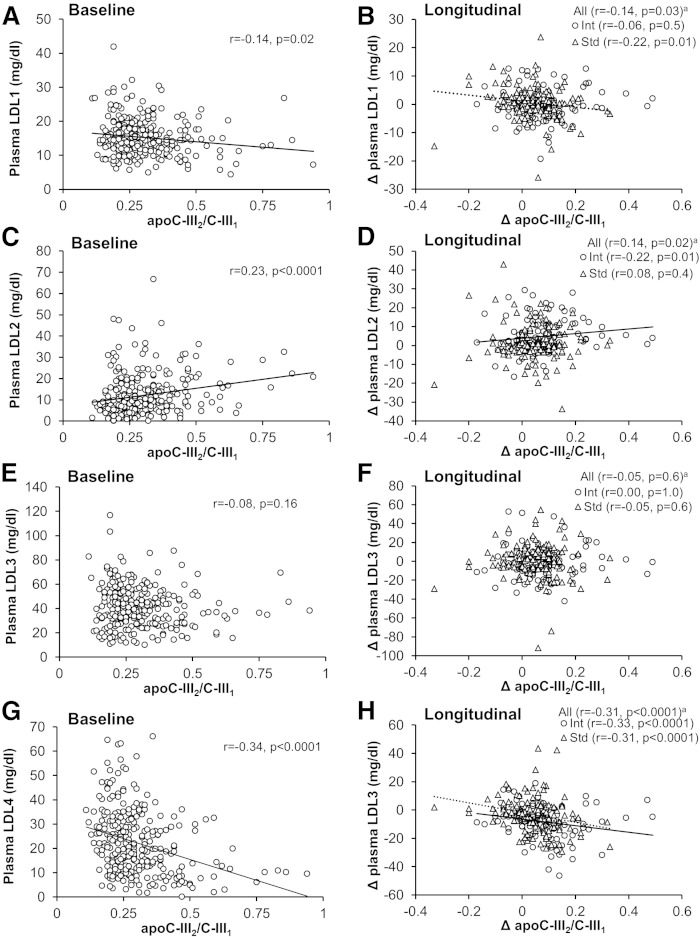
apoC-III2/apoC-III1 and LDL subclasses
LDL cholesterol subclasses were measured in plasma samples of the RACED cohort. Higher apoC-III2/apoC-III1 at baseline was associated with lower LDL1 (largest LDL) and LDL4 (smallest LDL), and higher LDL2 (Fig. 3). After adjusting for plasma triglycerides, apoC-III2/apoC-III1 was still negatively associated with LDL1 (P < 0.0001) and LDL4 (P = 0.002), but not significantly associated with LDL2. Association between apoC-III2/apoC-III1 and LDL4 remained significant after adjusting for lipid-lowering therapy or total apoC-III concentrations (P < 0.0001 for both).
Fig. 3.
Spearman correlations between baseline values (n = 258) and longitudinal changes (Δ, follow-up minus baseline, n = 253) of apoC-III2/apoC-III1 ratio and fasting LDL-cholesterol subclass concentrations [LDL1 (A, B); LDL2 (C, D); LDL3 (E, F); LDL4 (G, H)] in the RACED cohort. Data are Spearman correlation coefficients. apartially adjusted for treatment. Int, intensive glucose-lowering therapy; Std, standard glucose-lowering therapy.
Longitudinally, changes in apoC-III2/apoC-III1 were also inversely associated with changes in LDL1 and LDL4, and positively associated with changes in LDL2 (Fig. 3). Among individual proteoforms, higher LDL4 was associated with lower RPAs of apoC-III2 and higher apoC-III1 both at baseline and longitudinally, and with higher apoC-III0b at baseline (supplementary Table 2).
apoC-III2 and cardiovascular risk
To examine whether the uniquely favorable associations of apoC-III2 with metabolic risk factors may have an impact on CVD, we explored its association with major adverse cardiovascular events (MACEs; myocardial infarction, stroke, or CVD death) in the RACED cohort. A total 40 MACEs occurred during the 5 year median follow-up.
Among demographic and clinical characteristics, a baseline UKPDS risk score was the strongest predictor of incident MACEs (supplementary Table 3). Incident MACEs were also associated with baseline HbA1c in the whole group, baseline fasting triglycerides in the standard group, and older age in the intensive group (supplementary Table 3).
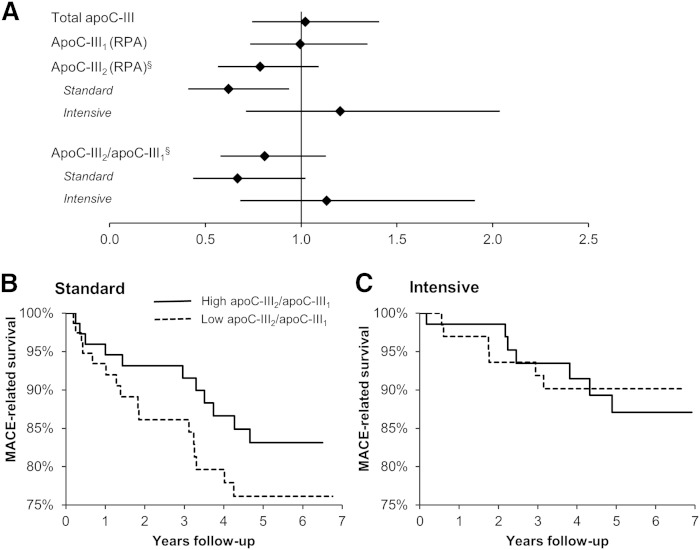
Incident MACEs trended negatively with both the RPA of apoC-III2 and the apoC-III2/apoC-III1 ratio in treatment-adjusted models (Fig. 4A). In the treatment-stratified analysis, a significant association of apoC-III2 (P = 0.02), and a trend for a significant association of apoC-III2/apoC-III1 (P = 0.06) with incident MACEs was found in the standard glucose-lowering group (Fig. 4B, C). In contrast, total apoC-III or apoC-III1 showed no association with incident MACEs (Fig. 4A).
Fig. 4.
Associations between apoC-III proteoforms and MACEs in the RACED cohort. A: Cox proportional HRs and 95% CIs for treatment-adjusted effects of total apoC-III, RPAs of apoC-III1 and apoC-III2, and apoC-III2/apoC-III1 ratio. §P < 0.15 interaction with treatment. B, C: Kaplan-Meier curves of the time to MACE in the standard and intensive glucose lowering groups of the RACED cohort by median apoC-III2/apoC-III1 ratio.
The association between apoC-III2/apoC-III1 and MACEs in the standard group was significant after adjusting for the UKPDS risk score {hazard ratio (HR) 0.54 [95% confidence interval (CI) 0.32–0.90], P = 0.02}, was slightly weaker after adjusting for HbA1c (HR 0.69 [95% CI 0.45–1.05], P = 0.08) or age (HR 0.66 [95% CI 0.42–1.04], P = 0.08), and disappeared after adjusting for fasting triglycerides (HR 0.88 [95% CI 0.54–1.46], P = 0.6).
VLDL uptake and LPL activity ex vivo
To explore potential mechanisms underlying the inverse association between relative apoC-III2 abundance and plasma triglycerides, VLDL was isolated from pooled plasma from nondiabetic participants whose plasma apoC-III2/apoC-III1 was within the upper or lower quartile. VLDL from subjects with a higher apoC-III2/apoC-III1 contained less total apoB, apoC-III, apoE, triglycerides, and cholesterol than VLDL from subjects within the lower quartile of plasma apoC-III2/apoC-III1 (Table 4). The differences in apoC-III, apoE, triglycerides, and cholesterol were less prominent after normalization to apoB concentrations.
TABLE 4.
Concentrations of apoC-III, apoB, and apoE, and total cholesterol and triglyceride content on VLDL isolated from pooled plasma of subjects within the upper and lower quartiles of the plasma apoC-III2/apoC-III1 ratio
| apoB (μg/ml) | apoC-III (μg/ml) | apoC-III (per apoB) | apoE (per apoB) | apoE (per apoB) | TG (μg/ml) | TG (per apoB) | TC (μg/ml) | TC (per apoB) | |
| Lo | 247 | 100 | 0.41 | 0.55 | 0.022 | 704 | 2.86 | 290 | 1.18 |
| Hi | 120 | 73 | 0.61 | 0.23 | 0.019 | 242 | 2.01 | 105 | 0.88 |
TC, total cholesterol; Hi, upper quartile; Lo, lower quartile.
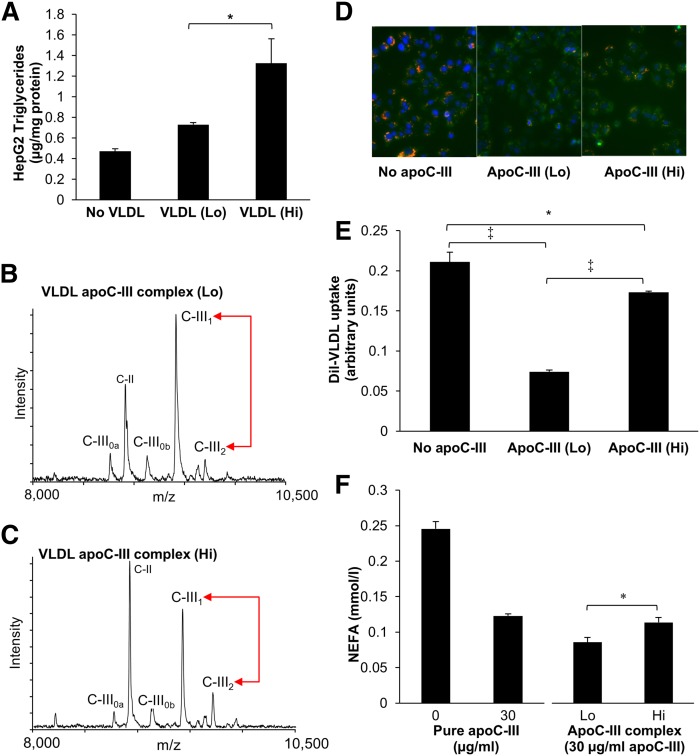
HepG2 cells incubated with VLDL from the upper quartile of plasma apoC-III2/apoC-III1 had higher triglyceride content than HepG2 cells incubated with VLDL from the lower quartile of plasma apoC-III2/apoC-III1, indicating increased triglyceride delivery (Fig. 5A).
Fig. 5.
Effect of higher apoC-III2/apoC-III1 ratio on VLDL uptake and LPL activity. VLDL was isolated from pooled plasma of subjects within the lower (Lo) and upper (Hi) quartiles of plasma apoC-III2/apoC-III1. A: Triglyceride content in HepG2 cells incubated with isolated VLDL in the presence of LPL. B, C: Mass spectra of apoC-III proteoforms in immunoprecipitated apoC-III complexes from Lo and Hi apoC-III2/apoC-III1 VLDL pools. D: Images obtained by fluorescent microscopy of HepG2 cells after addition of DiI-control VLDL without and with apoC-III complexes from Lo and Hi VLDL pools. DiI appears in orange, the lysosomes are stained in green and the nucleus in blue. E: Quantification of HepG2 DiI-VLDL uptake. F: LPL-mediated lipolysis in control VLDL without addition of apoC-III, and with addition of 30 μg/ml pure apoC-III and apoC-III complexes prepared from Lo and Hi VLDL pools. Data are mean ± SD, n = 4 [n = 2 (F)] repeats. *P < 0.05, ‡P < 0.001.
To further delineate the specific effect of apoC-III on VLDL uptake and LPL-mediated lipolysis, apoC-III was immunoprecipitated from VLDL of both high and low apoC-III2/apoC-III1 plasma pools. The difference in apoC-III2/apoC-III1 between these two pools was confirmed by MSIA (Fig. 5B, C). The apoC-III complex from the upper quartile of plasma apoC-III2/apoC-III1 also showed a more prominent intensity peak of the native apoC-II (Fig. 5B, C).
A previous study by Mann et al. (25) has shown that apoC-III sialylation may influence VLDL uptake via the LSR, a receptor for triglyceride-rich lipoproteins that is activated in the presence of fatty acids. We established an assay for LSR activity in HepG2 cells, measured as DiI-VLDL uptake in the presence of oleic acid, and confirmed that pure apoC-III inhibited DiI-VLDL uptake (supplementary Fig. 1). Using the apo-CIII isolated from VLDL, we found that the inhibition of DiI-VLDL uptake in the presence of oleic acid was less pronounced with the apoC-III isolated from VLDL obtained from those within the upper quartile of the plasma apoC-III2/apoC-III1 ratio, indicating a less inhibitory effect of higher relative apoC-III2 on VLDL uptake, as shown by immunofluorescence (Fig. 5D) and confirmed by quantitative fluorimetric analysis (Fig. 5E).
Inhibition of LPL activity was measured by incubating VLDL with the distinct apoC-III complexes containing 30 μg/ml of total apoC-III. The amount of NEFA released from VLDL was significantly higher after incubation with apoC-III prepared from plasma from individuals in the upper quartile of apoC-III2/apoC-III1 versus apoC-III from the lower quartile, indicating less prominent inhibition of LPL-mediated lipolysis (Fig. 5F).
DISCUSSION
In two separate large cohorts, we found strong cross-sectional and longitudinal inverse relationships between the relative abundance of apoC-III2 proteoform and plasma triglyceride concentrations. Importantly, the associations were very similar in the two current cohorts and our recently reported cross-sectional study (28), despite differences in their demographic and clinical characteristics (e.g., age, gender, ethnicity, glucose tolerance status, and prevalence of CVD). The association between relative apoC-III2 abundance and triglycerides remained present after adjustment for relevant demographic factors and even lipid lowering medications, suggesting that apoC-III2 abundance may be an independent determinant of plasma triglyceride levels. Furthermore, the inverse relationship between apoC-III2 abundance and plasma triglyceride levels remained whether apoC-III2 was expressed as the relative abundance among all apoC-III proteoforms, the ratio to the most abundant proteoform apoC-III1, or, in the RACED cohort, as the absolute apoC-III2 concentrations. In both cohorts, higher apoC-III2 relative abundance was also associated with a more favorable overall lipid profile, including lower total and LDL cholesterol and higher HDL cholesterol.
Increased prevalence of proatherogenic small dense LDL particles is commonly reported in patients with type 2 diabetes, particularly in those with poor glycemic control (38). apoC-III has been previously shown to facilitate production of small dense LDL (2). The current analyses in the RACED cohort showed distinct associations between relative amounts of apoC-III proteoforms and the smallest LDL4 particles. LDL4 was positively correlated with relative abundances of apoC-III0b and apoC-III1, and negatively correlated with apoC-III2 abundance. Higher relative apoC-III2 abundance was also associated with higher (buoyant) LDL2. The association between the apoC-III2/apoC-III1 ratio and LDL4 persisted after adjusting for plasma triglyceride concentrations, suggesting potentially favorable effects of apoC-III2 on LDL4 production that were separate from the effects on triglyceride levels. Interestingly, the relative abundance of apoC-III2 was negatively associated with the most buoyant, but very low abundant, LDL1. This negative association may reflect preferential removal of the larger LDL1 particles, which are most rapidly cleared, as previously noted in studies with LDL cholesterol-lowering agents (39). Importantly, these associations between relative apoC-III2 abundance and LDL subclasses were generally consistent between cross-sectional and longitudinal analyses, providing further confirmation of the validity of these relationships and suggesting that they may be causally related.
Given these favorable associations between apoC-III2 abundance and the major lipid categories or LDL cholesterol subclasses, we examined the relationship between apoC-III2 and incident MACE outcomes during the VADT study. A reduced hazard ratio for apoC-III2 was most evident in the standard group, suggesting the possibility that improvement in other risk factors or the specific effects of diabetes medications in the intensive treatment group might have masked the association between the apoC-III2 relative abundance and MACEs. Although the number of MACEs was relatively small in this subcohort, we were able to find the expected increase in MACEs with higher values of standard risk factors represented by the UKPDS risk coefficient. Importantly, the association between the relative abundance of apoC-III2 and MACEs in the standard group was independent of the UKPDS risk coefficient, but not of fasting triglyceride concentrations, indicating that this effect was largely mediated by changes in triglyceride metabolism.
Several previous studies showed association between apoC-III concentrations on lipid particles and coronary artery disease (14–17). In the present study, we did not find a significant association between total apoC-III concentration in plasma and MACEs. There are several potential explanations of our results. First, total apoC-III in plasma may be a less sensitive biomarker of cardiovascular risk compared with apoC-III on specific lipid particles. In fact, no studies, to date, have shown a relationship between total plasma apoC-III concentrations and incident cardiovascular events. Second, our VADT subset cohort was modest in size, participants were relatively homogenous in their high cardiovascular risk, and were on multiple medications that could alter either cardiovascular risk and/or total apoC-III concentrations. Third, our data suggest that apoC-III proteoforms vary in their association with, and potential contribution to, plasma lipids and cardiovascular risk. Thus, a measure of total apoC-III in plasma may not reflect the overall risk of all the individual proteoforms.
This is the first analysis of the relationships between apoC-III proteoforms and plasma lipids, LDL-cholesterol subclasses, and cardiovascular outcomes in larger cohorts. This type of analysis has previously been limited by the time- and labor-intensive nature of the isoelectric focusing methodology that was used in previous studies. In two earlier small cross-sectional studies, the concentrations of sialylated apoC-III proteoforms in triglyceride-rich lipoproteins and their production rates correlated more strongly with plasma triglycerides than apoC-III0 (22, 26). The production rate of apoC-III2 also negatively correlated with LDL peak particle size (26). However, in both studies, the regression slopes between apoC-III proteoforms and plasma triglycerides were greater for apoC-III1 than for apoC-III2, indicating a less harmful relationship between apoC-III2 and triglycerides. The reasons for differences between results of these smaller studies and our findings are unclear, but may be explained by differences in study participants and methodologies. Participants in our cohorts demonstrated moderate or advanced levels of glucose intolerance, and had a high prevalence of hypertension and use of glucose-lowering agents. In addition, isoelectric focusing does not distinguish among the many nonsialylated proteoforms. For example, two major nonsialylated proteoforms showed distinct associations with several demographic and metabolic outcomes in our study.
apoC-III inhibits both lipolysis in vitro and cellular uptake of triglyceride-rich lipoproteins (3, 4). Prior in vitro studies indicated that apoC-III sialylation may have a substantial functional effect on both VLDL uptake and LPL-mediated lipolysis (25, 40). Of particular relevance to our study, Mann et al. (25) reported that, compared with apoC-III1 or apoC-III0, apoC-III2 is a less effective inhibitor of VLDL uptake by the hepatic LSR. The LSR has been proposed as a rate-limiting factor for the clearance of triglycerides during the postprandial period (41, 42). LSR heterozygous mice display increased postprandial triglyceride levels, decreased clearance of lipid particles, and increased levels of proatherogenic lipoproteins following a Western diet (35). Silencing of hepatic LSR in mice has also been shown to lead to hypertriglyceridemia (43). We observed an increase in DiI-VLDL uptake in HepG2 cells in the presence of oleic acid that was inhibited by apoC-III. This inhibition was less pronounced with apoC-III isolated from subjects with high plasma apoC-III2/apoC-III1, as compared with that observed with the same amount of apoC-III isolated from subjects with low apoC-III2/apoC-III1. This is consistent with the previously demonstrated effect of apoC-III proteoforms on uptake of triglyceride-rich lipoproteins (25). As the conditions of the VLDL uptake experiments favored the LSR pathway, i.e., including pretreatment with leptin, shorter duration of cells starvation time, and coincubation with oleic acids, these experiments did not permit exploration of other important mechanisms contributing to clearance of triglyceride-rich lipoproteins. These additional pathways include the LDL-receptor (44), LDL receptor-related protein 1 (45), and heparan sulfate proteoglycans (46, 47).
Our ex vivo data also showed less efficient inhibition of LPL-mediated lipolysis for apoC-III with higher apoC-III2/apoC-III1 ratio. This may be in contrast with older studies showing either no effect (48, 49) or greater inhibition of LPL activity (40, 50) with increased apoC-III sialylation. The discrepancy may be due to methodological differences in the isolation of apoC-III isoforms, the effect of neuraminidase used for desialylation in some studies, the distinct action of the two sialylated proteoforms rather than sialylation in general, differences in the amount of apoC-III used in the experiments, and differences in the clinical characteristics of apoC-III donors. The effective amount of apoC-III to inhibit LPL-mediated lipolysis in our study and in some previous studies (37, 40) was also substantially higher than apoC-III concentrations inhibiting VLDL uptake. Thus, it is possible that inhibition of lipolysis by apoC-III may contribute to increased triglyceride levels only in those individuals with very high apoC-III levels.
apoC-III glycosylation may affect metabolism of triglyceride-rich lipoproteins by several mechanisms. Glycosylation alters multiple apolipoprotein properties, including conformational stability, resistance to proteolysis, charge, water-binding, and biological recognition in protein targeting and cell to cell interactions (51). Greater sialylation may stabilize apoC-III complexes with other apolipoproteins, including apoA-II, apoB, apoC-I, apoC-II, and apoE, and thus potentially enhance their effects on lipid metabolism, including clearance of triglyceride-rich particles (33, 52–54). The higher native apoC-II abundance noted in association with precipitated apoC-III from individuals with higher apoC-III2/apoC-III1 ratios may represent one example of such a scenario. It is possible that greater activation of LPL-mediated lipolysis by increased amounts of apoC-II could help counter the inhibitory effect of apoC-III on these events.
There were several other notable findings related to glucose- and lipid-lowering therapy in this study. First, relative apoC-III2 abundance was increased after pioglitazone treatment in the ACT NOW patients. Relative apoC-III2 abundance also increased in the RACED cohort in both treatment groups, and they both received rosiglitazone. This suggests the possibility of a PPAR-γ agonist class effect on apoC-III2, which could be related to their known triglyceride lowering action. The positive cross-sectional association of apoC-III2/apoC-III1 with insulin sensitivity in our previous study (28) indicates that this effect may be related to the insulin-sensitizing action of PPAR-γ agonists. Second, the relative distribution of apoC-III proteoforms was modified by the use of lipid-lowering drugs, and there was evidence of proteoform-specific effects. Whereas the use of both fibrates and statins was associated with lower native apoC-III, the increase in apoC-III2 was more evident with fibrates. Relative apoC-III proteoform abundance also significantly differed between the races or ethnicities. In the ACT NOW cohort, higher relative apoC-III2 in African Americans appeared to account for more than 20% of the differences in plasma triglyceride concentrations between African Americans and both non-Hispanic whites and Hispanics, suggesting a possible contribution of this proteoform to the well-described lower triglyceride concentrations in African Americans (55). Aggressive use of lipid-lowering therapy per protocol in the entire cohort and a smaller number of African Americans may explain why we did not observe significant racial differences in the relative apoC-III2 abundance in the RACED cohort. These observations need to be validated in larger populations where lipid-lowering medication use is relatively low.
Several limitations of the study deserve mention. The study was a post hoc analysis of baseline and follow-up samples from two previously completed studies. Although the studies were well powered to examine associations between apoC-III and both baseline and longitudinal lipid values, the analysis relating apoC-III proteoforms and incident MACEs was not, and must be considered exploratory. We also did not measure apoC-III proteoforms in different lipoprotein fractions, which may permit further insight into their role in lipid metabolism. Samples for these analyses were stored for several years at −80°C before MSIA was performed. Previous investigation of the effects of storage, time, and freeze/thaw cycles on these assays has indicated that the measurements are relatively stable (27). Moreover, despite differences in the storage time of the samples in the two different cohorts, there was a striking consistency in the relationships between apoC-III proteoforms and plasma lipids. Because the apoC-III complexes in our ex vivo studies contained other apolipoproteins, we could not distinguish to what extent the observed effects resulted from modification of apoC-III action per se or effects on the amount and activity of other apolipoproteins. Thus, further studies are needed to identify the effects of individual apoC-III proteoforms. While the RACED cohort showed a weak but significant negative association between absolute apoC-III2 concentrations and plasma triglycerides, our previous study in obese adolescents showed no association between these two variables. The discrepancy may be explained by differences among the cohorts, with the RACED cohort larger in size, comprised of diabetes patients, and having a broader range of triglyceride concentrations. Importantly, the associations of plasma triglycerides with total apoC-III or absolute concentrations of other major apoC-III proteoforms were positive in both the RACED cohort and the previous study, further supporting a different relationship of apoC-III2 with plasma triglycerides.
In conclusion, the present analyses provide the first evidence, from two independent large cohorts, of a strong and inverse association between the relative amounts of a disialylated apoC-III proteoforms and proatherogenic plasma lipid profiles in individuals with abnormal glucose metabolism. These relationships are distinct from those of total apoC-III and other major apoC-III proteoforms, and, together with our ex vivo data, support the concept that apoC-III proteoforms may have different effects on lipid metabolism. Measuring relative amounts of apoC-III variants may add to the risk assessment that can be obtained through measurement of total plasma apoC-III. Importantly, gender, race, and several classes of medications appear to influence the relative amounts of the potentially more favorable apoC-III2 proteoform, and this may help account for some of their effects on triglyceride and other lipid levels. These novel results also emphasize the need for further study of posttranslational modifications of apolipoproteins to clarify their role in lipid metabolism and cardiometabolic risk.
Supplementary Material
Footnotes
Abbreviations:
- CI
- confidence interval
- d
- des-Alanine
- DAPI
- 4′,6-diamidino-2-phenylindole
- Fuc
- fucose
- Gal
- galactose
- Gal-NAc
- N-acetygalactosamine
- HbA1c
- hemoglobin A1c
- Hi
- high apoC-III2/apoC-III1 ratio
- HR
- hazard ratio
- Int
- intensive glucose-lowering therapy
- Lo
- low apoC-III2/apoC-III1 ratio
- LPL
- lipoprotein lipase
- LSR
- lipolysis-stimulated receptor
- MACE
- major adverse cardiovascular event
- MSIA
- mass spectrometric immunoassay
- PBST
- phosphate buffered saline with Tween-20
- Pio
- pioglitazone
- Plc
- placebo
- RPA
- relative peak area Std, standard glucose-lowering therapy
- UKPDS
- United Kingdom Prospective Diabetes Study
This work was supported by the National Institutes of Health Grants R24-DK090958 (R.W.N./P.D.R.), R01-067690 (P.D.R.), R01-HL94775 (P.D.R.), R01-DK082542 (R.W.N.), and K23-HL107389 and 15BGIA25690024 from the American Heart Association (H.Y.), the Cooperative Studies Program of the Department of Veterans Affairs Office of Research and Development (#431), and Takeda Pharmaceuticals. The contents of this article do not represent the views of the Department of Veterans Affairs or the United States Government. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Ginsberg H. N., and Brown W. V.. 2011. Apolipoprotein CIII: 42 years old and even more interesting. Arterioscler. Thromb. Vasc. Biol. 31: 471–473. [DOI] [PubMed] [Google Scholar]
- 2.Mendivil C. O., Zheng C., Furtado J., Lel J., and Sacks F. M.. 2010. Metabolism of very-low-density lipoprotein and low-density lipoprotein containing apolipoprotein C-III and not other small apolipoproteins. Arterioscler. Thromb. Vasc. Biol. 30: 239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown W. V., and Baginsky M. L.. 1972. Inhibition of lipoprotein lipase by an apoprotein of human very low density lipoprotein. Biochem. Biophys. Res. Commun. 46: 375–382. [DOI] [PubMed] [Google Scholar]
- 4.Windler E., and Havel R. J.. 1985. Inhibitory effects of C apolipoproteins from rats and humans on the uptake of triglyceride-rich lipoproteins and their remnants by the perfused rat liver. J. Lipid Res. 26: 556–565. [PubMed] [Google Scholar]
- 5.Sundaram M., Zhong S., Khalil M. B., Links P. H., Zhao Y., Iqbal J., Hussain M. M., Parks R. J., Wang Y., and Yao Z.. 2010. Expression of apolipoprotein C-III in McA-RH7777 cells enhances VLDL assembly and secretion under lipid-rich conditions. J. Lipid Res. 51: 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito Y., Azrolan N., O’Connell A., Walsh A., and Breslow J. L.. 1990. Hypertriglyceridemia as a result of human apo CIII gene expression in transgenic mice. Science. 249: 790–793. [DOI] [PubMed] [Google Scholar]
- 7.Maeda N., Li H., Lee D., Oliver P., Quarfordt S. H., and Osada J.. 1994. Targeted disruption of the apolipoprotein C-III gene in mice results in hypotriglyceridemia and protection from postprandial hypertriglyceridemia. J. Biol. Chem. 269: 23610–23616. [PubMed] [Google Scholar]
- 8.Klein R. L., McHenry M. B., Lok K. H., Hunter S. J., Le N. A., Jenkins A. J., Zheng D., Semler A. J., Brown W. V., Lyons T. J., et al. 2004. Apolipoprotein C-III protein concentrations and gene polymorphisms in type 1 diabetes: associations with lipoprotein subclasses. Metabolism. 53: 1296–1304. [DOI] [PubMed] [Google Scholar]
- 9.Cohn J. S., Patterson B. W., Uffelman K. D., Davignon J., and Steiner G.. 2004. Rate of production of plasma and very-low-density lipoprotein (VLDL) apolipoprotein C-III is strongly related to the concentration and level of production of VLDL triglyceride in male subjects with different body weights and levels of insulin sensitivity. J. Clin. Endocrinol. Metab. 89: 3949–3955. [DOI] [PubMed] [Google Scholar]
- 10.Sacks F. M., Zheng C., and Cohn J. S.. 2011. Complexities of plasma apolipoprotein C-III metabolism. J. Lipid Res. 52: 1067–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olin-Lewis K., Krauss R. M., La Belle M., Blanche P. J., Barrett P. H. R., Wight T. N., and Chait A.. 2002. ApoC-III content of apoB-containing lipoproteins is associated with binding to the vascular proteoglycan biglycan. J. Lipid Res. 43: 1969–1977. [DOI] [PubMed] [Google Scholar]
- 12.Hiukka A., Ståhlman M., Pettersson C., Levin M., Adiels M., Teneberg S., Leinonen E. S., Hultén L. M., Wiklund O., Orešič M., et al. 2009. ApoCIII-enriched LDL in type 2 diabetes displays altered lipid composition, increased susceptibility for sphingomyelinase, and increased binding to biglycan. Diabetes. 58: 2018–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawakami A., Aikawa M., Alcaide P., Luscinskas F. W., Libby P., and Sacks F. M.. 2006. Apolipoprotein CIII induces expression of vascular cell adhesion molecule-1 in vascular endothelial cells and increases adhesion of monocytic cells. Circulation. 114: 681–687. [DOI] [PubMed] [Google Scholar]
- 14.Hodis H. N., Mack W. J., Azen S. P., Alaupovic P., Pogoda J. M., LaBree L., Hemphill L. C., Kramsch D. M., and Blankenhorn D. H.. 1994. Triglyceride- and cholesterol-rich lipoproteins have a differential effect on mild/moderate and severe lesion progression as assessed by quantitative coronary angiography in a controlled trial of lovastatin. Circulation. 90: 42–49. [DOI] [PubMed] [Google Scholar]
- 15.Sacks F. M., Alaupovic P., Moye L. A., Cole T. G., Sussex B., Stampfer M. J., Pfeffer M. A., and Braunwald E.. 2000. VLDL, apolipoproteins B, CIII, and E, and risk of recurrent coronary events in the Cholesterol and Recurrent Events (CARE) trial. Circulation. 102: 1886–1892. [DOI] [PubMed] [Google Scholar]
- 16.Lee S-J., Campos H., Moye L. A., and Sacks F. M.. 2003. LDL containing apolipoprotein CIII is an independent risk factor for coronary events in diabetic patients. Arterioscler. Thromb. Vasc. Biol. 23: 853–858. [DOI] [PubMed] [Google Scholar]
- 17.Mendivil C. O., Rimm E. B., Furtado J., Chiuve S. E., and Sacks F. M.. 2011. Low-density lipoproteins containing apolipoprotein C-III and the risk of coronary heart disease. Circulation. 124: 2065–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pollin T. I., Damcott C. M., Shen H., Ott S. H., Shelton J., Horenstein R. B., Post W., McLenithan J. C., Bielak L. F., Peyser P. A., et al. 2008. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 322: 1702–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jørgensen A. B., Frikke-Schmidt R., Nordestgaard B. G., and Tybjærg-Hansen A.. 2014. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N. Engl. J. Med. 371: 32–41. [DOI] [PubMed] [Google Scholar]
- 20.Caron S., Verrijken A., Mertens I., Samanez C. H., Mautino G., Haas J. T., Duran-Sandoval D., Prawitt J., Francque S., Vallez E., et al. 2011. Transcriptional activation of apolipoprotein CIII expression by glucose may contribute to diabetic dyslipidemia. Arterioscler. Thromb. Vasc. Biol. 31: 513–519. [DOI] [PubMed] [Google Scholar]
- 21.Chen M., Breslow J. L., Li W., and Leff T.. 1994. Transcriptional regulation of the apoC-III gene by insulin in diabetic mice: correlation with changes in plasma triglyceride levels. J. Lipid Res. 35: 1918–1924. [PubMed] [Google Scholar]
- 22.Kashyap M. L., Srivastava L. S., Hynd B. A., Gartside P. S., and Perisutti G.. 1981. Quantitation of human apolipoprotein C-III and its subspecie by radioimmunoassay and analytical isoelectric focusing: abnormal plasma triglyceride-rich lipoprotein apolipoprotein C-III subspecie concentrations in hypertriglyceridemia. J. Lipid Res. 22: 800–810. [PubMed] [Google Scholar]
- 23.Ito Y., Breslow J. L., and Chait B. T.. 1989. Apolipoprotein C-III0 lacks carbohydrate residues: use of mass spectrometry to study apolipoprotein structure. J. Lipid Res. 30: 1781–1787. [PubMed] [Google Scholar]
- 24.Wiggins R., Hicks S. J., Soothill P. W., Millar M. R., and Corfield A. P.. 2001. Mucinases and sialidases: their role in the pathogenesis of sexually transmitted infections in the female genital tract. Sex. Transm. Infect. 77: 402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mann C. J., Troussard A. A., Yen F. T., Hannouche N., Najib J., Fruchart J-C., Lotteau V., André P., and Bihain B. E.. 1997. Inhibitory effects of specific apolipoprotein C-III isoforms on the binding of triglyceride-rich lipoproteins to the lipolysis-stimulated receptor. J. Biol. Chem. 272: 31348–31354. [DOI] [PubMed] [Google Scholar]
- 26.Mauger J-F., Couture P., Bergeron N., and Lamarche B.. 2006. Apolipoprotein C-III isoforms: kinetics and relative implication in lipid metabolism. J. Lipid Res. 47: 1212–1218. [DOI] [PubMed] [Google Scholar]
- 27.Trenchevska O., Schaab M. R., Nelson R. W., and Nedelkov D.. 2015. Development of multiplex mass spectrometric immunoassay for detection and quantification of apolipoproteins C-I, C-II, C-III and their proteoforms. Methods. 81: 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yassine H. N., Trenchevska O., Ramrakhiani A., Parekh A., Koska J., Walker R. W., Billheimer D., Reaven P. D., Yen F. T., Nelson R. W., et al. 2015. The association of human apolipoprotein C-III sialylation proteoforms with plasma triglycerides. PLoS One. 10: e0144138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeFronzo R. A., Tripathy D., Schwenke D. C., Banerji M., Bray G. A., Buchanan T. A., Clement S. C., Henry R. R., Hodis H. N., Kitabchi A. E., et al. 2011. Pioglitazone for diabetes prevention in impaired glucose tolerance. N. Engl. J. Med. 364: 1104–1115. [DOI] [PubMed] [Google Scholar]
- 30.Reaven P. D., Moritz T. E., Schwenke D. C., Anderson R. J., Criqui M., Detrano R., Emanuele N., Kayshap M., Marks J., Mudaliar S., et al. 2009. Intensive glucose-lowering therapy reduces cardiovascular disease events in veterans affairs diabetes trial participants with lower calcified coronary atherosclerosis. Diabetes. 58: 2642–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duckworth W., Abraira C., Moritz T., Reda D., Emanuele N., Reaven P. D., Zieve F. J., Marks J., Davis S. N., Hayward R., et al. 2009. Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 360: 129–139. [DOI] [PubMed] [Google Scholar]
- 32.Koska J., Saremi A., Bahn G., Yamashita S., and Reaven P. D.; Veterans Affairs Diabetes Trial Investigators. 2013. The effect of intensive glucose lowering on lipoprotein particle profiles and inflammatory markers in the Veterans Affairs Diabetes Trial (VADT). Diabetes Care. 36: 2408–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng C., Khoo C., Ikewaki K., and Sacks F. M.. 2007. Rapid turnover of apolipoprotein C-III-containing triglyceride-rich lipoproteins contributing to the formation of LDL subfractions. J. Lipid Res. 48: 1190–1203. [DOI] [PubMed] [Google Scholar]
- 34.Huff M. W., Miller D. B., Wolfe B. M., Connelly P. W., and Sawyez C. G.. 1997. Uptake of hypertriglyceridemic very low density lipoproteins and their remnants by HepG2 cells: the role of lipoprotein lipase, hepatic triglyceride lipase, and cell surface proteoglycans. J. Lipid Res. 38: 1318–1333. [PubMed] [Google Scholar]
- 35.Yen F. T., Roitel O., Bonnard L., Notet V., Pratte D., Stenger C., Magueur E., and Bihain B. E.. 2008. Lipolysis stimulated lipoprotein receptor: a novel molecular link between hyperlipidemia, weight gain, and atherosclerosis in mice. J. Biol. Chem. 283: 25650–25659. [DOI] [PubMed] [Google Scholar]
- 36.Di Filippo M., Marçais C., Charrière S., Marmontel O., Broyer M., Delay M., Merlin M., Nollace A., Valéro R., and Lagarde M.. 2014. Post-heparin LPL activity measurement using VLDL as a substrate: a new robust method for routine assessment of plasma triglyceride lipolysis defects. PLoS One. 9: e96482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang C. S., McConathy W. J., Kloer H. U., and Alaupovic P.. 1985. Modulation of lipoprotein lipase activity by apolipoproteins. Effect of apolipoprotein C-III. J. Clin. Invest. 75: 384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garvey W. T., Kwon S., Zheng D., Shaughnessy S., Wallace P., Hutto A., Pugh K., Jenkins A. J., Klein R. L., and Liao Y.. 2003. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes. 52: 453–462. [DOI] [PubMed] [Google Scholar]
- 39.Bays H., Conard S., Leiter L., Bird S., Jensen E., Hanson M., Shah A., and Tershakovec A.. 2010. Are post-treatment low-density lipoprotein subclass pattern analyses potentially misleading? Lipids Health Dis. 9: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holleboom A. G., Karlsson H., Lin R. S., Beres T. M., Sierts J. A., Herman D. S., Stroes E. S., Aerts J. M., Kastelein J. J., Motazacker M. M., et al. 2011. Heterozygosity for a loss-of-function mutation in GALNT2 improves plasma triglyceride clearance in man. Cell Metab. 14: 811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mann C. J., Khallou J., Chevreuil O., Troussard A. A., Guermani L. M., Launay K., Delplanque B., Yen F. T., and Bihain B. E.. 1995. Mechanism of activation and functional significance of the lipolysis-stimulated receptor. Evidence for a role as chylomicron remnant receptor. Biochemistry. 34: 10421–10431. [DOI] [PubMed] [Google Scholar]
- 42.Yen F. T., Mann C. J., Guermani L. M., Hannouche N. F., Hubert N., Hornick C. A., Bordeau V. N., Agnani G., and Bihain B. E.. 1994. Identification of a lipolysis-stimulated receptor that is distinct from the LDL receptor and the LDL receptor-related protein. Biochemistry. 33: 1172–1180. [DOI] [PubMed] [Google Scholar]
- 43.Narvekar P., Berriel Diaz M., Krones-Herzig A., Hardeland U., Strzoda D., Stohr S., Frohme M., and Herzig S.. 2009. Liver-specific loss of lipolysis-stimulated lipoprotein receptor triggers systemic hyperlipidemia in mice. Diabetes. 58: 1040–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown M. S., and Goldstein J. L.. 1986. A receptor-mediated pathway for cholesterol homeostasis. Science. 232: 34–47. [DOI] [PubMed] [Google Scholar]
- 45.Rohlmann A., Gotthardt M., Hammer R. E., and Herz J.. 1998. Inducible inactivation of hepatic LRP gene by cre-mediated recombination confirms role of LRP in clearance of chylomicron remnants. J. Clin. Invest. 101: 689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ji Z. S., Brecht W. J., Miranda R. D., Hussain M. M., Innerarity T. L., and Mahley R. W.. 1993. Role of heparan sulfate proteoglycans in the binding and uptake of apolipoprotein E-enriched remnant lipoproteins by cultured cells. J. Biol. Chem. 268: 10160–10167. [PubMed] [Google Scholar]
- 47.MacArthur J. M., Bishop J. R., Stanford K. I., Wang L., Bensadoun A., Witztum J. L., and Esko J. D.. 2007. Liver heparan sulfate proteoglycans mediate clearance of triglyceride-rich lipoproteins independently of LDL receptor family members. J. Clin. Invest. 117: 153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stoline A. M., Saku K., Hynd B. A., and Kashyap M. L.. 1985. Effect of desialylation of very low-density lipoproteins on their catabolism by lipoprotein lipase. Metabolism. 34: 30–35. [DOI] [PubMed] [Google Scholar]
- 49.Catapano A. L. 1987. Activation of lipoprotein lipase by apolipoprotein C-II is modulated by the COOH terminal region of apolipoprotein C-III. Chem. Phys. Lipids. 45: 39–47. [DOI] [PubMed] [Google Scholar]
- 50.Holdsworth G., Stocks J., Dodson P., and Galton D. J.. 1982. An abnormal triglyceride-rich lipoprotein containing excess sialylated apolipoprotein C-III. J. Clin. Invest. 69: 932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paulson J. C. 1989. Glycoproteins: what are the sugar chains for? Trends Biochem. Sci. 14: 272–276. [DOI] [PubMed] [Google Scholar]
- 52.Davidson W. S., Silva R. A., Chantepie S., Lagor W. R., Chapman M. J., and Kontush A.. 2009. Proteomic analysis of defined HDL subpopulations reveals particle-specific protein clusters: relevance to antioxidative function. Arterioscler. Thromb. Vasc. Biol. 29: 870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Campos H., Perlov D., Khoo C., and Sacks F. M.. 2001. Distinct patterns of lipoproteins with apoB defined by presence of apoE or apoC-III in hypercholesterolemia and hypertriglyceridemia. J. Lipid Res. 42: 1239–1249. [PubMed] [Google Scholar]
- 54.Morita S. Y., Sakurai A., Nakano M., Kitagawa S., and Handa T.. 2011. Presence of apolipoprotein C-III attenuates apolipoprotein E-mediated cellular uptake of cholesterol-containing lipid particles by HepG2 cells. Lipids. 46: 323–332. [DOI] [PubMed] [Google Scholar]
- 55.Sumner A. E., Finley K. B., Genovese D. J., Criqui M. H., and Boston R. C.. 2005. Fasting triglyceride and the triglyceride-HDL cholesterol ratio are not markers of insulin resistance in African Americans. Arch. Intern. Med. 165: 1395–1400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.