Abstract
Elevated plasma concentrations of lipoprotein (a) [Lp(a)] have been determined to be a causal risk factor for coronary heart disease, and may similarly play a role in other atherothrombotic disorders. Lp(a) consists of a lipoprotein moiety indistinguishable from LDL, as well as the plasminogen-related glycoprotein, apo(a). Therefore, the pathogenic role for Lp(a) has traditionally been considered to reflect a dual function of its similarity to LDL, causing atherosclerosis, and its similarity to plasminogen, causing thrombosis through inhibition of fibrinolysis. This postulate remains highly speculative, however, because it has been difficult to separate the prothrombotic/antifibrinolytic functions of Lp(a) from its proatherosclerotic functions. This review surveys the current landscape surrounding these issues: the biochemical basis for procoagulant and antifibrinolytic effects of Lp(a) is summarized and the evidence addressing the role of Lp(a) in both arterial and venous thrombosis is discussed. While elevated Lp(a) appears to be primarily predisposing to thrombotic events in the arterial tree, the fact that most of these are precipitated by underlying atherosclerosis continues to confound our understanding of the true pathogenic roles of Lp(a) and, therefore, the most appropriate therapeutic target through which to mitigate the harmful effects of this lipoprotein.
Keywords: apolipoproteins, atherosclerosis, low density lipoprotein, lipoproteins, lipoproteins/kinetics, apolipoprotein (a), coagulation, fibrinolysis, thrombosis
Elevated plasma concentrations of lipoprotein (a) [Lp(a)] have been known to be a risk factor for cardiovascular disorders, such as coronary heart disease (CHD), for 40 years (1, 2). A great deal of excitement was generated when the remarkable homology between apo(a), the distinguishing protein component of Lp(a), and the fibrinolytic proenzyme, plasminogen, was discovered by protein and cDNA sequence analysis in the late 1980s (3, 4). It was immediately apparent that the unique structure of Lp(a) potentially constituted a molecular link between the processes of atherosclerosis (mediated by the LDL-like moiety) and thrombosis [mediated by the apo(a) moiety] that together precipitate events such as myocardial infarction (MI) and ischemic stroke. Indeed, some of the first functional studies of Lp(a) showed that it was able to compete with plasminogen for binding to endothelial cells and monocytes, a function that was mediated by apo(a) (5, 6). In the intervening years, a large body of data has been generated, principally through in vitro studies, that supports a procoagulant/antifibrinolytic function for apo(a), but precious little progress has been made in demonstrating that this function has pathophysiological relevance in humans. Moreover, there has been considerable disagreement about whether elevated plasma concentrations of Lp(a) are a risk factor for purely thrombotic disorders, such as venous thromboembolism, and whether these findings in any way inform our understanding of atherothrombotic events in the arterial tree. This review will delve into the mechanistic basis for the potential prothrombotic effects of Lp(a), emerging data on the effects of Lp(a) on fibrin clot structure, the latest epidemiological and genetic studies in human patients, and possible pathways forward that may bring a potential prothrombotic role of Lp(a) into greater focus.
BIOCHEMISTRY OF apo(a): HOMOLOGY TO PLASMINOGEN
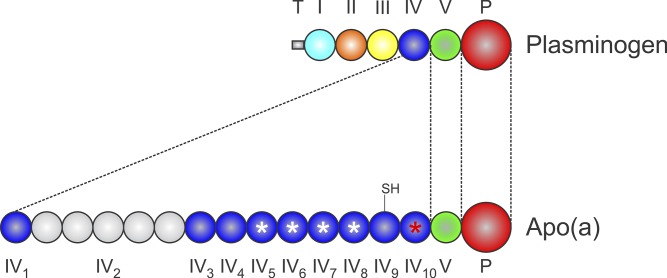
Plasminogen consists of an N-terminal tail domain, five different kringle domains, and a latent trypsin-like protease domain (Fig. 1) (7). Kringles are autonomously folding domains lacking any helical secondary structure and containing only a few short stretches of β-strand (8, 9). The overall kringle structure is defined as a tri-looped arrangement stabilized by the presence of three invariant disulfide bonds (8, 9). Kringles are present in several other proteases involved in coagulation and fibrinolysis, including prothrombin (two kringles), Factor XII (one kringle), tissue-type plasminogen activator (two kringles), and urokinase-type plasminogen activator (one kringle) (10). Several other proteins also contain kringles, most notably the nonprotease hepatocyte growth factor, which contains four (11). The apo(a) consists of 10 different types of kringle domains, differing in amino acid sequence, that are most homologous to plasminogen kringle IV (KIV), as well as a single plasminogen kringle V (KV)-like domain and a protease-like domain (Fig. 1) (4). Of the 10 KIV types in apo(a), 9 are present in single copy in all apo(a) isoforms (12), while KIV type 2 (KIV2) is encoded in a variable number of tandemly repeated copies by the apo(a) gene (LPA), giving rise to the existence of a series of differently-sized LPA alleles and, hence, apo(a) isoforms in the human population (13). Known alleles encode as few as 1 and as many as 34 KIV2 repeats, giving rise to apo(a) isoforms containing between 10 and 43 KIV-like domains, and polypeptide molecular masses between ∼200 and ∼800 kDa (14).
Fig. 1.
Structure of apo(a). The topologies of plasminogen and apo(a) are aligned to show regions of structural homology. The apo(a) lacks plasminogen kringles I–III and contains single copies of sequences similar to plasminogen KV and protease (P), although the protease domain of apo(a) is nonfunctional. The apo(a) contains 10 different types of kringle related to plasminogen KIV. The apo(a) KIV2 is present in differing numbers of copies in different apo(a) alleles. The apo(a) KIV9 contains a free cysteine (–SH) that mediates covalent coupling to apoB-100 in Lp(a). The apo(a) KIV5–KIV8 each contain a weak LBS (white asterisks) while KIV10 contains a strong LBS (red asterisk).
The biological role of kringles is considered to be in ligand interactions (8), often with lysine-containing substrates. Several of the kringles in plasminogen contain lysine binding sites (LBSs), defined structurally by a hydrophobic trough, lined by two or three key aromatic side chains, that binds the aliphatic backbone of the lysine side chain and that is flanked on either end by a cationic and anionic center (15, 16). LBSs can therefore bind to both internal lysines, as well as carboxyl-terminal lysines. Of the LBS in plasminogen, the LBS in kringle I has the highest affinity for lysine analogs, followed by KIV and KV (17). The LBSs in plasminogen have been shown to be important for both lysine-dependent interactions with substrates, such as fibrin and cell-surface receptors (18–20), as well as for intramolecular interactions that maintain the closed native conformation of plasminogen (21, 22).
Of the KIV types in apo(a), only KIV5, KIV6, KIV7, KIV8, and KIV10 have LBSs, with all the other KIV types containing one or more key amino acid substitutions that inactivate their LBSs (Fig. 1) (4, 23). However, the LBSs in KIV5–KIV8 have been demonstrated to be of a lower affinity than the one in KIV10, due to some conservative amino acid substitutions (24, 25). As such, the LBSs in apo(a) KIV10 and KIV5–KIV8 have been termed as “strong” and “weak” LBSs, respectively (Fig. 1), although all of these LBSs are of a lower affinity for lysine than the kringle I LBSs in plasminogen (26). Of the lysine-binding apo(a) kringles, only KIV10 is available for interaction with lysine-containing substrates. This is because the LBSs in KIV5–KIV8 are masked when bound to apoB-100 in the Lp(a) particle (27, 28), with KIV7–KIV8 having been explicitly shown to participate in noncovalent interactions with specific lysine residues on apoB-100 that precede covalent Lp(a) formation (29).
The protease-like domain in apo(a) is catalytically inactive, despite having an intact Ser-His-Asp catalytic triad (30). An Arg to Ser substitution at the location analogous to the site on plasminogen that is cleaved by plasminogen activators ensures that an activating cleavage of apo(a) cannot occur (4). In addition, several other amino acid substitutions relative to plasminogen, as well as a key nine-amino acid deletion in apo(a), have been proposed to render the protease-like domain in apo(a) inactive (30).
The gene encoding apo(a) has been proposed to have arisen from duplication of the gene encoding plasminogen comparatively late in primate evolution (Fig. 1) (31). Indeed, Lp(a) is only present in Old World monkeys, apes, and humans. The apo(a) from all these species generally shares the isoform size heterogeneity and kringle organization observed in humans, with the exception that some species lack KV (32–35). What is most interesting in examining the various sequences is that independently occurring mutations have ensured that the protease-like domain is inactive and, most relevant to the role of Lp(a) in thrombosis, KIV10 is not able to bind to lysine-containing substrates in the context of Lp(a). With respect to KIV10, some species, such as chimpanzees, have a key amino acid substitution that abolishes the LBSs (34). In other species, such as baboon, the LBS is intact (in most individuals), but the lack of KV in this species prevents binding of Lp(a) to lysine-Sepharose, perhaps by promoting a conformation of apo(a) within the Lp(a) particle that masks the KIV10 LBSs (36). Crucially, human Lp(a) can bind to lysine-Sepharose and other lysine-containing substrates, as it contains both KV and an intact LBS in KIV10, and is unique in this respect among the primates examined, except for orangutans (35). Therefore, it can be argued that human Lp(a) is uniquely pathogenic, although a correlation between Lp(a) levels and the extent of diet-induced atherosclerosis was observed in a study of rhesus and cynomolgus macaques (37). The true biological role of Lp(a) is unknown, although evolution has exerted pressure to maintain an inactive protease-like domain and, less so, a lack of lysine binding.
Because human Lp(a) lacks protease activity while retaining the ability to bind to lysine-containing substrates, it can readily be hypothesized that Lp(a) may interfere with the functions of plasminogen through molecular mimicry. A great many studies conducted in vitro have confirmed this concept, while offering mechanistic insights that may prove useful to test in vivo.
EVIDENCE FOR ANTI-FIBRINOLYTIC ROLES OF Lp(a): IN VITRO STUDIES
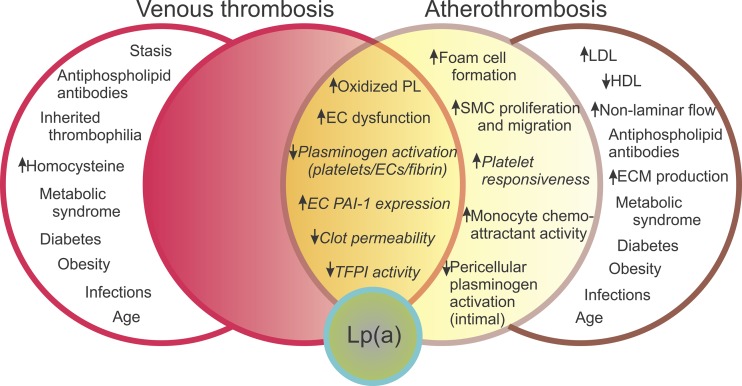
A large body of evidence has been accumulated concerning potential pathophysiological functions of Lp(a), both proatherosclerotic and prothrombotic (Fig. 2). It is notable, however, that none of these potential mechanisms has been explicitly proved to be occurring in human patients. Moreover, because atherosclerosis and subsequent thrombosis are mechanistically interlinked, it is not clear whether the direct procoagulant/antifibrinolytic effects of Lp(a) may be at play in increasing risk for atherothrombotic events (Fig. 2). In this section and the two subsequent sections, we summarize the evidence that Lp(a) is indeed procoagulant/antifibrinolytic.
Fig. 2.
Mechanistic basis of elevated Lp(a) as a risk factor for thrombosis as a complication of atherosclerosis. The Venn diagram depicts a series of factors that potentially contribute to venous thrombosis (left side) and arterial thrombosis in the setting of atherosclerosis (i.e., atherothrombosis; right side). Factors that are influenced by Lp(a) are contained in the filled circles; all the factors influenced by Lp(a) potentially contributing to both venous thrombosis and atherothrombosis are clustered in the center (orange zone), while those only influencing atherothrombosis are in the yellow zone. Factors influenced by Lp(a) that are directly prothrombotic/antifibrinolytic are italicized. Factors that contribute uniquely to venous thrombosis or atherosclerosis and are not influenced by Lp(a) are contained in the open circles. EC, endothelial cell; ECM, extracellular matrix; PL, phospholipids; SMC, smooth muscle cell. Adapted from reference (145).
Interference with functions of plasminogen
The lysine-binding function of plasminogen is crucial to its fibrinolytic role. Activation of plasminogen by tissue-type plasminogen activator (tPA) does not occur at a meaningful rate in the absence of a fibrin surface, whereas lysine-dependent interactions between plasminogen and tPA with fibrin result in the formation of a ternary complex that results in efficient production of plasmin (38). Plasminogen binding to fibrin converts the protein from a closed to an open conformation that makes it a better substrate for tPA (39). Moreover, partial degradation of fibrin by plasmin results in the formation of carboxyl-terminal lysine residues that mediate positive feedback in the fibrinolytic cascade by: i) promoting plasminogen binding (40); ii) promoting plasmin-mediated conversion of native Glu1-plasminogen to Lys77-plasminogen, which lacks the tail domain (see Biochemistry of apo(a): Homology to Plasminogen, above) and is a better substrate for tPA (41); and iii) binding to plasmin and thus protecting it from consumption by antiplasmin (42).
The first studies to examine the functional implications of the homology between apo(a) and plasminogen demonstrated the ability of Lp(a) and apo(a) to inhibit binding of plasminogen to cell surface receptors on monocytes and endothelial cells (5, 6). The presumed competition between apo(a)/Lp(a) and plasminogen for carboxyl-terminal lysine-containing cell surface receptors, such as α-enolase and annexin AII tetramer, was speculated to inhibit cell-surface (pericellular) plasminogen activation. Direct proof of an effect of apo(a)/Lp(a) on pericellular plasminogen activation was only very recently presented by our group (43); interestingly, we found that carboxyl-terminal lysines played essentially no role in the inhibition by apo(a). Inhibition of pericellular plasminogen activation by Lp(a) is probably not a large factor in thrombolysis in the context of atherothrombotic effects, as this would only generate plasmin on the periphery of the thrombus rather than on or in the thrombus. However, inhibition of pericellular plasminogen activation by apo(a) may contribute to the atherosclerotic process through persistence of mural thrombi or through effects in the vascular wall, such as extracellular matrix breakdown, cell migration, and angiogenesis (43). Embedded in this notion is an emerging theme that we will return to in this review: the difficulty of distinguishing, in an ontological sense, the proatherosclerotic and procoagulant/antifibrinolytic effects of Lp(a).
Studies performed in vitro indicate clearly that apo(a) and Lp(a) are capable of inhibiting tPA-mediated clot lysis and inhibiting tPA-mediated plasminogen activation (Fig. 3) (44–48). In addition, apo(a) is able to attenuate the positive feedback step of plasmin-mediated Glu- to Lys-plasminogen conversion in the context of fibrin (Fig. 3) (49). This last result is noteworthy in that it had been previously found that apo(a) was unable to inhibit fibrinolysis in a system of purified components in which the Glu- to Lys-plasminogen step was bypassed by the addition of only Lys-plasminogen (46). In addition, apo(a) was not able to influence the activity of plasmin itself in degrading fibrin (46). These findings are supported by studies of thrombolysis in rabbit jugular vein (50) and transgenic mice expressing apo(a) (51). In addition, induction of carotid artery thrombosis in cynomolgus monkeys showed a relationship between Lp(a) levels in the animals and the extent of cessation of flow (52).
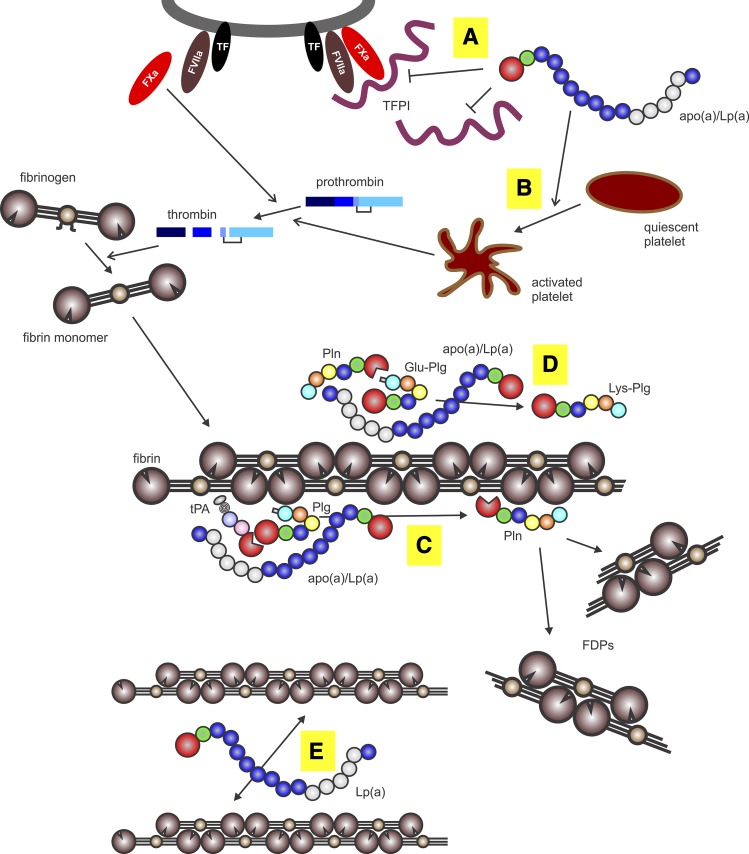
Fig. 3.
Procoagulant and antifibrinolytic mechanisms of apo(a). The apo(a) binds to either free or cell-bound TFPI (A), thereby promoting prothrombin activation by Factor Xa formed by the tissue factor (TF)-Factor VIIa complex. The apo(a) promotes platelet aggregation and release of granule contents through the thrombin receptor (B), promoting both platelet plug formation and enhanced prothrombin activation. Thrombin formed from prothrombin cleaves fibrinopeptides (A, B) from soluble fibrinogen to form fibrin monomers, which then polymerize to form insoluble fibrin. The apo(a) forms a ternary complex with fibrin, plasminogen (Plg), and tPA to prevent activation of plasminogen to form plasmin (Pln) (C). Plasmin proteolyzes fibrin to form soluble fibrin degradation products (FDPs). The apo(a) prevents plasmin-mediated conversion of Glu-plasminogen to Lys-plasminogen (D), the latter of which is a better substrate for tPA. Lp(a) alters fibrin structure to yield thinner and more dense fibers, potentially by binding to the αC domain of fibrin and, hence, preventing lateral association of fibrin fibrils (E).
Studies aimed at uncovering the mechanism underlying the effect of apo(a) on tPA-mediated plasminogen activation have been somewhat in conflict, with some reports suggesting that apo(a) directly competes with plasminogen for binding to fibrin (53, 54), while another described a mechanism whereby apo(a) forms a quaternary complex with plasminogen, tPA, and fibrin that has a much lower turnover number than the ternary complex lacking apo(a) (Fig. 3) (55).
Structural determinants on apo(a) for anti-fibrinolytic effects
The modular structure of apo(a) has given rise to many studies that have attempted to ascribe specific functions to particular domains in apo(a), largely through the use of deletion mutants and point mutants of apo(a). In particular, our own laboratory has amassed a large collection of recombinant apo(a) variants that allows us to systematically screen the various domains in apo(a) for functional roles. Through these studies, we have found that several domains are required for maximally-efficient inhibition of tPA-mediated plasminogen activation or of plasmin-mediated Glu- to Lys-plasminogen conversion (47, 49). The strong LBSs in KIV10, as well as the KV, are required for both inhibitory activities, and additional sequences within KIV5–KIV8 are also required to inhibit plasminogen activation. Although the exact role of each of these domains has not been uncovered, it is likely that apo(a), by virtue of its complex modular structure, makes a series of contacts with fibrin, plasminogen, and, possibly, tPA to effect its antifibrinolytic functions (55).
An additional area of interest has been to assess the role of apo(a) isoform size on its antifibrinolytic ability. There is a general inverse correlation between apo(a) isoform size and plasma Lp(a) concentrations (56). Several epidemiological studies have attempted to determine whether small apo(a) isoforms are more harmful in their own right, i.e., independent of their association with elevated plasma Lp(a) concentrations. While evidence both for and against this notion has been presented (57–59), epidemiological studies remain inherently ambiguous about the mechanism underlying any associations. A possible exception to this is the frequently cited example of the Bruneck study, which determined that small apo(a) isoform sizes predicted advanced atherogenesis (as assessed by ultrasonically determined carotid stenosis) even after adjustment for Lp(a) concentrations (57). However, only Lp(a) concentrations predicted early atherogenesis, and then only in the presence of elevated LDL-cholesterol levels (57). It was concluded that the effects of Lp(a) on advanced atherogenesis reflected plaque thrombosis [and thus the antifbrinolytic role of Lp(a)], while the effects on early atherogenesis reflected purely atherogenic effects, possibly exacerbating the effects of LDL. However, a more recent 15 year follow-up of the Bruneck subjects revealed that Lp(a) isoforms did not add to the predictive power of Lp(a) concentrations for CVD events (60). It was speculated that the discordance between the two studies may be explained by increased statin use among subjects with smaller Lp(a) isoforms.
Meanwhile, some biochemical studies have sought to directly assess whether smaller apo(a)/Lp(a) isoforms have greater pathogenic effects. In fact, the majority of such studies have focused on the antifibrinolytic effects of Lp(a). It has been reported that Lp(a) containing smaller apo(a) isoforms has a higher affinity for fibrin (53, 54, 61, 62), is more effective in inhibiting plasminogen binding to fibrin, and inhibits plasminogen activation on fibrin to a greater extent (53, 63). Interestingly, however, the effect of apo(a) isoform size on affinity for fibrin was only seen in the context of Lp(a) particles, as there was no difference in fibrin affinity seen in a panel of recombinant apo(a) species varying in KIV2 repeat number, but not complexed to apoB-100 (54). These findings are in agreement with other functional studies in which isoform size did not affect the ability of apo(a) alone to inhibit plasmin-mediated conversion of Glu-plasminogen to Lys-plasminogen (49). Likewise, binding of Lp(a) to mononuclear cells (THP-1 cell line) was influenced by apo(a) isoform size, with a smaller isoform displaying a higher affinity and a greater ability to inhibit plasminogen binding (64). On the other hand, recombinant apo(a) variants of two different sizes showed no differences in their ability to inhibit plasminogen activation on the surface of THP-1 monocytes, THP-1 macrophages, or human umbilical vein endothelial cells (43). In one study, however, it was reported that larger apo(a) isoforms, whether in the context of Lp(a) or not, resulted in a greater inhibition of plasmin generation in an in vitro fibrinolysis assay (48). It must be noted that many of these studies used a limited number of recombinant apo(a) isoforms or Lp(a) isoforms of various sizes in human subjects. Thus, it cannot always be concluded with certainty that the effect observed (or not observed) is not attributable to an insufficient range of apo(a) variants or subject-dependent variations in the properties of Lp(a).
Taken in sum, while it is clear that important mechanistic questions as well as the role of Lp(a) isoform size remain unresolved, the existing body of literature consistently demonstrates that Lp(a)/apo(a) can inhibit fibrinolysis as well as plasminogen activation in the context of fibrin clots or on the vascular cell surface.
EVIDENCE FOR PROTHROMBOTIC EFFECTS OF Lp(a): IN VITRO STUDIES
Several studies have investigated the possibility that Lp(a) or apo(a) might influence platelet aggregation. These studies were motivated by observations that some lipoproteins induce platelet aggregation and that apo(a) mediates the binding of Lp(a) to plasminogen receptors on the platelet surface (65, 66). The available studies, however, reflect contradictory findings. A study from our group found that apo(a) and Lp(a) enhanced platelet aggregation and granule release mediated by the thrombin receptor activating peptide SFLLRN, but not ADP (Fig. 3) (67). Another study found that apo(a) and Lp(a) evoke platelet aggregation in response to doses of arachidonic acid that would normally be subaggregant (68). These effects were the apparent result of specific binding of apo(a) to lysine-containing receptors on the platelet (68). On the other hand, several studies have shown that Lp(a) or apo(a) actually decreases platelet activation induced by collagen, ADP, or platelet-activating factor (69–72). One provocative conclusion from these studies might be that an anti-aggregation effect of Lp(a) may exist to counterbalance its antifibrinolytic effects (71). Overall, however, it appears that the effect of Lp(a) on platelet responsiveness depends on the balance of activating factors present.
A different prothrombotic effect of Lp(a) relates to its ability to bind to tissue factor pathway inhibitor (TFPI) (Fig. 3) (73). TFPI squelches the extrinsic pathway of coagulation through its ability to bind to Factor Xa and then the tissue factor/Factor VIIa complex (74). By binding of the apo(a) moiety to TFPI through lysine residues in the carboxyl-terminal portion of TFPI, Lp(a) is able to inhibit the activity of both free and cell-bound TFPI, thus reducing the anti-thrombotic effects of this inhibitor (Fig. 3) (73). Two studies found that plasma TFPI concentrations were higher in patients with elevated Lp(a) (75, 76), potentially reflecting the effects of their mutual interaction on clearance rates from plasma; one of these studies also found that TFPI activity was no different between subjects with high and low Lp(a), consistent with an inhibitory consequence of their interaction (76). Recent genome-wide association study data indicate a significant association between plasma Lp(a) levels and a small region of chromosome 2 that harbors the gene encoding TFPI (77). However, no association between 12 SNPs in the gene encoding TFPI and Lp(a) levels were detected, casting doubt on whether the chromosome 2 association is attributable to TFPI (77).
EFFECT OF Lp(a) ON FIBRIN CLOT PROPERTIES
Fibrin is the structural framework for fibrin clots in both normal physiology as well as thrombi formed as a result of vascular disease processes (78). Fibrin fibers grow both longitudinally and laterally, and branch to form a three-dimensional network (79). Initially, fibrin monomers assemble into half-staggered double-stranded protofibrils; in a second step, these protofibrils associate laterally to form fibrin fiber networks (80). A variety of factors have been shown to alter fibrin clot structure, including negatively charged substances (81). Variations in clot structure have been correlated with both atherothrombotic diseases, such as CHD, as well as purely thrombotic disorders, including venous thromboembolism (81, 82).
Clot structure is important in determining both the stability and fibrinolytic potential of the fibrin clot, which can, in turn, have implications for abnormal thrombolysis (83, 84). The most widely used measures of fibrin clot structure are fibrin fiber diameter and pore sizes within the fibrin network. Dense clots have been shown to be correlated with smaller pores and fibrin fibers of reduced diameter, and with increased fibrin stiffness (85). Because thinner fibers dissolve at a faster rate than larger diameter fibers, this suggests that the rate of fibrin clot lysis is largely a function of pore size. As such, smaller pores correlate with more dense clots and increased clot lysis times, which, in turn, has been associated with increased CHD risk.
Elevated Lp(a) levels have been associated with an altered fibrin clot structure that is, in turn, accompanied by reduced fibrin clot permeability and impaired fibrinolysis (86, 87). This has been interpreted to suggest a causal role for Lp(a) in altering clot structure in coronary artery disease (CAD) patients compared with healthy controls, thereby contributing to CAD risk. This may reflect the ability of the apo(a) component of Lp(a) to bind with high affinity to the fibrin(ogen) αC region (88). This interaction may disrupt the lateral association of fibrin protofibrils, thereby resulting in the formation of thinner fibrin fibers (Fig. 3) (83). Additionally, the negative charge of apo(a) (89) may also underlie the ability of Lp(a) to alter fibrin clot structure, as has been demonstrated for other negatively charged substances (90).
The LPA gene contains a SNP (rs3798220) that results in an Ile to Met substitution at amino acid 4399 within the protease-like domain of apo(a) (91). The allele encoding Met has been associated with elevated plasma Lp(a) levels, small apo(a) isoform sizes, and increased risk for CHD (91). Interestingly, in the Women’s Heart Study, individuals heterozygous for the Ile4399Met variant exhibited elevated Lp(a) levels and an increased risk for CAD, and benefitted more from aspirin therapy than noncarrier subjects (92). This has been interpreted to suggest a possible prothrombotic role for the Ile4399Met polymorphism. Recently, it has been reported that Caucasians heterozygous for the Ile4399Met variant exhibit elevated Lp(a) levels, increased clot density, and increased clot lysis times, while non-Causasian carriers showed increased clot permeability and shorter lysis times, with no significant increase in Lp(a) levels (93).
Clearly, mechanistic studies are required to determine the role of apo(a)/Lp(a), as well as the Ile4399Met variant, in altering fibrin clot architecture and the molecular basis for resultant alterations in clot lysis times. Demonstration that apo(a) overexpression in plasminogen knockout mice resulted in a higher incidence of thrombosis in these mice suggests a plasminogen-independent contribution of Lp(a) to prothrombotic events (94). As such, the ability of Lp(a) to alter fibrin clot architecture with attendant effects on clot lysis times merits further study in this context.
INDIRECT EFFECTS OF Lp(a) ON THROMBOSIS
As mentioned above, Lp(a) has many effects on cells and molecules of the vasculature (Fig. 2). Some of these effects could be considered directly prothrombotic (italicized items in Fig. 2), while others would be indirect. For example, we and others have shown that apo(a)/Lp(a) can promote endothelial cell dysfunction (95–100). This may serve to suppress the native anticoagulant function of the endothelium, while provoking a procoagulant phenotype. An important emerging functional determinant of Lp(a) pathogenicity is the presence of oxidized phospholipids (oxPLs) (101), which, in part, are phosphorylcholine-containing adducts covalently linked to, as-yet poorly defined, sequences in apo(a) (102, 103). It is not known whether the oxPLs on apo(a) influence its prothrombotic effects, a question complicated by the fact that variants of apo(a) lacking the key strong LBS in KIV10 specifically lack oxPL addition (35). Interestingly, plasminogen contains oxPLs (104, 105), and the presence of this adduct actually increases the fibrinolytic potency of plasminogen (105). However, there was no correlation between the extents of modification of plasminogen and Lp(a) by oxPLs.
EVIDENCE FOR ANTI-FIBRINOLYTIC ROLES OF Lp(a): IN VIVO STUDIES
Arterial thrombosis
While acute coronary syndromes almost all feature thrombotic complications of atherosclerotic disease, it remains a mystery as to whether Lp(a) contributes to the former or latter processes, or both (Fig. 2). Addressing this question in a direct way is a nontrivial undertaking, not least because a comparatively large number of subjects need to be included in a study to achieve sufficient statistical power with respect to Lp(a). Moreover, some of the most dangerous lesions, vulnerable plaques, are not readily detectable by many imaging methods, and it cannot be excluded at this time that Lp(a) does not have a strong association with the development of vulnerable plaques. In theory, though, it could be determined whether Lp(a) contributes directly to atherosclerosis, such as by detecting an association between Lp(a) concentrations and angiographically or ultrasonically detectable CAD. In fact, a relatively small number of studies have attempted to do such a study, albeit in the more easily monitored carotid artery. For example, Spence and coworkers found that Lp(a) was a significant predictor of carotid stenosis (presumably reflecting atherothrombotic events), but not total plaque area (presumably reflecting the underlying atherosclerotic process) (106). Gardener et al. (107) found that Lp(a) was a significant predictor of the number of lesions within the carotid, and just barely failed to be a significant predictor of the presence of plaque. LDL-cholesterol, however, was a significant predictor of both of these parameters (107). Clearly, additional studies that specifically capture the effect of Lp(a) on lesion development without the complication of atherothrombotic stenosis need to be performed.
Other studies have examined whether Lp(a) levels influence the spontaneous resolution of coronary thrombi (i.e., not aided by administration of thrombolytic therapy) through measurement of the patency of coronary arteries by angiography in survivors of MI. In an interesting and underappreciated study, Moliterno et al. (108) found that elevated Lp(a) was a significant predictor of resistance to endogenous thrombolysis. Notably, typical predictors of fibrinolytic potential were not significant predictors, including levels of plasminogen activator inhibitor type 1 (PAI-1) (108). Similar findings were reported by two other studies in the ensuing years (109, 110). However, one very small study (32 subjects) found that Lp(a) levels were higher in MI patients exhibiting intermittent occlusion of the infarct-related artery than patients with persistent occlusion, a result seemingly at odds with an anti-fibrinolytic role for Lp(a) (111). The rise of interventional cardiology likely means that larger prospective studies of the effect of Lp(a) on the evolution of coronary artery thrombosis will no longer be feasible, yet the available findings are a strong indicator that Lp(a) has an antifibrinolytic effect in arterial thrombosis in vivo.
Thrombolytic therapy
A potential opportunity to assess the specific antifibrinolytic effects of Lp(a) that could also represent a target for interfering with the effects of Lp(a) is in the setting of thrombolytic therapy. In the early 1990s, this question was addressed in studies of different thrombolytic agents in acute MI, most frequently derivatives of tPA, such as alteplase. Surprisingly, these studies uniformly reported that Lp(a) concentrations did not predict the success of thrombolysis in this setting (112–116). Given the strong in vitro findings that apo(a) can interfere with tPA-mediated fibrinolysis and plasminogen activation (see above), it is fair to ask why these clinical results may have been observed. It is possible that at the very high local concentrations of thrombolytic agent achieved, any effect of apo(a) might be overwhelmed. It is also notable that the numbers of patients in the trials were quite small (rarely exceeding 50 per arm). Therefore, there would not have been many patients with elevated Lp(a), or with the very high concentrations of Lp(a) that may be required to overcome the effects of a large concentration of thrombolytic agent. One study using anistreplase found that although Lp(a) did not affect the outcome of thrombolysis, higher levels of Lp(a) were associated with smaller decreases in plasminogen levels after therapy; this is consistent with Lp(a) promoting an antifibrinolytic state under these conditions (116).
While most studies used derivatives of tPA, two studies examined the impact of Lp(a) on thrombolysis by streptokinase (115, 117). This is interesting because tPA-mediated plasminogen activation, one of the reactions that apo(a) inhibits, would not be a significant contributor. On the other hand, plasmin-mediated conversion of Glu-plasminogen to Lys-plasminogen, which is also inhibited by apo(a) (49), would still be occurring and contributing to positive feedback. However, Lp(a) levels did not affect the outcome of thrombolytic therapy in this setting either (115, 117); although, in one study, a correlation between Lp(a) concentrations and the atherosclerotic burden was noted (117).
One of the complications of thrombolytic therapy is bleeding elsewhere in the vasculature, most notably and devastatingly in the brain. To our knowledge, however, the impact of Lp(a) concentrations on the rate of such complications was never examined.
A more recently-emerging indication for thrombolytic therapy is for the treatment of acute ischemic stroke. One study found a trend toward higher Lp(a) levels in patients who did not achieve recanalization 1 h after initiation of thrombolysis, although this did not achieve significance; notably, levels of another fibrinolysis inhibitor, PAI-1, were significantly associated with recanalization (118). This result is interesting, as a study regarding spontaneous recanalization in coronary arteries, cited above (108), found that Lp(a) levels, but not PAI-1 levels, were significantly associated with decreased recanalization. It has also been reported that Lp(a) levels are not associated with the rate of occurrence of the most feared complication of thrombolytic therapy for stroke, symptomatic intracranial hemorrhage (119). It is fair to conclude, based on the small number of studies reported to date and the relatively small numbers of patients involved in the reported studies, that further work to definitively rule out a role for Lp(a) in influencing the outcome of thrombolytic therapy is warranted.
Venous thrombosis and thromboembolism
Thrombosis in the venous compartment, observed clinically as deep vein thrombosis or venous thromboembolism, has a very different etiology to thrombosis in large arteries. Venous thrombosis normally is not precipitated by atherosclerotic disease, as the veins are generally resistant to atherosclerosis. Compared with arterial thrombi, venous thrombi are: i) more fibrin-rich and platelet-poor; ii) generally the result of stasis or as a complication of surgery, vascular access complications, or trauma; and iii) better predicted by risk factors for thrombophilia, such as Factor V Leiden or antithrombin deficiency (120). Against this backdrop, it might be expected that elevated plasma concentrations of Lp(a) would be a risk factor for various types of venous thrombosis. Historically, the results of such studies have been mixed, with evidence both for (121–123) and against (124–128) a role for elevated Lp(a) as a risk factor for different kinds of venous thrombosis in adult populations.
More recently, however, genetic approaches have provided strong evidence against a role for Lp(a) in venous thrombosis (129–131). Because certain SNPs, such as rs3798220 (Ile4399→Met) and rs10455872 (intronic A/G polymorphism), are strongly associated with plasma Lp(a) concentrations, they can serve as surrogate markers for Lp(a) concentrations while also facilitating measurement in very large prospective cohorts where measurement of Lp(a) concentrations would be either cost-prohibitive or otherwise impossible. Moreover, being genetic markers, use of these SNPs reflects lifelong elevation in plasma Lp(a) while eliminating the artifact of “reverse causality” where the condition under study, in fact, affects Lp(a) levels, rather than the converse. Therefore, positive results from “Mendelian randomization” and similar types of studies using these informative SNPs have been considered to provide evidence that Lp(a) is a causal risk factor for CHD and other atherosclerotic and atherothrombotic disorders (132). When applied to assessment of associations between genetically elevated Lp(a) levels and different forms of venous thrombosis, however, the results have been uniformly negative (129–131). These findings quite definitely rule out a role for elevated Lp(a) in the etiology of venous thrombosis.
In the wake of these findings, it is natural to ask why, despite strong in vitro evidence, Lp(a) does not influence this set of “pure” thrombotic disorders? A strong possibility is that venous thrombosis reflects impaired anti-coagulation/accelerated coagulation rather than impaired fibrinolysis, and likewise, that Lp(a) does not promote coagulation in vivo as much as it impairs fibrinolysis. In the genetic studies that were negative for venous thrombosis, genetically elevated Lp(a) concentrations were a strong risk factor for atherosclerotic burden in a variety of locations in the arterial tree (129, 130). It has been concluded, therefore, that elevated Lp(a) promotes atherosclerotic events through promotion of atherosclerosis, not through promotion of thrombosis/inhibition of fibrinolysis. However, the notable differences in the nature of venous and arterial thrombi suggest that this conclusion be tempered.
Children
While thrombosis of any kind is rare in children, these subjects represent a special case from the point of view of understanding risk. Genetic risk factors would play a greater role in children, as other types of risk factors related to age and lifestyle (smoking, diabetes, etc.) would not yet be at play. This may explain why studies of the role of Lp(a) in thrombosis in children have consistently shown positive associations (133–135). The forms of thrombosis studied included both venous (venous thromboembolism) and arterial (incident and recurrent ischemic stroke, where the thromboembolism would not have an atherosclerotic origin). A study that did not find a significant association between elevated Lp(a) and recurrent venous thrombosis in children did, however, report that a combination of elevated Lp(a) and at least one other thrombophilic risk factor was a significant predictor of risk (136). This finding may reflect the fact that because Lp(a) affects fibrinolysis more than coagulation, its effects would only be observed when some other factor precipitated thrombosis. In other words, elevated Lp(a) may affect the penetrance of procoagulant thrombophilic risk factors. One recent study reported that elevated Lp(a) was associated with risk for recurrent, but not incident, stroke (137). Another study found no relationship between elevated Lp(a) and thromboembolism (of either venous or arterial origin) in Thai children (138). In general, the majority of the epidemiological studies on Lp(a) have focused on Caucasian populations, with some also focusing on persons of sub-Saharan African descent. As differences in the genetic architecture of LPA between different ethnic groups (such as prevalence of differently sized isoforms) are incompletely understood (139), it will be important to consider this variable in the design of future studies.
PERSPECTIVES FOR THE FUTURE
While a role for Lp(a) in venous thrombosis in adults can largely be ruled out, there remains considerable evidence that Lp(a) may contribute to atherothrombotic disease, both through promotion of atherosclerosis and promotion of thrombosis/inhibition of fibrinolysis. It cannot have escaped the attention of the reader that many of the basic science and clinical/epidemiological studies cited in this review are more than 20 years old. This reflects, in part, a long fallow period in Lp(a) research prior to the publication of large genetic studies that pointed to a causal role for Lp(a) in the development of CAD (132). The time is now ripe to revisit the prothrombotic/antifibrinolytic potential of Lp(a) with a view toward understanding its mechanistic contribution to atherothrombotic events. Specific foci for future research are as follows.
The development of better animal models for Lp(a) to better address crucial biochemical and pathophysiological issues
Mice expressing high levels of bona fide human Lp(a) were reported; these animals express both human apo(a) (a truncated 8-kringle version of the protein) and human apoB-100 from transgenes (140). The effect of the expression of Lp(a) on atherosclerosis in these animals has not yet been reported. A recent study of similar mice containing a 17-kringle apo(a) transgene found that expression of human Lp(a) resulted in an increase in aortic root atherosclerosis in the setting of uremia (141). Crucially, mouse models of the thrombotic complications of atherosclerosis have recently been developed, where plaque rupture in carotid arteries of fat-fed Apoe−/− mice is provoked by ultrasound and thrombosis monitored by intravital fluorescence microscopy (142). These studies have illuminated roles for both platelets and the intrinsic pathway of coagulation in atherothrombosis (142–144).
More effective identification of individuals at greater risk of events due to elevated Lp(a)
Further study of the relationship of elevated Lp(a) to the development of atherosclerosis per se (including “vulnerable” plaques) is required (106). This may pave the way for the development of a prognostic “signature” specific for Lp(a), in conjunction with certain biomarkers (such as elevated coagulation factors). It may also allow for assessment of the contribution of elevated Lp(a) to events after adjustment for its effect on atherosclerosis.
Development of therapeutic interventions targeted at Lp(a) that might mitigate the contribution of this lipoprotein to events
This will require a better elucidation of the mechanisms by which Lp(a) promotes coagulation and inhibits fibrinolysis, in order to illuminate a viable therapeutic target [for example, fibrin binding by Lp(a)]. Clearly, there remains much work to be done in clarifying the interactions between Lp(a) and coagulation and fibrinolysis while, ultimately, providing proof that promotion of thrombosis is an authentic mechanism through which Lp(a) causes atherothrombotic events.
Footnotes
Abbreviations:
- CAD
- coronary artery disease
- CHD
- coronary heart disease
- KIV
- kringle IV
- KIV2–10
- KIV types 2–10
- KV
- kringle V
- LBS
- lysine binding site
- Lp(a)
- lipoprotein (a)
- MI
- myocardial infarction
- oxPL
- oxidized phospholipids
- PAI-1
- plasminogen activator inhibitor type 1
- TFPI
- tissue factor pathway inhibitor
- tPA
- tissue-type plasminogen activator
REFERENCES
- 1.Berg K., Dahlén G., and Frick M. H.. 1974. Lp(a) lipoprotein and pre-beta1-lipoprotein in patients with coronary heart disease. Clin. Genet. 6: 230–235. [DOI] [PubMed] [Google Scholar]
- 2.Dahlén G., Berg K., Gillnäs T., and Ericson C.. 1975. Lp(a) lipoprotein/pre-beta1-lipoprotein in Swedish middle-aged males and in patients with coronary heart disease. Clin. Genet. 7: 334–341. [DOI] [PubMed] [Google Scholar]
- 3.Eaton D. L., Fless G. M., Kohr W. J., McLean J. W., Xu Q. T., Miller C. G., Lawn R. M., and Scanu A. M.. 1987. Partial amino acid sequence of apolipoprotein(a) shows that it is homologous to plasminogen. Proc. Natl. Acad. Sci. USA. 84: 3224–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLean J. W., Tomlinson J. E., Kuang W. J., Eaton D. L., Chen E. Y., Fless G. M., Scanu A. M., and Lawn R. M.. 1987. cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature. 330: 132–137. [DOI] [PubMed] [Google Scholar]
- 5.Miles L. A., Fless G. M., Levin E. G., Scanu A. M., and Plow E. F.. 1989. A potential basis for the thrombotic risks associated with lipoprotein(a). Nature. 339: 301–303. [DOI] [PubMed] [Google Scholar]
- 6.Hajjar K. A., Gavish D., Breslow J. L., and Nachman R. L.. 1989. Lipoprotein(a) modulation of endothelial cell surface fibrinolysis and its potential role in atherosclerosis. Nature. 339: 303–305. [DOI] [PubMed] [Google Scholar]
- 7.Forsgren M., Raden B., Israelsson M., Larsson K., and Heden L. O.. 1987. Molecular cloning and characterization of a full-length cDNA clone for human plasminogen. FEBS Lett. 213: 254–260. [DOI] [PubMed] [Google Scholar]
- 8.Patthy L., Trexler M., Vali Z., Banyai L., and Varadi A.. 1984. Kringles: modules specialized for protein binding. Homology of the gelatin-binding region of fibronectin with the kringle structures of proteases. FEBS Lett. 171: 131–136. [DOI] [PubMed] [Google Scholar]
- 9.Tulinsky A. 1991. The structures of domains of blood proteins. Thromb. Haemost. 66: 16–31. [PubMed] [Google Scholar]
- 10.Patthy L. 1985. Evolution of the proteases of blood coagulation and fibrinolysis by assembly from modules. Cell. 41: 657–663. [DOI] [PubMed] [Google Scholar]
- 11.Ichinose A. 1992. Multiple members of the plasminogen-apolipoprotein(a) gene family associated with thrombosis. Biochemistry. 31: 3113–3118. [DOI] [PubMed] [Google Scholar]
- 12.van der Hoek Y. Y., Wittekoek M. E., Beisiegel U., Kastelein J. J., and Koschinsky M. L.. 1993. The apolipoprotein(a) kringle IV repeats which differ from the major repeat kringle are present in variably-sized isoforms. Hum. Mol. Genet. 2: 361–366. [DOI] [PubMed] [Google Scholar]
- 13.Lackner C., Cohen J. C., and Hobbs H. H.. 1993. Molecular definition of the extreme size polymorphism in apolipoprotein(a). Hum. Mol. Genet. 2: 933–940. [DOI] [PubMed] [Google Scholar]
- 14.Marcovina S. M., Albers J. J., Wijsman E., Zhang Z., Chapman N. H., and Kennedy H.. 1996. Differences in Lp[a] concentrations and apo[a] polymorphs between black and white Americans. J. Lipid Res. 37: 2569–2585. [PubMed] [Google Scholar]
- 15.Hoover G. J., Menhart N., Martin A., Warder S., and Castellino F. J.. 1993. Amino acids of the recombinant kringle 1 domain of human plasminogen that stabilize its interaction with omega-amino acids. Biochemistry. 32: 10936–10943. [DOI] [PubMed] [Google Scholar]
- 16.McCance S. G., Menhart N., and Castellino F. J.. 1994. Amino acid residues of the kringle-4 and kringle-5 domains of human plasminogen that stabilize their interactions with omega-amino acid ligands. J. Biol. Chem. 269: 32405–32410. [PubMed] [Google Scholar]
- 17.Castellino F. J., and McCance S. G.. 1997. The kringle domains of human plasminogen. Ciba Found. Symp. 212: 46–60; discussion 60–65. [DOI] [PubMed] [Google Scholar]
- 18.Váli Z., and Patthy L.. 1984. The fibrin-binding site of human plasminogen. Arginines 32 and 34 are essential for fibrin affinity of the kringle 1 domain. J. Biol. Chem. 259: 13690–13694. [PubMed] [Google Scholar]
- 19.Wu T. P., Padmanabhan K., Tulinsky A., and Mulichak A. M.. 1991. The refined structure of the epsilon-aminocaproic acid complex of human plasminogen kringle 4. Biochemistry. 30: 10589–10594. [DOI] [PubMed] [Google Scholar]
- 20.Fleury V., and Angles-Cano E.. 1991. Characterization of the binding of plasminogen to fibrin surfaces: the role of carboxy-terminal lysines. Biochemistry. 30: 7630–7638. [DOI] [PubMed] [Google Scholar]
- 21.Violand B. N., Byrne R., and Castellino F. J.. 1978. The effect of alpha-,omega-amino acids on human plasminogen structure and activation. J. Biol. Chem. 253: 5395–5401. [PubMed] [Google Scholar]
- 22.Cockell C. S., Marshall J. M., Dawson K. M., Cederholm-Williams S. A., and Ponting C. P.. 1998. Evidence that the conformation of unliganded human plasminogen is maintained via an intramolecular interaction between the lysine-binding site of kringle 5 and the N-terminal peptide. Biochem. J. 333: 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guevara J. Jr., Jan A. Y., Knapp R., Tulinsky A., and Morrisett J. D.. 1993. Comparison of ligand-binding sites of modeled apo[a] kringle-like sequences in human lipoprotein. [a] Arterioscler. Thromb. 13: 758–770. [DOI] [PubMed] [Google Scholar]
- 24.Ye Q., Rahman M. N., Koschinsky M. L., and Jia Z.. 2001. High-resolution crystal structure of apolipoprotein(a) kringle IV type 7: insights into ligand binding. Protein Sci. 10: 1124–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahman M. N., Becker L., Petrounevitch V., Hill B. C., Jia Z., and Koschinsky M. L.. 2002. Comparative analyses of the lysine binding site properties of apolipoprotein(a) kringle IV types 7 and 10. Biochemistry. 41: 1149–1155. [DOI] [PubMed] [Google Scholar]
- 26.Marti D. N., Hu C. K., An S. S., von Haller P., Schaller J., and Llinas M.. 1997. Ligand preferences of kringle 2 and homologous domains of human plasminogen: canvassing weak, intermediate, and high-affinity binding sites by 1H-NMR. Biochemistry. 36: 11591–11604. [DOI] [PubMed] [Google Scholar]
- 27.Ernst A., Helmhold M., Brunner C., Petho-Schramm A., Armstrong V. W., and Muller H. J.. 1995. Identification of two functionally distinct lysine-binding sites in kringle 37 and in kringles 32-36 of human apolipoprotein(a). J. Biol. Chem. 270: 6227–6234. [DOI] [PubMed] [Google Scholar]
- 28.Gabel B. R., May L. F., Marcovina S. M., and Koschinsky M. L.. 1996. Lipoprotein(a) assembly. Quantitative assessment of the role of apo(a) kringle IV types 2-10 in particle formation. Arterioscler. Thromb. Vasc. Biol. 16: 1559–1567. [DOI] [PubMed] [Google Scholar]
- 29.Becker L., Cook P. M., Wright T. G., and Koschinsky M. L.. 2004. Quantitative evaluation of the contribution of weak lysine-binding sites present within apolipoprotein(a) kringle IV types 6–8 to lipoprotein(a) assembly. J. Biol. Chem. 279: 2679–2688. [DOI] [PubMed] [Google Scholar]
- 30.Gabel B. R., and Koschinsky M. I.. 1995. Analysis of the proteolytic activity of a recombinant form of apolipoprotein(a). Biochemistry. 34: 15777–15784. [DOI] [PubMed] [Google Scholar]
- 31.Lawn R. M., Boonmark N. W., Schwartz K., Lindahl G. E., Wade D. P., Byrne C. D., Fong K. J., Meer K., and Patthy L.. 1995. The recurring evolution of lipoprotein(a). Insights from cloning of hedgehog apolipoprotein(a). J. Biol. Chem. 270: 24004–24009. [DOI] [PubMed] [Google Scholar]
- 32.Tomlinson J. E., McLean J. W., and Lawn R. M.. 1989. Rhesus monkey apolipoprotein(a). Sequence, evolution, and sites of synthesis. J. Biol. Chem. 264: 5957–5965. [PubMed] [Google Scholar]
- 33.Hixson J. E., Britten M. L., Manis G. S., and Rainwater D. L.. 1989. Apolipoprotein(a) (Apo(a)) glycoprotein isoforms result from size differences in Apo(a) mRNA in baboons. J. Biol. Chem. 264: 6013–6016. [PubMed] [Google Scholar]
- 34.Doucet C., Huby T., Chapman J., and Thillet J.. 1994. Lipoprotein[a] in the chimpanezee: relationship of apo[a] phenotype to elevated plasma Lp[a] levels. J. Lipid Res. 35: 263–270. [PubMed] [Google Scholar]
- 35.Leibundgut G., Scipione C., Yin H., Schneider M., Boffa M. B., Green S., Yang X., Dennis E., Witztum J. L., Koschinsky M. L., et al. 2013. Determinants of binding of oxidized phospholipids on apolipoprotein (a) and lipoprotein (a). J. Lipid Res. 54: 2815–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belczewski A. R., Ho J., Taylor F. B. Jr., Boffa M. B., Jia Z., and Koschinsky M. L.. 2005. Baboon lipoprotein(a) binds very weakly to lysine-agarose and fibrin despite the presence of a strong lysine-binding site in apolipoprotein(a) kringle IV type 10. Biochemistry. 44: 555–564. [DOI] [PubMed] [Google Scholar]
- 37.Nachman R. L., Gavish D., Azrolan N., and Clarkson T. B.. 1991. Lipoprotein(a) in diet-induced atherosclerosis in nonhuman primates. Arterioscler. Thromb. 11: 32–38. [DOI] [PubMed] [Google Scholar]
- 38.Hoylaerts M., Rijken D. C., Lijnen H. R., and Collen D.. 1982. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J. Biol. Chem. 257: 2912–2919. [PubMed] [Google Scholar]
- 39.Urano T., Sator de Serrano V., Gaffney P. J., and Castellino F. J.. 1988. Effectors of the activation of human [Glu1]plasminogen by human tissue plasminogen activator. Biochemistry. 27: 6522–6528. [DOI] [PubMed] [Google Scholar]
- 40.Suenson E., and Petersen L. C.. 1986. Fibrin and plasminogen structures essential to stimulation of plasmin formation by tissue-type plasminogen activator. Biochim. Biophys. Acta. 870: 510–519. [DOI] [PubMed] [Google Scholar]
- 41.Suenson E., and Thorsen S.. 1988. The course and prerequisites of Lys-plasminogen formation during fibrinolysis. Biochemistry. 27: 2435–2443. [DOI] [PubMed] [Google Scholar]
- 42.Wiman B., and Collen D.. 1978. Molecular mechanism of physiological fibrinolysis. Nature. 272: 549–550. [DOI] [PubMed] [Google Scholar]
- 43.Romagnuolo R., Marcovina S. M., Boffa M. B., and Koschinsky M. L.. 2014. Inhibition of plasminogen activation by apo(a): role of carboxyl-terminal lysines and identification of inhibitory domains in apo(a). J. Lipid Res. 55: 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loscalzo J., Weinfeld M., Fless G. M., and Scanu A. M.. 1990. Lipoprotein(a), fibrin binding, and plasminogen activation. Arteriosclerosis. 10: 240–245. [DOI] [PubMed] [Google Scholar]
- 45.Edelberg J. M., Gonzalez-Gronow M., and Pizzo S. V.. 1990. Lipoprotein(a) inhibition of plasminogen activation by tissue-type plasminogen activator. Thromb. Res. 57: 155–162. [DOI] [PubMed] [Google Scholar]
- 46.Sangrar W., Bajzar L., Nesheim M. E., and Koschinsky M. L.. 1995. Antifibrinolytic effect of recombinant apolipoprotein(a) in vitro is primarily due to attenuation of tPA-mediated Glu-plasminogen activation. Biochemistry. 34: 5151–5157. [DOI] [PubMed] [Google Scholar]
- 47.Hancock M. A., Boffa M. B., Marcovina S. M., Nesheim M. E., and Koschinsky M. L.. 2003. Inhibition of plasminogen activation by lipoprotein(a): critical domains in apolipoprotein(a) and mechanism of inhibition on fibrin and degraded fibrin surfaces. J. Biol. Chem. 278: 23260–23269. [DOI] [PubMed] [Google Scholar]
- 48.Knapp J. P., and Herrmann W.. 2004. In vitro inhibition of fibrinolysis by apolipoprotein(a) and lipoprotein(a) is size- and concentration-dependent. Clin. Chem. Lab. Med. 42: 1013–1019. [DOI] [PubMed] [Google Scholar]
- 49.Feric N. T., Boffa M. B., Johnston S. M., and Koschinsky M. L.. 2008. Apolipoprotein(a) inhibits the conversion of Glu-plasminogen to Lys-plasminogen: a novel mechanism for lipoprotein(a)-mediated inhibition of plasminogen activation. J. Thromb. Haemost. 6: 2113–2120. [DOI] [PubMed] [Google Scholar]
- 50.Biemond B. J., Friederich P. W., Koschinsky M. L., Levi M., Sangrar W., Xia J., Buller H. R., and ten Cate J. W.. 1997. Apolipoprotein(a) attenuates endogenous fibrinolysis in the rabbit jugular vein thrombosis model in vivo. Circulation. 96: 1612–1615. [DOI] [PubMed] [Google Scholar]
- 51.Palabrica T. M., Liu A. C., Aronovitz M. J., Furie B., Lawn R. M., and Furie B. C.. 1995. Antifibrinolytic activity of apolipoprotein(a) in vivo: human apolipoprotein(a) transgenic mice are resistant to tissue plasminogen activator-mediated thrombolysis. Nat. Med. 1: 256–259. [DOI] [PubMed] [Google Scholar]
- 52.Williams J. K., Bellinger D. A., Nichols T. C., Griggs T. R., Bumol T. F., Fouts R. L., and Clarkson T. B.. 1993. Occlusive arterial thrombosis in cynomolgus monkeys with varying plasma concentrations of lipoprotein(a). Arterioscler. Thromb. 13: 548–554. [DOI] [PubMed] [Google Scholar]
- 53.Anglés-Cano E., de la Peña Díaz A., and Loyau S.. 2001. Inhibition of fibrinolysis by lipoprotein(a). Ann. N. Y. Acad. Sci. 936: 261–275. [DOI] [PubMed] [Google Scholar]
- 54.Kang C., Dominguez M., Loyau S., Miyata T., Durlach V., and Angles-Cano E.. 2002. Lp(a) particles mold fibrin-binding properties of apo(a) in size-dependent manner: a study with different-length recombinant apo(a), native Lp(a), and monoclonal antibody. Arterioscler. Thromb. Vasc. Biol. 22: 1232–1238. [DOI] [PubMed] [Google Scholar]
- 55.Sangrar W., Gabel B. R., Boffa M. B., Walker J. B., Hancock M. A., Marcovina S. M., Horrevoets A. J., Nesheim M. E., and Koschinsky M. L.. 1997. The solution phase interaction between apolipoprotein(a) and plasminogen inhibits the binding of plasminogen to a plasmin-modified fibrinogen surface. Biochemistry. 36: 10353–10363. [DOI] [PubMed] [Google Scholar]
- 56.Boerwinkle E., Leffert C. C., Lin J., Lackner C., Chiesa G., and Hobbs H. H.. 1992. Apolipoprotein(a) gene accounts for greater than 90% of the variation in plasma lipoprotein(a) concentrations. J. Clin. Invest. 90: 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kronenberg F., Kronenberg M. F., Kiechl S., Trenkwalder E., Santer P., Oberhollenzer F., Egger G., Utermann G., and Willeit J.. 1999. Role of lipoprotein(a) and apolipoprotein(a) phenotype in atherogenesis: prospective results from the Bruneck study. Circulation. 100: 1154–1160. [DOI] [PubMed] [Google Scholar]
- 58.Kamstrup P. R., Tybjaerg-Hansen A., Steffensen R., and Nordestgaard B. G.. 2009. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 301: 2331–2339. [DOI] [PubMed] [Google Scholar]
- 59.Lamon-Fava S., Marcovina S. M., Albers J. J., Kennedy H., Deluca C., White C. C., Cupples L. A., McNamara J. R., Seman L. J., Bongard V., et al. 2011. Lipoprotein(a) levels, apo(a) isoform size, and coronary heart disease risk in the Framingham Offspring Study. J. Lipid Res. 52: 1181–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Willeit P., Kiechl S., Kronenberg F., Witztum J. L., Santer P., Mayr M., Xu Q., Mayr A., Willeit J., and Tsimikas S.. 2014. Discrimination and net reclassification of cardiovascular risk with lipoprotein(a): prospective 15-year outcomes in the Bruneck Study. J. Am. Coll. Cardiol. 64: 851–860. [DOI] [PubMed] [Google Scholar]
- 61.Hervio L., Durlach V., Girard-Globa A., and Angles-Cano E.. 1995. Multiple binding with identical linkage: a mechanism that explains the effect of lipoprotein(a) on fibrinolysis. Biochemistry. 34: 13353–13358. [DOI] [PubMed] [Google Scholar]
- 62.Hervio L., Girard-Globa A., Durlach V., and Angles-Cano E.. 1996. The antifibrinolytic effect of lipoprotein(a) in heterozygous subjects is modulated by the relative concentration of each of the apolipoprotein(a) isoforms and their affinity for fibrin. Eur. J. Clin. Invest. 26: 411–417. [DOI] [PubMed] [Google Scholar]
- 63.Falcó C., Estellés A., Dalmau J., España F., and Aznar J.. 1998. Influence of lipoprotein (a) levels and isoforms on fibrinolytic activity–study in families with high lipoprotein (a) levels. Thromb. Haemost. 79: 818–823. [PubMed] [Google Scholar]
- 64.Kang C., Durlach V., Soulat T., Fournier C., and Angles-Cano E.. 1997. Lipoprotein(a) isoforms display differences in affinity for plasminogen-like binding to human mononuclear cells. Arterioscler. Thromb. Vasc. Biol. 17: 2036–2043. [DOI] [PubMed] [Google Scholar]
- 65.Desai K., Bruckdorfer K. R., Hutton R. A., and Owen J. S.. 1989. Binding of apoE-rich high density lipoprotein particles by saturable sites on human blood platelets inhibits agonist-induced platelet aggregation. J. Lipid Res. 30: 831–840. [PubMed] [Google Scholar]
- 66.Ezratty A., Simon D. I., and Loscalzo J.. 1993. Lipoprotein(a) binds to human platelets and attenuates plasminogen binding and activation. Biochemistry. 32: 4628–4633. [DOI] [PubMed] [Google Scholar]
- 67.Rand M. L., Sangrar W., Hancock M. A., Taylor D. M., Marcovina S. M., Packham M. A., and Koschinsky M. L.. 1998. Apolipoprotein(a) enhances platelet responses to the thrombin receptor-activating peptide SFLLRN. Arterioscler. Thromb. Vasc. Biol. 18: 1393–1399. [DOI] [PubMed] [Google Scholar]
- 68.Martínez C., Rivera J., Loyau S., Corral J., González-Conejero R., Lozano M. L., Vicente V., and Anglés-Cano E.. 2001. Binding of recombinant apolipoprotein(a) to human platelets and effect on platelet aggregation. Thromb. Haemost. 85: 686–693. [PubMed] [Google Scholar]
- 69.Barre D. E. 1998. Lipoprotein (a) reduces platelet aggregation via apo(a)-mediated decreases in thromboxane A(2)production. Platelets. 9: 93–96. [DOI] [PubMed] [Google Scholar]
- 70.Barre D. E. 2003. Apolipoprotein (a) mediates the lipoprotein (a)-induced biphasic shift in human platelet cyclic AMP. Thromb. Res. 112: 321–324. [DOI] [PubMed] [Google Scholar]
- 71.Barre D. E. 2007. Arginyl-glycyl-aspartyl (RGD) epitope of human apolipoprotein (a) inhibits platelet aggregation by antagonizing the IIb subunit of the fibrinogen (GPIIb/IIIa) receptor. Thromb. Res. 119: 601–607. [DOI] [PubMed] [Google Scholar]
- 72.Tsironis L. D., Mitsios J. V., Milionis H. J., Elisaf M., and Tselepis A. D.. 2004. Effect of lipoprotein (a) on platelet activation induced by platelet-activating factor: role of apolipoprotein (a) and endogenous PAF-acetylhydrolase. Cardiovasc. Res. 63: 130–138. [DOI] [PubMed] [Google Scholar]
- 73.Caplice N. M., Panetta C., Peterson T. E., Kleppe L. S., Mueske C. S., Kostner G. M., Broze G. J. Jr., and Simari R. D.. 2001. Lipoprotein (a) binds and inactivates tissue factor pathway inhibitor: a novel link between lipoproteins and thrombosis. Blood. 98: 2980–2987. [DOI] [PubMed] [Google Scholar]
- 74.Broze G. J. Jr., Warren L. A., Novotny W. F., Higuchi D. A., Girard J. J., and Miletich J. P.. 1988. The lipoprotein-associated coagulation inhibitor that inhibits the factor VII-tissue factor complex also inhibits factor Xa: insight into its possible mechanism of action. Blood. 71: 335–343. [PubMed] [Google Scholar]
- 75.Bilgen D., Sonmez H., Ekmekci H., Ulutin T., Ozturk Z., Kokoglu E., Bayram C., Soner A., and Domanic N.. 2005. The relationship of TFPI, Lp(a), and oxidized LDL antibody levels in patients with coronary artery disease. Clin. Biochem. 38: 92–96. [DOI] [PubMed] [Google Scholar]
- 76.Di Nisio M., ten Wolde M., Meijers J. C., and Buller H. R.. 2005. Effects of high plasma lipoprotein (a) levels on tissue factor pathway inhibitor and the protein C pathway. J. Thromb. Haemost. 3: 2123–2125. [Erratum. 2007. J. Thromb. Haemost. 5: 218.] [DOI] [PubMed] [Google Scholar]
- 77.López S., Buil A., Ordoñez J., Souto J. C., Almasy L., Lathrop M., Blangero J., Blanco-Vaca F., Fontcuberta J., and Soria J. M.. 2008. Genome-wide linkage analysis for identifying quantitative trait loci involved in the regulation of lipoprotein a (Lpa) levels. Eur. J. Hum. Genet. 16: 1372–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mosesson M. W. 2005. Fibrinogen and fibrin structure and functions. J. Thromb. Haemost. 3: 1894–1904. [DOI] [PubMed] [Google Scholar]
- 79.Ariëns R. A. 2011. Elevated fibrinogen causes thrombosis. Blood. 117: 4687–4688. [DOI] [PubMed] [Google Scholar]
- 80.Chernysh I. N., Nagaswami C., and Weisel J. W.. 2011. Visualization and identification of the structures formed during early stages of fibrin polymerization. Blood. 117: 4609–4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Undas A., and Ariens R. A.. 2011. Fibrin clot structure and function: a role in the pathophysiology of arterial and venous thromboembolic diseases. Arterioscler. Thromb. Vasc. Biol. 31: e88–e99. [DOI] [PubMed] [Google Scholar]
- 82.Fogelson A. L., and Keener J. P.. 2010. Toward an understanding of fibrin branching structure. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 81: 051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lord S. T. 2011. Molecular mechanisms affecting fibrin structure and stability. Arterioscler. Thromb. Vasc. Biol. 31: 494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Collet J. P., Allali Y., Lesty C., Tanguy M. L., Silvain J., Ankri A., Blanchet B., Dumaine R., Gianetti J., Payot L., et al. 2006. Altered fibrin architecture is associated with hypofibrinolysis and premature coronary atherothrombosis. Arterioscler. Thromb. Vasc. Biol. 26: 2567–2573. [DOI] [PubMed] [Google Scholar]
- 85.Collet J. P., Park D., Lesty C., Soria J., Soria C., Montalescot G., and Weisel J. W.. 2000. Influence of fibrin network conformation and fibrin fiber diameter on fibrinolysis speed: dynamic and structural approaches by confocal microscopy. Arterioscler. Thromb. Vasc. Biol. 20: 1354–1361. [DOI] [PubMed] [Google Scholar]
- 86.Undas A., Stepien E., Tracz W., and Szczeklik A.. 2006. Lipoprotein(a) as a modifier of fibrin clot permeability and susceptibility to lysis. J. Thromb. Haemost. 4: 973–975. [DOI] [PubMed] [Google Scholar]
- 87.Undas A., Plicner D., Stepien E., Drwila R., and Sadowski J.. 2007. Altered fibrin clot structure in patients with advanced coronary artery disease: a role of C-reactive protein, lipoprotein(a) and homocysteine. J. Thromb. Haemost. 5: 1988–1990. [DOI] [PubMed] [Google Scholar]
- 88.Tsurupa G., Ho-Tin-Noe B., Angles-Cano E., and Medved L.. 2003. Identification and characterization of novel lysine-independent apolipoprotein(a)-binding sites in fibrin(ogen) alphaC-domains. J. Biol. Chem. 278: 37154–37159. [DOI] [PubMed] [Google Scholar]
- 89.Koschinsky M. L., Tomlinson J. E., Zioncheck T. F., Schwartz K., Eaton D. L., and Lawn R. M.. 1991. Apolipoprotein(a): expression and characterization of a recombinant form of the protein in mammalian cells. Biochemistry. 30: 5044–5051. [DOI] [PubMed] [Google Scholar]
- 90.Mutch N. J., Engel R., Uitte de Willige S., Philippou H., and Ariens R. A.. 2010. Polyphosphate modifies the fibrin network and down-regulates fibrinolysis by attenuating binding of tPA and plasminogen to fibrin. Blood. 115: 3980–3988. [DOI] [PubMed] [Google Scholar]
- 91.Luke M. M., Kane J. P., Liu D. M., Rowland C. M., Shiffman D., Cassano J., Catanese J. J., Pullinger C. R., Leong D. U., Arellano A. R., et al. 2007. A polymorphism in the protease-like domain of apolipoprotein(a) is associated with severe coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 27: 2030–2036. [DOI] [PubMed] [Google Scholar]
- 92.Chasman D. I., Shiffman D., Zee R. Y., Louie J. Z., Luke M. M., Rowland C. M., Catanese J. J., Buring J. E., Devlin J. J., and Ridker P. M.. 2009. Polymorphism in the apolipoprotein(a) gene, plasma lipoprotein(a), cardiovascular disease, and low-dose aspirin therapy. Atherosclerosis. 203: 371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rowland C. M., Pullinger C. R., Luke M. M., Shiffman D., Green L., Movsesyan I., Devlin J. J., Malloy M. J., Kane J. P., and Undas A.. 2014. Lipoprotein (a), LPA Ile4399Met, and fibrin clot properties. Thromb. Res. 133: 863–867. [DOI] [PubMed] [Google Scholar]
- 94.Sha J., McCullough B., Hart E., Nassir F., Davidson N. O., and Hoover-Plow J.. 2005. Apo(a) promotes thrombosis in a vascular injury model by a mechanism independent of plasminogen. J. Thromb. Haemost. 3: 2281–2289. [DOI] [PubMed] [Google Scholar]
- 95.Pellegrino M., Furmaniak-Kazmierczak E., LeBlanc J. C., Cho T., Cao K., Marcovina S. M., Boffa M. B., Cote G. P., and Koschinsky M. L.. 2004. The apolipoprotein(a) component of lipoprotein(a) stimulates actin stress fiber formation and loss of cell-cell contact in cultured endothelial cells. J. Biol. Chem. 279: 6526–6533. [DOI] [PubMed] [Google Scholar]
- 96.Haque N. S., Zhang X., French D. L., Li J., Poon M., Fallon J. T., Gabel B. R., Taubman M. B., Koschinsky M., and Harpel P. C.. 2000. CC chemokine I-309 is the principal monocyte chemoattractant induced by apolipoprotein(a) in human vascular endothelial cells. Circulation. 102: 786–792. [DOI] [PubMed] [Google Scholar]
- 97.Allen S., Khan S., Tam S., Koschinsky M., Taylor P., and Yacoub M.. 1998. Expression of adhesion molecules by lp(a): a potential novel mechanism for its atherogenicity. FASEB J. 12: 1765–1776. [DOI] [PubMed] [Google Scholar]
- 98.Sorensen K. E., Celermajer D. S., Georgakopoulos D., Hatcher G., Betteridge D. J., and Deanfield J. E.. 1994. Impairment of endothelium-dependent dilation is an early event in children with familial hypercholesterolemia and is related to the lipoprotein(a) level. J. Clin. Invest. 93: 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tsurumi Y., Nagashima H., Ichikawa K., Sumiyoshi T., and Hosoda S.. 1995. Influence of plasma lipoprotein (a) levels on coronary vasomotor response to acetylcholine. J. Am. Coll. Cardiol. 26: 1242–1250. [DOI] [PubMed] [Google Scholar]
- 100.Schachinger V., Halle M., Minners J., Berg A., and Zeiher A. M.. 1997. Lipoprotein(a) selectively impairs receptor-mediated endothelial vasodilator function of the human coronary circulation. J. Am. Coll. Cardiol. 30: 927–934. [DOI] [PubMed] [Google Scholar]
- 101.Taleb A., Witztum J. L., and Tsimikas S.. 2011. Oxidized phospholipids on apoB-100-containing lipoproteins: a biomarker predicting cardiovascular disease and cardiovascular events. Biomark. Med. 5: 673–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bergmark C., Dewan A., Orsoni A., Merki E., Miller E. R., Shin M. J., Binder C. J., Horkko S., Krauss R. M., Chapman M. J., et al. 2008. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J. Lipid Res. 49: 2230–2239. [DOI] [PubMed] [Google Scholar]
- 103.Edelstein C., Pfaffinger D., Hinman J., Miller E., Lipkind G., Tsimikas S., Bergmark C., Getz G. S., Witztum J. L., and Scanu A. M.. 2003. Lysine-phosphatidylcholine adducts in kringle V impart unique immunological and potential pro-inflammatory properties to human apolipoprotein(a). J. Biol. Chem. 278: 52841–52847. [DOI] [PubMed] [Google Scholar]
- 104.Edelstein C., Pfaffinger D., Yang M., Hill J. S., and Scanu A. M.. 2010. Naturally occurring human plasminogen, like genetically related apolipoprotein(a), contains oxidized phosphatidylcholine adducts. Biochim. Biophys. Acta. 1801: 738–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Leibundgut G., Arai K., Orsoni A., Yin H., Scipione C., Miller E. R., Koschinsky M. L., Chapman M. J., Witztum J. L., and Tsimikas S.. 2012. Oxidized phospholipids are present on plasminogen, affect fibrinolysis, and increase following acute myocardial infarction. J. Am. Coll. Cardiol. 59: 1426–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Klein J. H., Hegele R. A., Hackam D. G., Koschinsky M. L., Huff M. W., and Spence J. D.. 2008. Lipoprotein(a) is associated differentially with carotid stenosis, occlusion, and total plaque area. Arterioscler. Thromb. Vasc. Biol. 28: 1851–1856. [DOI] [PubMed] [Google Scholar]
- 107.Gardener H., Della Morte D., Elkind M. S., Sacco R. L., and Rundek T.. 2009. Lipids and carotid plaque in the Northern Manhattan Study (NOMAS). BMC Cardiovasc. Disord. 9: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Moliterno D. J., Lange R. A., Meidell R. S., Willard J. E., Leffert C. C., Gerard R. D., Boerwinkle E., Hobbs H. H., and Hillis L. D.. 1993. Relation of plasma lipoprotein(a) to infarct artery patency in survivors of myocardial infarction. Circulation. 88: 935–940. [DOI] [PubMed] [Google Scholar]
- 109.Matsuda S., Arima M., Ohigawa T., Tanimoto K., Takagi A., Kanoh T., Yamagami S., and Daida H.. 2004. Relation between serum lipoprotein (a) and residual lesion stenosis of coronary artery after myocardial Infarction without reperfusion therapy. Jpn. Heart J. 45: 397–407. [DOI] [PubMed] [Google Scholar]
- 110.Kim J. W., Seo H. S., Suh S. Y., Choi C. U., Kim E. J., Rha S. W., Park C. G., and Oh D. J.. 2008. Relationship between lipoprotein(a) and spontaneous recanalization of infarct-related arteries in the early phase of acute myocardial infarction. Clin. Cardiol. 31: 211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Haider A. W., Andreotti F., Thompson G. R., Kluft C., Maseri A., and Davies G. J.. 1996. Serum lipoprotein(a) level is related to thrombin generation and spontaneous intermittent coronary occlusion in patients with acute myocardial infarction. Circulation. 94: 2072–2076. [DOI] [PubMed] [Google Scholar]
- 112.Armstrong V. W., Neubauer C., Schutz E., and Tebbe U.. 1990. Lack of association between raised serum Lp(a) concentration and unsuccessful thrombolysis after acute myocardial infarction. Lancet. 336: 1077. [DOI] [PubMed] [Google Scholar]
- 113.von Hodenberg E., Kreuzer J., Hautmann M., Nordt T., Kubler W., and Bode C.. 1991. Effects of lipoprotein (a) on success rate of thrombolytic therapy in acute myocardial infarction. Am. J. Cardiol. 67: 1349–1353. [DOI] [PubMed] [Google Scholar]
- 114.Tranchesi B. Jr., Chamone D. F., Cobbaert C., Van de Werf F., Vanhove P., and Verstraete M.. 1991. Coronary recanalization rate after intravenous bolus of alteplase in acute myocardial infarction. Am. J. Cardiol. 68: 161–165. [DOI] [PubMed] [Google Scholar]
- 115.MBewu A. D., Durrington P. N., Mackness M. I., Hunt L., Turkie W. H., and Creamer J. E.. 1994. Serum Lp(a) lipoprotein concentration and outcome of thrombolytic treatment for myocardial infarction. Br. Heart J. 71: 316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brügemann J., van der Meer J., Hillege H. L., van Boven A. J., van Doormaal J. J., de Graeff P. A., and Lie K. I.. 1994. Lipoprotein(a) levels in patients with myocardial infarction treated with anistreplase: no prediction of efficacy but inverse correlation with plasminogen activation in non-patency. Int. J. Cardiol. 45: 109–113. [DOI] [PubMed] [Google Scholar]
- 117.Tokgözoğlu S. L., Ozmen F., Kabukçu M., Karanfil A., Aytemir K., Ozkuyumcu C., and Uğurlu S.. 1995. Does increased lipoprotein (a) impair the effectiveness of thrombolysis with streptokinase? Angiology. 46: 727–731. [DOI] [PubMed] [Google Scholar]
- 118.Ribo M., Montaner J., Molina C. A., Arenillas J. F., Santamarina E., and Alvarez-Sabin J.. 2004. Admission fibrinolytic profile predicts clot lysis resistance in stroke patients treated with tissue plasminogen activator. Thromb. Haemost. 91: 1146–1151. [DOI] [PubMed] [Google Scholar]
- 119.Ribo M., Montaner J., Molina C. A., Arenillas J. F., Santamarina E., Quintana M., and Alvarez-Sabin J.. 2004. Admission fibrinolytic profile is associated with symptomatic hemorrhagic transformation in stroke patients treated with tissue plasminogen activator. Stroke. 35: 2123–2127. [DOI] [PubMed] [Google Scholar]
- 120.Franchini M., and Mannucci P. M.. 2008. Venous and arterial thrombosis: different sides of the same coin? Eur. J. Intern. Med. 19: 476–481. [DOI] [PubMed] [Google Scholar]
- 121.von Depka M., Nowak-Gottl U., Eisert R., Dieterich C., Barthels M., Scharrer I., Ganser A., and Ehrenforth S.. 2000. Increased lipoprotein (a) levels as an independent risk factor for venous thromboembolism. Blood. 96: 3364–3368. [PubMed] [Google Scholar]
- 122.Sofi F., Marcucci R., Abbate R., Gensini G. F., and Prisco D.. 2007. Lipoprotein (a) and venous thromboembolism in adults: a meta-analysis. Am. J. Med. 120: 728–733. [DOI] [PubMed] [Google Scholar]
- 123.Grifoni E., Marcucci R., Ciuti G., Cenci C., Poli D., Mannini L., Liotta A. A., Miniati M., Abbate R., and Prisco D.. 2012. The thrombophilic pattern of different clinical manifestations of venous thromboembolism: a survey of 443 cases of venous thromboembolism. Semin. Thromb. Hemost. 38: 230–234. [DOI] [PubMed] [Google Scholar]
- 124.Lippi G., Bassi A., Brocco G., Manzato F., Marini M., and Guidi G.. 1999. Lipoprotein(a) concentration is not associated with venous thromboembolism in a case control study. Haematologica. 84: 726–729. [PubMed] [Google Scholar]
- 125.Libourel E. J., Bank I., Meinardi J. R., Baljé-Volkers C. P., Hamulyak K., Middeldorp S., Koopman M. M., van Pampus E. C., Prins M. H., Buller H. R., et al. 2002. Co-segregation of thrombophilic disorders in factor V Leiden carriers; the contributions of factor VIII, factor XI, thrombin activatable fibrinolysis inhibitor and lipoprotein(a) to the absolute risk of venous thromboembolism. Haematologica. 87: 1068–1073. [PubMed] [Google Scholar]
- 126.Tsai A. W., Cushman M., Rosamond W. D., Heckbert S. R., Polak J. F., and Folsom A. R.. 2002. Cardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiology. Arch. Intern. Med. 162: 1182–1189. [DOI] [PubMed] [Google Scholar]
- 127.Rodger M. A., Le Gal G., Carrier M., Betancourt M. T., Kahn S. R., Wells P. S., Anderson D. A., Lacut K., Chagnon I., Solymoss S., et al. 2010. Serum lipoprotein (a) levels in patients with first unprovoked venous thromboembolism is not associated with subsequent risk of recurrent VTE. Thromb. Res. 126: 222–226. [DOI] [PubMed] [Google Scholar]
- 128.van Schouwenburg I. M., Mahmoodi B. K., Gansevoort R. T., Muntinghe F. L., Dullaart R. P., Kluin-Nelemans H. C., Veeger N. J., and Meijer K.. 2012. Lipid levels do not influence the risk of venous thromboembolism. Results of a population-based cohort study. Thromb. Haemost. 108: 923–929. [DOI] [PubMed] [Google Scholar]
- 129.Kamstrup P. R., Tybjaerg-Hansen A., and Nordestgaard B. G.. 2012. Genetic evidence that lipoprotein(a) associates with atherosclerotic stenosis rather than venous thrombosis. Arterioscler. Thromb. Vasc. Biol. 32: 1732–1741. [DOI] [PubMed] [Google Scholar]
- 130.Helgadottir A., Gretarsdottir S., Thorleifsson G., Holm H., Patel R. S., Gudnason T., Jones G. T., van Rij A. M., Eapen D. J., Baas A. F., et al. 2012. Apolipoprotein(a) genetic sequence variants associated with systemic atherosclerosis and coronary atherosclerotic burden but not with venous thromboembolism. J. Am. Coll. Cardiol. 60: 722–729. [DOI] [PubMed] [Google Scholar]
- 131.Danik J. S., Buring J. E., Chasman D. I., Zee R. Y., Ridker P. M., and Glynn R. J.. 2013. Lipoprotein(a), polymorphisms in the LPA gene, and incident venous thromboembolism among 21483 women. J. Thromb. Haemost. 11: 205–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kronenberg F., and Utermann G.. 2013. Lipoprotein(a): resurrected by genetics. J. Intern. Med. 273: 6–30. [DOI] [PubMed] [Google Scholar]
- 133.Nowak-Göttl U., Junker R., Hartmeier M., Koch H. G., Münchow N., Assmann G., and von Eckardstein A.. 1999. Increased lipoprotein(a) is an important risk factor for venous thromboembolism in childhood. Circulation. 100: 743–748. [DOI] [PubMed] [Google Scholar]
- 134.Nowak-Göttl U., Strater R., Heinecke A., Junker R., Koch H. G., Schuierer G., and von Eckardstein A.. 1999. Lipoprotein (a) and genetic polymorphisms of clotting factor V, prothrombin, and methylenetetrahydrofolate reductase are risk factors of spontaneous ischemic stroke in childhood. Blood. 94: 3678–3682. [PubMed] [Google Scholar]
- 135.Sträter R., Becker S., von Eckardstein A., Heinecke A., Gutsche S., Junker R., Kurnik K., Schobess R., and Nowak-Göttl U.. 2002. Prospective assessment of risk factors for recurrent stroke during childhood–a 5-year follow-up study. Lancet. 360: 1540–1545. [DOI] [PubMed] [Google Scholar]
- 136.Nowak-Göttl U., Junker R., Kreuz W., von Eckardstein A., Kosch A., Nohe N., Schobess R., and Ehrenforth S.; Childhood Thrombophilia Study Group. 2001. Risk of recurrent venous thrombosis in children with combined prothrombotic risk factors. Blood. 97: 858–862. [DOI] [PubMed] [Google Scholar]
- 137.Goldenberg N. A., Bernard T. J., Hillhouse J., Armstrong-Wells J., Galinkin J., Knapp-Clevenger R., Jacobson L., Marcovina S. M., and Manco-Johnson M. J.. 2013. Elevated lipoprotein (a), small apolipoprotein (a), and the risk of arterial ischemic stroke in North American children. Haematologica. 98: 802–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sirachainan N., Chaiyong C., Visudtibhan A., Sasanakul W., Osatakul S., Wongwerawattanakoon P., Kadegasem P., and Chuansumrit A.. 2011. Lipoprotein(a) and the risk of thromboembolism in Thai children. Thromb. Res. 127: 100–104. [DOI] [PubMed] [Google Scholar]
- 139.Dubé J. B., Boffa M. B., Hegele R. A., and Koschinsky M. L.. 2012. Lipoprotein(a): more interesting than ever after 50 years. Curr. Opin. Lipidol. 23: 133–140. [DOI] [PubMed] [Google Scholar]
- 140.Schneider M., Witztum J. L., Young S. G., Ludwig E. H., Miller E. R., Tsimikas S., Curtiss L. K., Marcovina S. M., Taylor J. M., Lawn R. M., et al. 2005. High-level lipoprotein [a] expression in transgenic mice: evidence for oxidized phospholipids in lipoprotein [a] but not in low density lipoproteins. J. Lipid Res. 46: 769–778. [DOI] [PubMed] [Google Scholar]
- 141.Pedersen T. X., McCormick S. P., Tsimikas S., Bro S., and Nielsen L. B.. 2010. Lipoprotein(a) accelerates atherosclerosis in uremic mice. J. Lipid Res. 51: 2967–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kuijpers M. J., Gilio K., Reitsma S., Nergiz-Unal R., Prinzen L., Heeneman S., Lutgens E., van Zandvoort M. A., Nieswandt B., Egbrink M. G., et al. 2009. Complementary roles of platelets and coagulation in thrombus formation on plaques acutely ruptured by targeted ultrasound treatment: a novel intravital model. J. Thromb. Haemost. 7: 152–161. [DOI] [PubMed] [Google Scholar]
- 143.Kuijpers M. J., van der Meijden P. E., Feijge M. A., Mattheij N. J., May F., Govers-Riemslag J., Meijers J. C., Heemskerk J. W., Renne T., and Cosemans J. M.. 2014. Factor XII regulates the pathological process of thrombus formation on ruptured plaques. Arterioscler. Thromb. Vasc. Biol. 34: 1674–1680. [DOI] [PubMed] [Google Scholar]
- 144.van Montfoort M. L., Kuijpers M. J., Knaup V. L., Bhanot S., Monia B. P., Roelofs J. J., Heemskerk J. W., and Meijers J. C.. 2014. Factor XI regulates pathological thrombus formation on acutely ruptured atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 34: 1668–1673. [DOI] [PubMed] [Google Scholar]
- 145.Spence J. D., and Koschinsky M.. 2012. Mechanisms of lipoprotein(a) pathogenicity: prothrombotic, proatherosclerotic, or both? Arterioscler. Thromb. Vasc. Biol. 32: 1550–1551. [DOI] [PubMed] [Google Scholar]