Abstract
The non-destructive ex vivo determination of haemoglobin (Hgb) concentration offers the capability to conduct multiple red blood cell haematological measurements on a single sample, an advantage that current optical techniques are unable to offer. Here, a microwave method and device for the accurate and non-destructive determination of Hgb concentration in microlitre blood samples are described. Using broadband microwave spectroscopy, a relationship is established between the dielectric properties of murine blood and Hgb concentration that is utilized to create a technique for the determination of Hgb concentration. Subsequently, a microwave dielectric resonator-microfluidic system is implemented in the analysis of 52 murine samples with microlitre volumes and Hgb concentrations ranging from 0 to 17 g dL−1. Using the characterized relationship, independent and minimally invasive Hgb measurements are made on nine healthy mice as well as seven with mutations in the Adenomatous polyposis coli (APC) gene that leads to colorectal cancer and consequently anaemia.
1. Introduction
Haemoglobin (Hgb), serum protein, and electrolyte levels have long been recognized to be of medical importance in the diagnosis and monitoring of many diseases. In a recent study worldwide, anaemia prevalence was reported to be nearly a quarter of the global population,[1] and consequently Hgb counts are one of the most commonly requested haematological measurements. All current Hgb measurements are optical techniques with CO-oximeters and cyanomethaemoglobin methods representing the gold standard.[2–4] The principle behind the cyano/azide-methaemoglobin technique is the lysing of red blood cells (RBCs) and the chemical binding of cyanide/azide to Hgb. Hgb, cyano/azide-methaemoglobin, oxyglobin, and other related complexes exhibit unique optical spectral features that allow the calculation of concentration on the basis of absorption at multiple frequencies.[5] Non-invasive techniques such as CO-oximeters provide point of care (POC) capabilities as well as oxygenation information, however, the accuracy of such methods for Hgb concentration determination is still subject to debate.[6–9]
Here, we present a non-optical technique capable of conducting non-destructive and accurate Hgb concentration measurements, as well as providing information on total serum protein and electrolyte concentrations, with no requirement of lysing or chemical binding. To achieve this, we implement a high quality factor (Q) 9.4 GHz dielectric resonator with an integrated microfluidic system, which exploits the high-frequency (200 MHz to 40 GHz) microwave dielectric response of Hgb in serum. The resonator is highly sensitive to the concentration of proteins and electrolytes present in blood, therefore allowing measurements to be conducted on volumes as low as 500 nL. Initial studies are conducted on a range of healthy murine samples, paving the way for an assessment of Hgb levels in anaemic APCmin /+ mice. The non-destructive approach presented offers distinct advantages for integration with other haematological devices. An additional ability enabled by this design is the measurement of serum protein and electrolyte levels by one device on one sample, complimenting Hgb measurements in POC monitoring of patients.
2. Results and Discussion
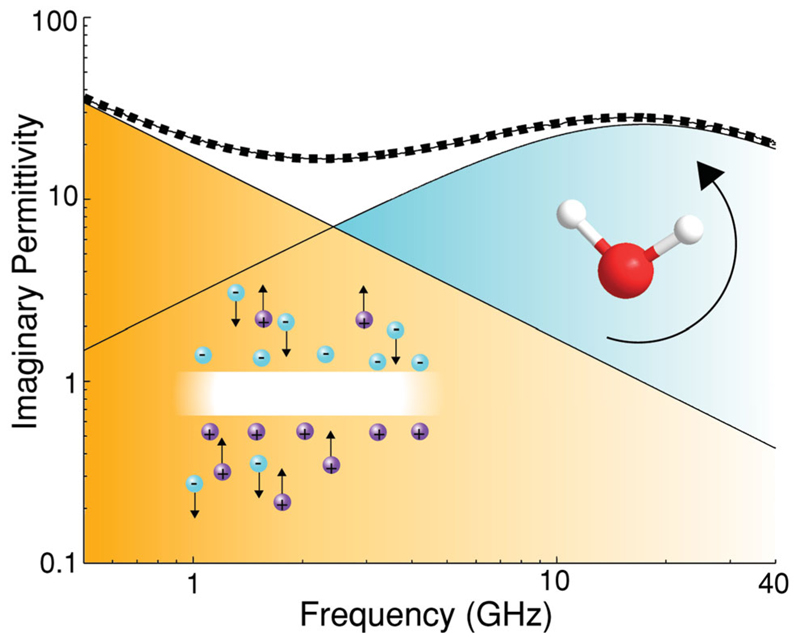
The complex dielectric properties of whole blood from single hertz to terahertz frequencies is primarily governed by three mechanisms; electrolyte concentration (ionic conductivity), cell membranes (Maxwell–Wagner/Looyenga polarization) and water molecules (rotational molecular polarization–Debye relaxation) (Figure 1). Electrolytes contribute to solution losses with an inverse frequency dependence of the imaginary permittivity that at physiological levels becomes negligible at frequencies above 40 GHz. Cell membranes, in conjunction with electrolytes, produce a Maxwell–Wagner polarization, which shows features described by Cole–Cole relaxation and contributes to the real dielectric spectrum up to ≈2 GHz.[10] The response of water, owing to the high density of electric dipole moments, contributes throughout the low-frequency dielectric spectrum but is orders of magnitude smaller than the Maxwell–Wagner and conductivity effects.[11] Bulk water has a relaxation time of 10 ps that equates to a relaxation frequency of 16 GHz at room temperature, around which, the permittivity response undergoes Debye relaxation (Figure 1). It is well known that hydration water in the immediate vicinity of proteins has different dynamics to bulk water that manifests as a relaxation at lower frequencies.[12,13] The magnitude of the dielectric contribution from bound water is sufficiently small that it has little significance in this report. The introduction of organic solutes effectively displaces water and reduces the dipole density, leading to a decrease in permittivity as given in mixed medium theories.[14] A comprehensive dielectric study on whole blood focusing on the described processes from 1 Hz to 50 GHz was recently reported by Wolf et al.[15]
Figure 1.
The imaginary permittivity of blood is composed of the dielectric contributions from Maxwell–Wagner/electrolyte conductivity and bulk water polarization. The dotted line is experimental results and the peaks are the deconvoluted dielectric contribution from each mechanism.
According to mixed medium theories, the primary contributors to the complex dielectric response at microwave frequencies relate almost directly to the solutes and solutions that occupy the greatest volume.[14] This may be understood by the rotationally polarisable water molecules being displaced by the predominantly non-polar proteins, thereby reducing the average dipole density in the solution and thus permittivity. Water is well known to make up ≈75% of whole blood though Hgb is the most abundant organic component in blood (≈15 g dL−1) followed by serum proteins, of which albumin and immunoglobulin are the most substantial (≈7 g dL−1). The remaining volume is mostly composed of electrolytes, white blood cells and platelets. Assuming that capacitive effects of Maxwell–Wagner polarization play no role at high frequencies, then the dielectric response of whole blood is primarily dependent on the levels of Hgb, serum proteins and electrolytes. Comparative measurements of water, serum, and whole blood may therefore be used to yield information on physiological levels of these three constituents.
2.1. Microwave Dielectric Response of Serum, Haemoglobin, and Red Blood Cells
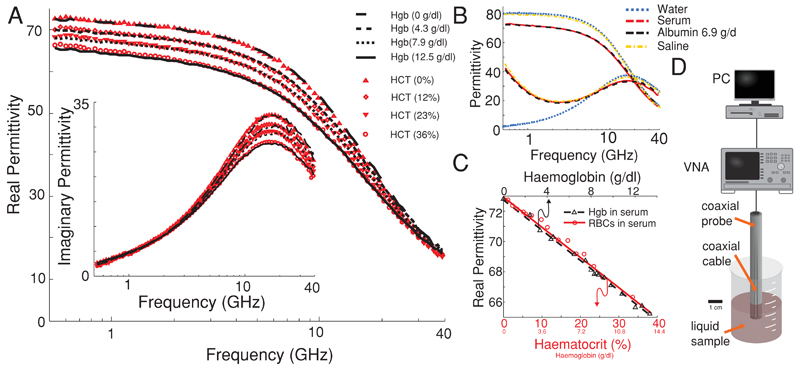
To determine the microwave dielectric response of the various constituents of whole blood, measurements were made on a range of concentrations of RBCs, electrolytes, serum proteins, and lyophilized Hgb in water or serum. Dielectric spectroscopy was carried out using a vector network analyser (VNA[HP 8722ET]) with a commercially available dielectric probe (Agilent probe kit 85070E) between the frequencies of 200 MHz and 40 GHz on sample volumes of 5 mL or greater (Figure 2D). Systematic studies were conducted to examine the dielectric contribution from the most abundant organic components in whole blood. The impact of serum proteins in water was examined by the addition of lyophilized albumin protein to an electrolyte with physiological salt levels and compared with serum. To confirm that Hgb plays an important role in the high-frequency dielectric response of blood, we measured and compared RBCs at various concentrations in serum with Hgb proteins suspended in the same serum.
Figure 2.
A) The dielectric response of serum with increasing Hgb and RBC concentration with conductivity removed from the imaginary permittivity. B) A comparison of the complex permittivity of water, serum, physiological saline and 6.9 g dL−1 albumin in physiological saline. C) The decrease in permittivity as a function of RBC and Hgb concentration. D) The experimental setup comprising of a PC, VNA, and coaxial probe immersed in a liquid sample.
The addition of 6.9 g dL−1 of lyophilized albumin protein to an electrolyte with physiological salt levels was found to have a similar dielectric spectrum to serum. By centrifuging the serum to separate the serum proteins from the solution and assuming a protein density of 1350 kg m−3, the concentration of proteins was found to be ≈6.5 g dL−1. The close match between the spectra of serum and protein solution at similar protein concentrations suggests that proteins are the primary contributor to the decrease in dielectric response. The difference in protein concentrations at matching permittivities may be attributed to small differences in the permittivity of proteins present in serum such as globulins and fibrinogens compared to albumin.
Microwave dielectric spectroscopy is incapable of differentiating various ions when dissolved in water, however, the deconvolution of the imaginary permittivity reveals a conductivity and Maxwell–Wagner contribution that encompasses all of the conductive contributions from the various salts present. The conductivity contribution to the imaginary permittivity has an inverse frequency dependence, seen in the lower frequency regime, that increases with linear proportionality to electrolyte concentration. The displacement of water molecules by ions results in a small decrease to the real permittivity with increasing salt concentration (Figure 2B). Across healthy physiological ranges of salt, the real permittivity remains unchanged and the imaginary permittivity shows differences up until 10 GHz (Figure S1, Supporting Information). Though non-selective and unable to distinguish the concentration of different ions measurements of the conductivity may be utilized to monitor the total salt levels in a blood sample.
The broadband permittivity as a function of RBC concentration [haematocrit (HCT)] was studied by the addition of pure RBCs from a centrifuged sample to serum. The findings demonstrate a clear correlation between the reduction in permittivity and an increased HCT, in good agreement with Wolf et al.[15] (Figure 2A). Furthermore, by dissolving lyophilized Hgb in serum, we observe the same decreasing dielectric response for increasing Hgb protein levels in serum. The Hgb concentration at each HCT value was calculated using a Sysmex F-820 with the mean Hgb cell concentration given as ≈35 g dL−1. The most important finding of this study was that in the case of Hgb concentrations of the whole RBC sample and the suspended Hgb sample matching the complex dielectric values were found to be in excellent agreement, thereby confirming Hgb as the main contributor to the dielectric response of RBCs (Figure 2A,C). Our findings also demonstrate that the change in the real permittivity across much of the microwave frequency range is linearly correlated to Hgb concentration. A small deviation in the real permittivity is observed between higher concentrations of Hgb and RBC samples from 500 MHz to 2 GHz, which we attribute to the tail-end of the Maxwell–Wagner response in RBCs. Using the dielectric response of blood, the Hgb concentration may be accurately ascertained by a relative measurement between serum and whole blood so as to remove the influence of serum protein and electrolyte levels. The microwave dielectric response of blood at frequencies above ≈2 GHz has been demonstrated to be almost entirely governed by the Hgb, serum protein and electrolyte concentration (Figure 2).
To develop a viable Hgb analysis method requires low sample volumes and the careful selection of a frequency with minimal influence from other constituents and dielectric mechanisms in blood. The high permittivity contrast between water and organic molecules makes frequencies below 40 GHz (ideally 10 GHz) preferable for Hgb and serum protein measurements. Additionally, the effects of Maxwell–Wagner polarization and losses from electrolytes make frequencies above 2 GHz preferential for accurate protein measurements especially if analysis is made with a single-frequency technique, thereby preventing deconvolution of a spectrum. The remaining frequency range presents a unique window to analyse organic solutes in blood that may otherwise be obscured or the dielectric contribution be negligible at other frequency ranges.
2.2. Dielectric Resonator
Sensing with an electromagnetic resonance proves advantageous when conducting measurements on volumes much smaller than a wavelength and have been demonstrated to be viable techniques for biological solution analysis.[16–20] Whispering gallery mode resonators and dielectric resonators are commonly used for dielectric and conductivity measurements in the microwave community[21] and have been exploited to measure single viruses and proteins in the optical regime owing to their high-quality resonances.[22] Two parameters are used in resonant measurements, the resonant frequency (f) that is regarded as the natural frequency of the device and depends upon the surrounding environments real permittivity[17] and the quality factor (Q), which is dependent on the losses in the system and is proportional to the ratio of energy lost per cycle to the total energy stored in the system.
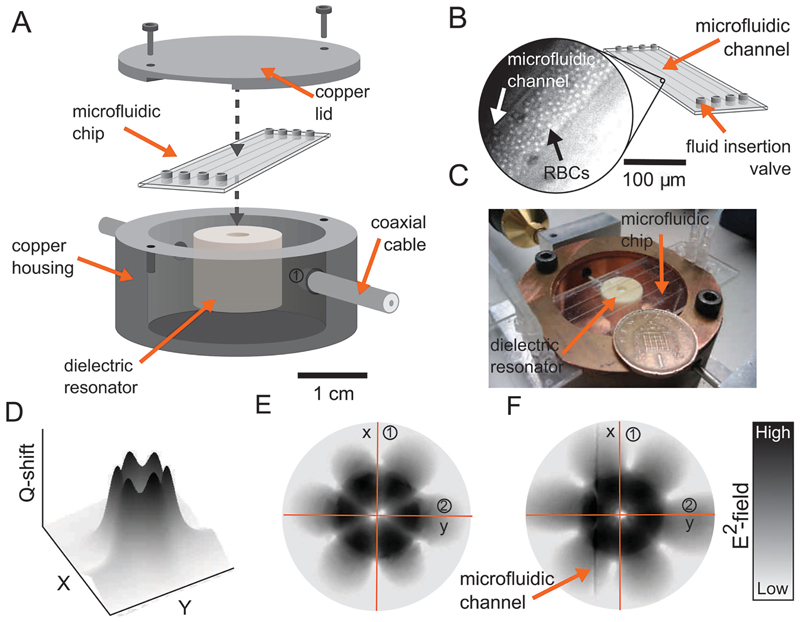
Our dielectric resonator consists of a high permittivity dielectric puck made of barium zirconium titanate (ε = 28) mounted on a Teflon support, which is contained within a pure copper housing (Figure 3). Two coaxial cables are terminated in coupling loops to allow excitation and measurement of the resonant modes within the dielectric puck. The resonator is operated with an EH603 mode, a low azimuthal index whispering gallery mode with predominant axial electric field at 9.4 GHz. The high-order resonance is used due to the increased concentration of electric fields at the dielectric puck–microfluidic chip interface, thereby increasing the sensitivity to samples present. Preliminary experimental field mapping helped identify the mode and to aid microfluidic positioning (Figure 3C). A microfluidic chip provided by Microfluidic Chip Shop is integrated above the dielectric puck with four channels of 200 μm diameter. The choice of microfluidic positioning and channel was optimized to obtain the largest reduction in Q with deionized water. Figure 3E,F depicts the electric field pattern with and without water in a channel, respectively. Electric fields that lay outside of the dielectric puck are predominantly evanescent and are highly responsive to changes in the permittivity of the space they occupy, which in turn effects the resonator Q and f. In the case of a low permittivity sample and a small interaction volume, the perturbation of f and Q has been reported to be linearly dependent on the real dielectric permittivity and losses, respectively.[23] In the case of higher permittivity, f and Q take on a more complex dependence on both the dielectric permittivity and losses,[24] however, over small changes in permittivity and losses the response of the resonant properties remain linear (Figure S2, Supporting Information). The use of a microwave resonator and a microfluidic system permits accurate dielectric measurements to be conducted on liquid sample volumes in the microlitre range with sub-1 s analysis times. Resonant results are given as the difference in resonant frequency and inverse quality factor between a reference sample R (i.e. serum, water) and sample S (i.e. blood) such that
| (1) |
| (2) |
Figure 3.
A) A diagram of the microwave dielectric resonator microfluidic assembly within a metal (copper) housing. B) The microfluidic chip with inset of a photograph of a blood-filled microfluidic channel visualising the red blood cells. C) The experimental setup in an open-lid configuration. D) The measured Q-shift at the surface of the resonator using a field mapping technique. E,F) Simulated (CST Microwave Studio) electric field distribution of the selected whispering gallery mode (HE036 mode) at the surface of the BZT puck with and without water present in the channel.
By conducting relative measurements between whole blood and serum, we are able to remove the influence of serum protein, salt, or temperature variations within the sample. Similarly, conducting serum measurements with a reference sample of water removes the influence of temperature.
2.3. Haemoglobin, Electrolyte, and Serum Protein Measurements
Studies were conducted on murine blood samples to demonstrate that the outlined resonator device provides reproducible and accurate results on low sample volumes. Samples were developed to determine sensitivity to variations in Hgb concentration, total serum protein concentration, and abnormal physiological electrolyte levels.
A study into the impact of electrolyte levels on the resonant parameters revealed increases in the Δf and decreases in ΔQ as a function of increasing ionic concentration. Preliminary studies suggest that the shift in resonant parameters, caused by the change in electrolyte concentration, is independent of RBC count, serum protein concentration, or existing electrolyte levels (Figure 4E,F).
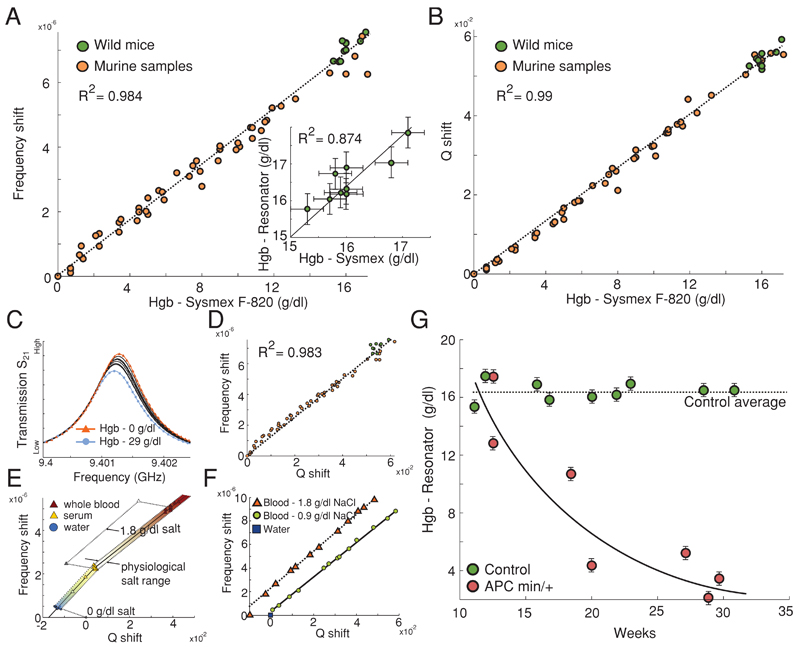
Figure 4.
A,B) Δf and ΔQ referenced against serum and with respect to Hgb values given by a Sysmex F-820, respectively. A,inset) Hgb values of healthy mice calculated by Sysmex and resonant methods. C) The change in the resonant transmission curve as a function of Hgb in serum. D) The resonant frequency plotted against inverse Q-factor as a function of Hgb concentration. E) The impact of salt and serum proteins on the resonant frequency and inverse Q-factor. F) The resonant frequency plotted against inverse Q-factor as a function of Hgb concentration in serum with varied electrolyte levels. G) The response of APCmin/+ mice Hgb levels with respect to age. Each data point is from a different mouse of a given age.
Sensitivity to serum protein content was conducted by the variation of serum albumin protein concentration in a physiological saline solution and measured relative to the resonant response of pure water. Δf and ΔQ showed linear proportionality to serum protein concentration when referenced against water (Figure 4E), demonstrating that measurements of whole serum may provide information on the total serum protein concentration. The resonant shift from variations in electrolyte levels can be differentiated from protein concentration variations owing to differing directions of ΔQ factor with respect to Δf (Figure 4E,F).
Resonant measurements were conducted on 52 murine samples made from the recombination of murine RBCs with serum from 10 healthy mice at Hgb concentrations between 0 and 17 g dL−1 and nine healthy wild mice. The dielectric resonator was excited with an operating Q of 14 100 at a f of 9.4021 GHz with no sample present in the microfluidic channel and in a closed-lid configuration. The temperature was maintained at 21 ± 0.5 °C throughout all measurements. Resonator measurements, Δf and ΔQ, were made by comparison of whole blood against a reference of serum, separated by centrifuge and recombined after measurement. Each resonant measurement was made three times and the average taken as the absolute value, for which the standard deviation of the resonant values was deemed negligible so long as the liquid within the microfluidic channel remained stationary. A study into the reproducibility of the Sysmex F-820 system across a number of hours showed an average standard deviation from the given value of ≈0.34 g dL−1. Errors in the resonant system are calculated from the standard deviation of the Δf and ΔQ Hgb values from the average Hgb value.
The resonant response was recorded as a function of Hgb concentration values obtained using a Sysmex F-820 (Figure 4C) with ΔQ and Δf, showing linear dependence and a strong correlation with respect to the 52 Hgb concentration values (Figure 4A,B,D). Errors in the resonant technique may arise from temperature variations between measuring the reference and sample as well as from the concentration of white blood cells and platelets. The dielectric contribution of all white blood cells and platelets is however less than 1% of the dielectric response of blood, so variations in concentration are likely to be negligible. Using fitted lines of the resonant parameter response to Hgb concentration allowed the resonant device to be calibrated so as to conduct independent Hgb measurements. All further Hgb measurements by the resonant technique were based upon the average of a ΔQ and Δf Hgb calculation. Subsequently, the technique was validated by the determination of the Hgb concentrations of nine healthy mice that showed clear correlation to the values given by the Sysmex F-820 (Figure 4A inset).
Having demonstrated the viability of this technique for Hgb measurements, we undertook a study into the Hgb response of a group of mice with mutations in the tumour-suppressor gene Adenomatous polyposis coli (APC).[25] Mutation of the APC gene leads to the uncontrolled growth of polyps on the epithelial cell lining of the small or large intestine. Polyps have a high chance of becoming malignant, thereby leading to further uncontrolled growth, rupturing of blood vessels near the surface of the cell lining and evolving towards colorectal carcinoma. Assessment of the colon and rectum of mice with mutated APC genes (APCmin/+) shows polyps to be present in the small bowel in numbers as great as 30–70, each potentially causing internal haemorrhaging.[25] Intermittent measurements of Hgb levels or haematocrit levels may therefore be used as a useful means for monitoring the progression of the disease on the assumption that a greater number or increased size of polyps is likely to lead to increased internal bleeding.[26] Minimally invasive measurements using small sample volumes (≤10 μL) are advantageous for reducing the level of stress on the mouse, thereby allowing the unimpeded development of the mouse and cancer. Blood samples from the genetically mutated murine model APCmin/+ were measured using the resonant technique over an age range of 10–28 weeks against a control group of APC+/+ (tumour-free wild-type) mice. Our findings with the resonant technique demonstrate a significant decrease in the murine samples Hgb count with respect to age when compared with a control, concurrent with expected trends.[26,27] In addition to the observed trend an onset of severe anaemia (Hgb ≤ 5 g dL−1) was seen to occur ≈ 22 weeks after birth (Figure 4F).
3. Conclusion
The microwave dielectric response of whole blood was shown to depend primarily on water, serum proteins, electrolytes and Hgb. A microwave resonant system has been demonstrated as a viable technique for the non-destructive and accurate determination of Hgb concentration in blood. This method has been validated by conducting minimally invasive independent measurements on healthy and anaemic APCmin/+ mice with colorectal cancer. In addition to the ability to measure Hgb levels, we outlined a technique, allowing the measurement of total serum protein and electrolyte levels by the comparative measurement of serum and water. By utilising existing microfluidic or on-chip centrifuging separation techniques and a portable breadboard VNA, future devices may be hand-held with envisaged analysis times from sample acquisition to results in the order of seconds. The non-destructive approach is appealing for integration with other haematological analysis techniques allowing multiple measurements on a single sample. This Hgb measurement technique has implications for medical POC diagnostics as well as potential use in patient care.
4. Experimental Section
Mouse Colony
APCmin/+ mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and genotyped as previously described.[28] Mice were bred and maintained in the animal facility at Imperial College London in a specific pathogen-free environment. Animal work was carried out in compliance with the British Home Office Animals Scientific Procedures Act 1986 (Project licence number 70/7129).
Sample Preparation
Broadband dielectric measurements were made on a blood sample composed of blood from 14 healthy mice. A sample (10 mL) was prepared with the anticoagulant heparin (10 000 U mL−1) and centrifuged for 5 min at 3000 RPM. We observed no effects on the dielectric properties of serum or blood in the presence of low levels of heparin. Platelets and white blood cells (WBCs) were removed and the serum and RBCs separated. A serum sample was measured to which pure RBCs were added to achieve 15 different concentrations between 0% and 40% v/v. The RBC count and Hgb concentration were measured using a Sysmex F-820. 18 Hgb solutions were prepared using the donors serum mixed with lyophilized Hgb and purchased from Sigma-Aldrich.
Samples measured with the dielectric resonator came from 10 healthy mice that were combined and prepared with heparin (10 000 U mL−1) and centrifuged for 5 min at 3000 RPM to separate the plasma and RBCs. The 52 murine samples used for the resonant parameter curve were prepared with RBC concentrations between 0% and 90% v/v and the Hgb values obtained on a Sysmex F-820. Nine murine samples from healthy mice were used for comparative Hgb measurements in which the samples were collected by intravenous methods. The mice were warmed to 37 °C for ≈ 5–10 min before the tail vein was punctured with a needle and the blood collected. Murine samples were acquire through intravenous methods in the tails of the mice. The separation of serum from the APCmin/+ blood samples was achieved using capillary centrifuging owing to the low levels of blood present.
Dielectric Measurements
To access the dielectric response of blood, a vector network analyser (VNA [HP 8722ET]) with a commercially available Agilent dielectric probe (85070E high temperature probe and performance probe) was used from 500 MHz to 40 GHz. The broadband permittivity measurements are made by calculating the change in amplitude and phase of the reflection (S11) from the coaxial-sample interface and uses calibration standards of air, deionized water and a metallic short. Fitting routines for extracting conductivity values were carried out using a third-order Debye equation (N = 3),
| (3) |
where ε∞ is the high-frequency permittivity, Δε is the dielectric contribution from a polarization mechanism, k is the number of relaxations, ω is the angular frequency, and τ is the relaxation time. Data fitting was conducted according to a least-means-square routine such that the residual is given by,
| (4) |
where S is the sum of the squared difference between the measured value and theoretically calculated value at M discrete frequencies, f. The residual was calculated separately for the real and imaginary component and then summed.
Resonator Measurements
The transmission (S21) measurement of the resonator was fitted to using a custom-built algorithm described elsewhere.[29] Samples were pumped into the microfluidic channel at a flow rate of 3 μL min−1 before being held stationary for measurement.
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Acknowledgements
This work was funded by the Leverhulme Emerging Discipline Award, the EPSRC APLAS Active Plasmonics Grant and the Wellcome trust (N.G. Research Career Development Fellowship 088381/Z/09/Z).
Contributor Information
Toby H. Basey-Fisher, Department of Materials, Imperial College London, London SW7 2AZ, UK.
Nadia Guerra, Division of Cell and Molecular Biology, Imperial College London, London, SW7 2AZ, UK.
Chiara Triulzi, Division of Cell and Molecular Biology, Imperial College London, London, SW7 2AZ, UK.
Andrew Gregory, National Physical Laboratory, Teddington, Middlesex, TW11 0LW, UK.
Stephen M. Hanham, Department of Materials, Imperial College London, London SW7 2AZ, UK
Molly M. Stevens, Department of Materials, Imperial College London, London SW7 2AZ, UK The Institute of Biomedical Engineering, Imperial College London, London SW7 2AZ, UK; Department of Bioengineering, Imperial College London, London SW7 2AZ, UK.
Stefan A. Maier, Department of Physics, Imperial College London, London, SW7 2AZ, UK
Norbert Klein, Department of Materials, Imperial College London, London SW7 2AZ, UK.
References
- 1.McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Public Health Nutr. 2009;12:444. doi: 10.1017/S1368980008002401. [DOI] [PubMed] [Google Scholar]
- 2.Zwart A, Buursma A, Oeseburg B, Zijlstra WG. Clin Chem. 1981;27:1903. [PubMed] [Google Scholar]
- 3.Kapoor SK, Kapil U, Dwivedi SN, Anand K, Pathak P, Singh P. Indian Pediatr. 2002;39:743. [PubMed] [Google Scholar]
- 4.Von Schenck H, Falkensson M, Lundberg B. Clin Chem. 1986;32:526. [PubMed] [Google Scholar]
- 5.Zijlstra WG, Buursma A, Van Assendelft OW. Visible and Near Infrared Absorption Spectra of Human and Animal Haemoglobin: Determination and Application. CRC Press; USA: 2000. [Google Scholar]
- 6.Lamhaut L, Apriotesei R, Combes X, Lejay M, Carli P, Vivien B. Anesthesiology. 2011;115:548. doi: 10.1097/ALN.0b013e3182270c22. [DOI] [PubMed] [Google Scholar]
- 7.Frasca D, Dahyot-Fizelier C, Catherine K, Levrat Q, Debaene B, Mimoz O. Crit Care Med. 2011;39:2277. doi: 10.1097/CCM.0b013e3182227e2d. [DOI] [PubMed] [Google Scholar]
- 8.Macknet MR, Allard M, Applegate RL, II, Rook J. Anesth Analg. 2010;111:1424. doi: 10.1213/ANE.0b013e3181fc74b9. [DOI] [PubMed] [Google Scholar]
- 9.Vos JJ, Kalmar AF, Struys MMRF, Porte RJ, Wietasch JKG, Scheeren TWL, Hendriks HGD. Br J Anaesth. 2012;109:522. doi: 10.1093/bja/aes234. [DOI] [PubMed] [Google Scholar]
- 10.Bordi F, Cametti C, Gili T. J Non-Cryst Solids. 2002;305:278. [Google Scholar]
- 11.Archer DG, Wang P. J Phys Chem Ref Data. 1990;19:371. [Google Scholar]
- 12.Wolf M, Gulich R, Lunkenheimer P, Loidl A. Biochim Biophys Acta. 2012;1824:723. doi: 10.1016/j.bbapap.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Cametti C, Marchetti S, Gambi CMC, Onori G. J Phys Chem B. 2011;115:7144. doi: 10.1021/jp2019389. [DOI] [PubMed] [Google Scholar]
- 14.Ruppin R. Opt Commun. 2000;182:273. [Google Scholar]
- 15.Wolf M, Gulich R, Lunkenheimer P, Loidl A. Biochim Biophys Acta. 2011;1810:727. doi: 10.1016/j.bbagen.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 16.Shaforost EN, Klein N, Vitusevich SA, Offenhausser A, Barannik AA. J Appl Phys. 2008;104:074111. [Google Scholar]
- 17.Shaforost EN, Klein N, Vitusevich SA, Barannik AA, Cherpak NT. Appl Phys Lett. 2009;94:112901. [Google Scholar]
- 18.Lonappan A, Thomas V, Bindu G, Rajasekaran C, Mathew KT. J Electromagn Waves Appl. 2007;21:1131. [Google Scholar]
- 19.Lonappan A. Microw Opt Technol Lett. 2012;54:1212. [Google Scholar]
- 20.Dobson R, Wu R, Callaghan P. Electron Lett. 2012;48:905. [Google Scholar]
- 21.Baker-Jarvis J, Janezic M, Degroot D. Instru Meas Mag IEEE. 2010;13:24. [Google Scholar]
- 22.Vollmer F, Arnold S, Keng D. Proc Natl Acad Sci U S A. 2008;105:20701. doi: 10.1073/pnas.0808988106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waldron RA. Proc IEEE-Part C Monogr. 1960;107:272. [Google Scholar]
- 24.Stockhausen M, Kessler M. J Phys E Sci Instru. 1980;13:732. [Google Scholar]
- 25.Moser AR, Pitot HC, Dove WF. Science. 1990;247:322. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 26.Rakoff-Nahoum S, Medzhitov R. Science. 2007;317:124. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Fernald AA, Anastasi J, Le Beau MM, Qian Z. Blood. 2010;115:3481. doi: 10.1182/blood-2009-11-251835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moser AR, Luongo C, Gould KA, MK McNeley, Shoemaker AR, Dove WF. Eur J Cancer. 1995;31:1061. doi: 10.1016/0959-8049(95)00181-h. [DOI] [PubMed] [Google Scholar]
- 29.Gregory AP. NPL Report MAT. 2013;58 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.