Abstract
We present neuroimaging markers of the remitted state of major depressive disorder (rMDD) during facial emotion perception in 84 individuals during fMRI. Participants comprised 47 individuals (aged 18–23) diagnosed with rMDD and 37 healthy controls (HCs). Participants classified emotional faces or animals (control condition) in the Facial Emotion Perception Test (FEPT) during fMRI. Behavioural performance on the FEPT did not differ significantly between groups. During fMRI, both groups demonstrated significant blood oxygen level-dependent (BOLD) activity in bilateral inferior frontal gyri for the faces minus animals (F−A) contrast. The rMDD group additionally showed BOLD activity during F−A in numerous regions, including the bilateral paracingulate gyri, middle temporal gyri and right amygdala. The rMDD group exhibited significantly greater activity than the HC group in regions including the bilateral middle temporal gyri and left superior frontal gyrus. Although the rMDD group did not manifest the behavioural performance deficits on facial emotion recognition tasks that have been observed in actively depressed individuals, the rMDD group nevertheless showed increased BOLD activity compared with never-depressed controls during F-A in multiple posterior brain regions, suggesting that persistent effects of illness or possible trait vulnerabilities may distinguish individuals with rMDD from never-depressed controls.
Keywords: emotion perception, major depressive disorder, remitted, state, amygdala
Introduction
Individuals with active major depressive disorder (aMDD) perform worse than healthy controls (HCs) on tasks of facial emotion recognition (Feinberg et al., 1986; Zuroff and Colussy, 1986; Rubinow and Post, 1992; Persad and Polivy, 1993; Langenecker et al., 2005, 2007; Csukly et al., 2009). Often, these patients display a negativity bias as they rate facial expressions less positively and/or more negatively than HCs (Gur et al., 1992; Hale, 1998; Hale et al., 1998; Surguladze et al., 2004). Inaccuracies or biases in the evaluation of social cues such as facial expressions can have a large impact on the interpretation of social interactions and subsequent emotional responses (Joormann and D’Avanzato, 2010). For example, if someone fails to detect a smile or misinterprets a neutral expression as negative due to an affective processing bias, they may be less likely to initiate social interaction and thereby experience the positive feedback of gratifying social interactions. Such an altered social perception may also lead the individual to misinterpret the event as social rejection and thus induce them to withdraw from social interactions. In this way, an impairment in facial emotion perception, a form of social cognition, could explain the interpersonal difficulties experienced by adults with MDD (Klerman and Weissman, 1992; Persad and Polivy, 1993) and contribute to the maintenance and reoccurrence of the disease (Joormann and Gotlib, 2006).
It has been speculated that alterations in facial emotion perception may signify a trait risk for MDD observed outside symptomatic episodes (LeMoult et al., 2009). The high recurrence rate of MDD (Mueller et al., 1999; Solomon et al., 2000) also suggests that this condition is associated with persistent vulnerability factors, which may include facial emotion perception biases. The severity of depression symptoms has been correlated with poor discrimination of sad emotional expressions (Gur et al., 1992), and with the persistence of MDD across time (Bouhuys et al., 1996; Hale, 1998) and risk of relapse in longitudinal studies (Bouhuys et al., 1999), suggesting that such perceptual abnormalities may associate with both state and residual ‘scar’ factors in MDD. Studies have attempted to disentangle state versus trait factors by studying individuals in the remitted state of MDD (rMDD). Such studies have found emotional processing impairments in this population using a variety of tasks, including a dot probe task with emotional faces (Joormann and Gotlib, 2007), an emotional Stroop task with faces (Strand et al., 2013), and recognition memory for emotional faces (Mikhailova et al., 1996). These studies lend support to the argument that emotion processing deficits reflect a trait-like vulnerability underlying the development of MDD.
Numerous fMRI studies of neural responses to emotional faces have reported increased amygdala activity, particularly in the left hemisphere, in patients with MDD relative to HCs (Sheline et al., 2001; Surguladze et al., 2005; Fu et al., 2004, 2008a,b), even for negative faces presented below conscious awareness (Victor et al., 2010). However, some studies have failed to find increased amygdala activity in response to emotional faces in MDD (Lawrence et al., 2004; Lee et al., 2008; Frodl et al., 2009; Demenescu et al., 2011). Increased amygdala activity in response to emotional faces has been found to predict depressive symptom reduction 8 months later (Canli et al., 2005) and to distinguish between unipolar and bipolar depression (Almeida et al., 2010; Fournier et al., 2013). Increased subgenual anterior cingulate cortex (sgACC) activity has also been reported in MDD in response to sad faces (Gotlib et al., 2005), and has been found to be associated with worse emotion perception performance (Briceño et al., 2013). Activity in the sgACC in response to sad but not happy faces has been associated with depression symptom scores (Keedwell et al., 2009). Amygdala and sgACC hyperactivity in response to emotional stimuli has been reported to normalize following treatment of MDD (Mayberg et al., 1999; Sheline et al., 2001; Fu et al., 2004; Victor et al., 2013). Unfortunately, these studies are confounded to some extent as it is unknown whether the normalization of the blood oxygen level-dependent (BOLD) response is due to medication effects or treatment response.
Few fMRI studies have examined facial emotion processing in individuals in the remitted state of MDD (Norbury et al., 2010; Victor et al., 2010; Kerestes et al., 2012). Studies of this type may prove informative for addressing state versus trait-like or scar effects (Lewinsohn et al., 1981; Bhagwagar and Cowen, 2008; Just et al., 2001). Moreover, by conducting such studies in unmedicated, young adults, the potentially confounding influence of medications, residual symptoms and chronic illness recurrence on observed results can be reduced. We aimed to disentangle these state and trait/residual markers in MDD by investigating facial emotion perception in a sample of young adults who had experienced few episodes of MDD but were euthymic (rMDD) whilst undergoing fMRI. First, we hypothesized that the rMDD group would demonstrate impaired facial emotion perception relative to the HC group. Second, based on previous findings in aMDD (e.g. Briceño et al., 2013), we hypothesized that the rMDD group would demonstrate greater amygdala and sgACC activity during facial emotion perception than the HC group.
Methods
Participants
Total participants enrolled in the study included 100 individuals aged 18–23 years. The rMDD group comprised 57 (18 male) individuals with a history of MDD (1–3 prior episodes1) who were in full remission (rMDD) at the time of the study, as defined by DSM-IV-TR criteria. The mean number of years well was 2.93 (SD = 1.72).
The HC group comprised 43 participants (17 male). Participants were recruited from two sites: The University of Michigan (UM, n = 35, 16 HC) and the University of Illinois at Chicago (UIC, n = 65, 27 HC). Diagnosis was assessed via clinical interview (the Diagnostic Interview for Genetic Studies) and confirmed with parent/guardian interview using a modified Family Interview for Genetic studies or treatment records for past MDD diagnosis. One rMDD and one HC participant were left-handed. Exclusion criteria comprised substance abuse or dependence within the past year, psychoactive medication (other than psychostimulant) use within the past 30 days, psychostimulant use within the past 2 days, regular smoking, major chronobiological disruption or phase shift in the preceding month (e.g. transmeridian travel), suicide attempt within the past 6 months or hospitalization for suicidal intent within the past 3 months, neurological condition, personal or family history of psychosis or other contraindications for MRI (e.g. metallic implants). Depression severity was assessed using the Hamilton Depression Rating Scale (HDRS), using a cut-off score of 7 (Zimmerman et al., 2013), excluding eight people with HDRS scores >7 for this report.
Individuals who had movement >1.5 mm or uncorrected artifacts (after despiking using AFNI in >33% of their data were removed from the sample (8 individuals), resulting in a final sample size of 84 (47 rMDD), of which 57 were from UIC.
Due to differences between sites in behavioural performance, IQ, demographic characteristics (see Supplementary Materials) and MRI acquisition (see below), this article presents results from the UIC sample, with replication of fMRI results from the UM sample. Characteristics of and results from the UM sample are presented in the Supplementary Materials , along with comparisons between sites.
Table 1 shows that between groups there was no significant difference in the proportion of males and females, mean age, mean years of education or mean estimated verbal IQ (VIQ) values. A Fisher’s exact test found no significant group difference in the proportion of participants of different races, P = 0.64.
Table 1.
Demographic comparisons between groups
| HC (n = 25) | rMDD (n = 32) | |
|---|---|---|
| Sex | M 11/F 14 | M 11/F 21 |
| Age | 21.12 (1.83) | 21.53 (1.57) |
| Hamilton depressiona | 0.36 (0.91) | 1.77 (1.89)*** |
| Age at onset | N/A | 16.39 (3.90) |
| Education (years) | 14.68 (1.44) | 14.56 (1.41) |
| VIQ | 103.48 (9.31) | 103.30 (9.82) |
Note. ***P = 0.001. aHamilton Depression data is excluding one rMDD participant, with an acutely elevated value (30, death of father) at clinical interview. At time of scanning, Beck Depression Inventory (8) and Personal Health Questionnaire (4) scale were within normal limits.
Measures
Facial emotion perception test
The Facial Emotion Perception Test (FEPT; Rapport et al., 2002; Langenecker et al., 2005, 2007) has been used in previous fMRI studies of aMDD to assess emotion perception and processing (Briceño et al., 2013). This task rapidly presents faces (and animals in the control condition) and requires participants to categorize them by emotion (or animal type). In each trial, a fixation cross is presented for 500 ms, followed by a face for 300 ms expressing either anger, happiness, sadness, fear or neutral emotion, followed by a visual grey-scale mask for 100 ms to prevent visual afterburn phenomena (see Supplementary Materials). This square-shaped mask was a randomized computer scramble of an animal stimulus, and was matched to the facial and animal stimuli for brightness and contrast. In the version used in this study, participants identified which emotion they perceived, and responded in the subsequent 3100 ms using a 5-button response ‘claw’ (Psychology Software Tools). The face stimuli consisted of colour pictures from the MacBrain Foundation set (Tottenham et al., 2009). A pre-scanner computerized practice version used face stimuli from the Ekman and Friesen (1976) set. A control condition required participants to identify animals (primates, cats, dogs, fish and birds) to control for activity related to visual processing, praxis, response selection and execution. Blocks of faces and animals were interspersed with rest blocks across five runs of 4 min, 20 s each. Each run had one or two animal blocks spread amongst four or five faces blocks. Accuracy for correct responses to faces and animals was recorded.
The emotions were counterbalanced so that the response for each emotion and animal had a different finger for different participants. Participants were randomly assigned to a task version, identified by which finger was allocated to ‘happy’. There were no significant differences in the proportions of participants from each group that completed the different task versions, χ2(4) = 1.29, P = 0.86, and task version was included as a covariate in the analyses.
Procedures
Written informed consent was obtained according to the guidelines of the Institutional Review Boards of UM and UIC and consistent with the Declaration of Helsinki (BMJ 1991;302:1194). Participants were compensated for their participation.
Participants completed a practice trial of the FEPT prior to fMRI scanning. During scanning the FEPT was completed during the second half of a 90-min fMRI session. Participants completed other tasks earlier in the scanning session; however, the results of these tasks will be reported separately.
MRI acquisition
Whole-brain imaging was performed at two sites, each using a 3T GE scanner. These details are included in the Supplementary Materials and only briefly reviewed here. The UM scanner was a 3T Signa (release VH3, General Electric, USA) and used a reverse spiral sequence to acquire 36 slices, 3.5-mm thick. The UIC scanner was a 3T GE Discovery and acquired 44 slices, 3-mm thick with a gradient-echo axial echo-planar imaging sequence. To avoid the potential confound of scanner difference, we analyzed the larger UIC fMRI dataset, then visually confirmed results with the UM sample.
MRI processing
SPM8 (http://www.fil.ion.ucl.ac.uk/spm/), AFNI (http://afni.nimh.nih.gov/afni/) and FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) were used to preprocess fMRI data. UM data were despiked using FSL by the technicians at the scanner. Data from UIC were despiked using AFNI at the beginning of our preprocessing protocol, as follows. All data were slice-time corrected in SPM, then realigned to the 10th volume in FSL using MCFLIRT (Jenkinson et al., 2002). Brain extraction of anatomical images was performed with FSL’s Brain Extraction Tool (Smith, 2002) then co-registered to functional images and spatially normalized to Montreal Neurological Institute (MNI) space in SPM, with a final reconstructed spatial resolution of 2 × 2 × 2. Smoothing was completed in SPM with a full-width at half-maximum filter of 5 mm. The subtraction method was used to create contrast images in SPM8. The BOLD signal for the animal blocks was subtracted from the faces blocks (‘faces minus animals contrast’, F−A) to examine facial emotion processing controlling for complex visual processing, motor selection and responses. The second level model was also built in SPM8.
Statistical analysis
For the performance analyses, a 2 (condition) × 2 (group) repeated measures Analysis of Covariance (ANCOVA) was calculated for accuracy, with group as the independent variable, and sex and task version as covariates.
For the neuroimaging data, the rMDD and HC groups were compared on F−A using an ANCOVA, with group as the independent variable, and accuracy on facial emotion and animal trials, sex and task version as covariates. We proceeded with the strategy of analyzing the larger UIC neuroimaging dataset first, and visually confirming with the UM dataset. Additional behavioural and imaging comparisons for the UM sample only, and comparing the UIC and UM sites are explored and reported in the Supplement. The threshold of significance reported for the fMRI analyses was P < 0.005 and k = 55 (P < 0.05 whole brain, after applying corrections for multiple testing using the AFNI program, AlphaSim).
In addition to the whole brain analysis, we also conducted region of interest (ROI) analyses to test specific hypotheses regarding the sgACC and the amygdala. The Marsbar toolbox in SPM8 was used to calculate four ROI 5-mm radius spheres centered on the right and left amygdala (MNI ±23, −5, −19) and sgACC (MNI ±4, 21, −8). The magnitude of the BOLD signal (beta weight) was extracted for each ROI from the second level analysis and compared between groups using a P < 0.05 (uncorrected) threshold. Correlations of activation within these ROIs were also computed with number of previous episodes, VIQ and accuracy for both Faces and Animals. Extent of activity was also calculated for these ROIs by computing the number of voxels in which the t-value exceeded the significance threshold within each ROI.
Finally, correlations between illness variables (number of previous episodes and HDRS score), performance accuracy and ROI variables (magnitude and extent of amygdala activity) were calculated separately for each group.
Results
Performance results
We hypothesized that the rMDD group would show impaired facial emotion perception and not animal categorization relative to the control group; however, there was no significant group by condition interaction, F(1, 53) = 0.01, P = 0.92, η2 < 0.001. There was no significant main effect of group F(1, 53) = 0.06 P = 0.80, η2 < 0.001, but a significant effect of condition, F(1, 53) = 14.73, P < 0.001, η2 = 0.22, with animals more accurately identified than facial emotions. There were no other significant interactions (P > 0.05).
Neuroimaging results
Whole-brain analysis with UIC sample
Table 2 shows the results from the UIC sample only for the BOLD activity during facial emotion perception minus animal identification (F−A contrast) for the HC and rMDD groups separately, and for the rMDD > HC contrast. Both HC and rMDD groups exhibited activation in similar networks (e.g. bilateral inferior frontal gyrus). There were no regions that were significantly more active in the HC than the rMDD group.
Table 2.
fMRI results of F–A contrast for UIC only
| Contrast | Lobe/gyrus | BA | MNI coordinates | peak | UIC cluster | UM on UIC cluster mask | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | Z | mm3 | mm3 | |||
| HC only | Frontal | |||||||
| Inferior frontal | 45 | 58 | 24 | 14 | 4.17 | 4168 | 1240, 8 | |
| 44 | −54 | 16 | 22 | 3.70 | 5176 | 3048 | ||
| Precentral | 4 | 44 | 4 | 44 | 3.46 | 664 | 248, 152 | |
| Superior frontal | 6 | −6 | 18 | 46 | 3.26 | 584 | 408 | |
| rMDD only | Frontal | |||||||
| Inferior frontal | 44 | −48 | 16 | 20 | 5.75 | 24168 | 9032, 120, 96, 8 | |
| 45 | 46 | 22 | 18 | 5.21 | 21328 | 7392, 208, 224, 56, 8, 8 | ||
| Superior frontal | 6 | −8 | 22 | 42 | 5.65 | 5816 | 4272 | |
| 6 | 4 | 14 | 52 | 4.78 | 2632 | 1888 | ||
| Temporal | ||||||||
| Inferior temporal | 20 | 44 | −40 | −20 | 4.82 | 744 | 224 | |
| 20 | 38 | −2 | −40 | 4.69 | 3320 | 248 | ||
| Middle temporal | 22 | 52 | −50 | 4 | 4.46 | 8448 | 3136 | |
| 22 | −58 | −52 | 2 | 4.17 | 3168 | 224 | ||
| Superior temporal | 38 | 46 | 10 | −20 | 4.91 | 840 | ||
| Supramarginal | 40 | −62 | −52 | 28 | 3.32 | 592 | ||
| Occipital | ||||||||
| Inferior occipital | 18 | −24 | −92 | −10 | 4.03 | 3800 | 160 | |
| Lingual | 17 | 22 | −96 | 2 | 3.34 | 2984 | ||
| rMDD > HC | Frontal | |||||||
| Superior frontal | 8 | −4 | 40 | 38 | 3.74 | 2136 | ||
| 6 | −10 | 32 | 56 | 3.44 | 520 | |||
| Temporal | ||||||||
| Fusiform cortex | 37 | 40 | −32 | −18 | 3.46 | 528 | 32 | |
| Superior temporal | 38 | 54 | 0 | −16 | 3.38 | 592 | ||
| Middle temporal | 21 | −58 | −10 | −12 | 3.28 | 736 | 16 | |
| 21 | 54 | 2 | −36 | 3.20 | 1072 | |||
| 21 | −56 | −44 | −4 | 3.06 | 864 | 184 | ||
| Parietal | ||||||||
| Superior lobule | 7 | −30 | −62 | 60 | 3.35 | 480 | ||
| Supramarginal | 40 | −48 | −42 | 44 | 3.14 | 608 | ||
| Occipital | ||||||||
| Lateral cortex, superior | 19 | −30 | −74 | 38 | 2.95 | 608 | 384 | |
We also took an individual differences approach to understanding the relationship between emotion perception skill and functional activation by extracting the magnitude of activation for each significant cluster in the rMDD > HC contrast for each individual, and correlating this with their facial emotion perception accuracy (by group). There were no significant correlations between activation in any of these clusters and performance, in either the rMDD or HC group, and these are reported in the Supplementary Materials.
Replication of activation effects for UM sample in UIC ROIs
We used a simple visual confirmation strategy to display UM activation differences in the framework of significant clusters observed within the UIC sample (Figure 1), given the site differences in IQ and performance, and the smaller UM sample. The clusters of image voxels in which t-values were significant at the whole brain corrected threshold in the UIC sample were used to create a binary mask. We then displayed the UM results through this mask using no threshold. Thus in the figures, the areas where there is any colour indicates regions that were significant in the UIC group at p = .005, k = 55. The warm colours indicate areas of convergent validity between the two studies (cf. t bars), and cool colours indicate regions where the UM data did not support the UIC data. We also calculated the statistics for the UM sample using the whole-brain corrected UIC mask, with a corrected threshold of P = 0.05 as a direct, voxel by voxel replication. These results are shown in the final column of Table 2.
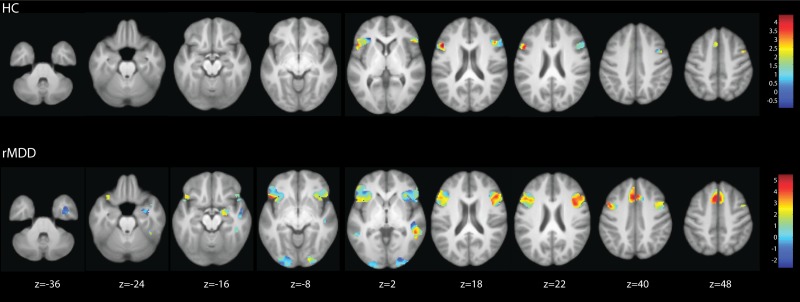
Fig 1.
F−A contrast for HC group (top panel) and remitted MDD group (bottom panel): UM sample shown on mask of significant whole brain corrected clusters for UIC sample.
As shown by the extent of activity in the top panel of Figure 1, for the HC group only, there was significant activity in the bilateral ventro lateral prefrontal cortex, peaking in the inferior frontal gyrus and extending into the right precentral gyrus. The strongest area of convergence (warm colours) between sites in the HC group was the left inferior frontal gyrus, with less strong support in the right precentral gyrus (Figure 1, top panel). The extent of activity in the lower panel of Figure 1 shows that the UIC rMDD group had stronger and more extensive activity in the areas that were significant in the HC group, with the activity in the inferior frontal gyrus extending into the orbitofrontal cortex. There were additional peaks of activity in the bilateral paracingulate gyri, extending into the superior frontal gyri. There was significant activity in the bilateral occipital lobes, the bilateral inferior and middle temporal gyri, the right superior temporal gyrus and the left supramarginal gyrus. The large cluster in the right inferior temporal lobe extended down to include the right amygdala. For the rMDD group, the strongest areas of convergence across sites were in the bilateral paracingulate gyrus, inferior frontal gyrus, right middle temporal gyrus the right amygdala (Figure 1 lower panel).
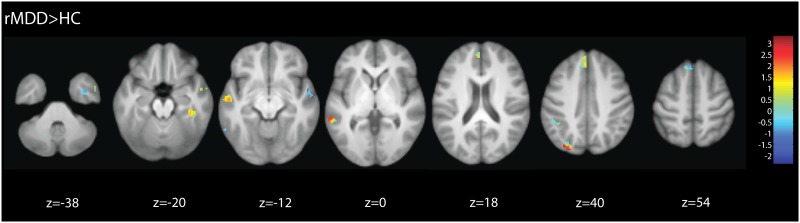
There were no significantly greater foci in HC relative to rMDD. As shown by the extent of activity in Figure 2, in the UIC sample the rMDD group exhibited greater activation than the HC group, with peaks in the bilateral middle temporal gyri, right temporal fusiform cortex and superior temporal gyrus, left superior parietal lobule and supramarginal gyri and the left superior frontal gyrus. For the rMDD > HC contrast, the strongest convergent support across sites was for the left superior lateral occipital cortex/superior parietal lobule and middle temporal gyrus, and right temporal fusiform cortex (Figure 2).
Fig 2.
F−A contrast for remitted MDD minus HC group: UM sample shown on mask of significant whole brain corrected clusters for UIC sample.
We also reversed this convergent validity check by performing an analysis on the UM sample, creating a binary mask of the clusters that were significant at the whole brain corrected threshold for the rMDD greater than HC contrast. These results are reported in the Supplementary Materials.
ROI analysis
Given the substantial literature demonstrating significant effects of active disease in the amygdala and sgACC, we used specific ROIs for these analyses, with an uncorrected significance threshold (P < 0.05). We found that in the left amygdala ROI, the rMDD subjects showed elevated BOLD activity relative to the HC subjects in a cluster of 12 contiguous voxels with peak voxel z = 2.00, located at x = −24, y = −2, z = −18, P = 0.02, uncorrected. Furthermore, in the right amygdala the rMDD subjects showed elevated BOLD activity relative to controls in a cluster of 29 voxels with peak z = 2.06, located at x = 20, y = −2, z = −20, P = 0.02, uncorrected). The ROI analyses for the rMDD > HC contrast found no significant group difference in mean BOLD activity in the left or right sgACC. We also extracted the mean beta weights from the second level model for each ROI for each individual. There were no significant group differences in height of activity for any of the four ROIs.
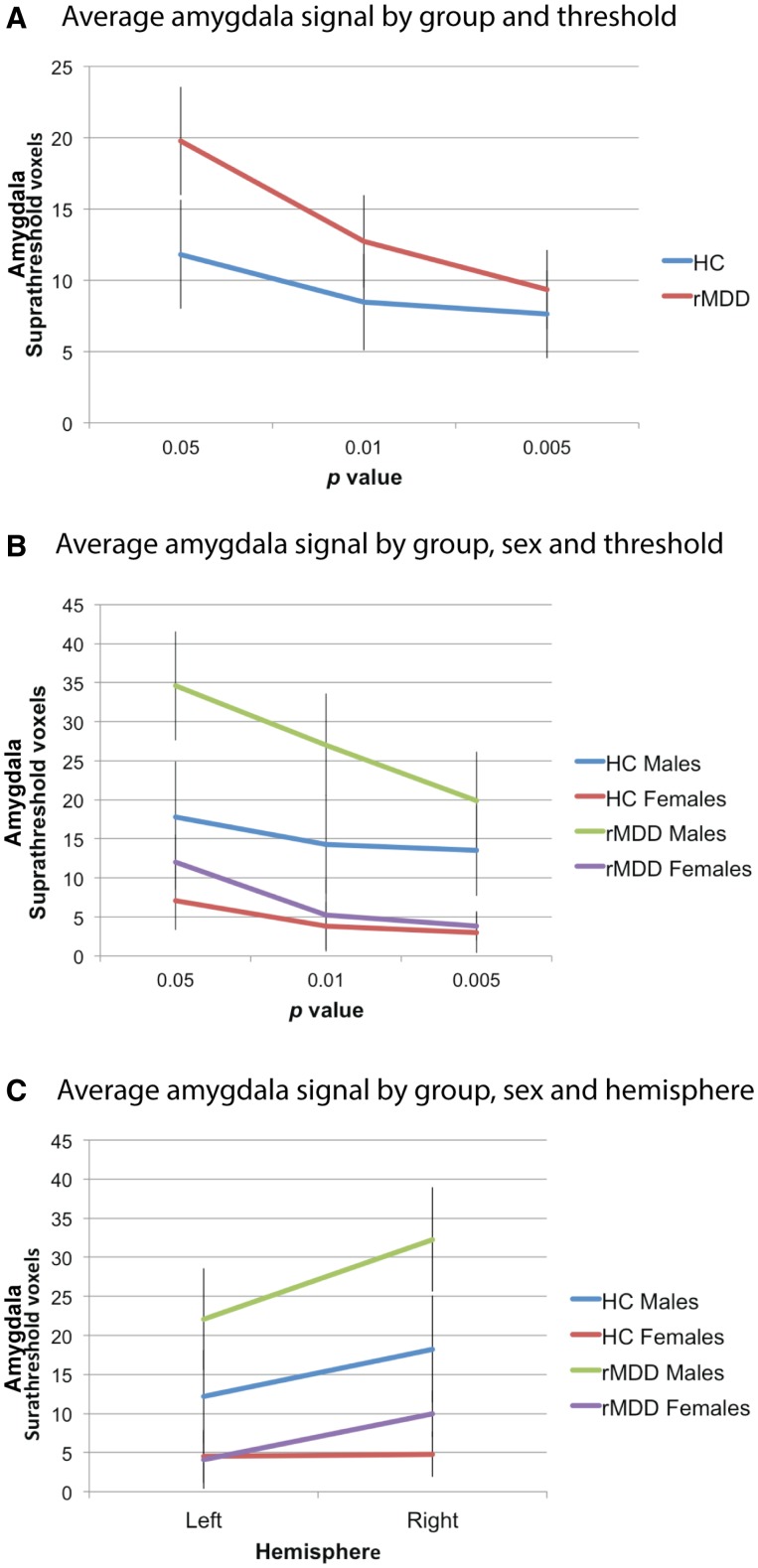
A second strategy looking at the spatial extent of activity within each ROI was also employed, as we compared the number of significant voxels within the ROIs for different statistical thresholds (P = 0.05, 0.01, 0.005). This was achieved using two repeated measures ANCOVAs (one for amygdala and one for sgACC), with group and sex as between subjects factors, threshold and hemisphere as within-subjects factors and task version and Faces and Animals accuracy as covariates. For the sgACC there was no significant effect of group, F(1, 50) = 1.08, P = 0.30, η2 = 0.02 and no significant threshold by group interaction, F(Huynh-Feldt 1.16, 58.13) = 0.44, P = 0.54, η2 < 0.01. For the amygdala analysis, there was a significant hemisphere by sex interaction, F(Huynh-Feldt 785.15, 9752.56) = 4.03, P = 0.05, η2 = 0.08, so separate ANCOVAs were calculated for each hemisphere. For the left hemisphere ROIs there was a significant sex by threshold interaction, F(Huynh-Feldt 274.02, 3672.86) = 3.73, P = 0.04, η2 = 0.07, and a threshold by group interaction, F(Huynh-Feldt 326.26, 3672.86) = 4.44, P = 0.02, η2 = 0.08. This suggests that the difference between the rMDD and HC groups may be contingent upon statistical height thresholds and power. A separate repeated measures ANCOVA was also calculated for the right hemisphere ROIs. There were also a significant threshold by group interaction, F(Huynh-Feldt 362.02, 3348.25) = 5.41, P = 0.01, η2 = 0.10, and a significant main effect of sex, F(1, 50) = 17.61, P < 0.001, η2 = 0.26. These interaction effects are presented in Figure 3, which shows that the rMDD group had a greater number of voxels than the HC group at several P-value thresholds (Panel A), that the rMDD males had the most suprathreshold voxels, followed by the HC males, with the two female groups showing relatively comparable activity (Panel B). Panel C shows that there were more suprathreshold voxels in the right hemisphere than the left for all groups except the HC females, who showed similarly low levels across hemispheres.
Fig 3.
Mean number of suprathreshold voxels at different thresholds for amygdala ROIs.
Correlations with illness variables
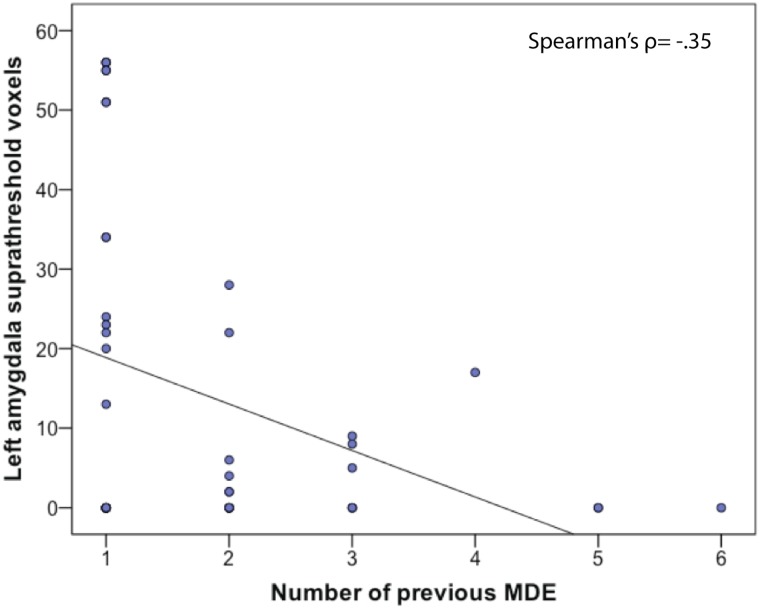
There were no significant within-group correlations for either group between magnitude of the BOLD activity in any ROI and VIQ, performance accuracy or illness variables. We also calculated within-group correlations between extent of ROI activity (calculated at P = 0.05), VIQ, illness and performance variables. For the rMDD group, there was a significant negative correlation between number of previous episodes and extent of activity in the left amygdala, Spearman’s ρ = −0.35, P = 0.05. This correlation is shown in Figure 4.
Fig 4.
Correlation between number of previous episodes of MDD and extent of activity in left amygdala ROI.
Discussion
We demonstrated that numerous brain regions were hyperactive in rMDD during facial emotion processing compared with a HC group. Our study is unique in that we conducted a within-study replication of findings using independent samples collected at different sites. Thus, with added confidence from convergent validity across samples, we conclude that compared with HCs, during facial emotion processing (minus animal perception), individuals with rMDD show increased activity in the bilateral middle temporal gyrus, left superior frontal gyrus and left lateral occipital cortex. Furthermore, there were no significant behavioural differences between groups, which was unexpected. These results are consistent with a previous meta-analysis of emotional face processing (Sabatinelli et al., 2011), and suggest that individuals with rMDD require increased compensatory neural activity to achieve similar levels of behavioural performance to HCs.
When we examined F-A just in individuals with rMDD, we observed hyperactivity in the bilateral ventrolateral prefrontal, paracingulate and superior temporal cortices, and the right amygdala, with strong convergent validity across sites. Thus we found weak support for the hypothesis of increased amygdala activity in rMDD relative to HC. The overall claim of relative amygdala hyperactivity is supported by the ROI analyses, but the absence of a significant effect in the rMDD > HC contrast limits the conclusion within the whole-brain analyses. The interactions depicted in Figure 3 suggest a significant group effect in the whole brain analysis may have oversimplified group by sex by hemisphere interactions. Intriguingly, it appeared that males with rMDD were more likely to have increased amygdala activation relative to same sex controls. Many previous studies have included insufficient numbers of males to evaluate sex differences; however, so our finding merits further investigation.
Areas that were significant at the whole-brain corrected threshold in the UIC sample but that had less convergent validity from the UM sample included the bilateral occipital poles and the right temporal pole. There was significant inferior frontal gyrus activity in both groups. This is a similar region that was reported as showing relatively less left hemisphere activation in aMDD relative to HC reported in Briceño et al. (2013). The inferior frontal gyrus forms part of the ventrolateral prefrontal cortex (VLPFC), which has frequently been implicated in depression (reviewed in Price and Drevets, 2010). The VLPFC, which includes the lateral orbitofrontal cortex more inferiorly, is important for cognitive control (Levy and Wagner, 2011), particularly the inhibitory control of emotion (Hooker and Knight, 2006). For example, lateral orbitofrontal activity has been found to be associated with successful decrease of negative affect (Ochsner et al., 2004) in response to emotional images. The paracingulate segment of the superior frontal gyrus was also active in the rMDD group, and this region of the medial prefrontal cortex is important for social cognition, including inferring the thoughts, feelings and emotions of others (Frith and Frith, 1999; Gallagher et al., 2002). The amygdala and superior frontal gyrus have both been found to be involved in cognitive aspects of emotional processing (Drevets and Raichle, 1998; Gusnard et al., 2001), and functional neuroimaging evidence suggests the dorsomedial prefrontal cortex is involved in the attenuation of amygdala activity during emotional processing (Shin et al., 2004; Phan et al., 2005). Areas of the lateral occipital visual cortex were also significantly active in the rMDD group, consistent with meta-analytic findings of emotional face processing (Sabatinelli et al., 2011). This is likely to reflect the amygdala’s role in increasing the direction of visual attention to potentially threatening stimuli (Morris et al., 1998; Surguladze et al., 2003), and it’s reciprocal anatomical connections with sensory association areas and feedback to early visual processing areas (Tigges et al., 1983; Amaral and Price, 1984; Iwai and Yukie, 1987).
The regions identified in the rMDD group form part of an extended circuit that has been implicated in MDD (Price and Drevets, 2012). In particular, imaging, deep-brain stimulation and histopathological studies have identified the amygdala and medial prefrontal cortex as crucially involved in mood disorders (Price and Drevets, 2010). These two areas have strong anatomical connections (Öngür and Price, 2000), and extend to involve a broader network that includes the rostral and medial temporal and dorsal prefrontal cortex (Price and Drevets, 2010). This network is closely related to a ventrolateral/orbitofrontal prefrontal network that is also anatomically connected to the amygdala, however is argued to have overlapping, but distinct functions to the medial network that are more closely related to assessing reward value of stimuli (Price and Drevets, 2012; Jenkins et al., 2014). However, the rMDD > HC contrast did not demonstrate activity in this extended network implicated in mood disorders, and the hypothesis that there would be significantly greater sgACC activity in the rMDD group relative to the HC group was also not supported. Our results suggest that state or chronic illness burden effects may primarily drive many of the affective processing findings in aMDD studies. However, a prospective study is needed to determine whether this finding suggests trait vulnerability for developing MDD or residual ‘scar’ effects of disease.
The behavioural results of this study suggest that emotion perception difficulties in aMDD do not persist or may be diminished during the remitted state. Our results support those of previous studies that also failed to find significant behavioural differences between patients with rMDD and HCs during emotional tasks (Kerestes et al., 2012; Norbury et al., 2010) and contrast those of previous findings with aMDD patients (e.g. Cooley and Nowicki, 1989; Langenecker et al., 2005; Kohler et al., 2011). These data thus are consistent with the hypothesis of state specific normalization of behavioural performance on the FEPT. For example, our results offer behavioural support for the Differential Activation Hypothesis (Teasdale, 1988), which posits that individuals in a depressed mood state have disrupted emotional processing that is normal in a remitted or asymptomatic state.
Other cognitive theories of depression suggest that enduring negative biases are the primary trait vulnerability factors (e.g. Beck, 1976; Mathews and Macleod, 1994). However, we did not find a significant difference between groups in performance; therefore the results of this study do not offer support for such theories. We did find significant group differences in neuronal activity in multiple brain regions. These group differences could represent residual effects of illness or underlying trait vulnerabilities, however since we did not test individuals at high risk for developing MDD, we cannot determine whether they represent risk factors. Amygdala hyper-reactivity during the processing of emotionally expressive faces appears to be at least a residual marker in those predisposed to MDD, showing similar results in our rMDD group as the heightened amygdala activity during aMDD (Sheline et al., 2001; Surguladze et al., 2005; Fu et al., 2004, 2008a,b). Our findings are also consistent with reports that activity in the sgACC normalizes following treatment of MDD (e.g. Victor et al., 2013), with no significant group difference in this region.
One intriguing result here is that in the absence of active treatments plus no significant symptoms, there is still evidence of amygdala hyperactivation. Thus, the normalization of amygdala hyperactivity following pharmaceutical treatment of MDD may be treatment, and not remission specific (Mayberg et al., 1999; Sheline et al., 2001; Fu et al., 2004, 2008a; Victor et al. 2010). Our results have the benefit of not being confounded by current medication use, unlike most previous studies. It remains unclear if amygdala hyperactivity was attenuated in these rMDD participants relative to active illness states, as the study was cross-sectional. Particularly, as the number of previous episodes went up, the extent of activity in the left amygdala in the rMDD group went down. Given the variability of fMRI data obtained in studies of MDD, it’s possible that previously reported differences in amygdala and sgACC activity may relate to variables such as number of episodes, age, symptoms and IQ.
Some limitations of the present study exist. Although we required participants to be medication-free for at least one month, this minimum period is shorter than that of some other studies (e.g. Norbury et al., 2010 used 8 months, Kerestes et al., 2012 used 4 months). The data were collected from two sites, and sampling error meant that the participants tested at the UIC and UM sites were not equivalent in estimated IQ or FEPT task performance. We did not equate the signal to noise ratio between the two scanners, as data were collected at UM prior to transfer to UIC. Technological error meant that data from each site were despiked using different programs. Finally, the two sites used different MRI sequences. This may have led to susceptibility artifact differences and precluded effective analysis of the role of more ventral aspects of the sgACC in some of the UIC participants. To address these issues, we used the UM sample for confirmation and replication analyses, which provided within-study convergent validity. Effects of site and site by diagnosis interactions are reported in the Supplementary Materials. Furthermore, the fMRI F−A analyses cannot determine whether differences arose from the animal or face identification condition. As mentioned, we cannot confidently conclude whether our observed group differences in fMRI measures represent early scar or underlying trait effects, however measuring individuals early in the course of illness can guard against repetitive scar as an explanation for group differences. Finally, the selection criteria of few episodes and current wellness likely indicate that this is a higher functioning group relative to the population of those who suffer from chronic MDD. In some respects this difference makes any results more conservative, but in others it may reflect aspects of flexibility and resilience that may not be present in the broader population of individuals that suffer from chronic MDD.
In this study, we used functional neuroimaging in an attempt to identify markers that could potentially represent biological intermediate phenotypes in individuals in the remitted state of MDD. Dorsomedial, posterior parietal activity, and to a lesser extent amygdala activity, but not sgACC hyperactivity, may be such traits. Middle temporal and lateral occipital hyperactivity may also be markers. The lack of significant group behavioural differences in facial emotion perception accuracy suggests that impaired emotion perception in MDD is a state phenomenon. We argue that emotion perception in individuals with MDD in the active phase involves both state factors and possibly either residual or trait vulnerabilities, whereas only the latter two possibilities are present in the remitted state. Future research should aim to further disentangle residual markers from trait vulnerabilities. Specifically, studies examining individuals at high risk for, but who have not yet developed a mood disorder will allow us to distinguish between residual markers and trait vulnerability factors for MDD. Longitudinal studies can also follow participants to the point of MDD relapse, to more carefully evaluate state vs trait effects. This is an important goal for future research because identifying intermediate phenotype vulnerability factors for MDD may allow for earlier detection and intervention, as well as secondary prevention, which will reduce risk of relapse, and illness burden on individuals and society.
Funding
This work was supported by NIMH grant to S.A.L. (mh091811).
Supplementary Material
Footnotes
1 mean = 2, median = 1 episode. However, one person with four episodes, two people with five episodes and one with six episodes were included with seasonal affective components to the illness.
References
- Almeida J. R. C., Versace A., Hassel S., Kupfer D. J., Phillips M. L. (2010). Elevated amygdala activity to sad facial expressions: a state marker of bipolar but not unipolar depression. Biological Psychiatry, 67(5), 414–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral D. G., Price J. L. (1984). Amygdalo-cortical projections in the monkey (macaca-fascicularis). Journal of Comparative Neurology , 230(4), 465–96. [DOI] [PubMed] [Google Scholar]
- Beck A. (1976). Cognitive Therapy and the Emotional Disorders. Madison, CT: International Universities Press. [Google Scholar]
- Bhagwagar Z., Cowen P. J. (2008). ‘It’s not over when it’s over’: persistent neurobiological abnormalities in recovered depressed patients. Psychological Medicine, 38, 307–13. [DOI] [PubMed] [Google Scholar]
- Bouhuys A. L., Geerts E., Gordijn M. C. M. (1999). Depressed patients’ perceptions of facial emotions in depressed and remitted states are associated with relapse: a longitudinal study. Journal of Nervous and Mental Disease , 187(10), 595–602. [DOI] [PubMed] [Google Scholar]
- Bouhuys A. L., Geerts E., Mersch P. P. A., Jenner J. A. (1996). Nonverbal interpersonal sensitivity and persistence of depression: perception of emotions in schematic faces. Psychiatry Research , 64(3), 193–203. [DOI] [PubMed] [Google Scholar]
- Briceño E. M., Weisenbach S. L., Rapport L. J., et al. (2013). Shifted inferior frontal laterality in women with major depressive disorder is related to emotion processing deficits. Psychological Medicine , 43(7), 1433–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T., Cooney R. E., Goldin P., et al. (2005). Amygdala reactivity to emotional faces predicts improvement in major depression. Neuroreport , 16(12), 1267–70. [DOI] [PubMed] [Google Scholar]
- Cooley E. L., Nowicki S. (1989). Discrimination of facial expressions of emotion by depressed subjects. Genetic Social and General Psychology Monographs , 115(4), 449–65. [PubMed] [Google Scholar]
- Csukly G., Czobor P., Szily E., Takacs B., Simon L. (2009). Facial expression recognition in depressed subjects: the impact of intensity level and arousal dimension. Journal of Nervous and Mental Disease , 197(2), 98–103. [DOI] [PubMed] [Google Scholar]
- Demenescu L. R., Renken R., Kortekaas R., et al. (2011). Neural correlates of perception of emotional facial expressions in out-patients with mild-to-moderate depression and anxiety: a multicenter fMRI study. Psychological Medicine , 41(11), 2253–64. [DOI] [PubMed] [Google Scholar]
- Dolan R. J., Fletcher P., Morris J., Kapur N., Deakin J. F. W., Frith C. D. (1996). Neural activation during covert processing of positive emotional facial expressions. Neuroimage , 4(3), 194–200. [DOI] [PubMed] [Google Scholar]
- Drevets W. C., Raichle M. E. (1998). Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: implications for interactions between emotion and cognition. Cognition and Emotion , 12(3), 353–85. [Google Scholar]
- Ekman P., Friesen P. (1976). Pictures of Facial Affect. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Feinberg T. E., Rifkin A., Schaffer C., Walker E. (1986). Facial discrimination and emotional recognition in schizophrenia and affective disorders. Archives of General Psychiatry , 43(3), 276–9. [DOI] [PubMed] [Google Scholar]
- Fournier J. C., Keener M. T., Almeida J., Kronhaus D. M., Phillip M. L. (2013). Amygdala and whole-brain activity to emotional faces distinguishes major depressive disorder and bipolar disorder. Bipolar Disorders , 15(7), 741–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith C. D., Frith U. (1999). Interacting minds: a biological basis. Science. 286(5445), 1692–5. [DOI] [PubMed] [Google Scholar]
- Frodl T., Scheuerecker J., Albrecht J., et al. (2009). Neuronal correlates of emotional processing in patients with major depression. World Journal of Biological Psychiatry , 10(3), 202–8. [DOI] [PubMed] [Google Scholar]
- Fu C. H. Y., Mourao-Miranda J., Costafrecla S. G., et al. (2008a). Pattern classification of sad facial processing: toward the development of neurobiological markers in depression. Biological Psychiatry , 63(7), 656–62. [DOI] [PubMed] [Google Scholar]
- Fu C. H. Y., Williams S. C. R., Cleare A. J., et al. (2004). Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Archives of General Psychiatry , 61(9), 877–89. [DOI] [PubMed] [Google Scholar]
- Fu C. H. Y., Williams S. C. R., Cleare A. J., et al. (2008b). Neural responses to sad facial expressions in major depression following cognitive behavioral therapy. Biological Psychiatry , 64(6), 505–12. [DOI] [PubMed] [Google Scholar]
- Gallagher H. L., Jack A. I., Roepstorff A., Frith C. D. (2002). Imaging the intentional stance in a competitive game. NeuroImage , 16(3 Pt 1), 814–21. [DOI] [PubMed] [Google Scholar]
- Gotlib I. H., Sivers H., Gabrieli J. D. E., et al. (2005). Subgenual anterior cingulate activation to valenced emotional stimuli in major depression. Neuroreport , 16(16), 1731–4. [DOI] [PubMed] [Google Scholar]
- Gur R. C., Erwin R. J., Gur R. E., Zwil A. S., Heimberg C., Kraemer H. C. (1992). Facial emotion discrimination II: behavioral findings in depression. Psychiatry Research , 42(3), 241–51. [DOI] [PubMed] [Google Scholar]
- Gusnard D. A., Akbudak E., Shulman G. L., Raichle M. E. (2001). Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America , 98(7), 4259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale W. W. (1998). Judgment of facial expressions and depression persistence. Psychiatry Research , 80(3), 265–74. [DOI] [PubMed] [Google Scholar]
- Hale W. W., Jansen J. H. C., Bouhuys A. L., van den Hoofdakker R. H. (1998). The judgment of facial expressions by depressed patients, their partners and controls. Journal of Affective Disorders , 47(1–3), 63–70. [DOI] [PubMed] [Google Scholar]
- Hooker C., Knight R. (2006). The role of the lateral orbital cortex in the inhibitory control of emotion. In: Zald D., Rauch S., editors. The Orbitofrontal Cortex. New York: Oxford University Press, 307–24. [Google Scholar]
- Iwai E., Yukie M. (1987). Amygdalofugal and amygdalopetal connections with modality-specific visual cortical areas in macaques (macaca-fuscata, m.-mulatta, and macaca-fascicularis). Journal of Comparative Neurology , 261(3), 362–87. [DOI] [PubMed] [Google Scholar]
- Jenkins L. M., Andrewes D. G., Nicholas C. L., et al. (2014). Social cognition in patients following surgery to the prefrontal cortex. Psychiatry Research: Neuroimaging, 224, 192–203. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady J. M., Smith S. M. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17(2), 825–41. [DOI] [PubMed] [Google Scholar]
- Joormann J., D’Avanzato C. (2010). Emotion regulation in depression: examining the role of cognitive processes. Cognition and Emotion , 24(6), 913–39. [Google Scholar]
- Joormann J., Gotlib I. H. (2006). Is this happiness I see? Biases in the identification of emotional facial expressions in depression and social phobia. Journal of Abnormal Psychology , 115(4), 705–14. [DOI] [PubMed] [Google Scholar]
- Joormann J., Gotlib I. H. (2007). Selective attention to emotional faces following recovery from depression. Journal of Abnormal Psychology , 116(1), 80–5. [DOI] [PubMed] [Google Scholar]
- Just N., Abramson L. Y., Alloy L. B. (2001). Remitted depression studies as tests of the cognitive vulnerability hypotheses of depression onset: a critique and conceptual analysis. Clinical Psychology Review , 21(1), 63–83. [DOI] [PubMed] [Google Scholar]
- Keedwell P., Drapier D., Surguladze S., Giampietro V., Brammer M., Phillips M. (2009). Neural markers of symptomatic improvement during antidepressant therapy in severe depression: Subgenual cingulate and visual cortical responses to sad, but not happy, facial stimuli are correlated with changes in symptom score. Journal of Psychopharmacology , 23(7), 775–88. [DOI] [PubMed] [Google Scholar]
- Kerestes R., Bhagwagar Z., Nathan P. J., et al. (2012). Prefrontal cortical response to emotional faces in individuals with major depressive disorder in remission. Psychiatry Reseach: Neuroimaging, 202, 30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerestes R., Ladouceur C. D., Meda S., et al. (2012). Abnormal prefrontal activity subserving attentional control of emotion in remitted depressed patients during a working memory task with emotional distracters. Psychological Medicine , 42(1), 29–40. [DOI] [PubMed] [Google Scholar]
- Klerman G. L., Weissman M. M. (1992). The course, morbidity, and costs of depression. Archives of general psychiatry , 49(10), 831–4. [DOI] [PubMed] [Google Scholar]
- Kohler C. G., Hoffman L. J., Eastman L. B., Healey K., Moberg P. J. (2011) Facial emotion perception in depression and bipolar disorder: a quantitative review. Psychiatry Research , 188, 303–9. [DOI] [PubMed] [Google Scholar]
- Langenecker S. A., Bieliauskas L. A., Rapport L. J., Zubieta J. K., Wilde E. A., Berent S. (2005). Face emotion perception and executive functioning deficits in depression. Journal of Clinical and Experimental Neuropsychology , 27, 320–33. [DOI] [PubMed] [Google Scholar]
- Langenecker S. A., Caveny A. F., et al. (2007). The sensitivity and psychometric properties of a brief computer-based cognitive screening battery in a depression clinic. Psychiatry Research , 152, 143–54. [DOI] [PubMed] [Google Scholar]
- Lawrence N. S., Williams A. M., Surguladze S., et al. (2004). Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biological Psychiatry , 55(6), 578–87. [DOI] [PubMed] [Google Scholar]
- Lee B. T., Seok J. H., Lee B. C., et al. (2008). Neural correlates of affective processing in response to sad and angry facial stimuli in patients with major depressive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry , 32(3), 778–85. [DOI] [PubMed] [Google Scholar]
- LeMoult J., Joormann J., Sherdell L., Wright Y., Gotlib I. H. (2009). Identification of emotional facial expressions following recovery from depression. Journal of Abnormal Psychology , 118(4), 828–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B. J., Wagner A. D. (2011). Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Annals of the New York Academy of Sciences, 1224(1), 40–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn P. M., Steinmetz J. L., Larson D. W., Franklin J. (1981). Depression-related cognitions: Antecedent or consequence. Journal of Abnormal Psychology , 90(3), 213–9. [DOI] [PubMed] [Google Scholar]
- Mathews A., Macleod C. (1994). Cognitive approaches to emotion and emotional disorders. Annual Review of Psychology, 45, 25–50. [DOI] [PubMed] [Google Scholar]
- Mayberg H. S., Liotti M., Brannan S. K., et al. (1999). Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. American Journal of Psychiatry , 156(5), 675–82. [DOI] [PubMed] [Google Scholar]
- Mikhailova E. S., Vladimirova T. V., Iznak A. F., Tsusulkovskaya E. J., Sushko N. V. (1996). Abnormal recognition of facial expression of emotions in depressed patients with major depression disorder and schizotypal personality disorder. Biological Psychiatry , 40, 697–705. [DOI] [PubMed] [Google Scholar]
- Morris J. S., Friston K. J., Buchel C., et al. (1998). A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain , 121, 47–57. [DOI] [PubMed] [Google Scholar]
- Mueller T. I., Leon A. C., Keller M. B., et al. (1999). Recurrence after recovery from major depressive disorder during 15 years of observational follow-up. American Journal of Psychiatry, 156(7), 1000–6. [DOI] [PubMed] [Google Scholar]
- Norbury R., Selvaraj S., Taylor M. J., Harmer C., Cowen P. J. (2010). Increased neural response to fear in patients recovered from depression: a 3T functional magnetic resonance imaging study. Psychological Medicine , 40(3), 425–32. [DOI] [PubMed] [Google Scholar]
- Ochsner K. N., Ray R. D., Cooper J. C., et al. (2004). For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage , 23, 483–99. [DOI] [PubMed] [Google Scholar]
- Öngür D., Price J. L. (2000). The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex , 10, 206–19. [DOI] [PubMed] [Google Scholar]
- Persad S. M., Polivy J. (1993). Differences between depressed and nondepressed individuals in the recognition of and response to facial emotional cues. Journal of Abnormal Psychology , 102(3), 358–68. [DOI] [PubMed] [Google Scholar]
- Phan K. L., Fitzgerald D. A., Nathan P. J., Moore G. J., Uhde T. W., Tancer M. E. (2005). Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biological Psychiatry , 57(3), 210–9. [DOI] [PubMed] [Google Scholar]
- Price J. L., Drevets W. C. (2010). Neurocircuitry of mood disorders. Neuropsychopharmacology Reviews , 35(1), 192–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J. L., Drevets W. C. (2012). Neural circuits underlying the pathophysiology of mood disorders. Trends in Cognitive Sciences , 16(1), 61–71. [DOI] [PubMed] [Google Scholar]
- Rapport L. J., Friedman S. R., Tzelepis A., Van Voorhis A. (2002). Experienced emotion and affect recognition in adult attention-deficit hyperactivity disorder. Neuropsychology , 16(1), 102–10. [DOI] [PubMed] [Google Scholar]
- Rubinow D. R., Post R. M. (1992). Impaired recognition of affect in facial expression in depressed patients. Biological Psychiatry , 31(9), 947–53. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D., Fortune E. E., Li Q., et al. (2011) Emotion perception: Meta-analysis of face and natural scene processing. Neuroimage, 54, 2524–33. [DOI] [PubMed] [Google Scholar]
- Sheline Y. I., Barch D. M., Donnelly J. M., Ollinger J. M., Snyder A. Z., Mintun M. A. (2001). Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biological Psychiatry , 50(9), 651–8. [DOI] [PubMed] [Google Scholar]
- Shin L. M., Orr S. P., Carson M. A., et al. (2004). Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Archives of General Psychiatry , 61(2), 168–76. [DOI] [PubMed] [Google Scholar]
- Smith S. M. (2002). Fast robust automated brain extraction. Human Brain Mapping , 17(3): 143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon D. A., Keller M. B., Leon A. C., et al. (2000). Multiple recurrences of major depressive disorder. American Journal of Psychiatry, 157(2), 229–33. [DOI] [PubMed] [Google Scholar]
- Strand M., Saetrevik B., Lund A., Hammar A. (2013). The relationship between residual symptoms of depression and emotional information processing. Nordic Journal of Psychiatry , 67(4), 233–9. [DOI] [PubMed] [Google Scholar]
- Surguladze S., Brammer M. J., Keedwell P., et al. (2005). A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biological Psychiatry , 57(3), 201–9. [DOI] [PubMed] [Google Scholar]
- Surguladze S. A., Brammer M. J., Young A. W., et al. (2003). A preferential increase in the extrastriate response to signals of danger. Neuroimage , 19(4), 1317–28. [DOI] [PubMed] [Google Scholar]
- Surguladze S. A., Young A. W., Senior C., Brebion G., Travis M. J., Phillips M. L. (2004). Recognition accuracy and response bias to happy and sad facial expressions in patients with major depression. Neuropsychology , 18(2), 212–8. [DOI] [PubMed] [Google Scholar]
- Teasdale J. D. (1988). Cognitive vulnerability to persistent depression. Cognition and Emotion , 2(3), 247–4. [Google Scholar]
- Tigges J., Walker L. C., Tigges M. (1983). Subcortical projections to the occipital and parietal lobes of the chimpanzee brain. Journal of Comparative Neurology , 220(1), 106–15. [DOI] [PubMed] [Google Scholar]
- Tottenham N., Tanaka J. W., Leon A. C., et al. (2009). The NimStim set of facial expressions: Judgements from untrained research participants. Psychiatry Research , 168(3), 242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor T. A., Furey M. L., Fromm S. J., Ohman A., Drevets W. C. (2010). Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Archives of General Psychiatry , 67(11), 1128–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor T. A., Furey M. L., Fromm S. J., Ohman A., Drevets W. C. (2013). Changes in the neural correlates of implicit emotional face processing during antidepressant treatment in major depressive disorder. International Journal of Neuropsychopharmacology , 16(10), 2195–208. [DOI] [PubMed] [Google Scholar]
- Zimmerman M., Martinez J. H., Young D., Chelminski I., Dalrymple K. (2013). Severity classification on the Hamilton Depression Rating Scale. Journal of Affective Disorders , 150(2), 384–8. [DOI] [PubMed] [Google Scholar]
- Zuroff D. C., Colussy S. A. (1986). Emotion recognition in schizophrenic and depressed inpatients. Journal of Clinical Psychology , 42(3), 411–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.