Abstract
Although it has been proposed that schizophrenia is characterized by impaired empathy, several recent studies found intact neural responses on tasks measuring the affective subdomain of empathy. This study further examined affective empathy in 21 schizophrenia outpatients and 21 healthy controls using a validated pain empathy paradigm with two components: (i) observing videos of people described as medical patients who were receiving a painful sound stimulation treatment; (ii) listening to the painful sounds (to create regions of interest). The observing videos component incorporated experimental manipulations of perspective taking (instructions to imagine ‘Self’ vs ‘Other’ experiencing pain) and cognitive appraisal (information about whether treatment was ‘Effective’ vs ‘Not Effective’). When considering activation across experimental conditions, both groups showed similar dorsal anterior cingulate cortex (dACC) and anterior insula (AI) activation while merely observing others in pain. However, there were group differences associated with perspective taking: controls showed relatively greater dACC and AI activation for the Self vs Other contrast whereas patients showed relatively greater activation in these and additional regions for the Other vs Self contrast. Although patients demonstrated grossly intact neural activity while observing others in pain, they showed more subtle abnormalities when required to toggle between imagining themselves vs others experiencing pain.
Keywords: empathy, schizophrenia, self-related processing, pain, affective empathy, social cognition
Introduction
Social neuroscience models define empathy as the ability to understand and share the thoughts and feelings of others. There is general agreement that empathy is a multidimensional construct, which includes distinct cognitive and affective processes (Decety and Jackson 2006; Lamm et al., 2007; Shamay-Tsoory, 2011; Zaki and Ochsner, 2012). Cognitive empathy refers to effortful, reflective mentalizing processes that include taking the perspective of others and making inferences about their mental states. In contrast, affective empathy refers to relatively automatic experience sharing processes through which observed actions and social/emotional cues trigger a shared neural response in the observer. These sub-processes involve separate neural systems and adaptive empathic responding is believed to involve coordinated interaction between them (Zaki and Ochsner, 2011).
Studies of empathy in schizophrenia have primarily examined cognitive empathy, reporting consistent evidence of diminished empathy across self-report, behavioral and fMRI tasks (Benedetti et al., 2009; Lee et al., 2010; Derntl et al., 2012, 2015; Horan et al., 2015; Smith et al., 2015). The more limited research on affective empathy provides a less consistent pattern. Some studies found diminished neural responses during motor action or facial expression execution–observation tasks using electroencephalogram (EEG), transcranial magnetic stimulation (TMS), or functional magnetic resonance imaging (fMRI) (see Mehta et al., 2014). However, others found that individuals with schizophrenia self-report normal or elevated scores on measures of affective empathy (e.g. personal distress) (see Michaels et al., 2014), and demonstrate normal or elevated neural activity during relevant EEG paradigms (e.g. McCormick et al., 2012; Horan et al., 2014a). Furthermore, a recent fMRI study found normal activation patterns in mirror neuron system regions during an action execution–observation task that included simple finger movements and complex facial emotion expressions (Horan et al., 2014b). To further examine the affective empathy subdomain, the current fMRI study focuses on pain empathy, a topic that has been extensively studied in healthy individuals but rarely considered in schizophrenia.
Empathy for physical pain
Pain empathy fMRI paradigms typically examine neural responses during the first hand experience of pain (e.g. painful shock or auditory stimulation) and while exposed to stimuli depicting or indicating that other people are in pain. With remarkable consistency in over 30 studies, an overlapping set of brain regions activate while processing both self- and other-related pain (Lamm et al., 2011). This includes a core network comprised of dorsal anterior cingulate cortex (dACC) and bilateral anterior insula (AI), which are involved in the affective and motivational aspects of processing of pain. This overlapping neural coding of the motivational-affective dimension of pain has been taken as evidence that ‘shared representations’ between self and other are central to affective sharing and empathy.
Although shared representations are fundamental to affective empathy, a complete overlap between one’s own pain and the pain of others would lead to overwhelming personal distress and actually hamper our ability to respond empathically (Decety and Lamm, 2006). In fact, a number of cognitive, emotional and social factors have been found to modulate our responses to the pain of others, ranging from personal characteristics (trait empathy, alexithymia), mood state, perceived fairness and in-group vs out-group status (Bernhardt and Singer, 2012; Bufalari and Ionta, 2013). The current study focuses on two such modulators, perspective taking and contextual appraisals, which have been found to impact activation patterns during pain empathy tasks.
Regarding perspective taking, adaptive empathic responding requires the ability to toggle back and forth between considering one’s own perspective vs another person’s perspective. This process has been manipulated in fMRI studies that instructed participants to imagine how another person would feel and how you would feel while viewing stimuli depicting others experiencing pain. Considerable social psychology research has examined the emotional and motivational consequences of perspective taking (Underwood and Moore, 1982; Batson et al., 1987, 1997; Batson, 2009). Focusing on another person’s feelings (imagine other) while viewing them in pain typically evokes empathic concern, an other-oriented response that is congruent with the perceived suffering of the person in need. In contrast, explicitly putting yourself in the shoes of the person in pain (imagine self) leads to both empathic concern and personal distress (a self-oriented aversive response). The capacity to consider another’s pain, while maintaining a boundary from the personal distress associated with one’s own pain, is thought to facilitate adaptive, prosocial responding to others in need of assistance. Consistent with these findings, viewing others in pain while imagining self has been found to elicit greater activation than imagining other in dACC and AI, as well as somatosensory cortex, the posterior part of the dACC, and the middle insula (Jackson et al., 2006; Lamm et al., 2007).
Regarding contextual appraisals, the ability to flexibly re-appraise a situation is critical for regulating one’s own emotions in order to adaptively respond to others who are experiencing distress (Ochsner and Gross, 2005; Buhle et al., 2014). fMRI studies indicate that regions such as the dACC and prefrontal cortical regions are sensitive to experimental manipulations of contextual appraisals. For example, in a study by Lamm et al. (2007), participants viewed videos of patients receiving a painful medical procedure, and contextual appraisals were manipulated by providing information about whether treatment was ultimately effective or not effective for each patient. Activity in the dACC was lower while viewing patients for whom the treatment was effective, suggesting that participants’ down-regulated their affective responses when they knew that the painful treatment had a beneficial effect. In summary, merely observing others in pain activates regions of the dACC and AI that are also activated during the first hand experience of pain, though activation levels in these regions can be modulated by perspective taking and contextual appraisal manipulations.
Pain empathy in schizophrenia
Despite the well-developed literature on pain empathy, only a few studies have considered this construct in schizophrenia. Behaviorally, individuals with schizophrenia show diminished ability to recognize painful expressions in others (see Wojakiewicz et al., 2013). One study used an event-related potential (ERP) pain empathy paradigm and found that while patients showed generally smaller early ERP components (N110, P180, N240; thought to reflect affect sharing) than controls while viewing pictures of hands in painful or non-painful situations, there were no significant interactions of group by stimulus type, suggesting sensitivity to pain-relevant stimuli was not different (Corbera et al., 2014).
In this study, an adapted version of the pain empathy task developed by Lamm et al. (2007) was administered to individuals with schizophrenia and healthy controls. The paradigm allowed us to examine neural activity in experienced vs observed pain, as well as the modulatory influences of perspective taking and contextual appraisals. Based on the meta-analysis by Lamm et al. (2011), we focused on the dACC and AI in this first study fMRI study of pain empathy in schizophrenia. Based on our recent finding that patients showed similar neural activation to controls during execution and observation of facial expressions, we predicted that both groups would show comparable overall activation in the dACC and AI during the observation of pain in others. However, evidence of impaired self-other discrimination and emotion regulation in schizophrenia (Brunet-Gouet and Decety, 2006; Harvey et al., 2011; Horan et al., 2013; O’Driscoll et al., 2014) led us to predict that patients would show diminished modulation of neural activity by the perspective taking (i.e. dACC and AI activation would be greater for the Self vs Other conditions in controls but not in patients) and contextual modulation (i.e. dACC and AI activation would be greater for the Not effective vs Effective conditions in controls but not in patients) task manipulations.
Materials and methods
Participants
Twenty-one outpatients meeting DSM-IV criteria for schizophrenia and 21 healthy controls participated. Patients were medicated at clinically determined dosages. Controls were recruited through flyers and website postings, and exclusion criteria included: (i) history of psychotic disorder, bipolar disorder, recurrent depression, dysthymia or substance dependence disorder; (ii) avoidant, paranoid, schizoid or schizotypal personality disorders; (iii) history of loss of consciousness >1 h and (iv) schizophrenia or other psychotic disorder in a first-degree relative. Inclusion criteria for both groups included: (i) 18–60 years of age, (ii) no current substance use disorder, (iii) no identifiable neurological disorder and (iv) sufficient English fluency. All participants provided written informed consent to participate. Additional information about recruitment, assessments and exclusion criteria are in the supplement.
Clinical measures
Clinical symptoms were assessed using the expanded Brief Psychiatric Rating Scale (BPRS) (Overall and Gorham, 1962; Lukoff et al., 1986; Kopelowicz et al., 2008). The BPRS total score as well as BPRS mean subscales for positive symptoms and negative symptoms were used to characterize the patient sample.
Activation paradigm
Stimuli
Two types of stimuli, aversive tones and video clips showing individuals listening to these tones, were used. These stimuli were developed and validated in healthy individuals in two behavioral experiments by Lamm et al. (2007). Twenty-four aversive 2 s tones were composed by mixing three highly dissonant tone pairs in a frequency range from 1300 to 11 000 Hz (to minimize interference with MR gradient noise).
Eighty 3.5 s video clips (without sound) showed the faces of 24 individuals (12 male and 12 female experienced actors) listening to the sounds (Figure 1). Each video displayed a frontal view of the individual’s whole head and parts of the shoulders. In order to imply that videos had been taken in a hospital environment, videos were taken against a light blue background curtain (as used in hospitals), and targets were wearing a white medical blouse and audiometric headphones. Each video began with the individual displaying a neutral face (500 ms) that transitioned to a facial expression of strong pain (3 s) in reaction to the sounds.
Fig. 1.
Sample frames from a video clip showing the transition from a neutral to painful facial expression triggered by the presentation of a noxious tone.
Procedure
The paradigm was described to all participants using a standardized audio-visual presentation procedure. Participants were informed that they would watch video clips of patients experiencing painful auditory stimulation due to a new experimental medical treatment. They were told that the patients were suffering from a neurological disease (Tinnitus aurium) that had been treated using the new therapy that required repeated stimulation by certain tones, resulting in great pain. A sample of the sounds was played, pointing out that the pain experienced by patients was considerably stronger due to their neurological illness. Participants were also told that since this was a new therapy, some of the patients benefited from it whereas others did not (further information about instructions and training procedures is provided in the Supplementary material).
Tone task
In the first functional run, the aversive tones were used to localize the neural network activated by first-hand experience of painful auditory stimulation. Aversive tones were presented in 6 On and 7 Off epochs. After each On epoch, participants had to evaluate the intensity of the pain evoked by the sounds. Each On block consisted of three consecutively presented 2 s tones (no inter-tone time gap) followed immediately by a screen (presented for 4 s) informing subjects to make their pain rating using a 1 (not at all painful) to 4 (extremely painful) rating scale. Each Off block consisted of a 6 s rest period (blank screen). MR-compatible headphones were used to present auditory stimuli at ∼95 dB.
Empathy task
The empathy task had two conditions that were crossed in a 2 × 2 design. One condition was ‘perspective’ in which participants were instructed to watch the video clips either imagining how they themselves would feel if they were receiving the treatment or imaging how the patients in the clips were feeling while receiving the treatment. The other condition was ‘effectiveness’ in which subjects were told that the experimental treatment was either effective or not. After watching the videos, participants made a series of pain ratings with a button box ranging from 1 (not painful at all) to 4 (extremely painful).
Several modifications were made to simplify the original Lamm et al. (2007) task design for use with schizophrenia patients, including eliminating the need to rapidly shift between perspectives within a functional run, providing more frequent instruction screens to minimize memory demands, and lengthening the time periods used for making pain ratings. The task was administered in four functional runs; two from the Self perspective and two from the Other perspective. We decided to use a fixed order starting with Self, which we consider an easier task. We then alternated between perspectives (i.e., Self, Other, Self, Other). Within each run, blocks of videos showing patients for whom treatment was Effective or Not effective were presented in a mixed, unpredictable order (using the same order across subjects).1
Within each run, a mixed blocked/event-related presentation mode was used. At the beginning of each run, participants were informed verbally and visually whether they were to make the ratings from the Self or Other Perspective. Each run included five 44 s blocks that each contained four videos (trials). At the beginning of each block, a 6 s instruction screen indicated whether the treatment was Effective or Not effective; the screen also repeated whether ratings were to be made from the Self or Other perspective. A centered fixation dot was presented during the inter-trial intervals (ITIs). Mean ITI duration was 4 s (range 3 – 5 s), and ITIs were randomly jittered to reduce stimulus predictability and to allow efficient event-related signal estimation (Donaldson and Buckner, 2001). Fixation was followed by a 2 s instruction screen that reminded participants of the perspective and effectiveness condition. After the last trial of each block, a 6 s instruction screen informed participants to make a pain intensity rating for the last video in the block, considering information about both perspective and effectiveness. The five blocks were interspersed among six 12 s rest periods (blank screen).
Across the four functional runs, participants viewed 80 videos, with equal numbers of male and female patient videos, equal mean pain ratings and standard deviations based on data from the original validation study, and identical ITI distributions. Visual stimuli were timed and presented with Presentation software (Neurobehavioral Systems, Albany, CA) through magnet-compatible LCD goggles. At the conclusion of the tasks participants were debriefed.
MRI data acquisition & processing
Images were acquired on a Siemens 3T (Erlangen, Germany) Trio MRI scanner. Image preprocessing and data analysis were performed with FMRI Software Library (FSL; (Smith et al., 2004). To facilitate multi-subject analyses, statistical images created for each subject were normalized to standard Montreal Neurological Institute (MNI) space using a 12-parameter linear affine transformation. Specific details of image acquisition, processing, and analysis can be found in the Supplementary material.
fMRI analyses
We used regions of interest (ROI) analyses, focusing on dACC and bilateral AI, to examine neural activation during the empathy task. The ROIs were based on data from the tone task, which assessed the direct, first-hand experience of pain while listening to aversive tones. To create the ROIs, we first examined activation during the painful tones in the combined sample of patients and controls at a threshold of Z = 1.9 (uncorrected). We chose to define the ROI’s based on data from the combined sample of patients and controls to minimize any bias that would be associated with potential group differences in activation and that could confound direct between-group comparisons. This functional activation map was then anatomically constrained using two masks based on templates from the Harvard–Oxford cortical atlas tool in FSL. For the dACC, the atlas includes a template for the anterior region of the cingulate cortex. For the bilateral AI, the insula template from the atlas was divided at its midpoint (y = 0), which corresponds roughly to the boundary between the dysgranular and granular sectors (Ongür et al., 2003; Bonthius et al., 2005). This process generated a priori, functionally coherent, anatomically constrained, and unbiased ROIs based on the current sample, which are described further and displayed below in section fMRI tone task.
Two primary sets of ROI analyses for the empathy task, modeled on the approach used by Lamm et al (2007), were conducted. For these analyses, mean beta values were extracted from each of the ROIs, and analyzed using the SPSS software package (with a threshold of P < 0.05). First, we evaluated between group differences in activation collapsed across all of the experimental factors for the two ROIs. Second, we conducted separate group X Perspective (Self, Other) X Effectiveness (Effective, Not Effective) Repeated-Measures analysis of variances (ANOVAs) for the two ROIs. These analyses address whether the groups differ in activation associated with the experimental perspective and effectiveness manipulations (i.e., Self/Effective, Self/Not Effective, Other/Effective, Other/Not Effective).
The ROI analyses were followed up with secondary whole brain analyses of the empathy task to more fully explore between-group and group X condition differences. To characterize neural activation in each group separately and to directly compare patients to controls for each contrast of interest in the whole brain analysis, a mixed-effects model (FLAME stage 1) (Beckmann et al., 2003; Woolrich et al., 2004) was performed. Resulting activation maps were thresholded at P < 0.05 with an extent threshold of 36 contiguous voxels, corresponding to a false-positive discovery rate of < 5% across the whole brain as estimated by Monte Carlo simulation (10 000 simulations) (Slotnick et al., 2003).
Results
Descriptive information
The groups did not significantly differ in sex, age or ethnicity (Table 1). Patients had lower personal education levels than controls but the groups did not differ in parental education. The schizophrenia group had a typical age of onset, was chronically ill, and showed mild to moderate levels of clinical symptoms at the time of testing. Correlational analyses within the patient group indicated that symptom levels, chlorpromazine equivalent units, age of onset and duration of illness were not significantly associated with pain ratings or fMRI activation levels.
Table 1.
Demographic and descriptive data
| Schizophrenia | Controls | Statistic | |
|---|---|---|---|
| Sex (% male) | 71.4% | 66.7% | X2 (1,42) = 0.11 |
| Age (s.d.) | 48.2 (10.4) | 46.5 (7.1) | t(40) = 0.63 |
| Ethnicity | X2 (4,42) = 1.53 | ||
| Hispanic | 23.8% | 9.5% | |
| Not Hispanic | 76.2% | 90.5% | |
| Race | X2 (4,42) = 1.61 | ||
| African American | 42.9% | 33.3% | |
| White | 21.4% | 66.7% | |
| Asian | 4.8% | ||
| Marital status | |||
| Never married | 65.2% | 34.8% | X2 (2,58) = 4.73** |
| Currently married | 8.7% | 26.1% | |
| Ever married | 26.1% | 39.1% | |
| Education (s.d.) | 13.3 (1.6) | 15.1 (1.6) | t(40) = − 3.63*** |
| Parental education (s.d.) | 13.2 (3.7) | 14.8 (3.0) | t(40) = 1.46 |
| Handedness (% right) | 90.5% | 85.7% | X2 (1,42) = 0.23 |
| Age of onset (s.d.) | 22.1 (4.8) | ||
| Duration of illness (s.d.) | 26.1 (12.2) | ||
| Chlorpromazine equivalent units (s.d.) | 282.51 (162.49) | ||
| BPRS | |||
| Positive symptoms (s.d.) | 1.5 (0.5) | ||
| Negative symptoms (s.d.) | 1.6 (0.8) | ||
| Total (s.d.) | 33.8 (6.5) |
Notes: **P < 0.01; ***P < 0.001. Chlorpromazine equivalent units based on the procedures described by Andreasen et al. (2010).
Behavioral data
Tone task
During the tone task, pain ratings for the aversive sounds were significantly higher in the schizophrenia group (M = 2.3; SE = 0.35) than the control group (M = 1.6; SE = 0.20), t(40) = 2.67, P < 0.05.
Empathy task
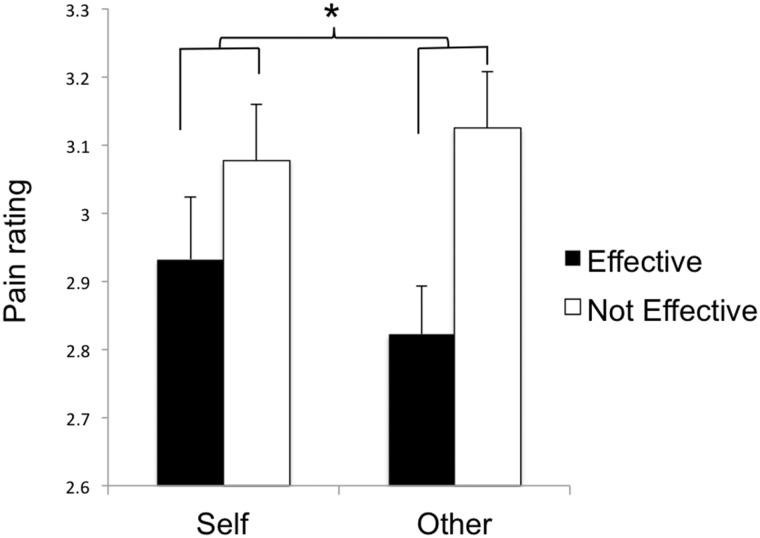
Since the groups differed on pain ratings during the tone task, the data were analyzed using a 2 (Perspective) × 2 (Effectiveness) × 2 (group) repeated-measured ANOVA with localizer task pain ratings included as a covariate. There was a significant Perspective × Effectiveness Interaction, F(1,39) = 4.29, P < 0.05 (Figure 2). Follow-up tests indicated that the Effective–Not effective difference score was significantly larger for the Other condition than for the Self condition, F(1,40) = 4.12, P < 0.05, although none of the simple effects (comparisons within the Perspective or Effectiveness conditions) was significant (all F’s < 2.93, P’s > 0.05). Aside from a trend-level Effectiveness × Group interaction, F(1,39) = 3.12, P = 0.09, there were no other significant main or interaction effects. Thus, there was a comparable overall pattern of pain ratings across groups during the empathy task.
Fig. 2.
Perspective × Effectiveness interaction for subjective pain ratings during the empathy task. Error bars reflect standard errors. *P < 0.05.
fMRI tone task
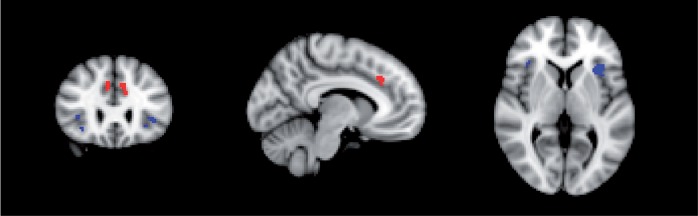
Since there were significant group differences in pain ratings during the tone task, we accounted for individual differences in subjective pain ratings when creating the masks for the primary ROI analyses. Specifically, in the combined sample of patients and controls, we included the de-meaned value of each individuals’ average pain rating as a regressor. The ROIs for the dACC and AI derived from the tone task are presented in Figure 3. The locations of these ROIs correspond to those previously identified during pain empathy tasks. Further, there were no significant patient vs control differences for mean beta values extracted from the dACC, t(40) = −0.04, P > 0.05, or the AI, t(40) = −0.50, P > 0.05, indicating comparable activation levels across the groups while listening to the aversive tones.
Fig. 3.
Locations of the regions of Interest (Z = 1.9, uncorrected) derived from the Tone task (from both groups) for the dACC (red) (right: 8,28,34; left: −8,28,36) and AI (blue) (right: 38,26,4; left: −34,20,4). Each ROI is shown centered on coordinates listed in MNI standard space.
fMRI Empathy task: ROI analyses
Group comparisons for activation across task conditions
Between group differences in activation (extracted beta values) collapsed across all of the experimental factors was evaluated separately for the two ROIs. For the dACC, the patient (M = 20.01; SE = 5.14) and control (M = 18.59; SE = 6.68) groups did not significantly differ, t(40) = 0.50, P > 0.05. Similarly, for the AI, there was not a significant difference between patients (M = 29.91; SE = 4.44) and controls (M = 30.41; SE = 6.85), t(40) = −0.10, P > 0.05. Thus, the patient and control groups showed comparable activation in the AI and dACC while obeserving others experiencing painful auditory stimulation.
Group comparisons for task conditions
For beta values from the dACC and AI, group differences were evaluated with separate 2 (Perspective) × 2 (Effectiveness) × 2 (Group) RM-ANOVAs (summarized in Table 2). For the dACC there was one significant interaction involving Group. The significant Perspective × Group interaction, F(1,40) = 7.49, P < 0.01, indicated that patients showed greater relative activation for Other than Self, whereas controls showed greater relative activation for Self than Other (Figure 4a); the between-group comparison of the Self-Other difference score was significant, t(40) = −2.73, P < 0.01, although the simple within-group and within-condition contrasts did not reach significance (all t’s < 1.80, all P’s > 0.05). There was also a non-significant trend level effect for Effectiveness, F(1,40) = 3.80, P < 0.10, which reflected numerically higher mean beta values for the Effective (M = 23.14, SE = 3.82) than the Not Effective (M = 16.64, SE = 4.94) condition.
Table 2.
Results of repeated-measures ANOVA’s on beta values from the dACC and AI during the fMRI empathy task
| Dorsal anterior cingulate F value | AI F value | |
|---|---|---|
| Perspective | 0.07 | 0.03 |
| Perspective × Group | 7.49** | 4.50* |
| Outcome | 3.80+ | 2.78 |
| Outcome × Group | 1.03 | 1.13 |
| Perspective × Outcome | 0.52 | 0.15 |
| Perspective × Outcome × Group | 0.03 | 0.41 |
Notes: df = 1,40; +P < 0.10; *P < 0.05; **P < 0.01.
Fig. 4.
Results of repeated-measures ANOVA’s on beta values from the dACC and AI for the Self and Other conditions during the fMRI empathy task. Error bars reflect standard errors. *P < 0.05. **P < 0.01. Panel a: Significant Group × Perspective interaction effect for the dACC. Panel b: Significant Group × Perspective interaction effect for the AI.
For AI, there was also a significant Perspective × Group interaction, F(1,40) = 4.50, P < 0.05. The interaction indicated that patients showed greater relative activation for Other than Self whereas controls showed greater relative activation for Self than Other (Figure 4b). The between-group comparison of the Self–Other difference score was significant, t(40) = −2.12, P < 0.05, although the simple within-group and within-condition contrasts did not reach significance (all t’s < 1.65, all P’s > 0.05). Thus, controls and patients showed opposing patterns of activation for self- vs other-related processing across both ROIs.
Empathy task whole brain analyses
Secondary whole brain analyses were performed to examine the key contrasts of interest (i) within each group separately and (ii) in direct comparisons of the two groups (i.e. main effect of condition within each group, and condition × group interactions). The first set of analyses focused on the Perspective condition: Self minus Other, Other minus Self contrasts. The second set of analyses focused on the Effectiveness condition: Effective minus Not Effective, Not Effective minus Effective contrasts. Select results are summarized here and the full results are presented in Supplementary Table S1.
For Perspective, consistent with the ROI analyses, controls showed greater relative activation for the Self condition, whereas patients showed greater relative activation in the Other condition. In particular, for the Self minus Other contrast, controls showed activation in the posterior cingulate and precuneus, patients showed no activation for this contrast, and direct between-group comparisons indicated that controls showed greater activation than patients in posterior cingulate and precuneus. For the Other minus Self contrast, controls showed no regions of significant activation and patients showed activation in right orbitofrontal cortex, frontal pole, inferior frontal gyrus, bilateral pre- and post-central gyrus, middle cingulate/juxtapositional lobule and left insula/central operculum. Direct between-group comparisons indicated that patients showed greater activation than controls in bilateral frontal pole (Supplementary Figure S1).
For Effectiveness, the Effective minus Not Effective contrast indicated that controls showed significant activation in the right frontal lobe (frontal pole, orbitofrontal cortex), right anterior cingulate/ paracingulate gyri and right AI. Patients showed activation for this contrast in midfrontal gyri. Direct between-group comparisons indicated that controls showed greater activation than patients in anterior cingulate/paracingulate gyri, while patients showed no areas of greater activation than controls. For the Not Effective minus Effective contrast, patients showed significant activation in the right frontal pole, though direct between-group comparisons did not reveal significant activation in this area (Supplementary Figures S2 and Supplementary Data).
Discussion
When considering activation across all conditions, controls and patients showed generally comparable overall activation in dACC and AI while viewing others in pain. However, when considering the modulatory impact of perspective taking, the groups showed different activation patterns. Controls showed a typical pattern of relatively increased dACC and AI activation for self- vs other-related processing, whereas patients showed the opposite pattern. Thus, although the patients demonstrated grossly intact neural sensitivity while observing others in pain, they showed subtler neural response abnormalities when toggling between imagining themselves vs others experiencing pain.
Behavioral responses
Patients reported significantly higher pain ratings to the noxious tones than controls during the localizer task, i.e. direct experience of pain. The ratings in both groups were somewhat lower than those found in the original Lamm et al. (2007) study using an undergraduate sample. These lower ratings could reflect differences in how we conducted pre-scanning training in that we used a relatively extensive training procedure to ensure that the participants fully understood the task. Speculatively, this different procedure may have resulted in habituation of controls’ pain ratings while in the scanner (Ernst et al., 1986; Jempa et al., 2014).
During the video viewing task, pain ratings were sensitive to both the Perspective and Effectiveness manipulations across both groups. As in Lamm et al. (2007) pain ratings were generally higher for the Not Effective vs Effective condition, though this effect was stronger when imagining another vs oneself experiencing pain. Notably, the pattern of pain ratings did not significantly differ between the patient and control groups. Although some prior studies suggest that schizophrenia is associated with elevated physical pain thresholds (see Wojakiewicz et al., 2013), we found no such evidence.
Direct vs observed experience of pain
By directly exposing participants to noxious tone stimuli, we could examine the correspondence between dACC and AI with the first-hand experience of pain vs merely observing others in pain. Both groups showed significant bilateral activation in ROI-defined dACC and AI regions while observing others in pain, which converges with a large body of research demonstrating a key role for these regions in the affective component of pain processing (Lamm et al., 2011). Overall activation levels did not significantly differ between groups for either ROI, suggesting that the schizophrenia groups’ neural responses were grossly intact while observing others experiencing physical pain.
The schizophrenia patients’ intact overall dACC and AI activation converges with several recent studies examining the affective subdomain of empathy (see Green et al., 2015). The only prior pain empathy study found that patients showed normal sensitivity to pictures depicting body parts in painful vs non-painful positions for early ERP components, which was interpreted as reflecting intact affective empathy (Corbera et al., 2014). Similarly, other studies have reported intact neural activity using tasks intended to measure mirror neuron system functioning using fMRI (Horan et al., 2014b) and EEG (Horan et al, 2014a). Other EEG studies also report largely normal or even hyper-active responses on such tasks in schizophrenia, though some fMRI, EEG and TMS studies have reported hypoactivation (see Mehta et al., 2014). Overall, the emerging literature suggests that affective empathy may be relatively well preserved as compared to the consistent, large impairments seen on measures of cognitive empathy, other social cognitive processes (e.g. facial affect identification), and real world community social functioning (Bellack et al., 2007; Savla et al., 2012; Horan et al., 2015).
Aberrant modulation by perspective taking
Although the patients’ overall neural sensitivity to observing others in pain appeared grossly intact, subtler abnormalities were detected when considering the impact of perspective taking instructions. Consistent with prior findings, controls demonstrated relatively greater dACC and AI activation when instructed to image oneself vs another experiencing pain (Jackson et al., 2006; Lamm et al., 2007). Further, whole brain analyses indicated greater self-related activation in controls in the posterior cingulate and precuneus, regions strongly implicated in self-related processing (Northoff et al., 2006). In stark contrast, patients showed relatively greater dACC and AI activation for the imagine Other vs Self contrast. The whole brain analyses revealed greater activation for this contrast in affective and somatosensory-motor regions of the pain matrix that are typically more strongly activated during first-hand experience of pain vs vicarious processing of others’ pain, as well as frontal regions associated with higher level cognitive and emotional functions (Bludau et al., 2014). Thus, the patients failed to show a typical pattern of self-relevant pain processing and instead appear to process other-related stimuli in a manner more closely associated with how self-relevant stimuli are typically processed.
This disturbance in self vs other related processing converges with behavioral and neuroimaging evidence that individuals with schizophrenia fail to show preferential self-referential processing. For example, patients fail to show a typical self-referential memory bias and demonstrate disturbances in source monitoring and agency detection on behavioral tasks (Harvey et al., 2011; Maeda et al., 2012; Renes et al., 2013). There is evidence that abnormalities in the structure and function of neural regions associated with self-related processing are detectable in individuals with schizophrenia (Brunet-Gouet and Decety, 2006; Backasch et al., 2014; Liu et al., 2014; Pauly et al., 2014), as well as those at heightened genetic and clinical high-risk for psychosis (Brent et al., 2014). Considered in this context, our findings suggest that individuals with schizophrenia may actually be hyper-sensitive when considering pain of others. This could result in a state of empathic over-arousal that hampers their ability to adaptively respond to others’ distress.
Modulation by effectiveness
Although the effectiveness manipulation was intended to assess the impact of frontally mediated emotion regulation processes on empathic responses, it did not produce clear and consistent effects in this study. The primary ROI analyses did not demonstrate dACC or AI sensitivity to the effectiveness manipulation, and the whole brain analyses revealed activation patterns that were not fully consistent with expectations. For example, although controls activated right frontal regions in the Effective vs Not Effective contrast, which is consistent with the functions associated with these regions during emotion regulation and findings by Lamm et al., there were also unexpected activations in cingulate/paracingulate and insular sub-regions for this contrast. It is unclear why the effectiveness manipulation did not elicit the effects found by Lamm et al. The most obvious difference concerns the age of our sample, which was over 20 years older than the participants in the Lamm et al. study and the actors who portrayed the medical patients in the video stimuli. Perhaps our sample’s older age impacted their understanding of the task instructions or their ability to relate to the actors’ portrayals of pain.
Conclusions and implications
The current findings suggest that a more nuanced interpretation of empathic disturbances in schizophrenia may be needed. Although individuals with schizophrenia showed relatively intact sensitivity to the pain of others, their ability to process this information in a manner that promotes adaptive responding appears to be impaired. This may be particularly true with regard to making a clear distinction between one’s own suffering and that of others. In future schizophrenia research it will be useful to move beyond consideration of mean activation levels to examine potential disturbances in connectivity between neural regions associated with experience sharing and regions associated with more top-down modulatory influences (e.g. subregions of the prefrontal context) in empathic responding (Eack et al., 2013; Ebisch et al., 2014; Green et al., 2015).
Interpretations should be considered in the context of some limitations. First, the sample sizes were relatively small and the apparent lack of significant between-group differences in the primary ROI analyses across experimental conditions must therefore be interpreted cautiously. The sample was, however, sufficiently large to detect group differences for some of the experimental conditions and was larger than many fMRI studies reporting disturbances in social cognition in schizophrenia (e.g. Sugranyes et al., 2011; Harvey et al., 2013; Thakkar et al., 2014). Second, we modified the task to make it more appropriate for use in schizophrenia. However, these simplifications came with some methodological limitations, particularly the fact that we were unable to systematically evaluate order effects in the fMRI task. Third, we studied medicated, chronically ill patients who were clinically stable and living in the community, and it is unclear whether our findings are generalizable to other types of samples (e.g. unmedicated patients, patients with higher symptom levels).
In terms of clinical implications, our findings contribute to emerging evidence that affective empathy may be a relatively preserved aspect of social cognition in schizophrenia, reflecting a strength to build on in social cognitive interventions. This may greatly facilitate patients’ efforts to relate to, and feel connected with, others in the course of rehabilitative efforts aimed at improving interpersonal communication and developing social networks. However, the current results also point to particular areas where such efforts may go awry, namely, distinguishing between one’s own and others’ experiences, and managing overwhelming emotions around others in distress. A number of recent studies support the possibility of enhancing social cognitive skills through psychosocial training approaches (Horan et al., 2011; Abu-Akel et al., 2014; Davis et al., 2014). The efficacy and effectiveness of these approaches may be enhanced by directly targeting higher-level perspective taking and emotion regulation skills. Interventions for these two areas have been developed for other neuropsychiatric conditions (Mackay et al., 2007; Khoury et al., 2013; Neacsiu et al., 2014), and they may be useful additions to social cognitive skills training approaches for schizophrenia.
Supplementary Material
Acknowledgements
The authors wish to thank Jean Decety and Claus Lamm for providing the task stimuli, and Poorang Nori, Amanda Bender, Michelle Dolinsky, Crystal Gibson, Cory Tripp and Katherine Weiner for assistance in data collection.
Footnotes
1 Order effects for runs 1 vs 3 (the Self Runs) and runs 2 vs 4 (the Other runs) were examined using separate 2 (group) by 2 (run) repeated-measures ANOVA’s for the Self and Other runs. The dependent variable was the extracted beta values from the dACC and AI collapsed across the Effective and Not effective blocks for each run. There were no significant group, run, or interaction effects, indicating that activation levels did not systematically differ by whether they occurred early or late in the task.
Funding
Support for this study came from a VA Career Development Award (William P. Horan, PhD.) and NIMH Grants MH065707 and MH43292 (Michael F. Green, PhD).
References
- Abu-Akel A., Fischer-Shofty M., Levkovitz Y., Decety J., Shamay-Tsoory S. (2014). The role of oxytocin in empathy to the pain of conflictual out-group members among patients with schizophrenia. Psychological Medicine, 44, 3523–32. [DOI] [PubMed] [Google Scholar]
- Andreasen N.C., Pressler M., Nopoulos P., Miller D., Ho B.C. (2010). Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biological Psychiatry, 67, 255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backasch B., Sommer J., Kloühn-Saghatolislam F., Müller M.J., Kircher T.J., Leube D.T. (2014). Dysconnectivity of the inferior frontal gyrus: implications an impaired self-other distinction in patients with schizophrenia. Psychiatry Research: Neuroimaging, 223, 202–9. [DOI] [PubMed] [Google Scholar]
- Batson C.D. (2009). Two forms of perspective taking: imagining how another feels and imagining how you would feel. In: Markman K.D.W.M.P.K., Suhr J.A., editors. Handbook of Imagination and Mental Simulation. New York: Psychology Press, 267–79. [Google Scholar]
- Batson C.D., Early S., Salvarini G. (1997). Perspectivetaking: imagining how another feels versus imagining how you would feel. Personality and Social Psychology Bulletin, 23, 751–8. [Google Scholar]
- Batson C.D., Fultz J., Schoenrade P.A. (1987). Distress and empathy: two qualitatively distinct vicarious emotions with different motivational consequences. Journal of Personality, 55, 19–39. [DOI] [PubMed] [Google Scholar]
- Beckmann C.F., Jenkinson M., Smith S.M. (2003). General multilevel linear modeling for group analysis in FMRI. Neuroimage, 20, 1052–63. [DOI] [PubMed] [Google Scholar]
- Bellack A.S., Green M.F., Cook J.A., et al. (2007). Assessment of community functioning in people with schizophrenia and other severe mental illnesses: a white paper based on an NIMH-sponsored workshop. Schizophrenia Bulletin, 33, 805–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F., Bernasconi A., Bosia M., et al. (2009). Functional and structural brain correlates of theory of mind and empathy deficits in schizophrenia. Schizophrenia Research, 114, 154–60. [DOI] [PubMed] [Google Scholar]
- Bernhardt B.C., Singer T. (2012). The neural basis of empathy. Annual Review of Neuroscience, 35, 1–23. [DOI] [PubMed] [Google Scholar]
- Bludau S., Eickhoff S.B., Mohlberg H., et al. (2014). Cytoarchitecture, probability maps and functions of the human frontal pole. NeuroImage, 93, 260–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthius D.J., Solodkin A., Van Hoesen G.W. (2005). Pathology of the insular cortex in Alzheimer disease depends on cortical architecture. Journal of Neuropathology and Experimental Neurology, 64, 910–22. [DOI] [PubMed] [Google Scholar]
- Brent B.K., Seidman L.J., Thermenos H.W., Holt D.J., Keshavan M.S. (2014). Self-disturbances as a possible premorbid indicator of schizophrenia risk: a neurodevelopmental perspective. Schizophrenia Research, 152, 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet-Gouet E., Decety J. (2006). Social brain dysfunctions in schizophrenia: a review of neuroimaging studies. Psychiatry Research, 148, 75–92. [DOI] [PubMed] [Google Scholar]
- Bufalari I., Ionta S. (2013). The social and personality neuroscience of empathy for pain and touch. Frontiers in Human Neuroscience, 7, 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle J.T., Silvers J.A., Wager T.D., et al. (2014). Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cerebral Cortex, 24, 2981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbera S., Ikezawa S., Bell M.D., Wexler B.E. (2014). Physiological evidence of a deficit to enhance the empathic response in schizophrenia. European Psychiatry, 29, 463–72. [DOI] [PubMed] [Google Scholar]
- Davis M.C., Green M.F., Lee J., et al. (2014). Oxytocin-augmented social cognitive skills training in schizophrenia. Neuropsychopharmacology, 39, 2070–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J., Jackson P.L. (2006). A social neuroscience perspective on empathy. Current Directions in Psychological Science, 15, 54–8. [Google Scholar]
- Decety J., Lamm C. (2006). Human Empathy through the Lens of Social Neuroscience. The Scientific World Journal, Ltd., 6, 1146–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derntl B., Finkelmeyer A., Voss B., et al. (2012). Neural correlates of the core facets of empathy in schizophrenia. Schizophrenia Research, 136, 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derntl B., Michel T.M., Prempeh P., et al. (2015). Empathy in individuals clinically at risk for psychosis: brain and behavior. The British Journal of Psychiatry, 207, 407–13. [DOI] [PubMed] [Google Scholar]
- Donaldson D.I., Buckner R.L. (2001). Effective Paradigm Design. In: Matthews P.M., Jezzard P., Smith S.M., editors. Functional Magnetic Resonance Imaging: An Introduction to Methods. Oxford: Oxford University Press. [Google Scholar]
- Eack S.M., Wojtalik J.A., Newhill C.E., Keshavan M.S., Phillips M.L. (2013). Prefrontal cortical dysfunction during visual perspective-taking in schizophrenia. Schizophrenia Research, 150, 491–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisch S.J., Mantini D., Northoff G., et al. (2014). Altered brain long-range functional interactions underlying the link between aberrant self-experience and self-other relationship in first-episode schizophrenia. Schizophrenia Bulletin, 40, 1072–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M., Lee M.H., Dworkin B., Zaretsky H.H. (1986). Pain perception decrement produced through repeated administration. Pain, 26, 221–31. [DOI] [PubMed] [Google Scholar]
- Green M.F., Horan W.P., Lee J. (2015). Social cognition in schizophrenia. Nature Reviews Neuroscience, 16, 620–31. [DOI] [PubMed] [Google Scholar]
- Harvey P.O., Lee J., Horan W.P., Ochsner K., Green M.F. (2011). Do patients with schizophrenia benefit from a self-referential memory bias?. Schizophrenia Research, 127, 171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey P.O., Zaki J., Lee J., Ochsner K., Green M.F. (2013). Neural Substrates of Empathic Accuracy in People with Schizophrenia. Schizophrenia Bulletin, 39, 617–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan W.P., Hajcak G., Wynn J.K., Green M.F. (2013). Impaired emotion regulation in schizophrenia: evidence from event-related potentials. Psychological Medicine, 43, 2377–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan W.P., Iacoboni M., Cross K.A., et al. (2014b). Self-reported empathy and neural activity during imitation and action observation in schizophrenia. Neuroimage Clinical, 23, 100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan W.P., Kern R.S., Tripp C., et al. (2011). Efficacy and specificity of social cognitive skills training for outpatients with psychotic disorders. Journal of Psychiatric Research, 45, 1113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan W.P., Pineda J.A., Wynn J.K., Iacoboni M., Green M.F. (2014a). Some markers of mirroring appear intact in schizophrenia: evidence from mu suppression. Cognitive, Affective, and Behavioral Neuroscience, 14:1049–60. [DOI] [PubMed] [Google Scholar]
- Horan W.P., Reise S.P., Kern R.S., Lee J., Penn D.L., Green M.F. (2015). Structure and correlates of self-reported empathy in schizophrenia. Journal of Psychiatric Research, 66–67, 60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson P.L., Brunet E., Meltzoff A.N., Decety J. (2006). Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia, 44(5), 752–61. [DOI] [PubMed] [Google Scholar]
- Jempa M., Jones M., Wager T.D. (2014). The Dynamics of pain: evidence for simultaneous site-specific habituation and site-nonspecific sensitization in thermal pain. The Journal of Pain, 7, 734–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury B., Lecomte T., Gaudiano B.A., Paquin K. (2013). Mindfulness interventions for psychosis: a metaanalysis. Schizophenia Research, 150, 176–84. [DOI] [PubMed] [Google Scholar]
- Kopelowicz A., Ventura J., Liberman R.P., Mintz J. (2008). Consistency of brief Psychiatric Rating Scale Factor structure across a broad spectrum of schizophrenia patients. Psychopathology, 41, 77–84. [DOI] [PubMed] [Google Scholar]
- Lamm C., Batson C.D., Decety J. (2007). The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. Journal of Cognitive Neuroscience, 19(1), 42–58. [DOI] [PubMed] [Google Scholar]
- Lamm C., Decety J., Singer T. (2011). Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage, 54, 2492–502. [DOI] [PubMed] [Google Scholar]
- Lee S.J., Kang D.H., Kim C., et al. (2010). Multi-level comparison of empathy in schizophrenia: an fMRI study of a cartoon task. Psychiatry Research: Neuroimaging, 181, 121–9. [DOI] [PubMed] [Google Scholar]
- Liu J., Corbera S., Wexler B.E. (2014). Neural activation abnormalities during self-referential processing in schizophrenia: an fMRI study. Psychiatry Research: Neuroimaging, 222, 165–71. [DOI] [PubMed] [Google Scholar]
- Lukoff D., Nuechterlein K.H., Ventura J. (1986). Manual for the expanded Brief Psychiatric Rating Scale. Schizophrenia Bulletin, 12, 578–602. [DOI] [PubMed] [Google Scholar]
- Mackay T., Knott F., Dunlop A.W. (2007). Developing social interaction and understanding in individuals with autism spectrum disorder: a groupwork intervention. Journal of Intellectual and Developmental Disability, 279–90. [DOI] [PubMed] [Google Scholar]
- Maeda T., Kato M., Muramatsu T., Iwashita S., Mimura M., Kashima H. (2012). Aberrant sense of agency in patients with schizophrenia: forward and backward over-attribution of temporal causality during intentional action. Psychiatry Research, 198, 1–6. [DOI] [PubMed] [Google Scholar]
- McCormick L.M., Brumm M.C., Beadle J.N., Paradiso S., Yamada T., Andreasen N. (2012). Mirror neuron function, psychosis, and empathy in schizophrenia. Psychiatry Research, 201, 233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta U.M., Thirthalli J., Aneelraj D., Jadhav P., Gangadhar B.N., Keshavan M.S. (2014). Mirror neuron dysfunction in schizophrenia and its functional implications: a systematic review. Schizophrenia Research, 160, 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels T.M., Horan W.P., Ginger E.J., Martinovich Z., Pinkham A.E., Smith M.E. (2014). Cognitive empathy contributes to poor social functioning in schizophrenia: evidence from a new self-report measure of cognitive and affective empathy. Psychiatry Research, 220, 803–10. [PubMed] [Google Scholar]
- Neacsiu A.D., Eberle J.W., Kramer R., Wiesmann T., Linehan M.M. (2014). Dialectical behavior therapy skills for transdiagnostic emotion dysregulation: a pilot randomized controlled trial. Behavior Research and Therapy, 59, 40–1. [DOI] [PubMed] [Google Scholar]
- Northoff G., Heinzel A., de Greck M., Bermpohl F., Dobrowolny H., Panksepp J. (2006). Self-referential processing in our brain–—a meta-analysis of imaging studies on the self. NeuroImage, 31, 440–57. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9, 242–9. [DOI] [PubMed] [Google Scholar]
- O’Driscoll C., Laing J., Mason O. (2014). Cognitive emotion regulation strategies, alexithymia and dissociation in schizophrenia, a review and meta-analysis. Clinical Psychology Review, 34, 482–95. [DOI] [PubMed] [Google Scholar]
- Ongür D., Ferry A.T., Price J.L. (2003). Architectonic subdivision of the human orbital and medial prefrontal cortex. The Journal of Comparative Neurology, 460:425–49. [DOI] [PubMed] [Google Scholar]
- Overall J.E., Gorham D.R. (1962). The Brief Psychiatric Rating Scale. Psychological Reports, 10, 799–812. [Google Scholar]
- Pauly K.D., Kircher T.J., Schneider F., Habel U. (2014). Me, myself and I: temporal dysfunctions during self-evaluation in patients with schizophrenia. Social Cognitive and Affective Neuroscience, 9, 1779–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renes R.A., Vermeulen L., Kahn R.S., Aarts H., van Haren N.E.M. (2013). Abnormalities in the establishment of feeling of self-agency in schizophrenia. Schizophrenia Research, 143, 50–4. [DOI] [PubMed] [Google Scholar]
- Savla G.N., Vella L., Armstrong C.C., Penn D.L., Twamley E.W. (2012). Deficits in Domains of Social Cognition in Schizophrenia: a meta-analysis of the empirical evidence. Schizophrenia Bulletin, 39, 979–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory S.G. (2011). The neural bases for empathy. Neuroscientist, 17, 18–24. [DOI] [PubMed] [Google Scholar]
- Slotnick S.D., Moo L.R., Segal J.B., Hart J. (2003). Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cognitive Brain Research, 17, 75–82. [DOI] [PubMed] [Google Scholar]
- Smith M.J., Schroeder M.P., Abram S.V., et al. (2015). Alterations in brain activation during cognitive empathy are related to social functioning in schizophrenia. Schizophrenia Bulletin, 41, 211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., et al. (2004). Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage, 23, (Suppl 1), S208–19. [DOI] [PubMed] [Google Scholar]
- Sugranyes G., Kyriakopoulos M., Corrigall R., Taylor R., Frangou S. (2011). Autism Spectrum Disorders and Schizophrenia: Meta-Analysis of the Neural Correlates of Social Cognition. PLoS One, 6(10): e25322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar K.N., Peterman J.S., Park S. (2014). Altered brain activation during action imitation and observation in schizophrenia: a translational approach for studying social dysfunction. American Journal of Psychiatry, 171(5): 539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood B., Moore B. (1982). Perspective-taking and altruism. Psychological Bulletin, 91, 143–73. [Google Scholar]
- Wojakiewicz A., Januel D., Braha S., Prkachin K., Danziger N., Bouhassira D. (2013). Alteration of pain recognition in schizophrenia. European Journal of Pain, 17(9), 1385–92. [DOI] [PubMed] [Google Scholar]
- Woolrich M.W., Behrens T.E., Beckmann C.F., Jenkinson M., Smith S.M. (2004). Multi-level linear modeling for fMRI group analysis using Bayesian inference. NeuroImage, 21, 1732–47. [DOI] [PubMed] [Google Scholar]
- Zaki J., Ochsner K. (2011). Reintegrating the study of accuracy into social cognition research. Psychological Inquiry, 22, 159–82. [Google Scholar]
- Zaki J., Ochsner K. (2012). The neuroscience of empathy: progress, pitfalls and promise. Nature Neuroscience, 15, 675–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.