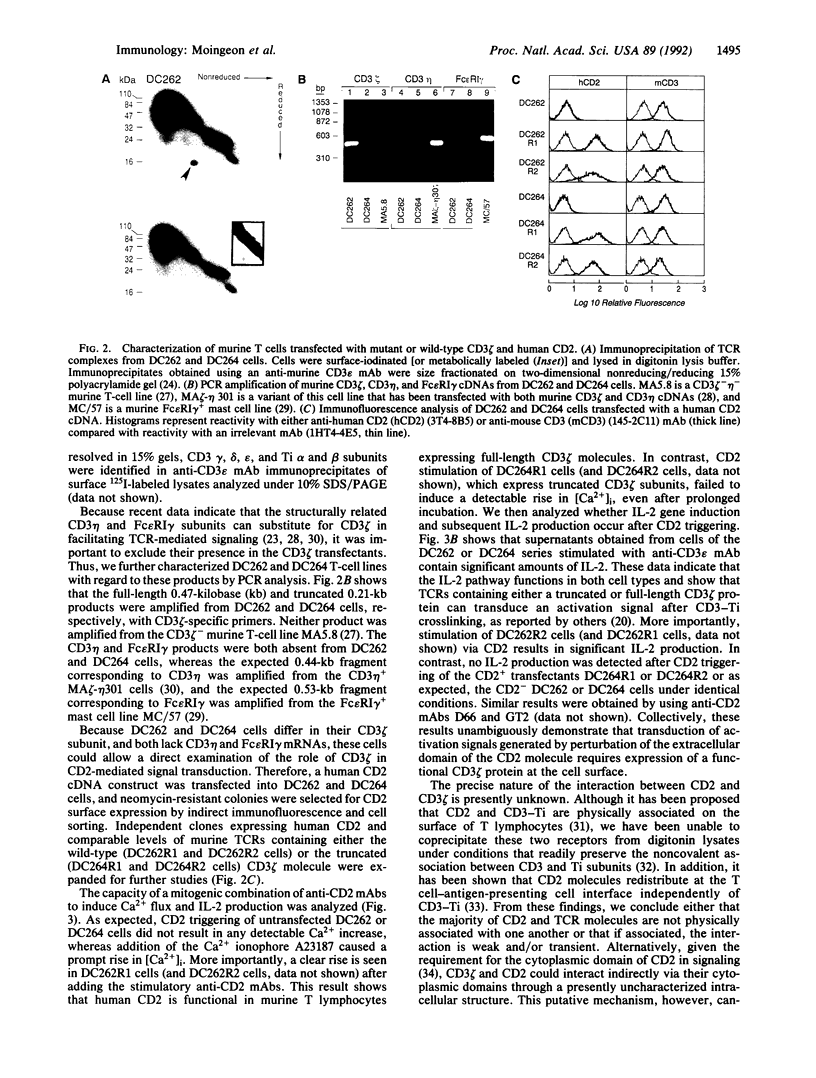
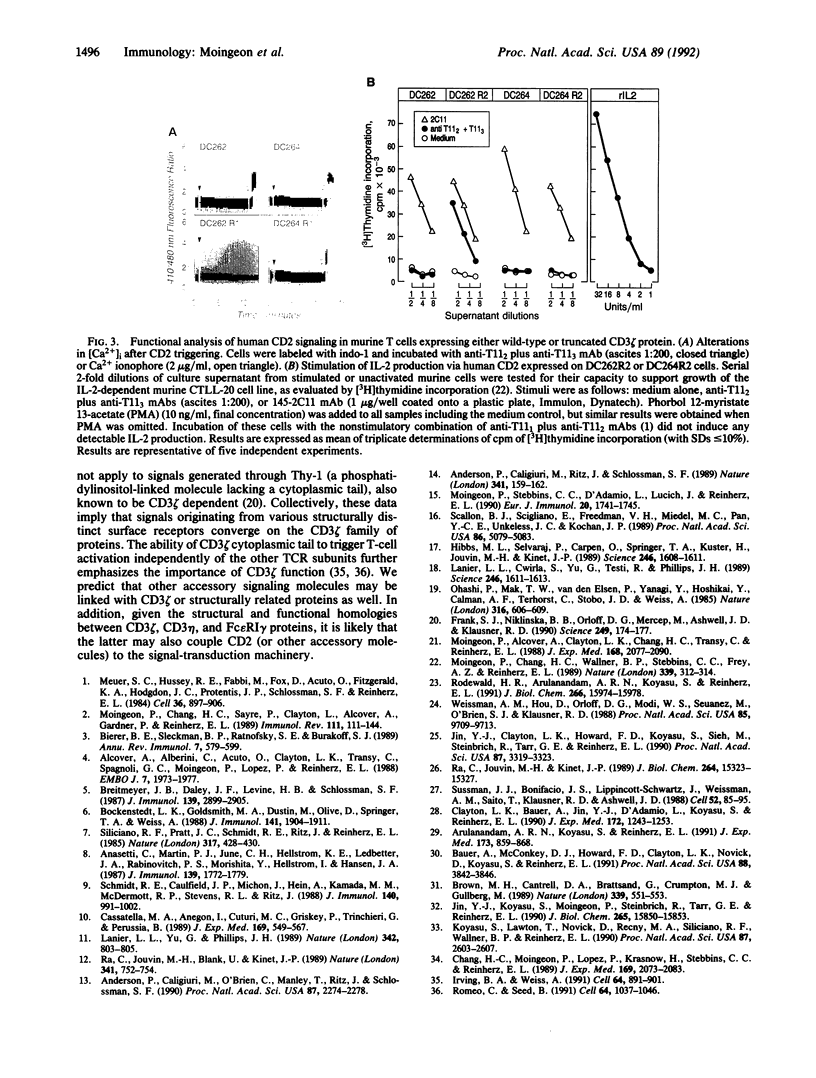
Abstract
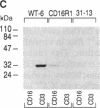
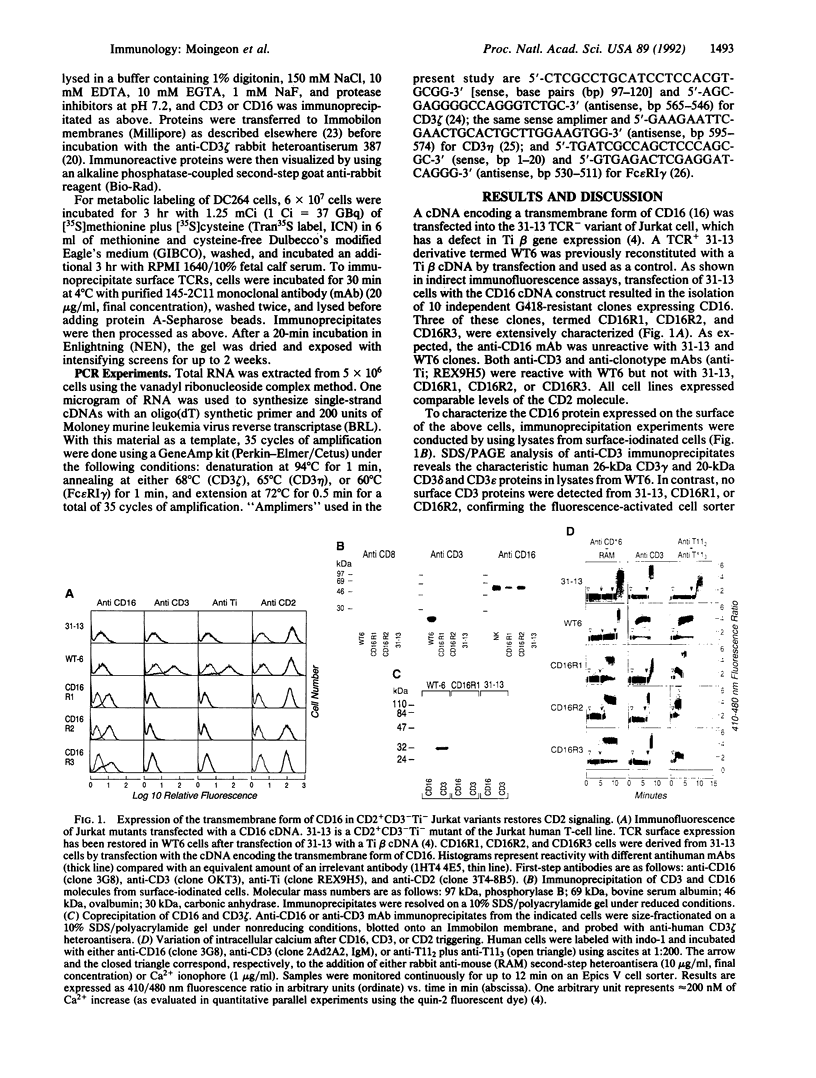
In T lymphocytes, signal transduction through the CD2 adhesion molecule requires surface expression of the CD3-Ti T-cell receptor (TCR) complex. In contrast, in natural killer (NK) cells, CD2 functions in the absence of a TCR. Because the TCR on T lymphocytes and the CD16 low-affinity IgG Fc receptor (Fc gamma receptor type III) complex on NK cells share a common CD3 zeta subunit, we reasoned that CD3 zeta may be critical for CD2 signaling in T lymphocytes and NK cells. Here we show that transfection of a cDNA encoding a transmembrane form of CD16 into TCR- variants of the Jurkat T-cell line results in CD16 expression in association with endogenous CD3 zeta homodimers and restores CD2 signaling function. To test directly the role of CD3 zeta in CD2 triggering, we then transfected human CD2 into each of two murine T-T hybridomas that express TCRs containing either a full-length CD3 zeta subunit or a truncated CD3 zeta subunit incapable of transducing signals. Despite expression of equivalent surface levels of TCR, CD2-mediated signaling is seen only in the T cells containing wild-type CD3 zeta. These findings show that (i) CD16 on NK cells is functionally equivalent to the TCR on T lymphocytes for coupling CD2 to signaling pathways and (ii) CD2 signal transduction depends upon the CD3 zeta subunit of the TCR complex and, by inference, the CD3 zeta subunit of the CD16 complex.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcover A., Alberini C., Acuto O., Clayton L. K., Transy C., Spagnoli G. C., Moingeon P., Lopez P., Reinherz E. L. Interdependence of CD3-Ti and CD2 activation pathways in human T lymphocytes. EMBO J. 1988 Jul;7(7):1973–1977. doi: 10.1002/j.1460-2075.1988.tb03035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anasetti C., Martin P. J., June C. H., Hellstrom K. E., Ledbetter J. A., Rabinovitch P. S., Morishita Y., Hellstrom I., Hansen J. A. Induction of calcium flux and enhancement of cytolytic activity in natural killer cells by cross-linking of the sheep erythrocyte binding protein (CD2) and the Fc-receptor (CD16). J Immunol. 1987 Sep 15;139(6):1772–1779. [PubMed] [Google Scholar]
- Anderson P., Caligiuri M., O'Brien C., Manley T., Ritz J., Schlossman S. F. Fc gamma receptor type III (CD16) is included in the zeta NK receptor complex expressed by human natural killer cells. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2274–2278. doi: 10.1073/pnas.87.6.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P., Caligiuri M., Ritz J., Schlossman S. F. CD3-negative natural killer cells express zeta TCR as part of a novel molecular complex. Nature. 1989 Sep 14;341(6238):159–162. doi: 10.1038/341159a0. [DOI] [PubMed] [Google Scholar]
- Arulanandam A. R., Koyasu S., Reinherz E. L. T cell receptor-independent CD2 signal transduction in FcR+ cells. J Exp Med. 1991 Apr 1;173(4):859–868. doi: 10.1084/jem.173.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A., McConkey D. J., Howard F. D., Clayton L. K., Novick D., Koyasu S., Reinherz E. L. Differential signal transduction via T-cell receptor CD3 zeta 2, CD3 zeta-eta, and CD3 eta 2 isoforms. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3842–3846. doi: 10.1073/pnas.88.9.3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierer B. E., Sleckman B. P., Ratnofsky S. E., Burakoff S. J. The biologic roles of CD2, CD4, and CD8 in T-cell activation. Annu Rev Immunol. 1989;7:579–599. doi: 10.1146/annurev.iy.07.040189.003051. [DOI] [PubMed] [Google Scholar]
- Bockenstedt L. K., Goldsmith M. A., Dustin M., Olive D., Springer T. A., Weiss A. The CD2 ligand LFA-3 activates T cells but depends on the expression and function of the antigen receptor. J Immunol. 1988 Sep 15;141(6):1904–1911. [PubMed] [Google Scholar]
- Breitmeyer J. B., Daley J. F., Levine H. B., Schlossman S. F. The T11 (CD2) molecule is functionally linked to the T3/Ti T cell receptor in the majority of T cells. J Immunol. 1987 Nov 1;139(9):2899–2905. [PubMed] [Google Scholar]
- Brown M. H., Cantrell D. A., Brattsand G., Crumpton M. J., Gullberg M. The CD2 antigen associates with the T-cell antigen receptor CD3 antigen complex on the surface of human T lymphocytes. Nature. 1989 Jun 15;339(6225):551–553. doi: 10.1038/339551a0. [DOI] [PubMed] [Google Scholar]
- Cassatella M. A., Anegón I., Cuturi M. C., Griskey P., Trinchieri G., Perussia B. Fc gamma R(CD16) interaction with ligand induces Ca2+ mobilization and phosphoinositide turnover in human natural killer cells. Role of Ca2+ in Fc gamma R(CD16)-induced transcription and expression of lymphokine genes. J Exp Med. 1989 Feb 1;169(2):549–567. doi: 10.1084/jem.169.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. C., Moingeon P., Lopez P., Krasnow H., Stebbins C., Reinherz E. L. Dissection of the human CD2 intracellular domain. Identification of a segment required for signal transduction and interleukin 2 production. J Exp Med. 1989 Jun 1;169(6):2073–2083. doi: 10.1084/jem.169.6.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton L. K., Bauer A., Jin Y. J., D'Adamio L., Koyasu S., Reinherz E. L. Characterization of thymus-derived lymphocytes expressing Ti alpha-beta CD3 gamma delta epsilon zeta-zeta, Ti alpha-beta CD3 gamma delta epsilon eta-eta or Ti alpha-beta CD3 gamma delta epsilon zeta-zeta/zeta-eta antigen receptor isoforms: analysis by gene transfection. J Exp Med. 1990 Oct 1;172(4):1243–1253. doi: 10.1084/jem.172.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S. J., Niklinska B. B., Orloff D. G., Merćep M., Ashwell J. D., Klausner R. D. Structural mutations of the T cell receptor zeta chain and its role in T cell activation. Science. 1990 Jul 13;249(4965):174–177. doi: 10.1126/science.2371564. [DOI] [PubMed] [Google Scholar]
- Hibbs M. L., Selvaraj P., Carpén O., Springer T. A., Kuster H., Jouvin M. H., Kinet J. P. Mechanisms for regulating expression of membrane isoforms of Fc gamma RIII (CD16). Science. 1989 Dec 22;246(4937):1608–1611. doi: 10.1126/science.2531918. [DOI] [PubMed] [Google Scholar]
- Irving B. A., Weiss A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991 Mar 8;64(5):891–901. doi: 10.1016/0092-8674(91)90314-o. [DOI] [PubMed] [Google Scholar]
- Jin Y. J., Clayton L. K., Howard F. D., Koyasu S., Sieh M., Steinbrich R., Tarr G. E., Reinherz E. L. Molecular cloning of the CD3 eta subunit identifies a CD3 zeta-related product in thymus-derived cells. Proc Natl Acad Sci U S A. 1990 May;87(9):3319–3323. doi: 10.1073/pnas.87.9.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y. J., Koyasu S., Moingeon P., Steinbrich R., Tarr G. E., Reinherz E. L. A fraction of CD3 epsilon subunits exists as disulfide-linked dimers in both human and murine T lymphocytes. J Biol Chem. 1990 Sep 15;265(26):15850–15853. [PubMed] [Google Scholar]
- Koyasu S., Lawton T., Novick D., Recny M. A., Siliciano R. F., Wallner B. P., Reinherz E. L. Role of interaction of CD2 molecules with lymphocyte function-associated antigen 3 in T-cell recognition of nominal antigen. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2603–2607. doi: 10.1073/pnas.87.7.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L. L., Cwirla S., Yu G., Testi R., Phillips J. H. Membrane anchoring of a human IgG Fc receptor (CD16) determined by a single amino acid. Science. 1989 Dec 22;246(4937):1611–1613. doi: 10.1126/science.2531919. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Yu G., Phillips J. H. Co-association of CD3 zeta with a receptor (CD16) for IgG Fc on human natural killer cells. Nature. 1989 Dec 14;342(6251):803–805. doi: 10.1038/342803a0. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Fabbi M., Fox D., Acuto O., Fitzgerald K. A., Hodgdon J. C., Protentis J. P., Schlossman S. F., Reinherz E. L. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984 Apr;36(4):897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- Moingeon P., Alcover A., Clayton L. K., Chang H. C., Transy C., Reinherz E. L. Expression of a functional CD3-Ti antigen/MHC receptor in the absence of surface CD2. Analysis with clonal Jurkat cell mutants. J Exp Med. 1988 Dec 1;168(6):2077–2090. doi: 10.1084/jem.168.6.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moingeon P., Chang H. C., Sayre P. H., Clayton L. K., Alcover A., Gardner P., Reinherz E. L. The structural biology of CD2. Immunol Rev. 1989 Oct;111:111–144. doi: 10.1111/j.1600-065x.1989.tb00544.x. [DOI] [PubMed] [Google Scholar]
- Moingeon P., Chang H. C., Wallner B. P., Stebbins C., Frey A. Z., Reinherz E. L. CD2-mediated adhesion facilitates T lymphocyte antigen recognition function. Nature. 1989 May 25;339(6222):312–314. doi: 10.1038/339312a0. [DOI] [PubMed] [Google Scholar]
- Moingeon P., Stebbins C. C., D'Adamio L., Lucich J., Reinherz E. L. Human natural killer cells and mature T lymphocytes express identical CD3 zeta subunits as defined by cDNA cloning and sequence analysis. Eur J Immunol. 1990 Aug;20(8):1741–1745. doi: 10.1002/eji.1830200818. [DOI] [PubMed] [Google Scholar]
- Ohashi P. S., Mak T. W., Van den Elsen P., Yanagi Y., Yoshikai Y., Calman A. F., Terhorst C., Stobo J. D., Weiss A. Reconstitution of an active surface T3/T-cell antigen receptor by DNA transfer. Nature. 1985 Aug 15;316(6029):606–609. doi: 10.1038/316606a0. [DOI] [PubMed] [Google Scholar]
- Ra C., Jouvin M. H., Blank U., Kinet J. P. A macrophage Fc gamma receptor and the mast cell receptor for IgE share an identical subunit. Nature. 1989 Oct 26;341(6244):752–754. doi: 10.1038/341752a0. [DOI] [PubMed] [Google Scholar]
- Ra C., Jouvin M. H., Kinet J. P. Complete structure of the mouse mast cell receptor for IgE (Fc epsilon RI) and surface expression of chimeric receptors (rat-mouse-human) on transfected cells. J Biol Chem. 1989 Sep 15;264(26):15323–15327. [PubMed] [Google Scholar]
- Rodewald H. R., Arulanandam A. R., Koyasu S., Reinherz E. L. The high affinity Fc epsilon receptor gamma subunit (Fc epsilon RI gamma) facilitates T cell receptor expression and antigen/major histocompatibility complex-driven signaling in the absence of CD3 zeta and CD3 eta. J Biol Chem. 1991 Aug 25;266(24):15974–15978. [PubMed] [Google Scholar]
- Romeo C., Seed B. Cellular immunity to HIV activated by CD4 fused to T cell or Fc receptor polypeptides. Cell. 1991 Mar 8;64(5):1037–1046. doi: 10.1016/0092-8674(91)90327-u. [DOI] [PubMed] [Google Scholar]
- Scallon B. J., Scigliano E., Freedman V. H., Miedel M. C., Pan Y. C., Unkeless J. C., Kochan J. P. A human immunoglobulin G receptor exists in both polypeptide-anchored and phosphatidylinositol-glycan-anchored forms. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5079–5083. doi: 10.1073/pnas.86.13.5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R. E., Caulfield J. P., Michon J., Hein A., Kamada M. M., MacDermott R. P., Stevens R. L., Ritz J. T11/CD2 activation of cloned human natural killer cells results in increased conjugate formation and exocytosis of cytolytic granules. J Immunol. 1988 Feb 1;140(3):991–1002. [PubMed] [Google Scholar]
- Siliciano R. F., Pratt J. C., Schmidt R. E., Ritz J., Reinherz E. L. Activation of cytolytic T lymphocyte and natural killer cell function through the T11 sheep erythrocyte binding protein. Nature. 1985 Oct 3;317(6036):428–430. doi: 10.1038/317428a0. [DOI] [PubMed] [Google Scholar]
- Sussman J. J., Bonifacino J. S., Lippincott-Schwartz J., Weissman A. M., Saito T., Klausner R. D., Ashwell J. D. Failure to synthesize the T cell CD3-zeta chain: structure and function of a partial T cell receptor complex. Cell. 1988 Jan 15;52(1):85–95. doi: 10.1016/0092-8674(88)90533-8. [DOI] [PubMed] [Google Scholar]
- Weissman A. M., Hou D., Orloff D. G., Modi W. S., Seuanez H., O'Brien S. J., Klausner R. D. Molecular cloning and chromosomal localization of the human T-cell receptor zeta chain: distinction from the molecular CD3 complex. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9709–9713. doi: 10.1073/pnas.85.24.9709. [DOI] [PMC free article] [PubMed] [Google Scholar]