Abstract
Stress exposure has been linked to increased rates of depression and anxiety in adults, particularly in females, and has been associated with maladaptive changes in the anterior cingulate cortex (ACC), which is an important brain structure involved in internalizing disorders. Coping styles are important mediators of the stress reaction by establishing homeostasis, and may thus confer resilience to stress-related psychopathology. Anatomical scans were acquired in 181 healthy participants at age 25 years. Positive coping styles were determined using a self-report questionnaire (German Stress Coping Questionnaire, SVF78) at age 22 years. Adult anxiety and depression symptoms were assessed at ages 22, 23 and 25 years with the Young Adult Self-Report. Information on previous internalizing diagnoses was obtained by diagnostic interview (2–19 years). Positive coping styles were associated with increased ACC volume. ACC volume and positive coping styles predicted anxiety and depression in a sex-dependent manner with increased positive coping and ACC volume being related to lower levels of psychopathology in females, but not in males. These results remained significant when controlled for previous internalizing diagnoses. These findings indicate that positive coping styles and ACC volume are two linked mechanisms, which may serve as protective factors against internalizing disorders.
Keywords: coping styles, perigenual anterior cingulate cortex, resilience, anxiety disorders, mood disorders
Introduction
Mood and anxiety disorders impose a serious burden in Western countries, with lifetime prevalences between 20.8% and 28.8%, respectively (Kessler et al., 2005). Evidence suggests that women are more likely to develop these disorders through the lifespan (Kessler et al., 2005; Mc Lean et al., 2011). Among other causes, such as familial disposition, stress exposure has been related to increased rates of depression and anxiety (Levitan et al., 2003). Therefore, coping styles, i.e. the way individuals try to restore the disturbed homeostasis in order to maintain well-being in stressful situations, may serve as a mediator between stress exposure and psychopathology, and may thus confer resilience (Feder et al., 2009). Coping styles can be positive, i.e. stress-reducing, or negative, i.e., stress-enhancing (Higgins and Endler, 1995; Wilkinson et al., 2000; Erdmann and Janke, 2007; Heck et al., 2009). Indeed, depression and anxiety have been linked to less positive and more negative coping (Zhang et al., 2009), making coping styles an attractive target for treatment intervention (Renaud et al., 2014).
The role of the anterior cingulate cortex (ACC) in conferring risk for mood and anxiety disorders has long been recognized (Etkin and Wager, 2007; Drevets et al., 2008). The ACC can be divided into five subregions: caudal, dorsal, rostral, perigenual and subgenual, with each of these parts covering distinct functions. While the more posterior parts of the ACC are mainly involved in cognitive processes such as error monitoring, the anterior parts cover emotional and social processes (Margulies et al., 2007; Kelly et al., 2009). For example, the perigenual ACC has been attributed a role in self-referential social processing (Amodio and Frith, 2006; Meyer-Lindenberg and Tost, 2012), while the subgenual part has been linked to emotion regulation (Meyer-Lindenberg and Tost, 2012), making these parts primary candidates for endophenotypes of internalizing disorders. Accordingly, a lower volume in these regions has specifically been observed in patients with major depression and comorbid anxiety, suggesting a shared vulnerability pathway between the two disorders (van Tol et al., 2010).
Notably, several studies have provided evidence for that the ACC is a convergence site of the effects of stress exposure (for a review see Holz et al., 2015). In detail, a decreased ACC volume has been demonstrated following harsh corporal punishment lasting for at least three years (Tomoda et al., 2009), following the attack on the World Trade Center on 11th September 2001 (Ganzel et al., 2008), and after exposure to cumulative adverse life events (Ansell et al., 2012) as well as exposure to childhood adversity (Gerritsen et al., 2012). Remarkably, during stress induction, the ACC has previously been associated with social-environmental risk factors acting in childhood, such as urban birth (Lederbogen et al., 2011) and ethnic minority status (Akdeniz et al., 2014), highlighting its importance in mediating the experience of psychosocial stress.
Using longitudinal data from an epidemiological cohort study of young adults followed since birth , the present investigation aimed to (i) examine the association between positive stress coping strategies and perigenual/subgenual ACC volume, and (ii) relate both to symptoms of anxiety and depression with a specific focus on sex-dependent effects (Kessler et al., 2005; Mc Lean et al., 2011).
Materials and Methods
Sample
This investigation was conducted in the framework of the Mannheim Study of Children at Risk, an ongoing epidemiological cohort study of the long-term outcome of early risk factors (for full details c.f. Laucht et al., 2000). Infants were recruited from two obstetric and six children’s hospitals in the Rhine-Neckar Region of Germany and were included consecutively into the sample according to a two-factorial design intended to enrich and to control the risk status of the sample [factor 1 varying the degree of obstetric complications, and factor 2 the degree of psychosocial adversity (for full details c.f. Laucht et al., 1997; Laucht et al., 2000)]. To control for confounding effects of family environment and infant medical status, only firstborn children with singleton births and German-speaking parents were enrolled. Assessments were conducted at the age of 3 months and at regular intervals throughout development (at the ages of 2, 4.5, 8, 11, 15, 19, 22, 23 and 25 years). Functional and structural magnetic resonance imaging was introduced in the most recent assessment at age 25 years. From the initial sample of 384 participants, 18 (4.7%) were excluded due to severe disabilities, and 57 (14.8%) were dropouts, leaving a final sample of 309 for the 25-year assessment. Of these, a subsample of N = 182 right-handed individuals were selected to participate in an MRI session investigating brain volume. Exclusion criteria were the usual contraindications for MRI (such as heart pacemaker, neurological abnormalities, history of seizures, unconsciousness or head trauma), current psychiatric disorders as assessed by the Structured Clinical Interview for DSM-IV (SCID; American Psychiatric Association, 1994; German version, Wittchen et al., 1997), and psychotropic medication. One participant was excluded due to systemic lupus erythematosus. The study was approved by the ethics committee of the University of Heidelberg and written informed consent was obtained from all participants.
Assessments
Coping styles were assessed with the German Stress Coping Questionnaire (Stressverarbeitungsfragebogen SVF78; Janke et al., 2002) at the participants’ age of 22 years. The SVF78 consists of 13 subscales comprising either positive, i.e. stress-reducing, or negative, i.e. stress-enhancing coping styles. Here, we focus on positive coping styles, which consist of three broad dimensions encompassing devaluation/defense (‘I tell myself that everything will turn out all right’), distraction (‘I try to distract myself’) and control (‘I tell myself that I can cope with this’). A correlation matrix of these subcomponents is provided in Supplementary Table S1. Internal consistency and reliability have been reported to be fairly high [Cronbach́s α = .77–.93; r = .74–.95 (split-half), r = .61–.74 (test–retest), respectively] and consistent construct validity and external validity have been confirmed (Ising et al., 2001; Hampel et al., 2002; Weyers et al., 2005; Israelski, 2008). Data were missing for two participants, leaving a final sample of N = 179 individuals.
Anxious and depressive behaviors during adulthood was assessed with the anxious/depressed subscale of the Young Adult Self-Report (YASR; Achenbach, 1991) at the participants’ ages of 22 (range 0–26; mean 5.1; SD: 4.9), 23 (range 0–22; mean 4.3; SD: 4.4) and 25 years (range 0–23; mean 4.7; SD: 4.7). A composite score was computed by summing up the z-standardized scores across assessments. Data were missing for one subject. In addition, to ensure that the relationship between coping styles and ACC volume still exists for current anxiety and depression, we reanalyzed the interactions with symptoms of anxiety and depression assessed at the age of 25 only.
Previous internalizing psychopathology (0 = not present; 1 = present) was assessed through diagnostic interviews with the parents (Mannheim Parent Interview; MPI Esser et al., 1989) until the age of 11 years, and with the offspring at the age of 15 years using the Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS-PL; Kaufman et al., 1996; German version, Delmo et al., 2000) and at the age of 19 years using the Structured Clinical Interview for DSM-IV (SCID; American Psychiatric Association, 1994; German version, Wittchen et al., 1997).
Voxel-Based Morphometry (VBM) and statistical analysis
We acquired 1 × 1 × 1 mm T1-weighted anatomical images with 192 slices covering the whole brain (matrix 256 × 256, repetition time = 2300 ms, echo time = 3.03 ms, 50% distance factor, field of view 256 × 256 × 192 mm, flip angle 9°) using a 3 T scanner (Magnetom TRIO, Siemens, Erlangen, Germany) with a standard 12-channel head coil. Images were bias-corrected and classified into gray matter, white matter, cerebrospinal fluid and non-brain tissue using Diffeomorphic Anatomical Registration through Exponentiated Lie algebra (DARTEL) registration toolbox (Ashburner, 2007). Data were segmented based on tissue probability maps, indexing the prior probability of any voxel in a registered image being gray or white matter or cerebrospinal fluid. An average template from the data was created, to which the images were registered. Additionally, the images were affine-transformed to MNI space and smoothed with a 6-mm FWHM kernel. Total intracranial volume was calculated by adding the tissue probabilities of gray matter, white matter and cerebrospinal fluid and used as a covariate. For group statistics of gray matter images, coping style was entered as a covariate of interest while additionally controlling for total intracranial volume and sex. To test our a priori hypothesis, anatomical region of interest (ROI) masks comprising the bilateral ACC were derived from the WFU PickAtlas v2.4 (Maldjian et al., 2003), where family-wise error (FWE) correction was applied with a threshold of p < .05. Additionally, exploratory whole brain analyses were performed with a p < .001 uncorrected threshold and a criterion of 10 adjacent voxels. For display purposes, the statistical threshold was set at p = .005 uncorrected in the ROI. To visualize and further analyse the effects using regression analysis (i.e., ACC by sex on depression/anxiety) and mediation analysis using the Sobel Test (Baron and Kenny, 1986), mean contrast values of each participant were extracted from the FWE-corrected significant cluster (45 voxel) and exported to SPSS Statistics 20 (IBM, Armonk, NY).
Results
Sample characteristics
As expected, females exhibited increased YASR anxiety and depression scores and were more likely to have a history of previous internalizing psychopathology at a trend level (Table 1). While no sex difference emerged with regard to positive coping styles in general, females made use of more distraction compared to males.
Table 1.
Sample characteristics by sex
| Sex | Males | Females | p-Value |
|---|---|---|---|
| No. (%) | 75 (41.44) | 106 (58.56) | |
| YASR anxiety and depression, mean (SD)a | −1.12 (1.98) | .25 (2.81) | <.001 b |
| Previous internalizing psychopathology, No. (%) | 17 (22.67) | 38 (35.85) | .06 c |
| Overall positive coping styles, mean (SD) | 12.81 (3.23) | 13.26 (2.40) | .28 b |
| Positive self-instruction mean (SD)d | 16.41 (5.05) | 16.58 (3.86) | .79 b |
| Denial of guilt, mean (SD)d | 11.27 (4.35) | 11.22 (3.32) | .93 b |
| Distraction, mean (SD)d | 11.23 (4.68) | 13.18 (3.83) | .003 b |
| Reaction control, mean (SD)d | 14.64 (4.46) | 15.30 (3.41) | .26 b |
Raw values for the different scales are presented.
a Sum of z-standardized values from age 22 years to 25 years.
b t-test.
c χ 2 -test.
d Subcomponent of positive coping styles.
VBM
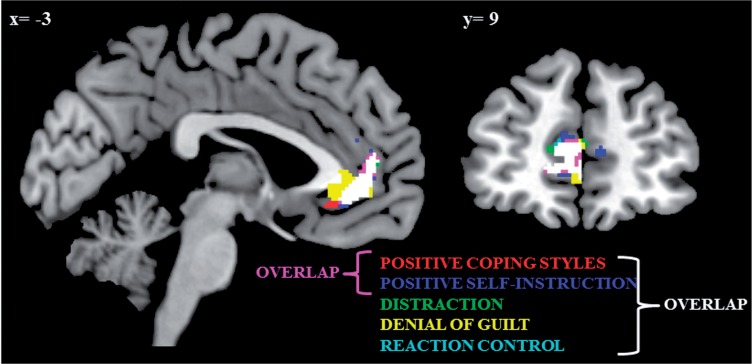
Positive coping styles were associated with increased volume in the perigenual ACC extending to the subgenual part (t(175) = 4.22, pFWE = .007; Figure 1). After exclusion of outliers, the results remained significant (t(173) = 3.92, pFWE = .02). Whole brain results are depicted in Supplementary Table S2. When controlling for previous psychopathology, the results remained unchanged (t(174) = 4.19; pFWE = .007). When parsing positive coping styles into its subcomponents, positive self-instruction (t(175) = 4.31, pFWE = .009) was the strongest predictor of ACC volume, followed by denial of guilt (t(175) = 3.96, pFWE = .02), distraction (t(175) = 3.65, pFWE = .04) and reaction control (t(175) = 3.67, pFWE = .04). The overlap between these effects is shown in Figure 2. In a second step, we tested for a possible interaction with sex on ACC volume, which reached significance only at an uncorrected level (positive coping styles by sex: t(172) = 3.03, puncorrected = .001).
Fig. 1.
Association between positive coping styles and ACC volume (peak –3 48 9, pFWE = .01). The association remained significant when outliers were excluded (pFWE = .02). Note that the scatter plot is adjusted for confounders and therefore negative levels of positive coping styles emerge.
Fig. 2.
Associations of different positive coping styles with ACC volume are overlaid, with their overlapping part in white.
Association with anxiety and depression
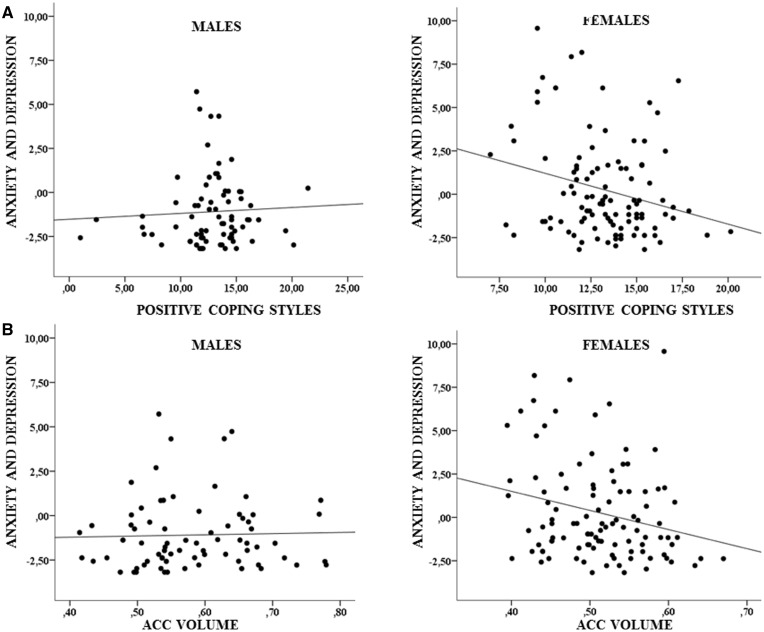
A significant interaction effect of positive coping styles with sex and of ACC volume with sex on the composite score of anxiety/depression during adulthood (covering age 22–25 years) was obtained (β = -.33, p = .02; β = -11.68, p = .03; respectively; Figure 3). In detail, positive coping styles and increasing ACC volume were associated with decreasing anxiety and depression in females (β = -.29, p = .01; β = -11.02, p = .01, respectively), while no such pattern was observed in males (β = .03, p = .64; β = .66, p = .80, respectively). The results remained unchanged when controlled for previous internalizing psychopathology. Further, results remained significant when outliers were excluded. In a further step, we examined a mediating effect of ACC volume on the relationship between positive coping and anxiety in females, which yielded a significant indirect effect (p = .047) with a significant path between positive coping and ACC volume (β = .006, p = .02), and between ACC volume and anxiety and depression (β = -8.95, p = .04). Moreover, the direct path between positive coping and anxiety remained significant, albeit at a lower threshold (β = -.24, p = .04), suggesting partial mediation. The results were similar when the outcome was current anxiety/depression at the age of 25 years (positive coping style by sex: β = -.51, p = .038; ACC volume by sex: β = -19.47, p = .036; mediation analysis: p = .05).
Fig. 3.
Interaction effect of positive coping styles with sex (A, p = .02) and of ACC volume with sex (B, p = .03) on YASR anxiety and depression.
Positive self-instruction was the subcomponent which was the strongest predictor of ACC volume and of positive coping styles (r = .79, p < .001). Likewise the interaction effect of positive self-instruction with sex on depression/anxiety was significant (β = -.29, p < .001), indicating a significant inverse association between positive coping styles and anxiety/depression in females (β = -.34, p < .001) but, again, not in males (p = .34). The same was true for the interaction between sex and denial of guilt (β = -.21, p = .03), but not for distraction (p = .22) or reaction control (p = .72).
Discussion
The present study investigated the association of coping styles with ACC volume and internalizing symptoms. Our findings from a prospective study over 25 years indicate that positive coping styles are associated with increased ACC volume. Both ACC volume and positive coping styles predicted symptoms of anxiety and depression in a sex-dependent manner, with inverse relationships emerging in females only.
Positive coping styles were associated with increased ACC volume, specifically with the perigenual extending to the subgenual part. This region is linked with social processing, the regulation of negative emotions and extinction learning (Etkin et al., 2011; Meyer-Lindenberg and Tost, 2012), thus supporting a potential role in conferring risk and resilience to stress-related disorders such as depression and anxiety. Interestingly, greater extinction memory has been associated with an increased thickness of the ventral medial prefrontal cortex (Milad et al., 2005), which is adjacent to the pACC, indicating that the size of this region might be directly related to the ability to modulate fear. Notably, activity in the pregenual ACC during attention interference in individuals at high familial risk has been suggested to play a role in assigning resilience to anxiety and depression (Peterson et al., 2014). Further, a twin study of combat-exposed subjects showed that a decreased gray matter volume in the ACC is unique to those who develop PTSD when compared to their non-affected twins (exposed and non-exposed and their co-twins), suggesting that the reduction may not be a possible familial vulnerability factor (Kasai et al., 2008). Previous studies investigating social evaluative stress processing have emphasized the vulnerability of this region to environmental stressors, including urbanicity (Lederbogen et al., 2011) and migration (Akdeniz et al., 2014). Moreover, it has been demonstrated that low subjective social status covaries with reduced gray matter volume in the pACC (Gianaros et al., 2007). Interestingly, the pACC is one of the key regions involved in the regulation of the HPA axis (Diorio et al., 1993), including the cortisol awakening response (Boehringer et al., 2015), thus providing a plausible biological mechanism linking susceptibility to stress with the ACC. Hence, a decreased volume and cortical thickness in the ACC along with increased activity during stress processing and a blunted response during attention interference might serve as risk endophenotypes of psychopathology. In sum, the risk and resilience trajectories of the ACC might point to adaptive emotional, behavioral and physiological response of this region to environmental and psychosocial stressors, and may thus represent an interesting target for intervention to reduce allostatic load. However, it is noteworthy that the pACC is tightly connected to other corticolimbic areas, including the amygdala and hippocampus, and is therefore unlikely to promote complex neurobehavioral processes independently.
Interestingly, while we observed a direct association of positive coping styles and ACC volume, an interaction with gender emerged when considering internalizing psychopathology. Females exhibited increased anxiety/depression symptoms in this sample, which echoes previous reports (Kessler et al., 2005; Mc Lean et al., 2011). Therefore, it seems plausible that protective factors such as increased positive coping styles, and thereby ACC volume, only emerge when a exceeding a specific psychopathological level or, in other words, only when homeostasis is disturbed and stress-reducing mechanisms are needed to restore allostatic load. Consequently, it might be the case that studies in clinical samples with a higher stress load across both sexes may not replicate a gender dependent effect but may rather confirm effects of positive coping styles and ACC volume on psychopathology for both genders.
A related concept to positive coping styles is reappraisal, which is defined as interpreting a potentially emotion-eliciting situation in a way that changes its emotional impact (Gross and John, 2003) either by reappraisal of stimuli in a positive way or reappraisal via perspective-taking (detached observer), both of which are widely used in emotion regulation tasks. So far, there have been several neuroimaging studies investigating the neural underpinnings of reappraisal. According to a recent meta-analysis, reappraisal in general has been related to activation in the semantic system including the inferior prefrontal gyrus, while reappraisal of stimuli additionally activated the dorsal attention system (medial prefrontal cortex and dorsal prefrontal cortex) (Messina et al., 2015). Interestingly, there is also evidence that increased dispositional cognitive reappraisal showed a higher activation of the right vmPFC and the lateral OFC over the course of symptom provocation in dental phobic individuals (Hermann et al., 2013). Consistent with these functional differences, cognitive reappraisal has been related to an increased volume in the dorsal region of the ACC (Giuliani et al., 2011) as well as to an increased ventromedial prefrontal cortex volume [(Welborn et al., 2009); but see Hermann et al. (2014)], the latter result partially overlapping with the outcome of the present study. Indeed, in analogy with findings showing that positive coping styles are important mediators between stress and resilience to depression and anxiety (Roohafza et al., 2014; Ren et al., 2015), there is also evidence that individuals using cognitive reappraisal exhibit less depressive symptoms (Cutuli, 2014).
In contrast to positive reappraisal, positive coping styles cover a rather broad range of stress reducing strategies, which involve two mechanisms of executive functioning recruitment. On the one hand, positive coping encompasses cognitive mechanisms such as positive self-instruction, which partly overlap with cognitive reappraisal, and, on the other hand, response modifications such as reaction control. While the first targets antecedent-focused strategies, i.e. the modification of the emotional stimuli before the emotional response, the latter is more a response-focused strategy, activated during the emotional response to a stimulus (Cutuli, 2014). Neuroimaging studies on positive coping styles have been rather sparse. Considering response modification mechanisms, Hermann et al. (2014), found expressive suppression to be negatively related to neuroticism and positively associated with dorsomedial and ventromedial PFC gray matter volume, with the latter overlapping with the present results. In sum, while functional data implicate more dorsal parts of the ACC in reappraisal, volume in the ventromedial part of the ACC seems to be a central mediator of both cognitive reappraisal and expressive suppression. This is in line with our results given that positive self-instruction, a subcomponent of cognitive reappraisal, and reaction control, a strategy of expressive suppression, are both predictors of pACC volume.
The acquisition of positive coping styles is a central aspect of cognitive behavioral therapy (CBT). Coping skills acquired during CBT and their independent implementation were found to predict the risk of relapse in the year following treatment termination in depressive individuals (Strunk et al., 2007). Similarly, mastery of active problem-focused coping strategies immediately after completion of therapy predicted better psychopathological and social outcome 12–18 months following CBT in schizophrenia (Andres et al., 2003). Likewise, symptom improvement after CBT was attributed to coping and the ability to reappraise misinterpretations rather than to clinical insight, duration of illness, gender or neuropsychological function (Premkumar et al., 2011), underlining the importance of this key therapeutic component. According to our results, the ability to attribute oneself with the capability and competence to control a stressor might be one of the most important aspects of positive coping strategies, followed by the degradation and the reappraisal of the stressor. Interestingly, apart from coping styles, ACC volume also seems to be critically involved in effective CBT. Evidence exists that CBT alters ACC activity to threat (Lipka et al., 2014) and ACC volume predicts treatment response (Bryant et al., 2008) and symptom improvement (Fujino et al., 2015) after CBT.
Our study should be considered in light of some limitations. First, coping styles were assessed prior to ACC volume, which does not allow us to make a definitive statement on the direction of the relationships. Both possibilities may be conceivable, i.e., that a positive coping style may increase ACC volume by decreasing stress-dependent atrophy, but also that positive coping styles arise due to an increased volume in the ACC possibly promoting a better capacity to regulate emotions. Second, functional data of the pACC during stress processing are not available. One candidate paradigm to address this may be the Montreal Imaging Stress Task (Lederbogen et al., 2011; Akdeniz et al., 2014), in which the stress-buffering effect of positive coping styles could be directly examined. Third, the multi-dimensional, dynamic nature of the resilience construct (Luthar et al., 2000) cannot be tested in our currently healthy sample given the lack of a group with a clinically significant level of depression or anxiety. The term resilience as used throughout the paper is therefore more reflective of a general protective factor (Luthar et al., 2000), i.e. an individual’s capacity to cope positively with stressful situations. Future studies are needed to test the promotion of resilience by positive coping skills using designs which include stressor-exposed individuals who develop a disease vs those who do not and/or, most elegantly, patients and their non-exposed twins.
Conclusion
In conclusion, increased ACC volume and positive coping styles are two linked mechanisms, which may represent possible protective factors for internalizing disorders. Since coping mechanisms can be modified, with our results underlining positive self-instruction as the most important strategy to control stressors, this may offer a more specific target for cognitive behavioral therapy to promote positive adaptation within the context of significant adversity.
Supplementary Material
Acknowledgements
The authors thank Sibylle Heinzel, Elisabeth Reichert, Erika Hohm, Katrin Zohsel, Anna Becker, Angelika Bocklage, Andrea Len, Daniel Megally and Elise Jezycki for conducting and supporting the assessments.
Conflicts of interest. T.B. served in an advisory or consultancy role for Hexal Pharma, Lilly, Medice, Novartis, Otsuka, Oxford outcomes, PCM scientific, Shire and Viforpharma. He received conference attendance support and conference support or received speaker’s fees from Lilly, Medice, Novartis and Shire. He has been involved in clinical trials conducted by Lilly, Shire & Viforpharma. A.M.L. received consultancy fees from Lundbeck International Neuroscience, Thieme Verlag Germany and Elsevier USA; for lectures including travel fees from Aula Médica Congresos, Groupo Ferrer International, Janssen-Cilag, Lilly Deutschland, Roche Pharma AG; and also grants from the Federal Office for Radiation Protection Germany and the Prix Roger de Spoelberch. All other authors declare that they have no biomedical financial interest or potential conflicts of interest. The present work is unrelated to the above grants and relationships.
Funding
This work was supported by grants from the German Research Foundation [grant number DFG LA 733/1-2] to [M.L., Da.B., A.M.L., T.B.]; the EC FP7 projects Aggressotype [FP7-Health-2013-Innovation-1 602805] and MATRICS [FP7-Health-2013-Innovation-1 603016] to [Da.B., T.B.]; and the ‘Kompetenzzentrum Aggression’ [AZ 42-04HV.MED(14)/14/1] to [Da.B., T.B.]. The funding source had no role in study design, in collection, analysis, and interpretation of data, in the writing of the report, and in the decision to submit the article for publication.
References
- Achenbach T. M. (1991). Young Adult Self Report. Burlington: University of Vermont, Department of Psychiatry. [Google Scholar]
- Akdeniz C., Tost H., Streit F., et al. (2014). Neuroimaging evidence for a role of neural social stress processing in ethnic minority-associated environmental risk. JAMA Psychiatry, 71(6), 672–80. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (1994). Diagnostic and Statistical Manual of Mental Disorders, 4th edn Washington, DC: American Psychiatric Association. [Google Scholar]
- Amodio D. M., Frith C. D. (2006). Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience, 7(4), 268–77. [DOI] [PubMed] [Google Scholar]
- Andres K., Pfammatter M., Fries A., Brenner H. D. (2003). The significance of coping as a therapeutic variable for the outcome of psychological therapy in schizophrenia. European Psychiatry, 18(4), 149–54. [DOI] [PubMed] [Google Scholar]
- Ansell E. B., Rando K., Tuit K., Guarnaccia J., Sinha R. (2012). Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biological Psychiatry, 72(1), 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. (2007). A fast diffeomorphic image registration algorithm. NeuroImage, 38(1), 95–113. [DOI] [PubMed] [Google Scholar]
- Baron R. M., Kenny D. A. (1986). The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology, 51(6), 1173–82. [DOI] [PubMed] [Google Scholar]
- Boehringer A., Tost H., Haddad L., et al. (2015). Neural correlates of the cortisol awakening response in humans. Neuropsychopharmacology, 40(9), 2278–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant R. A., Felmingham K., Whitford T. J., et al. (2008). Rostral anterior cingulate volume predicts treatment response to cognitive-behavioural therapy for posttraumatic stress disorder. Journal of Psychiatry & Neuroscience, 33(2), 142–6. [PMC free article] [PubMed] [Google Scholar]
- Cutuli D. (2014). Cognitive reappraisal and expressive suppression strategies role in the emotion regulation: an overview on their modulatory effects and neural correlates. Frontiers in Systems Neuroscience, 8, 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmo C., Weiffenbach O., Gabriel M., Poustka F. (2000). Diagnostisches Interview Kiddie-SADS-Present and Lifetime-Version (K-SADS-PL) Screening Interview. 3. Auflage der deutschen Forschungsversion, erweitert um ICD-10-Diagnostik. Frankfurt, Klinik für Psychiatrie und Psychotherapie des Kindes- und Jugendalters. [Google Scholar]
- Diorio D., Viau V., Meaney M. J. (1993). The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. Journal of Neuroscience, 13(9), 3839–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets W. C., Price J. L., Furey M. L. (2008). Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Structure & Function, 213(1–2), 93–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann G., Janke W. (2007). Handbuch zum Stressverarbeitungsfragebogen. Göttingen: Hogrefe. [Google Scholar]
- Esser G., Blanz B., Geisel B., Laucht M. (1989). [Mannheim Parent Interview – Structured interview for child psychiatric disorders]. Weinheim, Beltz. [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15(2), 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Wager T. D. (2007). Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry, 164(10), 1476–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder A., Nestler E. J., Charney D. S. (2009). Psychobiology and molecular genetics of resilience. Nature Reviews Neuroscience, 10(6), 446–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino J., Yamasaki N., Miyata J., et al. (2015). Anterior cingulate volume predicts response to cognitive behavioral therapy in major depressive disorder. Journal of Affective Disorders, 174, 397–9. [DOI] [PubMed] [Google Scholar]
- Ganzel B. L., Kim P., Glover G. H., Temple E. (2008). Resilience after 9/11: multimodal neuroimaging evidence for stress-related change in the healthy adult brain. NeuroImage, 40(2), 788–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerritsen L., Tendolkar I., Franke B., et al. (2012). BDNF Val66Met genotype modulates the effect of childhood adversity on subgenual anterior cingulate cortex volume in healthy subjects. Molecular Psychiatry, 17(6), 597–603. [DOI] [PubMed] [Google Scholar]
- Gianaros P. J., Horenstein J. A., Cohen S., et al. (2007). Perigenual anterior cingulate morphology covaries with perceived social standing. Social Cognitive & Affective Neuroscience, 2(3), 161–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani N. R., Drabant E. M., Bhatnagar R., Gross J. J. (2011). Emotion regulation and brain plasticity: expressive suppression use predicts anterior insula volume. NeuroImage, 58(1), 10–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J. J., John O. P. (2003). Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. Journal of Personality and Social Psychology, 85(2), 348–62. [DOI] [PubMed] [Google Scholar]
- Hampel P., Dickow B., Petermann F. (2002). Reliabilität und Validität des SVF-KJ. Zeitschrift für Differentielle und Diagnostische Psychologie, 23(3), 273–89. [Google Scholar]
- Heck A., Lieb R., Ellgas A., et al. (2009). Polymorphisms in the angiotensin-converting enzyme gene region predict coping styles in healthy adults and depressed patients. American Journal of Medical Genetics B Neuropsychiatric Genetics, 150B(1), 104–14. [DOI] [PubMed] [Google Scholar]
- Hermann A., Bieber A., Keck T., Vaitl D., Stark R. (2014). Brain structural basis of cognitive reappraisal and expressive suppression. Social Cognitive and Affective Neuroscience, 9(9), 1435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann A., Leutgeb V., Scharmuller W., Vaitl D., Schienle A., Stark R. (2013). Individual differences in cognitive reappraisal usage modulate the time course of brain activation during symptom provocation in specific phobia. Biology of Mood & Anxiety Disorders, 3(1), 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J., Endler N. (1995). Coping, life stress, and psychological and somatic distress. European Journal of Personality , 9, 253–70. [Google Scholar]
- Holz N. E., Laucht M., Meyer-Lindenberg A. (2015). Recent advances in understanding the neurobiology of childhood socioeconomic disadvantage. Current Opinion in Psychiatry, 28(5), 365–70. [DOI] [PubMed] [Google Scholar]
- Ising M., Weyers P., Janke W., Erdmann G. (2001). Die Gütekriterien des SVF78 von Janke und Erdmann, eine Kurzform des SVF120. Zeitschrift für Differentielle und Diagnostische Psychologie, 222, 79–89. [Google Scholar]
- Israelski K. A. (2008) Coping processes and their perceived effectiveness in siblings of people diagnosed with a pervasive developmental disorder. The Chicago School of Professional Psychology. [Google Scholar]
- Janke W., Erdmann G., Kallus K. W. (2002). Stressverarbeitungsfragebogen (SVF mit SVF 120 und SVF 78). Manual (3., erweiterte Auflage). Göttingen, Hogrefe. [Google Scholar]
- Kasai K., Yamasue H., Gilbertson M. W., Shenton M. E., Rauch S. L., Pitman R. K. (2008). Evidence for acquired pregenual anterior cingulate gray matter loss from a twin study of combat-related posttraumatic stress disorder. Biological Psychiatry, 63(6), 550–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Brent D., Rao U., Ryan N. (1996) Kiddie-Sads-Present and Lifetime Version (K-SADS_PL).http://www.psychiatry.pitt.edu/node/8233. [Google Scholar]
- Kelly A. M., Di Martino A., Uddin L. Q., et al. (2009). Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cerebral Cortex, 19(3), 640–57. [DOI] [PubMed] [Google Scholar]
- Kessler R. C., Berglund P., Demler O., Jin R., Merikangas K. R., Walters E. E. (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 593–602. [DOI] [PubMed] [Google Scholar]
- Laucht M., Esser G., Baving L., et al. (2000). Behavioral sequelae of perinatal insults and early family adversity at 8 years of age. Journal of the American Academy of Child & Adolescent Psychiatry, 39(10), 1229–37. [DOI] [PubMed] [Google Scholar]
- Laucht M., Esser G., Schmidt M. H. (1997). Developmental outcome of infants born with biological and psychosocial risks. Journal of Child Psychology and Psychiatry, 38(7), 843–53. [DOI] [PubMed] [Google Scholar]
- Lederbogen F., Kirsch P., Haddad L., et al. (2011). City living and urban upbringing affect neural social stress processing in humans. Nature, 474(7352), 498–501. [DOI] [PubMed] [Google Scholar]
- Levitan R. D., Rector N. A., Sheldon T., Goering P. (2003). Childhood adversities associated with major depression and/or anxiety disorders in a community sample of Ontario: issues of co-morbidity and specificity. Depression and Anxiety, 17(1), 34–42. [DOI] [PubMed] [Google Scholar]
- Lipka J., Hoffmann M., Miltner W. H., Straube T. (2014). Effects of cognitive-behavioral therapy on brain responses to subliminal and supraliminal threat and their functional significance in specific phobia. Biological Psychiatry, 76(11), 869–77. [DOI] [PubMed] [Google Scholar]
- Luthar S. S., Cicchetti D., Becker B. (2000). The construct of resilience: a critical evaluation and guidelines for future work. Child Development, 71(3), 543–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian J. A., Laurienti P. J., Kraft R. A., Burdette J. H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage, 19(3), 1233–39. [DOI] [PubMed] [Google Scholar]
- Margulies D. S., Kelly A. M., Uddin L. Q., et al. (2007). Mapping the functional connectivity of anterior cingulate cortex. NeuroImage, 37(2), 579–88. [DOI] [PubMed] [Google Scholar]
- Mc Lean C. P., Asnaani A., Litz B. T., Hofmann S. G. (2011). Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. Journal Psychiatric Research, 45(8), 1027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina I., Bianco S., Sambin M., Viviani R. (2015). Executive and semantic processes in reappraisal of negative stimuli: insights from a meta-analysis of neuroimaging studies. Frontiers in Psychology, 6, 956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A., Tost H. (2012). Neural mechanisms of social risk for psychiatric disorders. Nature Neuroscience, 15(5), 663–8. [DOI] [PubMed] [Google Scholar]
- Milad M. R., Quinn B. T., Pitman R. K., Orr S. P., Fischl B., Rauch S. L. (2005). Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proceedings of the National Academy of Sciences of the United States of America, 102(30), 10706–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson B. S., Wang Z., Horga G., et al. (2014). Discriminating risk and resilience endophenotypes from lifetime illness effects in familial major depressive disorder. JAMA Psychiatry, 71(2), 136–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar P., Peters E. R., Fannon D., Anilkumar A. P., Kuipers E., Kumari V. (2011). Coping styles predict responsiveness to cognitive behaviour therapy in psychosis. Psychiatry Research, 187(3), 354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J., Jiang X., Yao J., et al. (2015). Depression, Social Support, and Coping Styles among Pregnant Women after the Lushan Earthquake in Ya'an, China. PLoS ONE, 10(8), e0135809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud J., Dobson K. S., Drapeau M. (2014). Cognitive therapy for depression: Coping style matters. Counselling and Psychotherapy Research, 14(1), 42–7. [Google Scholar]
- Roohafza H. R., Afshar H., Keshteli A. H., et al. (2014). What's the role of perceived social support and coping styles in depression and anxiety? Research Journal of Medical Sciences, 19(10), 944–9. [PMC free article] [PubMed] [Google Scholar]
- Strunk D. R., DeRubeis R. J., Chiu A. W., Alvarez J. (2007). Patients' competence in and performance of cognitive therapy skills: relation to the reduction of relapse risk following treatment for depression. Journal of Consulting and Clinical Psychology, 75(4), 523–30. [DOI] [PubMed] [Google Scholar]
- Tomoda A., Suzuki H., Rabi K., Sheu Y. S., Polcari A., Teicher M. H. (2009). Reduced prefrontal cortical gray matter volume in young adults exposed to harsh corporal punishment. NeuroImage, 47(Suppl 2), T66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tol M. J., van der Wee N. J., van den Heuvel O. A., et al. (2010). Regional brain volume in depression and anxiety disorders. Archives of General Psychiatry, 67(10), 1002–11. [DOI] [PubMed] [Google Scholar]
- Welborn B. L., Papademetris X., Reis D. L., Rajeevan N., Bloise S. M., Gray J. R. (2009). Variation in orbitofrontal cortex volume: relation to sex, emotion regulation and affect. Social Cognitive and Affective Neuroscience, 4(4), 328–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyers P., Ising M., Janke W. (2005). Effects of imagined stress intensity on responses in a stress coping inventory. Anxiety, Stress & Coping: An International Journal, 18(2), 117–30. [Google Scholar]
- Wilkinson R., Walford W., Espnes G. (2000). Coping styles and psychological health in adolescents and young adults: A comparison of moderator and main effects models. Australian Journal of Psychology, 52(3), 155–62. [Google Scholar]
- Wittchen H. U., Zaudig M., Fydrich T. (1997). SKID - Strukturiertes Klinisches Interview DSM-IV Achse I und II. Göttingen (DE): Hogrefe. [Google Scholar]
- Zhang C. X., Tse L. A., Ye X. Q., Lin F. Y., Chen Y. M., Chen W. Q. (2009). Moderating effects of coping styles on anxiety and depressive symptoms caused by psychological stress in Chinese patients with Type 2 diabetes. Diabetic Medicine : a Journal of the British Diabetic Association, 26(12), 1282–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.