Abstract
Close social bonds are critical to a happy and fulfilled life and yet little is known, in humans, about the neurochemical mechanisms that keep individuals feeling close and connected to one another. According to the brain opioid theory of social attachment, opioids may underlie the contented feelings associated with social connection and may be critical to continued bonding. However, the role of opioids in feelings of connection toward close others has only begun to be examined in humans. In a double-blind, placebo-controlled, crossover study of naltrexone (an opioid antagonist), 31 volunteers took naltrexone for 4 days and placebo for 4 days (separated by a 10-day washout period). Participants came to the laboratory once on the last day of taking each drug to complete a task designed to elicit feelings of social connection. Participants also completed daily reports of feelings of social connection while on naltrexone and placebo. In line with hypotheses, and for the first time in humans, results demonstrated that naltrexone (vs placebo) reduced feelings of connection both in the laboratory and in daily reports. These results highlight the importance of opioids for social bonding with close others, lending support to the brain opioid theory of social attachment.
Keywords: brain opioid theory, μ-opioid antagonist, emotion, social reward, social attachments
Having and maintaining close social bonds is so critical to health, well-being and normal functioning (Bowlby, 1988; Uchino, 2004; Taylor, 2010) that bonding has been referred to as a basic need, much like the need for food and water (Baumeister and Leary, 1995). In fact, being deprived of care and support early in life results in severe cognitive, social, developmental and health consequences throughout development (Harlow, 1958; Bowlby, 1988; Gunnar, 2001). Although humans require close social bonds to survive and thrive, few studies have explored the experience of social connection and the resulting positive, contented feelings associated with being close to others. Furthermore, little is known about the neurochemical mechanisms that help support social bonds, especially in humans.
According to the brain opioid theory of social attachment, endogenous opioids, specifically μ-opioids, are released by experiences of social bonding and mediate the pleasant feelings stemming from social bonding and affiliation (Panksepp et al., 1980; Panksepp, 1981; Panksepp, 1998; Machin and Dunbar, 2011; Loseth et al., 2014). More specifically, opioids have been proposed to underlie the affiliative, interpersonal feelings (e.g. warmth, affection) that come from social connection (Panksepp, 1998; Depue and Morrone-Strupinsky, 2005). Furthermore, the theory states that experiences of social loss or separation lead to reduced opioid activity thus leading to feelings of disconnection and separation distress. Although few studies have examined the role of opioids in human attachment relationships, data from animal models support the theory that opioids are involved in social bonding (Panksepp et al., 1978; Herman and Panksepp, 1981; Panksepp, 1981; Panksepp and Bishop, 1981; Panksepp et al., 1981; Kalin et al., 1988; Keverne et al., 1989; Kalin et al., 1995; Burkett et al., 2011; Trezza et al., 2011).
For example, in chicks, blocking opioid activity via an opioid antagonist led to increased distress vocalizations when chicks were placed together in a group, suggesting that the chicks were no longer experiencing the comfort or pleasure from being in a group (Panksepp et al., 1980). Conversely, morphine, an opioid agonist, decreased distress vocalizations after social separation (Panksepp et al., 1980). Similarly, placebo-treated lambs showed a clear preference for their own mothers (vs an unknown mother) after birth, whereas naltrexone, an opioid antagonist, eliminated the preference for the mother (Shayit et al., 2003). Finally, μ-opioid receptor knockout mice (vs controls) showed no preference for bedding with their mother’s scent, again suggesting that the knockout mice no longer found the mother a source of comfort or derived as much pleasure from cues of her presence (Moles et al., 2004).
However, only a handful of studies have explored the role of opioids on perceptions of social rewards and social connection experiences in humans (although see Zubieta et al., 2003 for some work on opioids and social pain). With regard to the effect of opioids on social rewards, morphine (vs naltrexone) leads to higher attractiveness ratings for the most attractive faces presented, suggesting that opioids can enhance the social reward of certain stimuli (Chelnokova et al., 2014). In addition, increased desire for social interaction is associated with μ-opioid receptor binding in the left ventral striatum, a reward and affiliation-related region, in response to receiving social feedback that one is ‘liked’ by an opposite-sex stranger (vs baseline; Hsu et al., 2013). One early study (described in Depue and Morrone-Strupinsky, 2005 but not published on its own), more directly examined the role of opioids in feelings of social connection. Here, female participants were administered naltrexone (25 mg) and placebo (separated by a 4-day period) and were asked to rate their feelings of connection in response to watching two separate movie clips: an affiliative movie clip of a couple giving birth to their first child and a neutral film clip of a nature scene. In line with the brain opioid theory of social attachment, naltrexone (vs placebo) reduced the increases in warm, affectionate feelings to watching the affiliative clip (compared to the neutral clip) among those high in trait affiliation. Similarly, Schweiger et al. (2014) recently showed that a 25 mg dose of naltrexone (vs placebo) reduced affiliative feelings (cozy, comforting, liked, secure) after an economic trust game. Together, these results suggest that opioids may subserve feelings of connection. However, no studies have examined the effect of opioids on feelings of social connection to personally relevant social stimuli or to daily experiences of social connection.
Together, emerging evidence from the human and animal literatures points to the possibility that μ-opioids contribute to the experience of social bonding and connecting with close others. As such, the current study aimed to test the opioid theory of social attachment in a double-blind, placebo-controlled crossover study with the opioid-antagonist, naltrexone. Participants took naltrexone for 4 days (25 mg on days 1 and 2, 50 mg on days 3 and 4) and placebo for 4 days. On the fourth day of taking naltrexone or of taking placebo, participants completed a laboratory session in which feelings of social connection to an experimental task were assessed. Additionally, while on naltrexone or placebo, participants made ratings of their daily feelings of social connection each evening. We hypothesized that naltrexone, compared to placebo, would lead to reduced feelings of connection both in the laboratory as well as in day-to-day reports. Furthermore, in line with the possibility that opioids are specifically important for feelings of social connection in response to social stimuli, we hypothesized that opioid-blockade would reduce feelings of connection to a greater extent than general feelings of positive affect.
Method
Overview
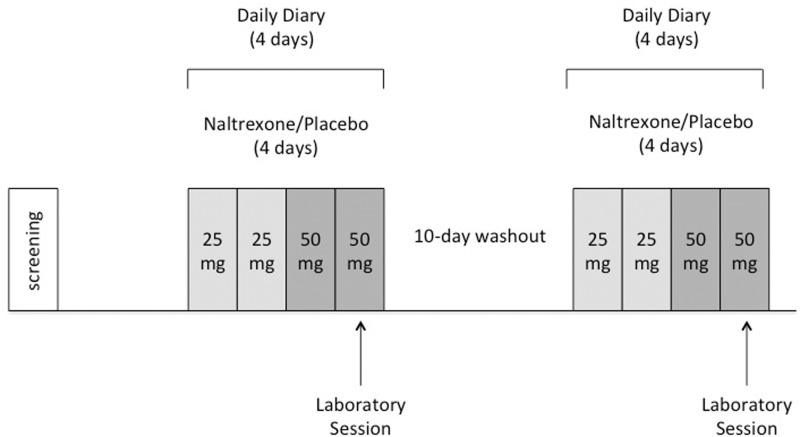
To assess the contribution of opioids to feelings of social connection, healthy participants completed a randomized, double-blind crossover study with the opioid-antagonist naltrexone (Figure 1). Based on a previously established titration schedule (Ray et al., 2011), participants received four administrations of naltrexone (25 mg on days 1 and 2 followed by 50 mg on days 3 and 4) as well as four administrations of placebo. Participants completed two laboratory sessions (one on day 4 of naltrexone (50 mg) and one on day 4 of placebo), separated by a 10-day washout period and completed daily diary reports on all four naltrexone and four placebo days. The study was registered on the U.S. National Institutes of Health Clinical Trials registry as NCT01672723 and all procedures were run (between December 2012 and February 2014) in accordance with UCLA’s IRB.
Figure 1.
Overview of procedures. After screening, participants completed a randomized, double-blind crossover study with the opioid-antagonist, naltrexone. Participants took four administrations of naltrexone, (25 mg on days 1 and 2 followed by 50 mg on days 3 and 4) as well as four administrations of placebo and completed two laboratory sessions (one on day 4 of naltrexone and one on day 4 of placebo), separated by a 10-day washout period. Daily diary reports were taken on all study drug days.
Screening and study participants
Participants were recruited via flyers. Prospective volunteers underwent a physical examination at UCLA’s Clinical & Translational Research Center (CTRC) where blood was drawn (assessed for liver functioning and pregnancy) and vital signs were collected (heart rate, blood pressure, height, weight). The experimenter then administered the Patient Health Questionnaire (PHQ-9; Spitzer et al., 1999) to assess depression levels and collected a urine sample to test for drug use [Tetrahydracannabinol (THC), Opiates, Cocaine, Amphetamines (AMP), Methamphetamines (mAMP)].
To be included in the study, participants needed to be in good health, between 18 and 35 years old, and fluent in English. Exclusion criteria were major physical health or psychiatric disorders (including a PHQ-9 score above a 13), medication use (prescription medications, except birth control, or over-the-counter medications), a positive drug test, a BMI greater than 35, a positive pregnancy test or clinically relevant abnormalities on the blood test.
After screening 50 individuals, 37 participants were enrolled. Of this sample, one participant asked to be removed from the study prior to scheduling, two participants were removed after being unresponsive to scheduling requests and three participants (all females) reported stomach discomfort at a severe level after the first day of taking naltrexone (25 mg) and were withdrawn by the study physician. Thirty-one participants in total completed the entire experimental protocol (21 females, M age = 21.55, SD = 3.34) with 38.7% Caucasian, 35.5% Asian, 12.9% Hispanic, 6.5% African American and 6.5% reporting Mixed Ethnicity. Participants were paid up to $160 for taking part in the study.
Study drug schedule
The opioid antagonist used in this study was oral naltrexone, an FDA-approved drug used to help manage alcoholism and opioid addiction (Ray et al., 2010). UCLA’s Investigational Drug Section dispensed tablets in a double-blind within-subjects crossover manner. Placebo consisted of microcrystalline cellulose and lactose. Each participant began taking their study drugs 3 days prior to the scheduled experimental session and took the fourth pill (50 mg) in the presence of the experimenter on the fourth day during their experimental session. The laboratory task (see below) began 60 min after taking the study drug when peak effects are known to occur (Lee et al., 1988). Drug compliance was assessed by packing the study drugs with 50 mg of riboflavin and analyzing urine samples for riboflavin content under a UV light at the start of each experimental session. All samples tested positive for riboflavin suggesting that all participants were compliant with the study drug schedule. In between each experimental session there was a 10-day washout period.
Laboratory task
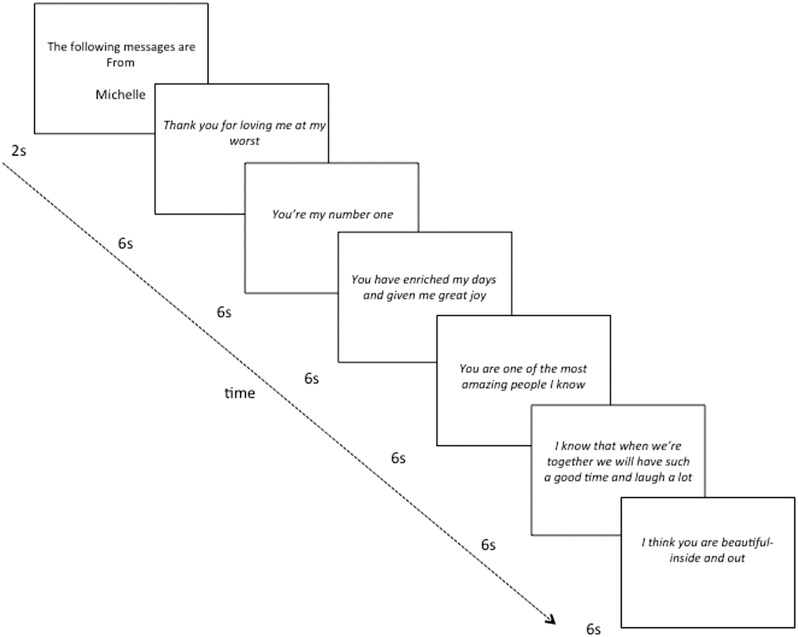
The experience of social connection is a powerful and personal experience. In order to bring this kind of personal experience into the laboratory, the experimenters asked participants to identify six people with whom they felt very close and who would be willing to be contacted in regards to the current study. The participants rated the closeness of these individuals on a scale ranging from (1) not at all close to (10) extremely close. Ratings of closeness were indeed high (M = 8.37, SD = .84, range 7–10). Experimenters then emailed the six close others (parents, grandparents, siblings, significant others, friends) to elicit help in creating the social connection laboratory task. Close others were asked to respond with brief sentences addressed to the participant that were positive in nature. To ensure that these messages were a surprise, we asked these close others to refrain from discussing what they had emailed to us. The emailed instructions included example messages to help guide writers as to the style and length of messages (e.g. I love you for being you, I appreciate that you are always there for me). Examples of messages received from actual close others included: ‘You have enriched my days and given me great joy,’ ‘Thank you for loving me at my worst,’ ‘You’re my number one,’ ‘I am so grateful to have you in my life and I completely don’t deserve you. You’re a gift!’
Once in the lab, participants read the messages from their close others. Messages were presented in sets of 6, beginning with a 2-s cue indicating who the messages were from (e.g. ‘The following messages are from Kim’), followed by the messages, one at a time, for 6-s each (Figure 2). After each set, participants rated their feelings of connection (how connected, touched and warm they felt, α = 0.93) and feelings of positive affect (how grateful they felt, as well as how good, pleasant, unpleasant (reversed) and enjoyable the messages were to read, α = 0.84). Ratings were made on a 1–7 scale anchored by ‘not at all’ and ‘very.’ As might be expected given the highly pleasant nature of the task, feelings of connection and positive affect in response to the laboratory task were correlated (r = 0.93, P < 0.001). However, the measures were kept separate in order to explore the theory that opioids might be especially relevant for social feelings (Panksepp et al., 1980; Panksepp, 1981; Panksepp, 1998; Depue and Morrone-Strupinsky, 2005; Machin and Dunbar, 2011).
Figure 2.
Social connection laboratory task. Participants read positive messages from close others (sample messages from actual friends and family displayed above) and then made ratings of their feelings of social connection and positive affect.
Messages from three of the participants’ close others were presented during the first experimental session and the remaining messages from a different 2–3 close others during the second experimental session. For four participants, only five close others responded to our request. Therefore, in the second session a message from one person that was presented in the first session was presented again. This procedure (to repeat the presentation of messages from a single close other) was only done for the four participants who were missing a sixth set of messages.
Differences between the first and second experimental lab sessions were also analyzed (using a repeated measures ANOVA to compare ratings (connection and positive affect) during session 1 to ratings during session 2) to check for potential habituation to the task. This analysis revealed a main effect of experimental session for self-reports to the messages, such that participants reported near ceiling levels of connection and positive affect during session 1 (M session 1 for combination of connection and positive affect = 6.68 on a scale of 1–7, SD = 0.40, range: 5.67–7.00) compared to session 2 where self-reports were significantly lower (M session 2 = 6.47, SD = 0.56, range: 4.67–7.00; F(1, 30) = 5.09, P = 0.03). In addition, there was a significant interaction between session and type of rating (connection vs positive affect; F(1, 30) = 16.05, P < 0.001) such that feelings of connection went down from session 1 to session 2 (F(1, 30) = 9.52, P = 0.004) but positive affect did not (F(1, 30) = 1.87, P = 0.18). Hence, perhaps because of the novel, surprising nature of the task (i.e. participants did not expect to see the loving messages from their close others the first time they came in for the study), self-reports were high during session 1, making it more difficult to observe drug effects during the first visit. Indeed, no main effect of drug (F(1, 29) = 1.81, P = 0.19) or drug × type of rating interaction (F(1, 29) = 0.63, P = 0.43) emerged for session 1. Therefore, analyses for the laboratory-based social connection task focused on session 2 alone.
Daily diary
To assess changes in day-to-day feelings of connection and general positive affect, daily diary responses were collected online at the end of each drug day. For 8 days (four while on placebo, four while on naltrexone), participants were asked to think back to the last 24 h and respond to how disconnected they felt (‘I felt out of touch and disconnected from others’) and how pleasant they felt (‘My mood was generally positive today’). For ease of interpretation, feelings of disconnection were reverse-coded to measure daily feelings of social connection. Unlike self-reports in response to the laboratory task, daily feelings of social connection and positive affect were not related to one another (r = 0.17, P = 0.36) further suggesting they are separable constructs. Daily diary responses were relatively stable across the 8 days (α feelings of connection = 0.72, α positive affect = 0.70).
Differences between the first set of daily self-reports and the second set were analyzed to check for differences across time using a repeated measures ANOVA comparing ratings (both connection and positive affect) from the first session to the second session. Although there was a novelty effect for ratings from the laboratory task (potentially because of the personal, powerful nature of the task), there was no such effect for the daily diary ratings. Hence, there were no significant differences between self-reports from the days surrounding session 1 (M of connection and positive affect = 5.51, SD = 0.85) and the days surrounding session 2 (M of connection and positive affect = 5.79, SD = 0.85; F(1, 30) = 2.31, P = 0.14) nor was there an interaction between session and type of rating (F(1, 30) = .19, P = 0.67). This is not surprising as, unlike the lab-based session, there was no specific task that participants were habituating to in their daily diary reports from their first to second set of ratings.
Participants also reported on the severity of the following physical symptoms at the end of each of the 8 days (0-no symptoms to 4-very severe symptoms): headache, dizziness/faintness, shortness of breath, rapid/irregular heartbeat, stomach/abdominal discomfort, nausea, appetite increase/decrease, difficulty urinating, muscle/bone/joint pain, fever, tiredness/fatigue. To further assess the subjective severity of the symptoms overall, participants were asked how distressing they found the symptoms on a 0 (not at all) to 7 (very) scale.1
Statistical analyses
Laboratory-based social connection task
In order to assess the effect of the pharmacological manipulation on self-reports to the social connection task during the second lab session, we conducted a 2 (drug: placebo vs naltrexone; between-subjects) × 2 (type of rating: connection vs positive affect; within-subjects) repeated measures analysis of variance (ANOVA) in SPSS. Since we are only examining session 2, drug condition is a between subjects factor for this analysis. Significant interactions were further interrogated to examine the effect of the drug on each rating type (connection or positive affect).
Daily diary assessment
Daily diary ratings were analyzed with a 2 (drug: placebo vs naltrexone) × 2 (type of rating: connection vs positive affect) × 2 (dose: 25 vs 50 mg) repeated measures ANOVA. Dosage was included as an additional variable because previous work has shown that the 50 mg dose of naltrexone (compared to baseline without naltrexone) completely blocks μ-opioid receptor binding in regions previously shown to activate to the social connection task used in the current study (including the VS, ACC, OFC and insula; Weerts et al., 2008; Inagaki and Eisenberger, 2013). However, there are no published data on receptor binding for the 25 mg dose leaving the possibility that receptors are not completely blocked at this lower dose. Significant interactions were further examined with repeated measures ANOVA and t-tests in SPSS for the effects at each dosage and for each rating type.
Results
Study drug blind and physical symptoms
When evaluating the reliability of the study drug blind, 61% of participants guessed correctly when on placebo and 55% guessed correctly when on naltrexone. These percentages were not significantly different from chance (50%) for either placebo (χ2 (1) = 0.35, P = 0.55) or naltrexone (χ2 (1) = 0.14, P = 0.71). Physical symptoms reported at the end of each day when on the study drugs revealed increases in headaches, dizziness/faintness, nausea and appetite increase/decrease when on naltrexone compared to placebo (P’s < 0.05), however reported symptoms were very mild for both drug conditions (M naltrexone = 0.39, M placebo = 0.10 on a 0–4 scale). Furthermore, there were no differences in how distressing participants found the symptoms when on naltrexone (M = 1.87, SD = 1.07) compared to placebo (M = 1.55, SD = .84, t(20) = 0.88, P = 0.39) and overall, levels of distress about the symptoms were low (ratings between 1 and 2 on a 1–7 scale) suggesting that the symptoms are unlikely to influence the main study outcomes.
Effect of naltrexone on feelings of connection in the lab
As noted earlier, there was a main effect of experimental session, such that participants reported significantly higher levels of social connection and positive affect during the first experimental session relative to the second experimental session. Thus, as might be expected based on the surprising, highly positive nature of the laboratory task, there was no main effect of drug (F(1, 29) = 1.81, P = 0.19) or drug × type of rating interaction (F(1, 29) = 0.63, P = 0.43) for session 1 when participants read the messages from their close others for the first time. Hence, analyses focused on session 2.
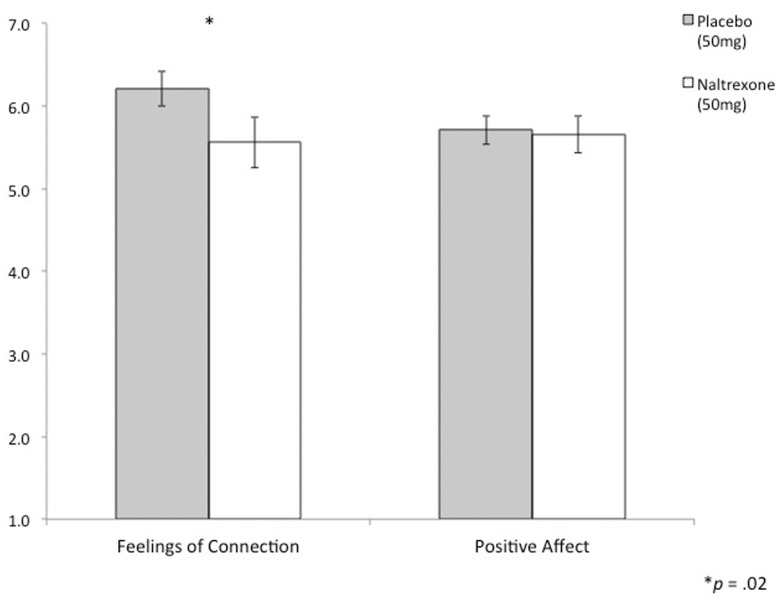
To assess whether naltrexone specifically altered feelings of connection to the lab task during session 2, participant self-reports were submitted to a 2 (drug) × 2 (type of rating: connection vs positive affect) repeated measures ANOVA. There was a main effect of type of rating (F(1, 29) = 41.18, P < 0.001), such that subjects reported higher levels of positive affect (M = 6.62, SD = 0.46) than feelings of connection (M = 6.20, SD = 0.77). In addition, those on naltrexone reported reduced ratings of social connection and positive affect to the messages (M = 6.20, SD = 0.83) compared to those who were on placebo (M = 6.61, SD = 0.80; F(1, 29) = 4.06, P = 0.05). This main effect was qualified by a marginal drug × type of rating interaction (F(1, 29) = 3.81, P = 0.06). In support of the main study hypothesis, naltrexone (vs placebo) reduced feelings of connection to reading the messages from close others (F(1, 29) = 4.32, P < 0.05, Figure 3) to a greater extent than it (marginally) reduced feelings of positive affect (F(1, 29) = 3.25, P = 0.08).
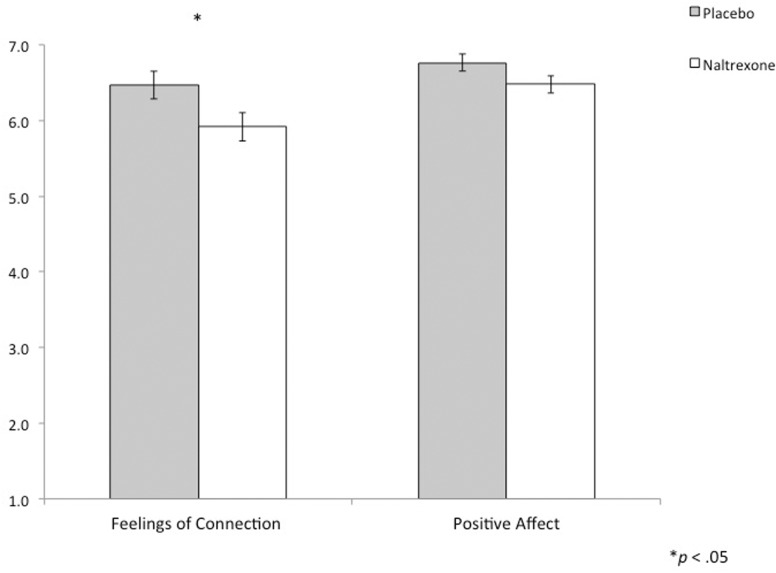
Figure 4.
Changes in feelings of connection and positive affect outside of the lab in day-to-day reports. Feelings of disconnection were reverse-coded such that higher numbers reflect more feelings of connection and lower numbers reflect less feelings of connection. Naltrexone (vs placebo) reduced feelings of connection when participants were on the 50 mg dose, but did not alter feelings of positive affect. Error bars reflect between-subject standard errors.
Figure 3.
The effect of naltrexone on feelings of connection to reading messages from close others in the lab. During experimental session 2, naltrexone (vs placebo) significantly reduced feelings of connection to messages from close others, but did not alter general positive affect.
To ensure that these effects were not driven by changes in self-reported symptoms, the association between symptoms (from the day of the naltrexone lab session) and feelings of connection and positive affect was evaluated. There was no association between symptoms and feelings of connection in response to the lab task (r = 0.02, P = 0.94) indicating that symptoms were not driving the reductions in feelings of connection. Symptoms were, however, associated with positive affect (r = 0.43, P = 0.02), although the association was in the opposite direction as one might expect if symptoms were driving the decreases in affective responses.
Hence, although naltrexone (vs placebo) reduced both feelings of social connection and positive affect overall (during the second experimental session), results showed that naltrexone reduced feelings of social connection to a greater extent than it reduced feelings of positive affect.
Effect of naltrexone on day-to-day feelings of connection
To assess whether naltrexone altered day-to-day feelings of connection from the daily diary reports, a 2 (drug) × 2 (type of rating) × 2 (dose) repeated measures ANOVA was run2. No main effects for drug, type of rating or dose were found (P’s > 0.25), nor was there a drug × dose interaction (F(1, 24) = 1.29, P = 0.27) but there was a drug × type of rating × dose interaction (F(1,24) = 6.67, P = 0.02). We then examined ratings for each dose (25 vs 50 mg) separately. As expected, there was no interaction between drug and type of rating at the lower 25 mg dose (F(1, 29) = 0.73, P = 0.40), but there was a significant drug × type of rating interaction when participants were on the full 50 mg dose (F(1, 25) = 6.51, P = 0.02, Figure 3). Specifically, while there was no effect of naltrexone (50 mg) on positive affect (t(25) = 0.33, P = 0.75), in support of the main study hypothesis, participants reported feeling less socially connected on the full 50 mg dose of naltrexone (t(25) = 2.56, P = 0.02) compared to comparable days of placebo.
Again, to ensure that physical symptoms were not driving the reductions in feelings of connection, we examined the relationship between self-reported symptoms across the 4 days when participants were on naltrexone and feelings of social connection. Although perceptions of symptom severity were low, physical symptoms could have been driving the reductions in feelings of connection. However, the average correlation between symptoms and feelings of connection was low (r = 0.07) and not significantly different from zero (P = 0.69) suggesting that reductions in feelings of connection were not driven by increases in physical symptoms. In addition, the average correlation between self-reported symptoms and positive affect was also low (r = −0.07) and not significantly different from zero (P = 0.63). Thus, blocking opioids reduced everyday feelings of social connection, but not general positive affect.
Discussion
As humans, we highly value and depend on close social bonds throughout life (Harlow, 1958; Durkheim, 1897/1951; Bowlby, 1988; Gunnar, 2001; Diener and Seligman, 2002). Therefore, it continues to be important to understand the neurobiological mechanisms that contribute to the experience of social connection. In support of the brain opioid theory of social attachment, the opioid antagonist, naltrexone, was found to reduce feelings of social connection in the lab and, for the first time, in daily reports, suggesting that opioids are a critical component of social bonding. This is the first study to highlight the importance of opioids for day-to-day social connection with close others in humans.
Importantly, the current study found a stronger effect of naltrexone on feelings of connection as compared to general positive feelings. That is, opioid antagonism (vs placebo) led to larger reductions in feelings of social connection, but altered ratings of more general positive affect to a lesser degree. These results are in line with theorizing that, while opioids play a general role in rewarding experiences, opioids may be particularly critical for social bonding and affiliation (Panksepp, 1998; Depue and Morrone-Strupinsky, 2005). However, more work in humans will be needed to replicate the current findings as the hypothesized effect was only found during the second experimental session (potentially due to the novelty of experiencing the social connection task, a highly pleasant and surprising task, during the first experimental session) and at the full 50 mg dose (when μ-opioid receptors are known to be completely blocked). Although naltrexone’s affinity for the µ-opioid receptor is the highest of all opioid receptors (Raynor et al., 1994), it also binds to other opioid receptors (Weerts et al., 2008) and thus we cannot definitively attribute the reported effects to the actions of µ-opioid receptors alone.
Although the brain opioid theory of social attachment emphasizes the role of opioids in social affect and social bonding, opioid-antagonism has previously been shown to reduce liking for nonsocial stimuli such as food (Yeomans and Gray, 1996, 1997) and gambling wins (Petrovic et al., 2008). Thus, it may not be the case that opioids are necessarily specific to social stimuli, but rather that opioids are specific to the most valued or greatest reward in the environment (Chelnokova et al., 2014). That is, opioid antagonism may affect one’s subjective experience in response to the greatest reward available. Social rewards, as presented in the current study, may be one such highly valuable reward. Future studies exploring the role of opioids in social affective experience may benefit from including ‘nonsocial’ rewards in order to clarify the specific role that opioids play in social processes.
There is emerging evidence that sensitivity to rewarding stimuli such as opiates or other drugs of abuse varies depending on a polymorphism in the μ-opioid receptor gene (OPRM1) (Matthes et al., 1996; Bond et al., 1998; Ray and Hutchison, 2004; Drakenberg et al., 2006; Dlugos et al., 2011). In particular, G allele carriers are more sensitive to β-endorphins (Bond et al., 1998), the rewarding effects of alcohol (Ray and Hutchinson, 2004), the reward-blunting effects of naltrexone (Ray and Hutchinson, 2007) and the painful effects of social exclusion (Way et al., 2009). Thus, to the extent that genetic variation confers sensitivity to social reward and pain more generally, allelic variation in OPRM1 may also contribute to the effects of opioid-blockade on feelings of social connection as measured here and may be an interesting future direction.
Naltrexone’s ability to reduce feelings of social connection may have implications for the use of this drug in addicted populations. Originally developed and prescribed to help treat opioid dependence and alcoholism (Martin et al., 1973; Anton et al., 1999), the current results suggest that an unintended side effect of naltrexone treatment may be reduced feelings of connection with others. Social support is especially important during times of need (Cohen and Wills, 1985), such as when struggling with an addiction and as such, any negative changes to how one perceives their network may introduce unintended barriers to recovery. Reduced feelings of connection may also partially contribute to why long-term compliance with naltrexone is somewhat low (Ray et al, 2010; Swift et al., 2011). Of course, in some cases, problems with social bonding or social connection may precede and follow addiction, however, moving forward it is worth understanding how naltrexone may affect feelings of social connection in addicted populations as well as healthy individuals, as studied here. Conversely, drugs that modulate the opioid system continue to be explored as a possible treatment option for other clinical disorders with social implications such as depression (Panksepp et al., 2014, Panksepp and Yovell, 2014) which is often accompanied by increases in social disconnection, as well as autism (Roy et al., 2014), which is characterized by deficits in social processes.
Humans have the capacity to form and maintain deeply emotional and meaningful bonds with others. Critical to these bonds are feelings of connection, the positive, contented feelings that come from being close to others, that have previously been hypothesized to involve the endogenous opioid system. In a direct test of this hypothesis, the current study found that naltrexone (vs a placebo) reduced feelings of connection both in the lab and in day-to-day reports, which furthers our understanding of the neurochemical mechanisms that contribute to social bonding and connection.
Acknowledgements
The authors wish to thank the staff and support of UCLA’s Clinical and Translational Science Institute (CTSI: UL1TR000124), Bill Hirokawa, of the UCLA Investigational Drug Section, Keely Muscatell, for assistance with drug randomization and the study blind, Spencer Bujarski for statistical advice and Cory Higgs and Molly Howland for assistance running the study.
Footnotes
1 How distressing participants found their daily symptoms was added partway into the study and obtained from the final 21 participants.
2 Six participants failed to complete daily diary responses for both days when on 25 mg of naltrexone (one participant), both days when on 50 mg of naltrexone (three participants), or comparable days when on placebo (two participants). Hence, daily diary responses are based on 25 participants.
Funding
We also acknowledge generous support from the National Institutes of Health (R01-AG034588; R01-AG026364; R01 CA160245-01; R01-CA119159; R01 HL095799; R01 DA032922-01; P30-AG028748 to M. R. I.) and the Cousins Center for Psychoneuroimmunology. Research was funded in part by a Dissertation Research Award from UC Berkeley’s Greater Good Science Center awarded to T.K.I.
Conflict of interest. None declared.
References
- Anton R. F., Moak D. H., Waid L. R., Latham P. K., Malcolm R. J., Dias J. K. (1999). Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: results of a placebo-controlled trial. American Journal of Psychiatry, 156, 1758–64. [DOI] [PubMed] [Google Scholar]
- Baumeister R. F., Leary M. R. (1995). The need to belong: desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin, 117, 497–529. [PubMed] [Google Scholar]
- Bond C., LaForge K. S., Tian M., et al. (1998). Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proceedings of the National Academy of Sciences USA, 95, 9608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowlby J. (1988). A Secure Base: Parent-Child Attachment and Healthy Human Development, Basic Books, USA. [Google Scholar]
- Burkett J. P., Spiegel L. L., Inoue K., Murphy A. Z., Young L. J. (2011). Activation of mu-opioid receptors in the dorsal striatum in necessary for adult social attachment in monogamous prairie voles. Neuropsychopharmacology, 36, 2200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelnokova O., Laeng B., Eikemo M., et al. (2014). Rewards of Beauty: The Opioid System Mediates Social Motivation in Humans. Molecular Psychiatry, 19, 746–7. [DOI] [PubMed] [Google Scholar]
- Cohen S., Wills T. A. (1985). Stress, social support, and the buffering hypothesis. Psychological Bulletin, 98, 310–57. [PubMed] [Google Scholar]
- Depue R. A., Morrone-Strupinsky J. V. (2005). A neurobehavioral model of affiliative bonding: implications for conceptualizing a human trait of affiliation. Behavioral and Brain Sciences, 28, 350–95. [DOI] [PubMed] [Google Scholar]
- Diener E., Seligman M. E. P. (2002). Very happy people. Psychological Science, 13, 81–4. [DOI] [PubMed] [Google Scholar]
- Dlugos A. M., Hamidovic A., Hodgkinson C., et al. (2011). OPRM1 gene variants modulate amphetamine-induced euphoria in humans. Genes, Brain and Behavior, 10, 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakenberg K., Nikoshokov A., Horvath M. C., et al. (2006). Mu opioid receptor A118G polymorphism is associated with striatal opioid neuropeptide gene expression in heroin abusers. Proceedings of the National Academy of Sciences U S A, 103, 7883–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkheim E. (1951). Suicide (J.A. Spaudling & G. Simpson, Trans. New York: Free Press. (Original work published in 1897) [Google Scholar]
- Gunnar M. R. (2001). Effects of early deprivation: Findings from orphanage-reared infants and children. In: Nelson C. A., Luciana M., editors. Handbook of Developmental Cognitive Neuroscience. Cambridge, MA: MIT Press, 617–29. [Google Scholar]
- Harlow H. F. (1958). The nature of love. American Psychologist , 13, 673–85. [DOI] [PubMed] [Google Scholar]
- Herman B. H., Panksepp J. (1981). Ascending endorphinergic inhibition of distress vocalization. Science, 211, 1060–2. [DOI] [PubMed] [Google Scholar]
- Inagaki T. K., Eisenberger N. I. (2013). Shared neural mechanisms underlying “social warmth” and physical warmth. Psychological Science, 24, 2272–80. [DOI] [PubMed] [Google Scholar]
- Kalin N. H., Shelton S. E., Barksdale C. M. (1988). Opiate modulation of separation-induced distress in non-human primates. Brain Res 440: 285–92. [DOI] [PubMed] [Google Scholar]
- Kalin N. H., Shelton S. E., Lynn D. E. (1995). Opiate systems in mother and infant primates coordinate intimate contact during reunion. Psychoneuroendocrinology, 20, 735–42. [DOI] [PubMed] [Google Scholar]
- Keverne E. B., Martensz N. D., Tuite B. (1989). Beta-endorphin concentrations in cerebrospinal fluid of monkeys are influenced by grooming relationships. Psychoneuroendocrinology, 14, 155–61. [DOI] [PubMed] [Google Scholar]
- Lee M. C., Wagner H. N., Tanada S., Frost J. J., Bice A. N., Dannals R. F. (1988). Duration of occupancy of opiate receptors by naltrexone. Journal of Nuclear Medicine, 29, 1207–11. [PubMed] [Google Scholar]
- Loseth G. E., Ellingsen D., Leknes S. (2014). State-dependent mu-opioid modulation of social motivation. Frontiers in Behavioral Neuroscience, 8, 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machin A. J., Dunbar R. (2011). The brain opioid theory of social attachment: a review of the evidence. Behaviour, 148, 985–1025. [Google Scholar]
- Martin W. R., Jasinski D. R., Mansky P. A. (1973). Naltrexone, an antagonist for the treatment of heroin dependence: effects in man. Archives of General Psychiatry, 28, 784–91. [DOI] [PubMed] [Google Scholar]
- Matthes H. W. D., Maldonado R., Simonin F., et al. (1996). Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature, 383, 819–23. [DOI] [PubMed] [Google Scholar]
- Moles A., Kieffer B. L., D’Amato F. R. (2004). Deficit in attachment behavior in mice lacking the mu-opioid receptor gene. Science, 304, 1983–6. [DOI] [PubMed] [Google Scholar]
- Panksepp J. (1998). Affective Neuroscience. Oxford University Press: New York, NY. [Google Scholar]
- Panksepp J., Bean N. J., Bishop P., Vilberg T., Sahley T. L. (1980). Opioid blockade and social comfort in chicks. Pharmacology Biochemistry and Behavior, 13, 673–83. [DOI] [PubMed] [Google Scholar]
- Panksepp J. (1981). Brain opioids: A neurochemical substrate for narcotic and social dependence. In Cooper S., editor. Progress in Theory in Psychopharmacology. London: Academic Press, 149–75. [Google Scholar]
- Panksepp J., Bishop P. (1981). An autoradiographic map of 3H diprenorphine binding in the rat brain: Effects of social interaction. Brain Research Bulletin, 7, 405–10. [DOI] [PubMed] [Google Scholar]
- Panksepp J., Yovell Y. (2014). Preclinical Modeling of Primal Emotional Affects (SEEKING, PANIC and PLAY): gateways to the development of new treatments for depression. Psychopathology, 47, 383–93. [DOI] [PubMed] [Google Scholar]
- Panksepp J., Herman B. H., Vilberg T., Bishop P., DeEskinazi F. G. (1980). Endogenous opioids and social behavior. Neuroscience and Biobehavioral Reviews, 1980, 4, 473–87. [DOI] [PubMed] [Google Scholar]
- Panksepp J., Herman B., Conner R., Bishop P., Scott J. P. (1987). The biology of social attachments: Opiates alleviate separation distress. Biological Psychiatry, 13, 607–18. [PubMed] [Google Scholar]
- Panksepp J., Wright J. S., Dobrossy M. D., Schlaepfer T. E., Coenen V. A. (2014). Affective Neuroscience strategies for understanding and treating depression: From preclinical models to three novel therapeutics. Clinical Psychology Science, 2, 472–94. [Google Scholar]
- Petrovic P., Pleger B., Seymour B., et al. (2008). Blocking central opiate function modulates hedonic impact and anterior cingulate response to rewards and losses. Journal of Neuroscience, 28, 10509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray L. A., Bujarski S., Chin P. F., Miotto K. (2011). Pharmacogenetics of naltrexone in asian americans: a randomized placebo-controlled laboratory study. Neuropsychopharmacology, 37, 445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray L. A., Chin P. F., Miotto K. (2010). Naltrexone for the treatment of alcoholism: clinical findings, mechanisms of action, and pharmacogenetics. CNS & Neurological Disorders - Drug Targets, 9, 13–22. [DOI] [PubMed] [Google Scholar]
- Ray L. A., Hutchison K. E. (2004). A polymorphism of the mu-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcoholism: Clinical and Experimental Research, 28, 1789–95. [DOI] [PubMed] [Google Scholar]
- Raynor K., Kong H., Chen Y., et al. (1994). Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors. Molecular Pharmacology , 45, 330–4. [PubMed] [Google Scholar]
- Roy A., Roy M., Deb S., Unwin G., Roy A. (2014). Are opioid antagonists effective in attenuating the core symptoms of autism spectrum conditions in children: a systematic review. Journal of Intellectual Disability Research, 59, 293–306. [DOI] [PubMed] [Google Scholar]
- Schweiger D., Stemmler G., Burgdorf C., Wacker J. (2014). Opioid receptor blockade and warmth-liking: effects on interpersonal trust and frontal asymmetry. Social Cognitive and Affective Neuroscience, 9, 1608–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayit M., Nowak R., Keller M., Weller A. (2003). Establishment of a preference by the newborn lamb for its mother: the role of opioids. Behavioral Neuroscience, 117, 446–54. [DOI] [PubMed] [Google Scholar]
- Spitzer R. L., Kroenke K., Williams J. B. W. (1999). Validation and utility of a self-report version of PRIME-MD: the PHQ Primary Care Study. The Journal of the American Medical Association, 282, 1737–44. [DOI] [PubMed] [Google Scholar]
- Swift R., Oslin D. W., Alexander M., Forman R. (2011). Adherence monitoring in naltrexone pharmacotherapy trials: a systematic review. Journal of Studies on Alcohol and Drugs, 72, 1012–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. E. (2010). Mechanisms linking early life stress to adult health outcomes. Proceedings of the National Academy of Sciences USA, 107, 8507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V., Damsteegt R., Marijke Achterberg E. J., Vanderschuren L. J. M. J. (2011). Nucleus accumbens μ-opioid receptors mediate social reward. Journal of Neuroscience, 31, 6362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino B. N. (2004). Social support and physical health: Understanding the health consequences of our relationships. Yale University Press: New Haven, CT. [Google Scholar]
- Way B. M., Taylor S. E., Eisenberger N. I. (2009). Variation in the mu-opioid receptor gene (OPRM1) is associated with dispositional and neural sensitivity to social rejection. Proceedings of the National Academy of Sciences U S A, 106, 15079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerts E. M., Kim Y. K., Wand G. S., et al. (2008). Differences in delta- and mu-opioid receptor blockade measured by positron emission tomography in naltrexone-treated recently abstinent alcohol-dependent subjects. Neuropsychopharmacology, 33, 653–65. [DOI] [PubMed] [Google Scholar]
- Yeomans M. R., Gray R. W. (1996). Selective effects of naltrexone on food pleasantness and intake. Physiology & Behavior, 60, 439–6. [DOI] [PubMed] [Google Scholar]
- Yeomans M. R., Gray R. W. (1997). Effects of naltrexone on food intake and changes in subjective appetite during eating: evidence for opioid involvement in the appetizer effect. Physiology & Behavior, 62, 15–21. [DOI] [PubMed] [Google Scholar]