Abstract
The habenula has been implicated in predicting negative events and in responding to unexpected negative outcomes. Animal models of depression have supported the hypothesis that perturbations in habenula activity contribute to the pathophysiology of Major Depressive Disorder (MDD), a psychiatric illness characterized by abnormalities in responding to negative feedback and by pessimism in evaluating the likelihood of future events. No research to date, however, has examined human habenula responses to potential and experienced negative outcomes in MDD. In this study, depressed and healthy control participants performed a probabilistic guessing task for monetary rewards and penalties during high-resolution functional magnetic resonance imaging of the habenula. In healthy adults, we observed a pattern of habenula activation consistent with its hypothesized role in predicting future losses and responding to suboptimal outcomes. In contrast, in depressed participants the left habenula was not activated significantly during the prediction or experience of monetary penalty. Complementing this group difference, attenuated habenula activation to negative feedback in control participants was associated with levels of shame and rumination. The results of this study suggest that depressed individuals are characterized by dysfunction in a neural system involved in generating expectations and comparing expectations with objective outcomes.
Keywords: depression, habenula, fMRI, dopamine, punishment
Introduction
Major Depressive Disorder (MDD) is among the most prevalent and debilitating of all psychiatric illnesses, affecting nearly 20% of the US population, or more than 30 million adults (Kessler and Wang, 2014). In addition to the diagnostic criteria established by the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), which include depressed mood, anhedonia, and a number of cognitive and somatic manifestations, MDD has been associated with maladaptive responses to negative events and feedback. For example, depressed individuals have a higher probability of committing further errors following error feedback than do healthy individuals (Beats et al., 1996; Douglas et al., 2009). Consistent with these behavioral results, investigators have also documented exacerbated neurophysiological responses to errors and negative feedback in MDD (Chiu and Deldin, 2007; Holmes and Pizzagalli, 2008). Such enhanced responses may erroneously signal that these events are highly significant, leading to increased recruitment of behavioral, affective, and cognitive processes that manifest as signs and symptoms of depression (Chiu and Deldin, 2007). Findings in this area are equivocal, however, as several investigators have reported blunting of neural responses to negative feedback in both currently depressed (Steele et al., 2007) and formerly depressed (Schiller et al., 2013) individuals. Thus, an alternative explanation for anomalous post-feedback performance in MDD is that attenuated responses to negative outcomes may preclude the recruitment of cognitive control resources necessary to implement appropriate behavioral adjustments. Importantly, depressed individuals are also characterized by pessimism when evaluating the probability of future negative events (Alloy and Ahrens, 1987; Strunk et al., 2006); therefore, it is possible that abnormal expectation of poor outcomes may amplify or attenuate responses to these events when they do occur. Taken together, this literature suggests that MDD is characterized by impairment in the neurobiological systems that support the prediction of, and response to, negative outcomes.
Recent animal research indicates that abnormalities of the habenula, a conserved component of the epithalamus, may contribute to both the anomalous prediction of, and response to, negative outcomes in MDD. The lateral habenula receives projections from the globus pallidus posited to carry information about reward expectancy and omission (Hong and Hikosaka, 2008; Shabel et al., 2012) and sends direct (Lammel et al., 2012) and indirect inhibitory (Jhou et al., 2009; Balcita-Pedicino et al., 2011) projections to the dopaminergic ventral tegmental area, thereby uniquely situating the structure to influence dopamine release as a function of both expected and observed outcomes. In contrast to midbrain dopaminergic neurons that fire physically when outcomes are unexpectedly rewarding (Schultz, 1998), habenula neurons increase their activity when an expected reward is omitted or when cues predict future non-rewarding outcomes (Matsumoto and Hikosaka, 2007). Such firing may give rise to the inhibition of dopaminergic neurons in the context of disappointing outcomes (Schultz, 1998). Importantly, habenula neurons respond not only to cues that signal future reward omission, but also to cues that predict aversive outcomes (Matsumoto and Hikosaka, 2008). Together, these data support the hypothesis that the habenula acts in parallel and in opposition to the reward-signaling midbrain dopamine system by registering the occurrence of unexpected reward omissions and predicting future suboptimal or aversive outcomes. Existing research suggests a similar role for the habenula in humans as in non-human primates; performance errors (Li et al., 2008), negative performance feedback (Ullsperger and Von Cramon, 2003; Shepard et al., 2006), primary punishment (Lawson et al., 2014; Hennigan et al., 2015) and omission of an expected reward (Salas et al., 2010) all increase blood oxygen-level dependent (BOLD) responses in the habenula. In addition, habenula activation increases when individuals view cues predictive of punishment, and corresponds to response time slowing (Lawson et al., 2014), further implicating the structure in the evaluation of, and response to, potentially disadvantageous outcomes.
Evidence for the involvement of the habenula in the pathophysiology of MDD comes primarily from studies of rodent models of depression. For example, elevations in regional glucose metabolism in the lateral habenula have been found in ‘learned helpless’ rats (Shumake et al., 2003) and accompany reduced exploration of new environments and decreased self-administration of rewarding electrical brain stimulation (Caldecott-Hazard et al., 1988). In contrast, inhibition of the habenula decreases learned helpless behavior (Winter et al., 2011) and improves performance on the forced swim task (Nair et al., 2012). Finally, deep-brain stimulation (DBS) of the habenula leads to acute reversal of learned helplessness and depression-like behaviors (Li et al., 2011; Meng et al., 2011). Given these findings, the habenula has emerged as a promising therapeutic target for MDD. Indeed, sustained clinical remission has been reported in one patient who received DBS in this region (Sartorius et al., 2010). These data provide initial support for a model in which ongoing habenula dysfunction contributes significantly to the clinical presentation of depression, due in part to the regulation of midbrain monoamine systems by this structure (Ji and Shepard, 2007; Zhao et al., 2015). Nevertheless, despite a considerable number of recent review articles that hypothesize links between habenula hyperactivity and the pathophysiology of human mood disorder (Lecca et al., 2014; Proulx et al., 2014; Benarroch, 2015; Zhao et al., 2015), researchers have not yet investigated whether depression is associated with abnormal phasic habenula responses during the prediction or the experience of negative outcomes.
Therefore, as a preliminary step in determining whether negative reward-related functionality of the habenula is compromised in depressed persons, we used high-resolution functional magnetic resonance imaging (fMRI) to examine habenula activation during the prediction and receipt of monetary penalties in a small sample of depressed and healthy participants. We hypothesized that in healthy individuals, both prediction and experience of monetary loss would elicit habenula activation; we also predicted that, in contrast, depressed participants would show exaggerated habenula responses to loss outcomes. In addition, based on documented depression-related biases in the prediction of future negative outcomes, we hypothesized that, in depressed individuals, habenula responses during the prediction of future outcomes would be dissociated from the objective probability of monetary penalty.
Method
Participants
Participants between 18 and 65 years of age were recruited through clinics in the Department of Psychiatry and Behavioral Science at Stanford University and through advertisements in local media. Individuals who met initial inclusion criteria were administered the Structured Clinical Interview for DSM-IV-TR (First et al., 2001) by trained clinical interviewers to yield a clinical diagnosis and assess history of psychopathology. Our team of interviewers has demonstrated excellent inter-rater reliability for both the MDD (κ = 0.93) and the non-psychiatric control (CTL; κ = 0.92) diagnoses. All depressed participants met criteria for a DSM-IV-TR diagnosis of current MDD; CTL participants did not meet criteria for current or lifetime Axis-I psychopathology. Exclusion criteria included learning disabilities, psychotic symptoms, bipolar disorder, current psychotropic drug use, history of routine gambling, regular cigarette smoking or substance abuse within the past 6 months.
Informed consent was obtained from all participants, and all aspects of this study complied with the American Psychological Association’s ethical standards for the treatment of human participants. Participants were paid $25/hour for diagnostic assessment and neuroimaging.
Protocol
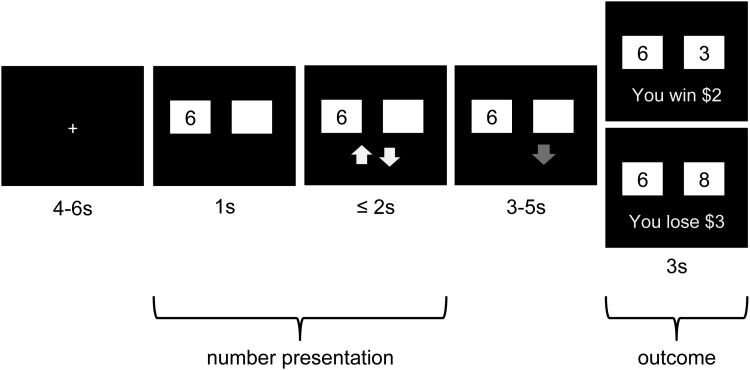
We adapted a paradigm previously found to elicit robust reward-related activation in the midbrain (D’Ardenne et al., 2008; Figure 1). At the start of each trial, participants were shown a number and a white square hiding a second number. Instructions presented prior to participants beginning the task indicated that all numbers would fall between 0 and 10 and would never be equal within a single trial. After 1000 ms, the appearance of yellow ‘up’ and ‘down’ arrows indicated that participants should guess within 2000 ms, via a button press, whether the hidden number was higher or lower than the first. Once a guess was entered, the corresponding arrow turned red and remained on the screen, along with the original number and white square, until the end of a variable delay period (3000–5000 ms). After the delay, the white box disappeared revealing the hidden number, and feedback regarding monetary gain (+$2) or monetary loss (−$3), corresponding to a correct or incorrect (or missed) guess, respectively, was presented for 3000 ms. In addition to allowing us to examine responses to negative feedback in general, this paradigm enables us to explore the extent to which habenula activation scales with the probability of a loss outcome (ranging from 0.5 when a ‘5’ is shown, to 0 when ‘0’ or ‘10’ are shown and participants guess according to a win-maximizing strategy), both before and after feedback is received. Thus, number pairings were pre-selected to adequately represent each level of probability and to ensure that each participant made a minimum number of incorrect guesses (i.e. 24 or 30% error rate). Participants completed 80 total trials over the course of two 10-min scanning blocks. To motivate performance, participants were told they would receive a percentage of their game earnings; all participants received an $8 bonus. Participants completed the Beck Depression Inventory-II (BDI-II; Beck et al., 1996), the Positive and Negative Affect Schedule (PANAS; Watson et al., 1988), the Ruminative Responses Scale (RRS; Treynor et al., 2003) and the Experience of Shame Scale (ESS; Andrews et al., 2002).
Fig. 1.
Schematic of paradigm structure. On each trial, participants saw a number between 0 and 10 appear on the left side of the screen, and were instructed to guess whether a second, hidden number was higher or lower than the visible number. Guesses could only be entered while yellow arrows were present on the screen. After guessing, a variable delay period was followed by feedback concerning the accuracy of the guess and corresponding monetary reward or penalty.
MRI acquisition procedures
MRI data were acquired using a 3.0 Tesla GE scanner (GE Medical systems, Waukesha, WI) and a 32-channel head coil. Using a T2*-sensitive gradient echo spiral sequence (TR = 2000 ms, TE = 33 ms, matrix = 148 × 148), high-resolution (1.5 mm3) BOLD signal data were acquired from 23 oblique slices centered about the epithalamus. Whole-brain T1-weighted spoiled gradient recalled (SPGR) images (0.9 mm3) were acquired to enable localization of the habenula.
Definition of habenula regions of interest
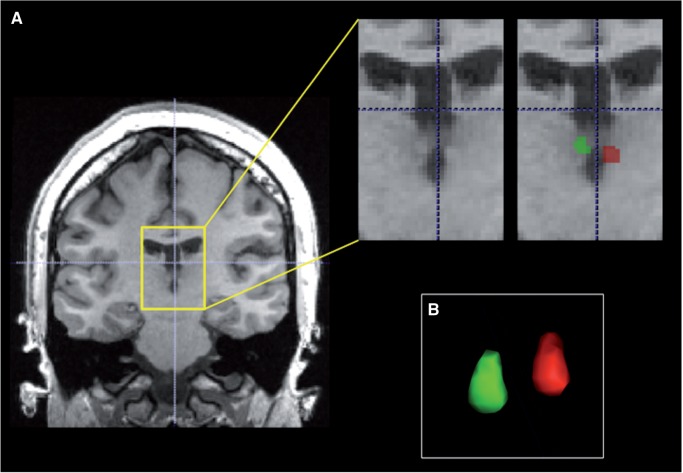
For each participant, the left and right habenula were manually segmented from SPGR images using Insight Toolkit’s SNAP program (www.itksnap.org; Figure 2). Because the lateral boundary of the habenula is difficult to delineate based on contrast differences alone, we adopted the geometrical approach described in detail by Lawson et al. (2012).
Fig. 2.
Manual segmentation of the habenula. (A) Boundaries of the habenula were defined in coronal slices anterior to the stalk of the pineal gland. In the representative anatomical image shown here, the left and right habenula are visible as protrusions into the cerebrospinal fluid of the third ventricle. (B) Representative 3D rendering of manually segmented habenula structures.
Data pre-processing and analysis
Pre-processing and analyses were implemented using AFNI software (Cox, 1996). Pre-processing of functional data included (i) removal of first three volumes to account for equilibration of the magnet; (ii) correction for effects of respiratory and cardiac noise (using 3dretroicor); (iii) slice-time correction; (iv) motion correction and co-registration of anatomical and functional data to first functional volume; (v) transformation to percent signal change; and (vi) spatial resampling to SPGR dimensions to allow for more fine-grained delineation of habenula structure. Any two timepoints between which movement exceeded 1 mm were censored from subsequent analysis. Following data pre-processing, habenula regions of interest (ROIs) were registered to functional data using the matrix specifications produced during co-registration.
For each participant, pre-processed BOLD data were analyzed using multiple regression. Our basic model included four regressors of interest corresponding to onset times for the following trial components, convolved with a gamma variate function of unknown amplitude: (i) presentation of first number until a guess was registered (‘number presentation’); (ii) delay period between guess and feedback onset; and (iii and iv) win and loss feedback presentation (modeled as instantaneous events). Four additional regressors modeled these components parametrically modulated by the probability of a loss outcome. For number presentation, probability varied as a function of the first number presented in each trial (e.g. ‘2’ corresponds to a loss probability of 0.2, given a win-maximizing guess strategy). For delay and feedback regressors, outcome probability factored in participants’ actual responses. Specifically, if participants made a guess that was inconsistent with what was most likely to yield a monetary gain (e.g. guessing that the second number will be >‘9’), outcome probability was updated accordingly (e.g. probability of loss outcome increased from 0.1 to 0.9). In addition, when a response was omitted, the probability of loss outcome was updated to 1.0.
To determine whether habenula activation at the time of loss feedback encodes the discrepancy between initially predicted and actual outcome, we conducted a series of analyses in which each instance of number presentation and loss outcome, respectively, was modeled with a separate regressor. First, we corrected for the effects of auto-correlation in the structure of time series noise with AFNI’s 3dREMLfit function. Next, for each participant, regression coefficients associated with each loss trial’s number and feedback presentation were extracted and correlated; this metric corresponds to the extent to which habenula activation at number presentation correlates with activation at outcome. Trials with missed responses were excluded from this analysis.
All models included the following nuisance variables: 0–5th order polynomial trends, 6 translational and rotational head motion estimates and their derivatives, and 13 regressors corresponding to components of physiological noise (computed with 3dretroicor). For two control participants, physiological data were unusable. Group-level statistical analyses were conducted on beta (or correlation) coefficients averaged across voxels in each habenula. Given known asymmetries of the habenula in non-human vertebrates (Concha and Wilson 2001; Bianco and Wilson 2009), we included laterality as a variable of interest in our analyses.
Results
Participants
Fifteen participants with current MDD and 13 never-disordered CTL participants completed the neuroimaging protocol. Participant characteristics, as well as group summary statistics for anatomical, performance, and questionnaire data, are presented in Table 1 and Supplementary Table S1. There were no significant differences between the depressed and non-depressed participants in head motion. Specifically, depressed and non-depressed participants did not differ in average magnitude of head shifting, computed as the mean sum of framewise motion in the X-, Y- and Z- directions (CTL: 0.03, MDD: 0.04; t(26) = 0.86, P > 0.05), or in average magnitude of head rotation, computed as the mean sum of framewise yaw, pitch, and roll (CTL: 0.04, MDD: 0.04; t(26) = 0.63, P > 0.05).
Table 1.
Participant characteristics
| Control (n = 13) |
MDD (n = 15) |
Between-group difference | |||
|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | t | |
| Age | 27.3 | 7.0 | 31.1 | 6.9 | 1.48 |
| Right habenula volume (in millimeters3) | 28.7 | 2.5 | 28.3 | 4.2 | 0.28 |
| Left habenula volume | 28.3 | 3.5 | 28.8 | 3.8 | 0.36 |
| Questionnaire measures | |||||
| BDI | 4.5 | 5.7 | 28.1 | 7.2 | 9.48* |
| Positive affect (PANAS) | 29.9 | 7.7 | 19.1 | 6.2 | 4.16* |
| Negative affect (PANAS) | 12.1 | 3.4 | 15.1 | 4.0 | 2.11* |
| Shame (ESS) | 1.7 | 0.4 | 2.8 | 0.7 | 5.07* |
| Trait rumination (RRS) | 2.0 | 0.7 | 2.6 | 0.5 | 2.46* |
| Behavioral variables | |||||
| Average response time (in milliseconds)a | 602.5 | 191.0 | 648.4 | 109.2 | 0.80 |
| Average increase in response time from low- to high-loss probabilityb | 225.5 | 141.4 | 227.1 | 159.5 | 0.03 |
| Average number of missed responses | 1.4 | 2.5 | 1.9 | 1.6 | 0.70 |
| Average number of non-maximizing responses | 4.2 | 3.6 | 5.6 | 4.0 | 1.00 |
Notes: adoes not include responses that were registered after allotted time window; bprobability corresponds to the objective likelihood of a negative outcome; low: 0–0.1, high: 0.4–0.5; *indicates significant group difference, P < 0.05.
Habenula activation to loss outcome
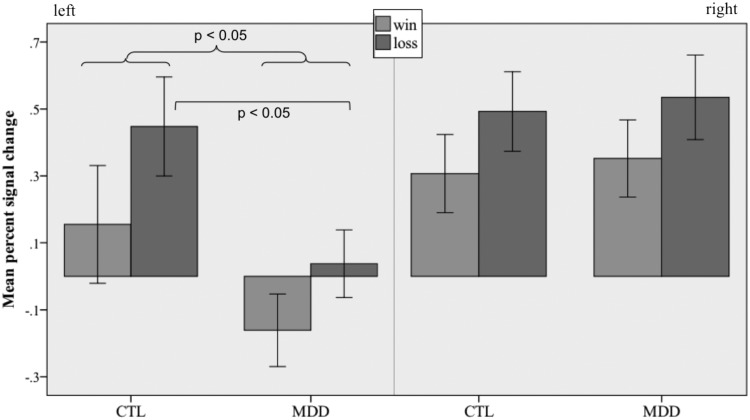
To examine differences between MDD and CTL groups in habenula activation in response to wins and losses, we conducted a three-way repeated-measures [group (MDD, CTL) × hemisphere (right, left) × outcome (win, loss)] ANOVA. This analysis yielded no main effect of group, F(1,26) = 1.82, P > 0.1, but a significant effect of outcome, F(1,26) = 9.25, P < 0.05. Both groups of participants activated the habenula more to loss than gain outcome. We also obtained a significant effect of hemisphere, F(1,26) = 8.89, P < 0.05, on habenula activation, but this result was qualified by a marginally significant interaction of group and hemisphere, F(1,26) = 4.04, P = 0.055; no other interaction was significant, all F(1,26) < 0.32, P > 0.1. Follow-up t-tests revealed greater activation across outcomes in CTL than in MDD participants for left [t(26) = 2.34, P < 0.05], but not right [t(26) = 0.24, P > 0.1] habenula. Given our a priori hypothesis of differential activation to loss in MDD, we conducted separate t-tests comparing CTL and MDD participants on loss and win outcomes in the left habenula; the two groups differed only in loss-related activation [loss: t(26) = 2.3, P < 0.05; win: t(26) = 1.6, P > 0.1; Figure 3]. In order to determine whether the observed pattern of results was specific to the habenula, we conducted the same set of between-group analyses for a nearby thalamic control region approximating the mediodorsal nucleus. To preserve variability attributable to differences in habenula ROI size, and consistent with the general approach implemented by Hennigan et al. (2015), we shifted each participant’s left and right habenula masks 10.8 mm in the anterior direction and 3.6 mm in the superior direction. A three-way repeated-measures [group (MDD, CTL) × hemisphere (right, left) × outcome (win, loss)] ANOVA on average activity in this control region yielded no main effect of group, F(1,26) = 0.16, outcome, F(1,26) = 0.002, or hemisphere, F(1,26) = 0.8, and no interaction of group and hemisphere, F(1,26) = 0.77, all Ps > 0.05. Independent t-tests comparing CTL and MDD participants on activation to loss and win outcomes in the control region confirmed that there were no significant group differences in either hemisphere, all t(26) ≤ 1.8, Ps > 0.05.
Fig. 3.
Percent signal change in the left and right habenula during win and loss outcomes. Bars represent SEM.
In CTL participants, habenula activation did not scale with the probability of loss given participants’ guesses [excluding misses; left: t(12) = 1.54, p > 0.1, right: t(12) = 0.01, P > 0.1]. No group differences or interactions were found in the scaling of habenula BOLD signal with loss probability at outcome, all F(1,26) < 3.2, Ps > 0.05. However, for CTLs, the correlation between activation during number presentation and activation at loss outcome was significant and negative for the left, t(12) = −2.29, P < 0.05, but not the right, t(12) = 0.18, P > 0.1, habenula; this metric was not significantly different from zero for either hemisphere in the MDD group [left: t(14) = −1.19, P > 0.1; right: t(14) = −1.56 P > 0.1].
Habenula activation during number presentation
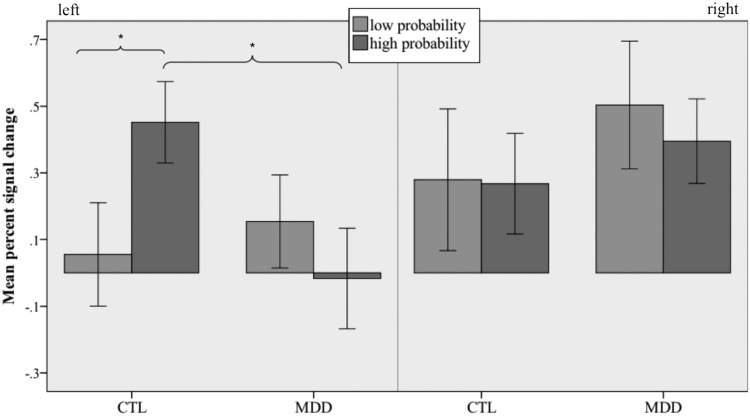
We first sought to determine whether activity in the habenula scales linearly with the probability of future loss in CTL participants. In neither hemisphere was habenula activation significantly parametrically modulated by the objective probability of loss outcome [left: t(12) = 1.65, P > 0.1; right: t(12) = −0.17, P > 0.1]. Given findings suggesting that the habenula responds preferentially to the worst-case scenario in a given context (17), we reasoned that the relation between probability and activation might not be linear, particularly within the lower range of loss probability. Thus, we examined whether the habenula activated more strongly to numbers associated with the highest (‘4’, ‘5’, ‘6’) than the lowest (‘0’, ‘1’, ‘9’, ‘10’) relative probabilities of a loss outcome. For each participant, we conducted another multiple regression analysis that included separate regressors corresponding to the convolved onset times for high, intermediate and low loss probability numbers. In CTLs, a two-way repeated-measures [hemisphere × probability (high, low)] ANOVA on the resulting activation estimates yielded no main effects of hemisphere, F(1,12) = 2.75, P > 0.1, or probability, F(1,12) = 0.02, P > 0.1, but did yield a significant interaction of hemisphere and probability, F(1,12) = 6.18, P < 0.05. Follow-up paired t-tests confirmed that high loss probability elicited greater activation than did low loss probability in left [paired t(12) = 3.53, P < 0.05] but not right [paired t(12) = 0.07, P > 0.1] habenula (Figure 4).
Fig. 4.
Percent signal change in the left and right habenula during presentation of numbers associated with low (0–0.1) and high (0.4–0.5) probabilities of loss outcome. Bars represent SEM; asterisk indicates significant difference, P < 0.05.
A two-way repeated-measures (group × probability) ANOVA conducted on left habenula activation did not yield main effects of group, F(1,26) = 1.18, P > 0.1, or probability, F(1,26) = 1.03, P > 0.1, but did yield a significant interaction of group and probability, F(1,26) = 6.55, P < 0.05. This effect was driven by less activation of left habenula to high loss probability in MDD than in CTL participants, t(26) = 2.37, P < 0.05 (Figure 4); no group difference was obtained for low probability trials, t(26) = 0.65, P > 0.1.
Correlation with response time
For each participant, we computed the change in response time between low and high loss probability trials (Table 1). CTL participants exhibited significant response time slowing, t(12) = 5.75, P < 0.05, that was correlated with differences in activation during number presentation between low and high loss probability trials in left (r = 0.61, P < 0.05), but not in right (r = 0.23, P > 0.1), habenula (Supplementary Figure S1). In the MDD group, response time slowing was also significant, t(14) = 5.52, P < 0.05, but was uncorrelated with changes in habenula activation (both rs < 0.2, Ps > 0.1).
Exploratory analyses: correlations with questionnaire and clinical variables
To better understand the clinical relevance of variation in habenula activation, we computed correlations between scores on the RRS, ESS and PANAS, and (i) left habenula response to loss outcome (excluding trials with omitted responses); and (ii) high vs low loss probability modulation of left habenula response during number presentation. In the CTL group, left habenula response to loss was inversely correlated with both RRS, r = −0.60, and ESS, r = −0.78, and positively correlated with positive affect, r = 0.64, all Ps < 0.05 (Supplementary Figures S2–S4); habenula response to loss was uncorrelated with negative affect, r = 0.10, P > 0.1. There were no significant correlations between self-report measures and probability modulation of the habenula in the CTL group (all |r|s < 0.45, P > 0.1). In the MDD group, none of the correlations between questionnaire measures and habenula activation was significant, all |r|s < 0.45, P > 0.09.
Discussion
Consistent with animal work suggesting a specialized role for the lateral habenula in responding to suboptimal outcomes (Matsumoto and Hikosaka, 2007, 2008), we observed greater activation of the habenula bilaterally in response to negative than to positive feedback. This result obtained in the context of secondary punishment contributes to the small but growing body of work demonstrating activation of the human habenula to unexpected primary (Salas et al., 2010) and secondary (Ullsperger and Von Cramon, 2003) reward omissions, receipt of negative performance feedback (Shepard et al., 2006; Ullsperger and Von Cramon, 2003), primary punishment (Lawson et al., 2014; Hennigan et al., 2015), errors (Li et al., 2008; Ide and Li, 2011) and pain (Sheltonet al., 2012). We also found, in healthy participants, an inverse correlation between left habenula responses to number presentation and to loss outcome, consistent with the hypothesis that loss-related habenula activation signals a discrepancy between predicted and actual outcome. Thus, when suboptimal outcomes are expected, the discrepancy between expected and experienced loss is diminished, thereby reducing the negative prediction error signal elicited by the outcome itself. Importantly, the metric used here was robust against departures from linear scaling with objective probability. Indeed, although we did not find linear scaling of habenula activation to feedback with the probability of a loss outcome, it is plausible that habenula responses to cues associated with the current range of loss probability do not scale linearly with expected outcome as they might with stimuli that are more predictive of loss, as is suggested by research with nonhuman primates (Matsumoto and Hikosaka, 2008).
Relative to CTLs, MDD participants exhibited a left-lateralized reduction in habenula activation to loss outcomes. We do not believe this difference resulted from differential engagement with the task: the two groups did not differ in the number of missed responses or in response times, or in the overall tendency to use a reward-maximizing guess strategy. One explanation for this surprising finding is that activity in the left habenula may be tonically elevated in MDD. Indeed, elevated habenula metabolism has been observed both in rodent models of depression (Shumake et al., 2003) and in formerly depressed individuals following tryptophan depletion (Roiser et al., 2009). Such dysregulation may interfere with the normal functionality of the structure or may impede our ability to detect relatively small task-related changes in activation. Future work dually examining cerebral metabolism or blood flow and loss-related BOLD activation is needed to better understand the relation between these two phenomena. In this context, however, it is important to note that, within our CTL sample, trait shame and rumination, both of which are associated with risk for depression (Nolen-Hoeksema and Morrow, 1991; Andrews et al., 2002), were also related to attenuated habenula response to loss outcomes; while it is not known whether these personality facets themselves are related to alterations in habenula metabolism, these findings support the hypothesis that abnormal habenula activation is associated with depressotypic responses to negative outcomes.
In addition to a role for the habenula in signaling the onset of non-rewarding and aversive outcomes, this structure has also been implicated in the prediction of such outcomes (Matsumoto and Hikosaka, 2008). Thus, we examined the extent to which BOLD response in the habenula varied as a function of the probability of future negative outcomes. In CTLs, the left habenula exhibited a pattern of responding consistent with this formulation, activating more strongly in response to higher than to lower probabilities of incorrect guesses. Notably, we observed such differential responding only when contrasting the extreme ends of the employed probability range, consistent with the proposal that the habenula is sensitive to the most unpleasant events in a given context (Matsumoto and Hikosaka, 2008). Although it is not surprising that response latencies varied as a function of outcome uncertainty, we observed a striking correlation between the degree to which response times slowed and the extent to which habenula responses rose with increasing probability of a negative outcome, a pattern consistent with the previously documented association between habenula activity and conditioned behavioral suppression (Lawson et al., 2014) and with the hypothesis that the habenula acts to suppress motor outputs that are likely to lead to suboptimal outcomes (Hikosaka, 2010).
In contrast, in depressed individuals the left habenula did not discriminate between outcome probabilities, suggesting abnormal encoding of the likelihood of aversive outcomes in MDD. This result complements the previous finding that, unlike healthy individuals, depressed participants do not activate dorsal anterior cingulate cortex, an area posited to integrate recent reinforcement history in the service of guiding choice behavior (Holroyd and Coles, 2008), with anticipation of increasing monetary loss (Knutson et al., 2008). This neural portrait is also consistent with the symptomatic presentation of indecision in depression, as well as with correlational (van Randenborgh et al., 2010) and experimental (Murphy et al., 2001; Must et al., 2006) accounts of suboptimal and punishment-insensitive decision-making in MDD.
The hemispheric asymmetry of patterns of habenula activation found in healthy participants, as well as in our between-group contrasts, is noteworthy. Although both number presentation and negative feedback elicited activation in the habenula bilaterally, signal modulation as a function of outcome probability was observed only in the left hemisphere, as was the correlation between habenula activation and behavior. Because all participants used their right hand to respond, it is possible that computations preceding such motor responses are localized to the contralateral habenula. Indeed, Lawson et al. (2014) observed right-lateralized habenula responses to cues predicting future shock when shocks were consistently administered to participants’ left hands. Asymmetries in habenula structure and connectivity have been well characterized in non-human species (Concha and Wilson 2001; Bianco and Wilson 2009); further work is needed to determine the task-dependent nature of habenula functional asymmetry.
We should note three limitations of this study. First, our sample, though carefully selected to minimize potential confounding effects on habenula activity (e.g. psychoactive medication status, cigarette use), is relatively small. Thus, our findings should be taken as preliminary evidence that habenula encoding of negative reward probability and/or receipt is disrupted in depressed individuals; these finding should be replicated in a larger sample both to better characterize the size and nature of the effects and to explore relations with depressive symptomatology and with changes following clinical remission. Second, in the current paradigm, prediction of loss and gain are confounded; therefore, it is not clear whether scaling of habenula activity during number presentation reflects the encoding of increasing probability of loss, decreasing probability of gain or general uncertainty. In addition, we manipulated outcome probability but not magnitude. Our failure to resolve linear scaling of the negative reward prediction error signal may stem from greater sensitivity of the habenula to unexpected variations in the magnitude of a loss than to loss probability alone. Third, as with the majority of existing fMRI studies of the habenula, our spatial resolution did not enable us to differentiate between the medial and lateral components, nor their respective subnuclei, raising the possibility that our results derive from an intermixing of signals from functionally distinct regions. Despite these limitations, this study contributes to the accumulating body of evidence that, as in behaving monkeys (Matsumoto and Hikosaka, 2007, 2008), the habenula in humans plays a role in both the prediction of, and response to, loss outcomes, and provides preliminary support for the hypothesis that abnormal phasic habenula activation is involved in the pathophysiology of depression.
Supplementary Material
Acknowledgements
We thank Meghan Vinograd, Emily Livermore, Brooke Gilbert, Arkadiy Maksimovsky, and Melissa Henry for their invaluable help in participant recruitment and data collection. This research was funded by National Institutes of Health (NIH)/National Institute of Mental Health (NIMH) grant MH59259 to I.H.G. and by an American Psychological Association Dissertation Research Award and a Stanford NeuroVentures program award to D.J.F.
References
- Alloy L.B., Ahrens A.H. (1987). Depression and pessimism for the future: biased use of statistically relevant information in predictions for self versus others. Journal of Personality and Social Psychology, 52(2), 366–78. [DOI] [PubMed] [Google Scholar]
- Andrews B., Qian M., Valentine J. D. (2002). Predicting depressive symptoms with a new measure of shame: the Experience of Shame Scale. British Journal of Clinical Psychology, 41(1), 29–42. [DOI] [PubMed] [Google Scholar]
- Balcita-Pedicino J.J., Omelchenko N., Bell R., Sesack S.R. (2011). The inhibitory influence of the lateral habenula on midbrain dopamine cells: ultrastructural evidence for indirect mediation via the rostromedial mesopontine tegmental nucleus. Journal of Comparative Neurology, 519(6), 1143–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beats B.C., Sahakian B.J., Levy R. (1996). Cognitive performance in tests sensitive to frontal lobe dysfunction in the elderly depressed. Psychological Medicine, 26, 591–603. [DOI] [PubMed] [Google Scholar]
- Beck A., Steer R.A., Brown G.K. (1996). Beck Depression Inventory-II (BDI-II). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Benarroch E.E. (2015). Habenula Recently recognized functions and potential clinical relevance. Neurology, 85(11), 992–1000. [DOI] [PubMed] [Google Scholar]
- Bianco I.H., Wilson S.W. (2009). The habenular nuclei: a conserved asymmetric relay station in the vertebrate brain. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1519), 1005–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldecott-Hazard S., Mazziotta J., Phelps M. (1988). Cerebral correlates of depressed behavior in rats, visualized using 14C-2-deoxyglucose autoradiography. The Journal of Neuroscience, 8(6), 1951–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu P., Deldin P. (2007). Neural evidence for enhanced error detection in major depressive disorder. American Journal of Psychiatry, 164(4), 608–16. [DOI] [PubMed] [Google Scholar]
- Concha M.L., Wilson S.W. (2001). Asymmetry in the epithalamus of vertebrates. Journal of Anatomy, 199(1–2), 63–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–73. [DOI] [PubMed] [Google Scholar]
- D’Ardenne K., McClure S.M., Nystrom L.E., Cohen J.D. (2008). BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science, 319(5867), 1264–7. [DOI] [PubMed] [Google Scholar]
- Douglas K.M., Porter R.J., Frampton C.M., Gallagher P., Young A.H. (2009). Abnormal response to failure in unmedicated major depression. Journal of Affective Disorders , 119, 92–9. [DOI] [PubMed] [Google Scholar]
- First M.B., Gibbon M., Spitzer R.L., Williams J. B. W. (2001). SCID-I-TR: Structured Clinical Interview for DSM-IV-TR Disorders. New York: Biometrics Research. [Google Scholar]
- Hennigan K., D’Ardenne K., McClure S.M. (2015). Distinct midbrain and habenula pathways are involved in processing aversive events in humans. The Journal of Neuroscience, 35(1), 198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O. (2010). The habenula: from stress evasion to value-based decision-making. Nature Reviews Neuroscience, 11(7), 503–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A.J., Pizzagalli D.A. (2008). Spatiotemporal dynamics of error processing dysfunctions in major depressive disorder. Archives of General Psychiatry, 65(2), 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd C.B., Coles M.G. (2008). Dorsal anterior cingulate cortex integrates reinforcement history to guide voluntary behavior. Cortex, 44(5), 548–59. [DOI] [PubMed] [Google Scholar]
- Hong S., Hikosaka O. (2008). The globus pallidus sends reward-related signals to the lateral habenula. Neuron, 60(4), 720–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide J.S., Li C.S.R. (2011). Error-related functional connectivity of the habenula in humans. Frontiers in Human Neuroscience, 5, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou T.C., Fields H.L., Baxter M.G., Saper C.B., Holland P.C. (2009). The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron, 61(5), 786–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H., Shepard P.D. (2007). Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABAA receptor-mediated mechanism. The Journal of Neuroscience, 27(26), 6923–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Wang P.S. (2014). Epidemiology of depression. In: Gotlib I.H., Hammen C.L., editors. Handbook of Depression, 3nd edn, pp. 7–24. New York: Guilford Press. [Google Scholar]
- Knutson B., Bhanji J.P., Cooney R.E., Atlas L.Y., Gotlib I.H. (2008). Neural responses to monetary incentives in major depression. Biological Psychiatry, 63(7), 686–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S., Lim B.K., Ran C., et al. (2012). Input-specific control of reward and aversion in the ventral tegmental area. Nature, 491(7423), 212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson R.P., Drevets W.C., Roiser J.P. (2012). Defining the habenula in human neuroimaging studies. NeuroImage, 64, 722–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson R.P., Seymour B., Loh E., et al. (2014). The habenula encodes negative motivational value associated with primary punishment in humans. Proceedings of the National Academy of Sciences of the United States of America, 111(32), 11858–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecca S., Meye F.J., Mameli M. (2014). The lateral habenula in addiction and depression: an anatomical, synaptic and behavioral overview. European Journal of Neuroscience, 39(7), 1170–8. [DOI] [PubMed] [Google Scholar]
- Li B., Piriz J., Mirrione M., et al. (2011). Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature, 470(7335), 535–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. S., Yan P., Chao H. A., et al. (2008). Error-specific medial cortical and subcortical activity during the stop signal task: a functional magnetic resonance imaging study. Neuroscience, 155(4), 1142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M., Hikosaka O. (2007). Lateral habenula as a source of negative reward signals in dopamine neurons. Nature, 447(7148), 1111–5. [DOI] [PubMed] [Google Scholar]
- Matsumoto M., Hikosaka O. (2008). Representation of negative motivational value in the primate lateral habenula. Nature Neuroscience, 12(1), 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H., Wang Y., Huang M., Lin W., Wang S., Zhang B. (2011). Chronic deep brain stimulation of the lateral habenula nucleus in a rat model of depression. Brain research, 1422, 32–8. [DOI] [PubMed] [Google Scholar]
- Murphy F.C., Rubinsztein J.S., Michael A., et al. (2001). Decision-making cognition in mania and depression. Psychological Medicine, 31(4), 679–93. [DOI] [PubMed] [Google Scholar]
- Must A., Szabó Z., Bódi N., Szász A., Janka Z., Kéri S. (2006). Sensitivity to reward and punishment and the prefrontal cortex in major depression. Journal of Affective Disorders, 90(2), 209–15. [DOI] [PubMed] [Google Scholar]
- Nair S.G., Strand N.S., Neumaier J.F. (2012). DREADDing the lateral habenula: a review of methodological approaches for studying lateral habenula function. Brain Research, 1511, 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S., Morrow J. (1991). A prospective study of depression and posttraumatic stress symptoms after a natural disaster: the 1989 Loma Prieta earthquake. Journal of Personality and Social Psychology, 61(1), 115–21. [DOI] [PubMed] [Google Scholar]
- Proulx C.D., Hikosaka O., Malinow R. (2014). Reward processing by the lateral habenula in normal and depressive behaviors. Nature Neuroscience, 17(9), 1146–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiser J.P., Levy J., Fromm S. J., et al. (2009). The effects of tryptophan depletion on neural responses to emotional words in remitted depression. Biological Psychiatry, 66(5), 441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R., Baldwin P., De Biasi M., Montague P.R. (2010). BOLD responses to negative reward prediction errors in human habenula. Frontiers in Human Neuroscience, 4, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorius A., Kiening K.L., Kirsch P., et al. (2010). Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biological Psychiatry, 67(2), e9–11. [DOI] [PubMed] [Google Scholar]
- Schiller C.E., Minkel J., Smoski M.J., Dichter G.S. (2013). Remitted major depression is characterized by reduced prefrontal cortex reactivity to reward loss. Journal of Affective Disorders, 151(2), 756–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. (1998). Predictive reward signal of dopamine neurons. Journal of Neurophysiology, 80(1), 1–27. [DOI] [PubMed] [Google Scholar]
- Shabel S.J., Proulx C.D., Trias A., Murphy R.T., Malinow R. (2012). Input to the lateral habenula from the basal ganglia is excitatory, aversive, and suppressed by serotonin. Neuron, 74(3), 475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton L., Pendse G., Maleki N., et al. (2012). Mapping pain activation and connectivity of the human habenula. Journal of Neurophysiology, 107(10), 2633–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard P.D., Holcomb H.H., Gold J.M. (2006). Schizophrenia in translation: the presence of absence: habenular regulation of dopamine neurons and the encoding of negative outcomes. Schizophrenia Bulletin, 32(3), 417–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumake J., Edwards E., Gonzalez-Lima F. (2003). Opposite metabolic changes in the habenula and ventral tegmental area of a genetic model of helpless behavior. Brain Research, 963(1–2), 274–81. [DOI] [PubMed] [Google Scholar]
- Steele J.D., Kumar P., Ebmeier K.P. (2007). Blunted response to feedback information in depressive illness. Brain, 130(9), 2367–74. [DOI] [PubMed] [Google Scholar]
- Strunk D.R., Lopez H., DeRubeis R.J. (2006). Depressive symptoms are associated with unrealistic negative predictions of future life events. Behaviour Research and Therapy, 44(6), 861–82. [DOI] [PubMed] [Google Scholar]
- Treynor W., Gonzalez R., Nolen-Hoeksema S. (2003). Rumination reconsidered: a psychometric analysis. Cognitive Therapy and Research, 27(3), 247–59. [Google Scholar]
- Ullsperger M., Von Cramon D.Y. (2003). Error monitoring using external feedback: specific roles of the habenular complex, the reward system, and the cingulate motor area revealed by functional magnetic resonance imaging. The Journal of Neuroscience, 23(10), 4308–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Randenborgh A., de Jong - Meyer R., Hüffmeier J. (2010). Decision making in depression: differences in decisional conflict between healthy and depressed individuals. Clinical Psychology and Psychotherapy, 17(4), 285–98. [DOI] [PubMed] [Google Scholar]
- Watson D., Clark L.A., Tellegen A. (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063–70. [DOI] [PubMed] [Google Scholar]
- Winter C., Vollmayr B., Djodari-Irani A., Klein J., Sartorius A. (2011). Pharmacological inhibition of the lateral habenula improves depressive-like behavior in an animal model of treatment resistant depression. Behavioural Brain Research, 216(1), 463–5. [DOI] [PubMed] [Google Scholar]
- Zhao H., Zhang B.L., Yang S.J., Rusak B. (2015). The role of lateral habenula–dorsal raphe nucleus circuits in higher brain functions and psychiatric illness. Behavioural Brain Research, 277, 89–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.