Abstract
Intimate partner violence (IPV) is a complex and global phenomenon that requires a multi-perspective analysis. Nevertheless, the number of neuroscientific studies conducted on this issue is scarce as compared with studies of other types of violence, and no neuroimaging studies comparing batterers to other criminals have been conducted. Thus, the main aim of this study was to compare the brain functioning of batterers to that of other criminals when they are exposed to IPV or general violence pictures. An fMRI study was conducted in 21 batterers and 20 other criminals while they observed IPV images (IPVI), general violence images (GVI) and neutral images (NI). Results demonstrated that batterers, compared with other criminals, exhibited a higher activation in the anterior and posterior cingulate cortex and in the middle prefrontal cortex and a decreased activation in the superior prefrontal cortex to IPVI compared to NI. The paired t-test comparison between IPVI and GVI for each group showed engagement of the medial prefrontal cortex, the posterior cingulate and the left angular cortices to IPVI in the batterer group only. These results could have important implications for a better understanding of the IPV phenomenon.
Keywords: intimate partner violence, neuroimaging, batterers
Introduction
Intimate partner violence (IPV) is a complex and global phenomenon that requires a multi-perspective analysis. According to the World Health Organization (WHO), IPV refers to any violent behavior within an intimate relationship. It includes physical aggression (e.g. slapping, hitting, kicking or beating), sexual force, psychological abuse (e.g. intimidation, constant belittling or humiliation) or any other controlling behavior by a current or former partner or spouse (Krug et al., 2002; World Health Organization, 2013). Many studies have pointed out that IPV is related to several factors including psychosocial, family, patriarchal or biological variables (Pinto et al., 2010; Corvo and Johnson, 2013), but the number of neuroscientific studies conducted on this issue are scarce as compared with the number of studies on other types of violence (Corvo, 2014).
A great number of neuroimaging studies on general violence (GV) have focused on the brain functioning of violent people. Furthermore, these results have been replicated in structural and functional studies using different techniques such as Computed tomography, positron emission tomography (PET), Single-photon emission computed tomograph, magnetic resonance imaging (MRI) and Event-related potential scans (Patrick, 2008; Schiltz et al., 2013). The prefrontal cortex, temporal cortex, insula, amygdala, hippocampus and cingulate gyrus have been key structures related to aggressive behavior (Blair, 2001; Siever, 2008; Blair and Lee, 2013). Nevertheless, brain structures are different according to the type of aggression. In reactive/impulsive aggression or aggression in response to a threating stimulus, activation in the amygdala, hypothalamus and periaqueductal gray (PAG) (Mobbs et al., 2007) has been consistently found. The same structures have been found to be involved in cases of frustration (Blair, 2010). In instrumental/ goal directed aggression, the motor cortex and cerebellum are involved (White et al., 2015). In the last type of aggression, the amygdala and ventromedial prefrontal cortex have been related to moral decisions about harming another person (Blair, 2007; Harenski et al., 2010). In the case of offenders with psychiatric conditions, other brain areas have been found to be involved. In a study conducted on antisocial offenders, differentiated according to borderline personality disorder (BPD) or high psychopathic traits (HPT), Bertsch et al. (2013) found that antisocial offenders with BPD showed gray matter reduction in the orbitofrontal and ventromedial prefrontal cortices (involved in emotion regulation and reactive aggression) and in the temporal pole (involved in cognitive empathy). On the other hand, antisocial offenders with HPT showed gray matter reduction in cortical midline structures, such as the dorsomedial prefrontal cortex, the postcentral gyrus, posterior cingulate cortex (PCC), and dorsal anterior and posterior precuneus, which are principally involved in the default mode network (DMN).
To our knowledge few studies have assessed the brain function of abusers (George et al., 2004; Lee et al., 2009). George et al. (2004) used PET to analyze glucose metabolism activity in the structures responsible for monitoring and mediating conditioned responses to fear associated with domestic violence. Findings show that perpetrators with alcohol abuse, compared with non-perpetrators with alcohol abuse and control participants, had lower glucose uptake in the hypothalamus. Interestingly, using an fMRI picture-viewing paradigm, Lee et al. (2009) demonstrated that batterers had an over-activation in the hippocampus, the fusiform gyrus, the PCC, the thalamus and the occipital cortex in response to threatening stimuli compared with neutral stimuli. On the other hand, specific higher activation was observed in batterers in the precuneus when they saw female aggression pictures versus neutral pictures.
However, no direct neuroimaging studies comparing batterers with other criminals have been conducted, and few studies have compared both populations while considering other psychological variables. For example, Moffitt et al. (2000) demonstrated that partner abuse and general crime represent different constructs that are moderately related but ‘not conceptually equivalent, even when performed by the same individual’, depending on his/her personality traits. General crime was related to high emotional negativity and low constraint, and IPV was also related to emotional negativity but not to low constraint. Boyle et al. (2008) found that general violent offenders showed more conduct disorder/delinquent behaviors, lifetime antisocial behaviors and disinhibition, and were more psychologically abusive than other violent participants. In this sense, comparing batterers’ brain functioning to that of other criminals could help in understanding the mechanism of IPV and its possible similarities with GV.
For these reasons, the main aim of this study is to compare the brain functioning of batterers with other criminals when they observe IPV or GV pictures to make progress from findings in previous studies (Lee et al., 2009). We also aimed to assess whether batterers have differences in brain functioning specific to IPV pictures that are not present to GV images. We hypothesized that batterers, relative to other criminals, will show a specific higher activation of the precuneus/PCC during the viewing of IPV pictures compared with neutral and GV pictures. This hypothesis is in line with the over-activation of these regions in the only fMRI study that has assessed brain activation in response to IPV pictures in batterers relative to controls (Lee et al., 2009). We also hypothesized that batterers will show higher activation of the occipital, posterior parietal and temporal cortices during the viewing of GV images compared with neutral images. This hypothesis is also congruent with the study completed by Lee et al. (2009) showing over-activation of these brain regions in response to threatening vs neutral stimuli.
Materials and methods
Participants
Forty-one men convicted of crimes were recruited from the Center for Social Insertion (CSI) (Centro de Inserción Social, CIS) ‘Matilde Cantos Fernández’, in Granada (Spain). They were divided into two groups: (i) 21 batterers (batterers group, BG) convicted for a crime of violence against women and (ii) 20 men convicted of crimes other than IPV (other criminal group, OCG).
In Spain, IPV crimes are regulated by a specific law (Law 1/2004, ‘Comprehensive Protection Law against Gender Violence’[Ley Orgánica 1/2004, de 28 de diciembre, de Medidas de Protección Integral contra la Violencia de Género]). This law states that a man may be convicted by a judge for several types of aggression including insults, threats, slaps or beatings, sexual abuse or murder. According to this law, first convictions for IPV without sexual or physical abuse are classified as a misdemeanor, which implies that the person is sent to an open facility (CSI) of the Ministry of Justice, but not to prison. In the CSI, batterers should attend IPV rehabilitation programs. In case of sexual or physical abuse with any physical injury, batterers go to prison.
Crime severity in Spaniard law is regulated by a Penal Code (article 33). According to this article, crimes sentences between 3 months and 5 years are classified as ‘less serious’. Given that all participants were recruited in the CSI, we guaranteed that (i) it was the first time that participants of both groups were convicted; and (ii) they were convicted for the similar sanction (‘less serious’).
Table 1 shows the socio-demographic and severity of crime information. Groups did not differ significantly in age, education level and intelligence quotient (IQ). All were right-handed males with native fluency in Spanish. The selection of participants included the following inclusion criteria: individuals 18 years old or older; for the BG, being convicted of an IPV crime, for the OCG being convicted of a crime other than IPV. The exclusion criteria for the two groups included: illiteracy, a history of serious antecedents of psychological and personality problems (measured through the Millon Multiaxial Personality Test III; Millon, 1994, Spaniard adaptation; Cardenal et al., 2007), head injury, neurological illness, infectious disease, history of drug abuse or dependence (including alcohol) (SCID/DSM-IV); American Psychiatric Association, 1994), systemic disease or any other diseases affecting the central nervous system (First et al., 1999), and the presence of significant abnormalities in MRI or any contraindications to MRI scanning (including claustrophobia or implanted ferromagnetic objects). Individuals in the OCG with a score greater than or equal to 11 on the severity scale of the CTS2 (Conflict Tactic Scales) (Straus et al., 1996) were excluded. This criterion was established in a previous study (Cohen et al., 2003) to rule out physical or psychological violence against partners.
Table 1.
Demographic and crime characteristics of batterers (BG) and other criminal groups (OCG)
| Variables (Mean (SD)) | BG | CG | P-value |
|---|---|---|---|
| Age | 38.38 (8.70) | 34.40 (8.66) | 0.15 |
| Years of education | 9.62 (3.90) | 9.45 (2.42) | 0.87 |
| IQ | 99.83 (14.29) | 92.85 (13.32) | 0.13 |
| Time of crime [%(n)] | |||
| Misdemeanor | IPV-PV= 38 % (8) | SCF/DD= 50% (10) | 0.44 |
| Felony | IPV-PPV= 62%(13) | GAR/VF= 50% (10) |
SD, standard deviation; IPV, intimate partner violence; PV, psychological violence; SCF, scams or crime of forgery; DD, dangerous driving; IPV-PPV, IPV- physical and psychological violence; GAR, grave assault/ robbery; VF, violent fight.
The study was approved by the Research Ethics Committee of the University of Granada, Spain. The participants were invited to collaborate in the study on a voluntary and anonymous basis. The confidentiality of personal information was guaranteed in accordance with the Spanish legislation on personal data protection (Organic Law 15/1999, December 13). All of the participants signed a written informed consent document and they received 25 euros for participating the study.
Materials
An interview evaluating socio-demographic information and the risk of serious couple violence was used (Echeburúa et al., 2008). This questionnaire measures the socio-demographic variables of the aggressor and victim, the relationship status of the couple (couple not living together, cohabitation, in the process of separating, separated, etc.), the types of violence, the profile of the aggressor (information about the formal complaint and emotions of the batterer in that moment) and vulnerability factors for the victim (i.e. substance use, economic dependence and lack of social support).
IPV severity
The CTS 2 Spanish version (Loinaz et al., 2012) of the original CTS2 Scales (Straus et al., 1996) was used to detect the existence of physical, psychological and/or sexual violence toward a partner in a relationship. It measures violence frequency and intensity in the relationship.
Intelligence quotient
The K-BIT (Brief Intelligence Test) (Kaufman et al., 1997): The K-BIT measures cognitive functions through two tests: verbal (vocabulary, composed of two tests), and non-verbal (matrix), which evaluates crystallized and fluid intelligence, and obtains a compound IQ.
fMRI task
The stimulus set comprised 72 pictures extracted from the International Affective Picture System database, divided equally into four categories: pleasant, unpleasant, GV and neutral. We also selected 18 pictures of IPV from Internet. For this study proposes, we focus on general and IPV images, using neutral images as the control condition. GV images included violent acts against humans and animals, such as fights, threats and injuries that lack women. IPV) images, in turn, involved a female victim being attacked by a man, or injured women. Neutral (N) images included general objects that were not related to violence, such as chairs, baskets and spoons. Each picture was presented in blocks of 15 s, with an individual picture duration of 5 s. Picture blocks were presented pseudo-randomly. The task was performed with the Presentation software (Neurobehavioral System Inc., San Francisco, CA). The items were presented through magnetic resonance-compatible liquid crystal display goggles (Resonance Technology, Northridge, CA.) equipped with various corrective lenses.
Participants were instructed to sustain the emotion elicited by the pictures displayed during IPV, GV and N images. After the functional imaging session, participant involvement was confirmed by asking the participants to rate images on three emotional components using the Self-Assessment Manikins (SAM) scales, with valence: from happy (9) to unhappy (1), arousal: from excited (9) to calm (1), and dominance: from controlled (1) to in control (9).
Imaging data acquisition and preprocessing
The equipment used was a 3.0 T clinical MRI scanner with an eight-channel phased-array head coil (Intera Achieva, Philips Medical Systems, Eindhoven, The Netherlands). During acquisition, a T2*-weighted echo-planar imaging (EPI) was obtained (Repetition time (TR) = 2000 ms, Echo time (TE) = 35 ms, Field of view (FOV) = 230 × 230 mm, 128 × 128 matrix, flip angle = 90°, twenty-one 4-mm axial slices, 1-mm gap, 315 scans). A sagittal three-dimensional T1-weighted turbo-gradient-echo sequence (160 slices, TR = 8.3 ms, TE = 3.8 ms, flip angle = 8°, FOV = 256 × 256, 1 mm3 voxels) was obtained in the same experimental session to check for gross anatomical abnormalities for each subject.
Brain images were analyzed using the Statistical Parametric Mapping (SPM8) software (Wellcome Department of Cognitive Neurology, Institute of Neurology, Queen Square, London, UK), running under Matlab R2009 (MathWorks, Natick, MA). Preprocessing steps included slice timing correction, re-slicing to the first image of the time series, normalization (using affine and smoothly non-linear transformations) to an EPI template in the Montreal Neurological Institute (MNI) space, and spatial smoothing by convolution with a 3D Gaussian kernel (full width at half maximum (FWHM) = 8 mm).
Procedure
In session 1, the initial interview and behavioral tasks were administered in the CSI. All participants were assessed in an individual and quiet room for approximately 1 h. In session 2, fMRI scans and image ratings were taken in the Centro Diagnóstico CEDISA (Granada, Spain) and each session lasted 1 h.
Statistical analyses
Behavioral analyses
Behavioral data were analyzed using the Statistical Package for the Social Sciences, version 22 (SPSS; Chicago, IL). Independent-sample t-tests or cross-tabulation analyses (depending on the type of variable) were conducted to compare the two groups with regard to demographics and severity of crime variables. We also performed a mixed-design ANOVA (2 groups ×3 types of images) to analyze group differences on emotional responses by type of image as recorded by the SAM.
Neuroimaging analyses
The BOLD response at each voxel was convolved with the SPM8 canonical hemodynamic response function (using a 128-s high-pass filter). Conditions were modeled for 15 s that each block appeared on the screen. To cover the study objectives, three contrasts of interest were defined at the first-level (single-subject): (i) ‘IPV vs N images’, (ii) ‘GV vs N images’ and (iii) ‘IPV vs GV images’. The resulting first-level contrast images were then used in the second-level random-effect analyses to assess for between-group differences. For the (i) ‘IPV vs N images’ and (ii) ‘GV vs N images’ contrasts two-sample t-test models were used to compare group activations. For the (iii) ‘IPV vs GV’ comparison, two approaches were used. First, a second-level paired t-test model, using contrast images from contrasts (i) and (ii), was used to sensitively explore group effects to each type of violence. Moreover, a collapsed across groups second-level t-test analysis using the ‘IPV vs GV’ contrast images was performed to identify brain activations uniquely associated with IPV images. For both of these latter statistical approaches, the signal eigenvariate of the significant brain regions (peak maxima) were extracted and group*condition interaction analyses were conducted in SPSS.
Threshold criteria
Significance in ANOVA models were established at a threshold of P<0.05. For the imaging analyses, the spatial extent threshold was determined by 1000 Monte Carlo simulations using AlphaSim as implemented in the SPM REST toolbox (Song et al., 2011; Ward, 2013). For one-sample t-tests, input parameters included a whole-brain brain mask of 283 654 voxels (2 mm × 2 mm × 2 mm), an individual voxel threshold probability of 0.005, a cluster connection radius of 5 mm, and the actual smoothness of the data. A minimum cluster extent (KE) of 173 voxels (1384 mm3) was estimated to satisfy a PFWE < 0.05. Significance in two-sample and paired t-tests was assessed using the same input parameters, masking results on the basis of activation and deactivation maps derived from the corresponding one-sample t-test contrasts for both study groups. Therefore, for contrasts 1 and 2, a minimum KE of 91 and 94 voxels (within masks of 38491 and 61538) were estimated to satisfy a PFWE < 0.05, respectively. For the paired t-test analyses, a KE of 99 voxels (within a mask of 69.598) were estimated.
Results
Differences between batterers and criminals in behavioral responses
Analyses showed no significant main effect for the group factor (batterers vs general crime), nor an interaction effect (group by type of imagine) in emotional ratings. These findings suggested that valence, arousal and dominance ratings were the same for batterers and criminals overall and across the type of image displayed in the fMRI task. As expected according to the selection criteria for images, a significant main effect for type of images (i.e. IPV, GV, N images) was observed for valence [F(2,74) =222.61; P = 0.000], arousal [F(2,74) = 40.26; P = 0.000], and dominance [F(2,74) = 14.60; P = 0.000]. For both groups, IPV images showed less valence, higher arousal and less dominance than GV images, and GV images showed less valence, higher arousal and less dominance than N images (see Table 2).
Table 2.
Descriptive scores and mixed-design ANOVA results for the valence, arousal and dominance affective dimensions
| Variables Mean (SD) | BG |
CG |
Main effect |
Interaction | |||||
|---|---|---|---|---|---|---|---|---|---|
| IPVI | GVI | NI | IPVI | GVI | NI | Group P-value | TI P-value | Group*TI P-value | |
| Valence | 1.44 (0.53) | 2.22 (0.91) | 5.76 (1.23) | 1.59 (0.64) | 2.54 (0.90) | 5.58 (1.05) | 0.57 | 0.000 | 0.67 |
| Arousal | 7.48 (1.80) | 6.53 (1.74) | 4.41 (1.39) | 7.08 (2.01) | 6.42 (1.60) | 4.48 (0.87) | 0.68 | 0.000 | 0.63 |
| Dominance | 5.14 (2.87) | 5.88 (2.20) | 6.51 (1.81) | 4.10 (2.50) | 5.16 (2.20) | 7.03 (1.48) | 0.45 | 0.000 | 0.33 |
SD, standard deviation; BG, batterers groups; CG, other criminals group; IPVI, intimate partner violence images; GVI, general violence images; NI, neutral images; TI, type of images.
Neuroimaging results
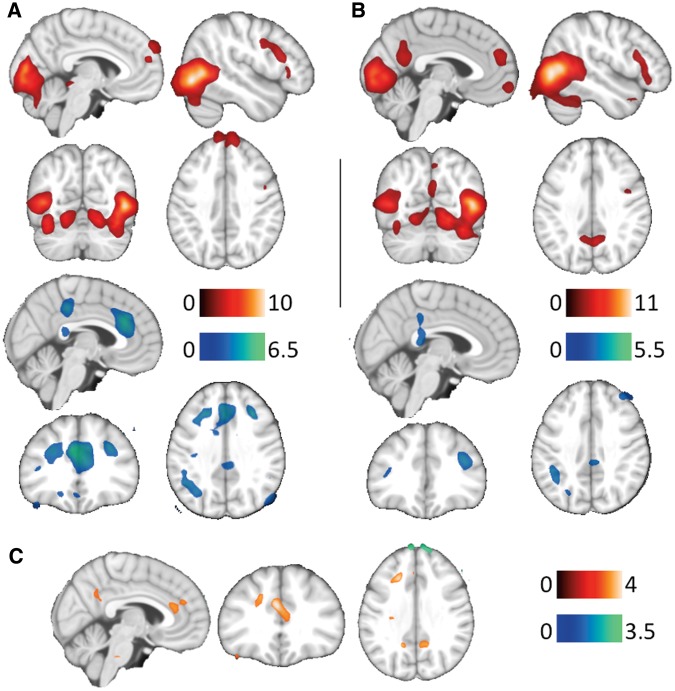
Intimate partner violence vs neutral images (IPV>N)
Group activations
Brain activation and deactivations for the IPV>N contrast in both groups are reported in Table 3. Criminals showed activation in the superior frontal gyrus. Batterers, on the other hand, showed additional activation in the orbitofrontal cortex and the PCC, and significant deactivation in the anterior cingulate cortex and the insula.
Table 3.
Brain activations and deactivations observed during IPV > N and GV > N contrasts in within-group (one-sample) whole brain analyses
| Brain region | Batterers |
Other Criminals |
||||
|---|---|---|---|---|---|---|
| x, y, z | kE | t value | x,y,z | kE | t value | |
| IPV > N | ||||||
| Activations | ||||||
| Superior PFC | ns | 8, 64, 30 | 973* | 4.7 | ||
| Inferior PFC | 42, 16, 20 | 484 | 4.2 | 60, 28, 34 | 706 | 3.9 |
| Medial PFC | 4, 50, 14 | 616 | 3.1 | −4, 54, 18 | 973* | 4.3 |
| OFC | 4, 62, −14 | 287 | 4.9 | ns | ||
| PCC | 4, −58, 30 | 1392 | 5.1 | ns | ||
| Temporal | 52, −68, 2 | 20891* | 11.0 | 50, −72, −4 | 20266* | 10.0 |
| −42, −76, −2 | 20891* | 10.7 | −42, −76, −2 | 20266* | 9.9 | |
| PAG | ns | 2,− 34, −8 | 238 | 3.7 | ||
| Occipital | −4, −90, 4 | 20891* | 7.2 | 8, −88, 4 | 20266* | 8.1 |
| Deactivations | ||||||
| ACC | ns | −2, 32, 28 | 2147* | 6.4 | ||
| Middle PFC | 38, 38, 18 | 784 | 5.2 | 32, 26, 34 | 452 | 5.4 |
| −32, 38, 10 | 199 | 3.9 | −26, 28, 32 | 2147* | 4.8 | |
| OFC | 30, 62, −6 | 294 | 4.2 | −14, 22, −16 | 181 | 3.7 |
| −38, 54, 2 | 202 | 3.5 | ns | |||
| Fusiform gyrus | 30, −54, 0 | 1055* | 4.4 | 32, −44, −6 | 177 | 3.8 |
| −34, −52, −6 | 379 | 3.6 | −20, −46, 2 | 530 | 3.4 | |
| Middle PCC | 6, −34, 40 | 1055* | 3.6 | −2, −36, 44 | 465 | 4.8 |
| Insula | ns | 48, −24 12 | 892 | 4.3 | ||
| Angular gyrus | ns | 54, −44, 66 | 942 | 3.8 | ||
| −44, −42, 38 | 228 | 4.0 | −46, −54, 36 | 351 | 4.4 | |
| GV > N | ||||||
| Activations | ||||||
| Superior PFC | ns | −10, 62, 36 | 1379 | 4.9 | ||
| Inferior PFC | 46, 16, 18 | 1044* | 4.3 | 50, 10, 32 | 1262 | 4.4 |
| OFC | 6, 70, −18 | 388 | 3.9 | ns | ||
| Precentral gyrus | 40, −2, 46 | 1044* | 4.2 | ns | ||
| −32, −12, 42 | 348 | 3.8 | ns | |||
| Amygdala-HPC | 20, −4, −20 | 36259* | 5.1 | 32, −16, −18 | 320 | 3.8 |
| −22, −8, −20 | 36259* | 3.8 | ns | |||
| Thalamus | 14, −26, −2 | 36259* | 5.5 | ns | ||
| −10, −24, −6 | 36259* | 4.1 | ns | |||
| Fusiform gyrus | 38, −56, −20 | 36259* | 6.9 | 38, −56, −16 | 27576* | 5.1 |
| −40, −44, −20 | 36259* | 5.1 | −49, −52, −22 | 27576* | 7.9 | |
| Precuneus | 6, −56, 44 | 36259* | 4.3 | ns | ||
| Superior Parietal | 26, −60, 48 | 36259* | 4.1 | ns | ||
| −26, −54, 46 | 207 | 3.4 | ns | |||
| Temporal | 50, −72, 0 | 36259* | 12.6 | 50, −72, −2 | 27576* | 11.7 |
| −44, −76, −2 | 36259* | 10.5 | −42, −74, −2 | 27576* | 9.8 | |
| Occipital | 16, −88, 6 | 36259* | 11.4 | 8, −86, −8 | 27576* | 13.8 |
| Deactivations | ||||||
| ACC | ns | 6, 34, 14 | 2198* | 4.3 | ||
| Orbitofrontal | −26, 58, 6 | 366 | 3.7 | −34, 54, 2 | 2198* | 4.0 |
| Middle PFC | ns | 34, 28, 36 | 399 | 4.4 | ||
| ns | −34, 12, 44 | 862 | 4.6 | |||
| SMA | ns | 30, −16, 58 | 528 | 5.2 | ||
| Insula | ns | 48, 8, −6 | 2323* | 4.9 | ||
| ns | −44, 18, −6 | 258 | 3.8 | |||
| Middle PCC | ns | −2, −18, 66 | 829 | 4.2 | ||
| Angular | 50, −54, 36 | 636 | 5.2 | 48, −50, 40 | 3303 | 5.8 |
| ns | −44, −58, 38 | 3117 | 4.5 | |||
| Temporal | ns | 64, −22, −10 | 2323* | 3.5 | ||
| −64, −30, −6 | 859 | 4.2 | ||||
x,y,z= MNI peak coordinates; kE, cluster extent in voxels; IPV, intimate partner violence; GV, general violence; N, neutral; ns, nonsignificant; *part of a larger cluster; PFC, prefrontal cortex; OFC, orbitofrontal cortex; PCC, posterior cingulate cortex; PAG, periaqueductal gray; ACC, anterior cingulate cortex; HPC, hippocampus; SMA, supplementary motor area.
Between-group differences
Batterers, relative to criminals, demonstrated significantly higher activation of the middle prefrontal cortex, and the anterior and PCC (Figure 1, Table 4). Criminals showed higher activation of the superior prefrontal cortex compared to batterers (Table 4).
Fig. 1.
Main group activations (red) and deactivations (blue) to IPV>N in criminals (A) and batterers (B). Between-group differences (C) show increased (red) and decreased (blue) brain activity in batterers. The right hemisphere corresponds to the right side of the axial and coronal views. Sagittal images show the right hemisphere in B and C views, and the left hemisphere in A view. The color bar indicates t-values.
Table 4.
Brain regions showing significantly differences between groups during IPV > N and GV > N contrasts
| Brain region | x, y, z | kE | t value |
|---|---|---|---|
| IPV > N | |||
| Other Criminals ≥ Batterers | |||
| Superior PFC | 10, 60, 40 | 156 | 3.4 |
| Batterers≥Other Criminals | |||
| Middle PFC | −20 26, 34 | 130 | 3.9 |
| ACC | −2, 30, 26 | 252 | 4.0 |
| PCC - Precuneus | 14, −52, 34 | 98 | 3.5 |
| GV> N | |||
| Other Criminals≥Batterers | |||
| Superior PFC | −8, 62, 34 | 145 | 3.7 |
| Batterers≥Other Criminals | |||
| Precentral | 28, −16, 62 | 360 | 5.1 |
| SMA-Precuneus | 6, −12, 52 | 696 | 4.0 |
| Middle PFC | −24, 28, 30 | 131 | 3.4 |
| Insula | 48, 8, −6 | 958 | 3.8 |
| −54, −8, 10 | 316 | 3.6 | |
x,y,z= MNI peak coordinates; kE, cluster extent in voxels; IPV, intimate partner violence; GV, general violence; N, neutral; PFC, prefrontal cortex; ACC, anterior cingulate cortex; PCC, posterior cingulate cortex;
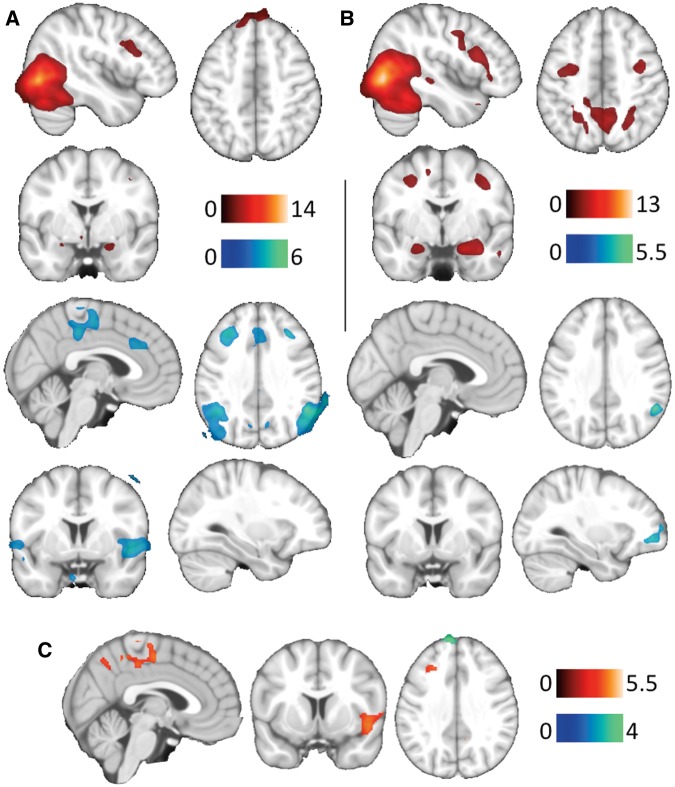
General violence vs neutral images (GV>N)
Group activations
Brain activation and deactivations for the GV>N contrast in both groups are reported in Table 3. Criminals showed activation in the superior frontal gyrus, and significant deactivation in the anterior cingulate cortex, middle frontal gyrus, insula, middle PCC and temporal cortex, which was not identified in batterers. Batterers, on the other hand, showed additional activation in the orbitofrontal cortex, the thalamus, the precuneus and the superior parietal.
Between-group differences
Relative to criminals, batterers demonstrated significantly higher activation of the middle prefrontal cortex, the SMA-Precuneus and the insula (Figure 2, Table 4). Criminals showed higher activation of the superior prefrontal cortex compared to batterers (Table 4).
Fig. 2.
Main group activations (red) and deactivations (blue) to GV>N in criminals (A) and batterers (B). Between-group differences (C) show increased (red) and decreased (blue) brain activity in batterers. The right hemisphere corresponds to the right side of the axial and coronal views, and sagittal images show the right hemisphere in all views. The color bar indicates t-values.
Intimate partner violence vs general violence (IPV > GV)
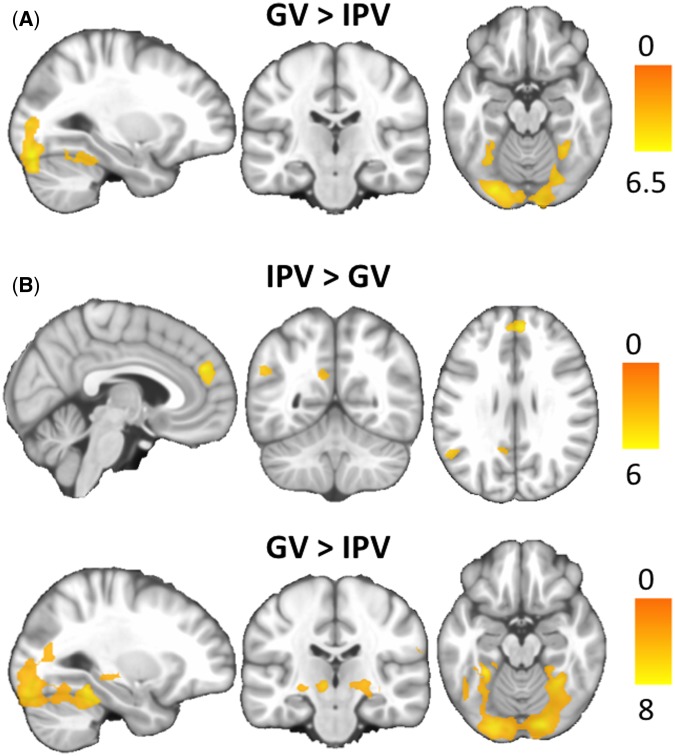
Group effects (paired t-test analysis)
Brain activation and deactivations for the IPV vs GV contrast in both groups are reported in Table 5. To IPV images, batterers showed activation in the medial prefrontal cortex, the PCC, and the left angular gyrus, which was not identified in the OCG (Supplementary Figure S1). Criminals showed no significant activations to IPV images. To GV images, both groups showed activation in the fusiform gyrus and the occipital cortex. Batterers showed additional activation in the thalamus, hippocampus and supramarginal gyrus not identified in the criminal group (Figure 3, Table 5). A group*condition interaction with the extracted signal eigenvariate of these brain regions did not yield significant findings (all Ps > 0.05). This was also the case for the PCC, that was initially hypothesized to show an increased activation to IPV compared to GV images in batterers relative to criminals.
Table 5.
Brain regions showing significantly differences within groups (paired t-test) between IPV and GV images
| Brain region | Batterers |
Brain region | Other criminals |
||||
|---|---|---|---|---|---|---|---|
| x, y, z | kE | t value | x, y, z | kE | t value | ||
| IPV≥GV | |||||||
| Medial PFC | 2, 48, 26 | 328 | 5.9 | ||||
| PCC | −4, −62, 24 | 111 | 3.3 | ||||
| Angular | −56, −56, 28 | 216 | 3.9 | ||||
| GV≥IPV | GV≥IPV | ||||||
| Fusiform gyrus | 30, −40, −20 | 8528* | 6.1 | Fusiform gyrus | 30, −60, 10 | 4635* | 4.8 |
| −28, −42, −22 | 8528* | 6.5 | −32, −60, −18 | 4635* | 4.5 | ||
| Occipital | −20, −88, −20 | 8528* | 8.0 | Occipital | −28, −86, −20 | 4635* | 6.3 |
| Thalamus | 24, −20, −2 | 178 | 4.6 | ||||
| −10, −20, −4 | 289* | 5.4 | |||||
| HPC | 24, −24, −6 | 178 | 3.2 | ||||
| −24, −24, −6 | 289* | 4.2 | |||||
| Supramarginal | 64, −24, 26 | 103 | 4.3 | ||||
x,y,z= MNI peak coordinates; kE, cluster extent in voxels; IPV, intimate partner violence; GV= general violence; *part of a larger cluster; PFC, prefrontal cortex; PCC, posterior cingulate cortex; HPC, hippocampus.
Fig. 3.
Brain regions showing significant differences in the comparison between GV and IPV conditions in criminals (A) and batterers (B). The right hemisphere corresponds to the right side of the axial and coronal views. Sagittal images show the right hemisphere in B ‘IPV>GV’ view, and the left hemisphere in the other sagittal views. The color bar indicates t-values.
IPV processing across groups (collapsed analysis)
The angular gyrus was the only region significantly associated with the processing of IPV images (MNI coordinates, x = −50, y = −60, z = 28, kE = 649, t = 3.4, P<0.005) across the study groups. A group*condition interaction with the extracted signal eigenvariate of the angular gyrus did not yield significant findings (F(1,39)=0.138, P=0.713).
Discussion
The main aim of this study is to compare the brain functioning of batterers with that of other criminals when they observe IPV or GV pictures. Results reveal that batterers, as compared to other criminals, show higher activation in the anterior and PCC to IPV images compared to neutral images. In addition, batterers demonstrate higher activation in the insula and parietal regions to GV images compared with neutral images. They also show a higher activation in the middle prefrontal cortex and a decreased activation in the superior prefrontal cortex to both IPV and GV images compared to neutral images. Nevertheless, batterers do not show the hypothesized higher activation of the PCC-precuneus during the viewing of IPV pictures compared to GV images when compared with the criminal group, although the PCC-precuneus is more activated in response to the IPV images in the batterers only. Therefore, our hypotheses were partially confirmed. This distinct brain functioning is observed regardless of differences in the subjective emotional responses between the study groups.
The finding of a higher activation in the PCC extending to the precuneus in response to IPV vs neutral images in batterers is consistent with our hypothesis and the study of Lee et al. (2009). In this study, the authors similarly found that batterers show an increased activation in the precuneus to aggressive-female vs neutral images, when compared to a sample of controls non-criminals. Our approach of comparing the batterers’ brain functioning to that of other criminals extends these findings, demonstrating that the activation of the PCC-precuneus specifically characterize the brain functioning of batterers, above those of other criminals, while processing IPV images. The PCC is key in episodic memory retrieval and emotional reasoning (Rekkas and Constable, 2005). For example, PCC activation has been reported following moral judgment of harmful actions and increased negative attitudes toward others (Greene et al., 2001; Bruneau et al., 2010). Furthermore, batterers also show a higher activation in the ACC to the IPV vs neutral images. At a functional level, the ACC has been involved in self-referential aspects of thinking, emotional contagion and affective perspective taking (Raichle et al., 2001; Raine and Yang, 2006; Harrison, 2008;), and its activation during the observation of pain have been predicted by individual differences in neuroticism (Cheetham et al., 2009). This observation may be directly related to findings showing that lower perspective taking abilities and higher levels of personal distress in reaction to the emotions of others are related to violence perpetration in batterers (Covell et al., 2007). Overall, increased activation in the PCC and ACC in batterers to intimate partner images may underlie the increased negative feelings of emotional distance that raise fears of abandonment from the significant other. This may in turn lead batterers to have maladaptive coping and regulation of affect in the form of obsessions about his/her partner and stalking, as documented by George et al. (2006).
The significant higher activation of the insula and the SMA-precuneus in the parietal cortex in batterers to the GV images, relative to other criminals, is also consistent with the brain over-activation to threatening situations found by Lee et al. (2009) in these individuals, and interpreted as a hyper-response to threatening stimuli. Hyperactivation of these brain regions is one of the most common neuroimaging findings across fear conditioning studies (Etkin and Wager, 2007; Fullana et al., 2015). Previous clinical and scientific work showing that batterers experience fear, autonomic activation and bias toward the processing of negative stimuli (George et al., 2000; Bitler et al., 1994; George et al., 1989; Chan et al., 2010) may be consistent with this neural over-activation to the GV images in batterers. Interestingly, the anterior insula has recently been associated particularly with perceived anxiety sensations independent from anxiety traits (Harrison et al., 2015). This finding may be consistent with the sudden affective instability in the form of increased anxiety, fearful mood states, anger or rage described by batterers when challenged by his/her partner (George et al., 2006). Furthermore, the decreased activation of the superior frontal and the increased activation of the middle frontal gyri to both IPV and GV images may also contribute to the affective instability and bias toward the processing of negative information in batterers (Kensinger and Schacter, 2006; Gross 2013). This is consistent with the deficient top-down regulatory control over excessive limbic activation already suggested by previous studies with batterers (Lee et al., 2009; George et al., 2004) and the preferential activation of the middle frontal gyrus to negative valence information (Kensinger and Schacter, 2006).
However, not all hypotheses were supported in this study. Specifically, the PCC was not preferentially activated in response to IPV images relative to the GV situations in batterers vs criminals. This was also the case for the angular gyrus, a region that shows a preferential activation in all participants during the viewing of IPV images and was only activated in batterers for the paired t-test. The angular gyrus is considered an important cerebral hub (Timoty and Volkow, 2011), consistently involved in semantic processing, attentional shifting, spatial cognition, episodic and autobiographical memory retrieval, DMN, conflict resolution and the theory of mind (Seghier, 2013). Therefore, the activation of the angular gyrus in the BG when they viewed IPV compared to GV images in paired analyses could be explained by the fact that IPV images activated autobiographical and episodic memory of past IPV events in batterers. Further studies with larger samples may be interested in investigating whether impairment of the angular gyrus in batterers is associated with their capacity to judge attempted harms as morally right or wrong.
Despite differences in brain functioning, there are a lack of differences between batterers and other criminals in the emotional behavioral responses. The absence of such differences may be related to the batterers’ response bias for social desirability. Social desirability has not been previously reported in emotional tasks, but it has been measured in personality questionnaires (Gibbons et al., 2011). This important concern, referred to as explicit subjective measures in batterers, has motivated the development of new implicit tasks to measure attitudes in batterers. Its inclusion may likely benefit further studies with batterer samples (Eckhardt et al., 2012).
Nevertheless, the generalization of our results is limited for several reasons. First, the sample size is relatively small, which may have made it difficult to reach statistical significance in some comparisons. Even so, our results are similar to other published articles that use smaller samples, and the sample size of this study is the largest to date. Second, categorizing types of crime is difficult due to the complex characteristics of each case. Another limitation was related to the representativeness of the IPV group. In order to reduce the influence of confounders in the MRI analyses, participants with a history of substance abuse or personality disorder were excluded. Third, we do not have objective evidence that the stimuli were attended to equally by both groups, although there were no differences between groups in the activation of the occipital cortex in the comparison between task conditions (Vuilleumier, 2005). Lastly, our research has been conducted in ‘first episode’ batterers with low severity of violence. Despite this limitation, the findings from this study indicate that even batterers who are not imprisoned show brain differences.
In sum, our results have shown that batterers have different brain functioning, as compared to other criminals, when they observe both IPV and GV images as compared to neutral images. Future studies should replicate our results in batterers who have committed more severe offenses.
Funding
This work was supported by the Spanish Ministry of Science and Innovation (Project: PSI 2009-13585), Ministry of Economy and Competitiveness (Project: PSI2013-42792-R), and Regional Ministry of Economy, Innovation and Science from Andalusian Government (Project: P2012-SEJ1723). A Sara Borrell postdoctoral fellowship (CD14/00246) of the Carlos III Health Institute to O.C.R.
Acknowledgment
We acknowledge the support of the General Secretary of Penitentiary Institutions, Ministry of the Interior, Spain.
Supplementary Material
References
- American Psychiatric Association (1994). Diagnostic and Statistical Manual of Mental Disorders, 4th ed Washington, DC: American Psychiatric Association. [Google Scholar]
- Blair R.J.R. (2001). Neurocognitive models of aggression, the antisocial personality disorders, and psychopathy. Journal of Neurology, Neurosurgery & Psychiatry, 71(6), 727–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair R.J.R. (2007). The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends in Cognitive Sciences, 11(9), 387–92. [DOI] [PubMed] [Google Scholar]
- Blair R.J.R. (2010). Neuroimaging of psychopathy and antisocial behavior: a targeted review. Current Psychiatry Reports, 12(1), 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair R.J.R., Lee T.M. (2013). The social cognitive neuroscience of aggression, violence, and psychopathy. Social Neuroscience, 8(2), 108–11. [DOI] [PubMed] [Google Scholar]
- Bertsch K., Grothe M., Prehn K., et al. (2013). Brain volumes differ between diagnostic groups of violent criminal offenders. European Archives of Psychiatry and Clinical Neuroscience, 263(7):593–606. [DOI] [PubMed] [Google Scholar]
- Bitler D.A., Linnoila M., George D.T. (1994). Psychosocial and diagnostic characteristics of individuals initiating domestic violence. The Journal of Nervous and Mental Disease, 182(10), 583–4. [DOI] [PubMed] [Google Scholar]
- Boyle D.J., O’Leary K.D., Rosenbaum A., Hassett-Walker C. (2008). Differentiating between generally and partner-only violent subgroups: lifetime antisocial behavior, family of origin violence, and impulsivity. Journal of Family Violence, 23(1):47–55. [Google Scholar]
- Cardenal V., Sánchez M.P., Ortiz-Tallo M. (2007). Adaptación y baremación al español del Inventario Clínico Multiaxial de Millon-III (MCMI-III). Madrid: TEA, Ediciones. [Google Scholar]
- Chan S.C., Raine A., Lee T.M.C. (2010). Attentional bias towards negative affect stimuli and reactive aggression in male batterers. Psychiatry Research, 176 (2-3), 246–9. [DOI] [PubMed] [Google Scholar]
- Cheetham M., Pedroni A.F., Antley A., Slater M., Jäncke L. (2009). Virtual milgram: empathic concern or personal distress? Evidence from functional MRI and dispositional measures. Frontiers in Human Neuroscience, 3, 29. doi:10.3389/neuro.09.029.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R.A., Brumm V., Zawacki T.M., Paul R., Sweet L., Rosenbaum A. (2003). Impulsivity and verbal deficits associated with domestic violence. Journal of the International Neuropsychological Society, 9(5), 760–70. [DOI] [PubMed] [Google Scholar]
- Corvo K. (2014). The role of executive function deficits in domestic violence perpetration. Partner Abuse, 5(3), 342–55. [Google Scholar]
- Corvo K., Johnson P. (2013). Sharpening Ockam's razor: the role of psychopathology and neuropsychopatology in the perpetration of domestic violence. Aggression and Violent Behavior , 18, 175–82. [Google Scholar]
- Covell C.N., Huss M.T., Langhinrichsen-Rohling J. (2007). Empathic deficits among male batterers: a multidimensional approach. Journal of Family Violence , 22(3), 165–74. [Google Scholar]
- Echeburúa E., Montalvo J.F., de Corral P. (2008). ¿Hay diferencias entre la violencia grave y la violencia menos grave contra la pareja?: un análisis comparativo?. International Journal of Clinical and Health Psychology , 8, 355–82. [Google Scholar]
- Eckhardt C.I., Samper R., Suhr L., Holtzworth-Munroe A. (2012). Implicit attitudes toward violence among male perpetrators of intimate partner violence. A preliminary investigation. Journal of Interpersonal Violence, 27(3), 471–91. [DOI] [PubMed] [Google Scholar]
- Etkin A., Wager T.D. (2007). Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry, 164(10), 1476–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibson M. (1999). Guía del usuario para la entrevista clínica estructurada para los trastornos de la personalidad del eje I del DSM-IV. Madrid: Masson. [Google Scholar]
- Fullana M.A., Harrison B.J., Soriano-Mas C., et al. (2015). Neural signatures of human fear conditioning: an updated and extended meta-analysis of fMRI studies. Molecular Psychiatry. doi: 10.1038/mp.2015.88. [DOI] [PubMed] [Google Scholar]
- George D.T., Anderson P., Nutt D.J., Linnoila M. (1989). Aggressive thoughts and behavior: another symptom of panic disorder? Acta Psychiatrica Scandinavica, 79(5), 500–2. [DOI] [PubMed] [Google Scholar]
- George D.T., Hibbeln J.R., Ragan P.W., et al. (2000). Lactate-induced rage and panic in a select group of subjects who perpetrate acts of domestic violence. Biological Psychiatry, 47(9), 804–12. [DOI] [PubMed] [Google Scholar]
- George D.T., Phillips M.J., Doty L., Umhau J.C., Rawlings R.R. (2006). A model linking biology, behavior and psychiatric diagnoses in perpetrators of domestic violence. Medical Hypotheses, 67(2), 345–53. [DOI] [PubMed] [Google Scholar]
- George D.T., Rawlings R.R., Williams W.A., Phillips M.J., Fong G., Kerich M. (2004). A select group of perpetrators of domestic violence: evidence of decreased metabolism in the right hypothalamus and reduced relationships between cortical/subcortical brain structures in position emission tomography. Psychiatry Research, 130(1), 11−25. [DOI] [PubMed] [Google Scholar]
- Gibbons P., Collins M., Reid C. (2011). How useful are indices of personality pathology when assessing domestic violence perpetrators? Psychological Assessment, 23, 164–73. [DOI] [PubMed] [Google Scholar]
- Greene J. D., Sommerville R. B., Nystrom L. E., Darley J. M., Cohen J. D. (2001). An fMRI investigation of emotional engagement in moral judgment Science, 293(5537), 2105–8. [DOI] [PubMed] [Google Scholar]
- Gross J.J., editor. (2013). Handbook of Emotion Regulation. Guilford publications. Spring Street, New York, NY. [Google Scholar]
- Harenski C. L., Antonenko O., Shane M. S., Kiehl K. A. (2010). A functional imaging investigation of moral deliberation and moral intuition. Neuroimage, 49(3), 2707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison B.J., Fullana M.A., Soriano-Mas C., et al. (2015). A neural mediator of human anxiety sensitivity. Human Brain Mapping, 36(10), 3950–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison B.J., Pujol J., López-Solà M., et al. (2008). Consistency and functional specialization in the default mode brain network. Proceedings of the National Academy of Sciences, 105(28), 9781–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman N.L., Cordero C., Calonge I. (1997). K-bit: Test breve de Inteligencia de Kaufman. TEA Ediciones. [Google Scholar]
- Kensinger E.A., Schacter D.L. (2006). Processing emotional pictures and words: effects of valence and arousal. Cognitive, Affective, & Behavioral Neuroscience, 6(2), 110–26. [DOI] [PubMed] [Google Scholar]
- Krug E.G., Mercy J.A., Dahlberg L.L., Zwi A.B. (2002). The world report on violence and health. The Lancet, 360(9339), 1083–8. [DOI] [PubMed] [Google Scholar]
- Lee T.M.C., Chan S.C., Raine A. (2009). Hyperresponsivity to threat stimuli in domestic violence offenders: A functional magnetic resonance imaging study. Journal Clinical Psychiatry, 70(1), 36–45. [DOI] [PubMed] [Google Scholar]
- Ley Orgánica 1/2004, de 28 de diciembre, de Medidas de Protección Integral contra la Violencia de Género. Boletín Oficial del Estado, 29 de diciembre de 2004, núm. 313. LO15/1999, de 13 de diciembre, de Protección de Datos de Carácter Personal. (BOE núm. 298, de 14-12-1999, 43088-99).
- Loinaz I., Echeburúa E., Ortiz-Tallo M., Amor P. J. (2012). Propiedades psicométricas de la Conflict Tactics Scales (CTS-2) en una muestra española de agresores de pareja. Psicothema, 24(1), 142–8. [PubMed] [Google Scholar]
- Mobbs D., Petrovic P., Marchant J.L., et al. (2007). When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science, 317(5841), 1079–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt T.E., Krueger R.F., Caspi A., Fagan J. (2000). Partner abuse and general crime: how are they the same? How are they different? Criminology, 38(1), 199–232. [Google Scholar]
- Patrick C.J. (2008). Psychophysiological correlates of aggression and violence: an integrative review. Philosophical Transactions of the Royal Society B: Biological Sciences, 363(1503), 2543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto L.A., Sullivan E.L., Rosenbaum A., Wyngarden N., Umhau J.C.M., Taft C.T. (2010). Biological correlates of intimate partner violence perpetration. Aggression and Violent Behavior, 15(5), 387–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences, 98(2), 676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A., Yang Y. (2006). Neural foundations to moral reasoning and antisocial behavior. Social Cognitive and Affective Neuroscience, 1(3), 203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekkas P.V., Constable R.T. (2005). Evidence that autobiographic memory retrieval does not become independent of the hippocampus: an fMRI study contrasting very recent with remote events. Journal of Cognitive Neuroscience, 17(12), 1950–61. [DOI] [PubMed] [Google Scholar]
- Schiltz K., Witzel J.G., Bausch-Holterhoff J., Bogerts B. (2013). High prevalence of brain pathology in violent prisoners: a qualitative CT and MRI scan study. European Archives of Psychiatry and Clinical Neuroscience, 263(7), 607–16. [DOI] [PubMed] [Google Scholar]
- Seghier M. L. (2013). The angular gyrus multiple functions and multiple subdivisions. The Neuroscientist , 19(1), 43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siever L. (2008). Neurobiology of aggression and violence. American Journal of Psychiatry, 165(4), 429–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X.W., Dong Z.Y., Long X.Y., et al. (2011). REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One, 6, e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus M.A., Hamby S.L., Boney-McCoy S., Sugarman D.B. (1996). The Revised Conflict Tactics Scales (CTS2): development and preliminary psychometric data. Journal of Family Issues, 17(3), 283–316. [Google Scholar]
- Vuilleumier P. (2005). How brains beware: neural mechanisms of emotional attention. Trends in Cognitive Sciences, 9(12), 585–94. [DOI] [PubMed] [Google Scholar]
- Ward B.D. (2013). AFNI and NIFTI server at NIMH in Bethesda, MD, USA. Simultaneous inference for FMRI data. Available: http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim. pdf. [Google Scholar]
- White S.F., Meffert H., Blair R.J.R. (2015). Functional neurobiology of aggression. Science in the Courtroom, 1(1), 2–11. [Google Scholar]
- World Health Organization. (2013). Global and regional estimates of violence against women: prevalence and health effects of intimate partner violence and non-partner sexualviolence. http://www.who.int/iris/bitstream/10665/85239/1/9789241564625_eng.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.