Abstract
Purpose
To evaluate the cost-effectiveness of combination chemotherapy, radiation, and surgery (CRS) versus definitive chemotherapy and radiation (CR) in clinical Stage IIIA non-small cell lung cancer (NSCLC) patients at academic and non-academic centers.
Methods
Patients with clinical stage IIIA NSCLC receiving CR or CRS from 1998–2010 were identified in the National Cancer Data Base (NCDB). Propensity score matching on patient, tumor, and treatment characteristics was performed. Medicare allowable charges were used for treatment costs. The incremental cost effectiveness ratio (ICER) was based on probabilistic 5-year survival and calculated as cost per life year gained.
Results
5,265 CR and CRS matched patient pairs were identified. Surgery imparted an increased effectiveness of 0.83 life years, with an ICER of $17,618. Among non-academic centers, 1,634 matched CR and CRS patients demonstrated a benefit with surgery of 0.86 life years gained, for an ICER of $17,124. At academic centers, 3,201 matched CR and CRS patients had increased survival of 0.81 life years with surgery, for an ICER of $18,144. Finally, 3,713 CRS patients were matched between academic and non-academic centers. Academic center surgical patients had an increased effectiveness of 1.5 months gained and dominated the model with lower surgical cost estimates associated with lower 30-day mortality rates.
Conclusions
In Stage IIIA NSCLC, the selective addition of surgery to chemoradiation is cost-effective compared to definitive chemoradiation therapy at both non-academic and academic centers. These conclusions are valid over a range of clinically meaningful variations in cost and treatment outcomes.
Introduction
The National Cancer Institute estimates that 226,160 lung cancer cases were diagnosed in the United States in 2012 and 160,340 patients died from lung cancer in the same period. It is estimated that the annual medical cost of lung cancer treatment exceeds $10 billion and that lost productivity costs society an additional $30 billion in the U.S. [1,2] As lung cancer presents most commonly in the elderly, costs are primarily absorbed by federal and state governments through Medicare and Medicaid programs and are expected to increase. [3]
For stage IIIA patients, the 5-year overall survival is typically less than 20%. [4,5] Stage IIIA NSCLC is usually treated with a combination of chemotherapy and radiation, while surgery may be offered to patients showing remission or lack of progression of tumor burden after induction therapy. Randomized trials have not shown a clear long-term benefit to surgery, but these studies have been criticized for suboptimal short-term outcomes after surgical resection. [6,7] In contrast, several single-center studies have reported improved long-term outcomes with the addition of surgery to chemotherapy and radiation. [8–11] A review of Stage IIIA NSCLC treatment outcomes from the National Cancer Data Base (NCDB) found improved overall survival for propensity matched patients receiving trimodality therapy including surgery versus definitive chemotherapy and radiation therapy. [12]
Multimodality treatment for stage IIIA NSCLC is associated with greater resource utilization and appropriate tailoring of evidence-based therapies is needed. Stage I NSCLC has been the focus of recent cost-effectiveness analyses, but treatment options for stage IIIA disease have not yet been examined in this manner. [13–15] The objective of this study was to compare the relative cost-effectiveness of chemotherapy and radiation alone (CR) versus chemotherapy, radiation, and surgery (CRS), in any sequence, for clinical stage IIIA NSCLC patients treated in academic and community settings.
Material and Methods
Using de-identified patient information from the NCDB participant user file, we abstracted patients with clinical stage IIIA NSCLC who received treatment between 1998 and 2010 that received CR or CRS, in any sequence. Information on patient, tumor, and treatment characteristics with short- and long-term outcomes was obtained. The Charlson/Deyo score was abstracted as a measure of comorbidity, and is recorded by the NCDB as 0, 1, or ≥2 (excluding points from a patient’s lung malignancy). Last known vital status and the time between diagnosis and follow-up were used to determine survival using a Kaplan-Meier analysis. All analyses were performed using SPSS (SPSS 21.0 for Windows, SPSS Inc, Chicago, IL).
To overcome the influence of selection bias in treatment allocation, patients in the CR group were matched to CRS patients using a propensity score technique. The propensity score between the CR and CRS groups was based on preoperative characteristics and was estimated using a backwards stepwise logistic regression model including age, gender, race, income, rural versus urban status, year of diagnosis, Charlson/Deyo comorbidity score, tumor size, and facility type. Patients for whom the propensity scores matched to the third decimal place (0.0001) were matched in 1:1 fashion. Automated matching was performed using the Fuzzy extension command in SPSS. [16] Subgroup analysis was performed by repeating these propensity matching techniques to identify CR and CRS patient pairs by institution type. A consort diagram is shown in Figure 1.
Figure 1.
Consort diagram demonstrating patient selection criteria and propensity matched analysis.
*CR and CRS patients were matched on age, gender, race, income, rural versus urban status, year of diagnosis, Charlson Deyo score, tumor size, and facility type (academic versus nonacademic).
† Subgroup analyses with academic and nonacademic CR and CRS patients were matched on age, gender, race, income, rural versus urban status, year of diagnosis, Charlson Deyo score, and tumor size.
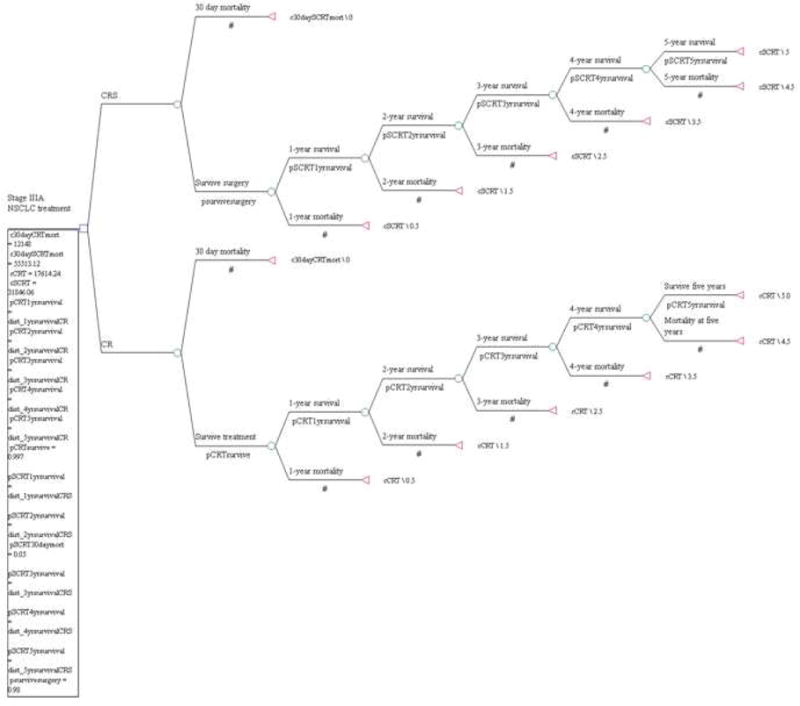
A decision analysis model was created using Tree Age Pro 2013 software (TreeAge Software, Inc, Williamstown, MA). Inputs to the model for probabilistic 5-year patient survival were obtained from the NCDB analysis described above. Such models have been previously described in thoracic surgery [17,18]. The model created for this analysis is shown in Electronic Figure 1.
Expenditures were based on the Medicare allowable costs (in 2014 US dollars). [19] The incremental cost effectiveness ratio (ICER) was calculated as the cost per additional life year gained over a five-year horizon. Length of survival (life years) was used as the measure of utility in the model. One- and two-way sensitivity analyses were performed where the value of each parameter was varied across a clinically relevant range and the model recalculated. To test the overall stability of the model we conducted probabilistic sensitivity analysis. Cost-effectiveness acceptability graphs were plotted.
Results
In the overall NCDB cohort, 51,979 (84.7%) patients received CR, while 9,360 (15.3%) patients received CRS. 5,265 matched patient pairs in the CR and CRS groups were identified, and used to determine survival probabilities for the decision analysis model. In the CRS arm, 3,708 (70.4%) of patients received a lobectomy, 682 (13.0%) received a pneumonectomy, and 875 (16.6%) were classified as ‘other’. Sequence of therapy was available for 4,535 (86%) of the CRS patients – 2,758 (60.8%) received neoadjuvant therapy, while 1,777 (39.2%) received adjuvant therapy. One year intervals of probabilistic survival for the matched CR and CRS groups from the NCDB over a five year period are shown in Table 1. Additional inputs to the model included the thirty-day surgical mortality of 2.2% for the CRS patients and thirty-day mortality for CR patients of 0.003%. Medicare allowable cost estimate calculations are shown in Table 2.
Table 1.
Five year probabilistic survival for CR and CRS patients.
| CR (95% CI) | CRS (95% CI) | |
|---|---|---|
| Probability of Survival at 1 | 66.4% (65.0 – 67.8%) | 83.4% (82.4 – 84.4%) |
| Probability of Survival at 2 years if surviving 1 year | 60.1% (57.7 – 62.5%) | 73.5% (71.6 – 75.4%) |
| Probability of Survival at 3 if surviving 2 years | 65.9% (61.8 – 70.0%) | 78.5% (75.4 – 81.6%) |
| Probability of Survival at 4 if surviving 3 years | 75.3% (68.8 – 81.8%) | 82.1% (77.9 – 86.3%) |
| Probability of Survival at 5 if surviving 4 years | 80.3% (71.4 – 89.2%) | 85.8% (80.2 – 91.4%) |
Five year survival, demonstrated for the chemotherapy and radiation (CR) group and the chemotherapy, radiation therapy, and surgery (CRS) group at one-year intervals, with 95% confidence intervals (CI).
Table 2.
Medicare allowable costs by treatment modality. These charges were used as base cost estimates for the decision analysis model.
| Base costs of treatment, without 30-day mortality | Medicare Allowable Cost Estimate (2014 US Dollars) |
|---|---|
|
| |
| CR group: |
|
| Definitive chemotherapya | $1,725
|
| Definitive radiation therapyb | $15,889.24
|
| Cost of 30 day mortality definitive therapyc | $12,148
|
| Total Cost definitive therapy | $17,614.24 |
|
| |
| CRS group: | |
| Induction chemoradiation therapya,b | $14,955.14 |
| Surgical resectiond | $16,890.91 |
| Cost of 30-day mortality from surgerye | $55, 513.12 |
| Total costs chemotherapy, radiation, and surgery | $31,846.06 |
Average Medicare allowable cost of docetaxel, etoposide/cisplatin, and paclitaxel/carboplatin regimens for induction and definitive chemotherapy protocols
Definitive and induction radiation oncology treatment costs were calculated using the Medicare allowable charges for both professional and technical fees, assuming one half of the patients were treated with intensity-modulated radiation therapy (IMRT) and one half with 3D conformal therapy.
Cost of 30-day mortality from induction chemotherapy was assumed to be primarily from aggressive treatment of febrile neutropenia, and this cost was estimated from the literature.[20]
Medicare allowable cost of mediastinoscopy, thoracotomy, and lobectomy, with a 5% complication rate with pneumonia or atrial fibrillation factored in.
Medicare allowable cost of 30-day mortality from pulmonary resection including pneumonia, atrial fibrillation, ventilator care, and death.
Based on Medicare allowable costs, the incremental cost increase of CRS compared to CR was $14,722. In this propensity matched population, there was an increase in effectiveness (patient survival) of 0.84 years (10.2 months) for patients in the CRS arm. The ICER is obtained by dividing the increased cost of a therapy by the incremental benefit that the therapy provides. Therefore, the ICER for CRS was $17,618. Results of this base cost-effectiveness analysis are shown in Table 3. The minimal increase in survival among CRS patients needed to obtain a traditional ICER of $50,000 is 0.29 years (3.5 months). Therefore, based on the NCDB propensity matched population, CRS was cost-effective with a relatively low ICER and a survival benefit substantially greater than that needed as a ‘cutoff’ value. A one-way sensitivity analysis was performed by varying the total cost of treatment in the CRS group from $30,000 to $100,000, while keeping the cost estimates of the CR group constant. Here, the model tolerated more than doubling of surgical costs (from a base cost of $16,891 to an increase of $40,352) with an ICER of $48,291.
Table 3.
Results of base cost effectiveness analysis. Total costs for CRS and CR modalities are adjusted by the model to include 30-day mortality rate and associated costs for each therapy modality.
| Strategy | Total Cost (2014 US $) | Incremental cost (2014 US$) | Total effectiveness (years) | Incremental effectiveness (years) | Incremental cost- effectiveness ratio |
|---|---|---|---|---|---|
| CRS | $32,319 | $14,722 | 2.93 | 0.83 | $17,617.80 |
| CR | $17,598 | 2.10 |
Total costs for CRS and CR modalities here include 30-day mortality risk cost adjustments.
An additional one-way sensitivity analysis was performed by varying the potential total cost of 30-day mortality after surgical resection from $50,000 to $250,000 (baseline cost of surgical mortality was $55,513), while keeping the expected mortality rate constant at the base case value of 2.2%. Even at a hypothetical cost of $250,000 for charges associated with 30-day mortality, the ICER was $22,273.
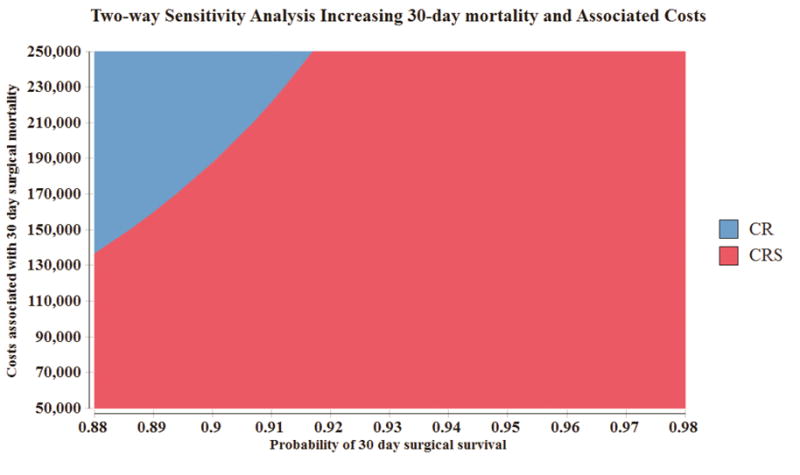
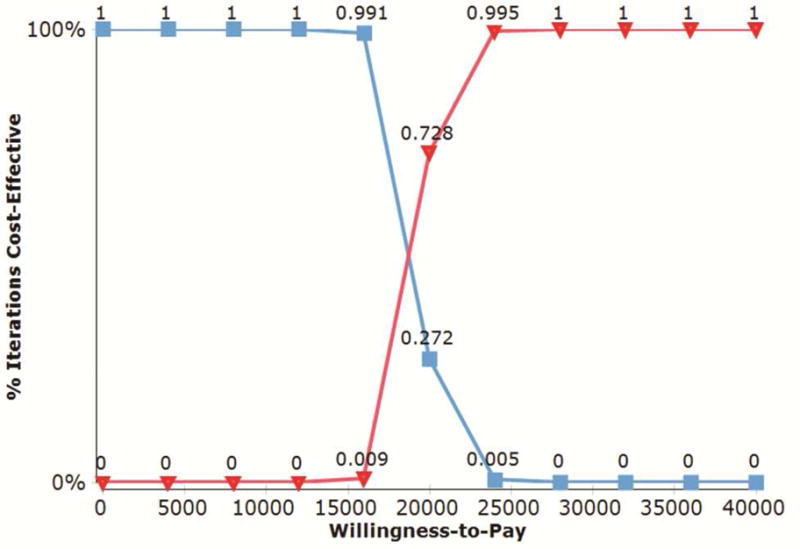
A two-way sensitivity analysis was performed varying both the risk of thirty-day mortality (from 2 to 12%) and associated costs of a 30-day mortality (from $50,000 to $250,000). With a 30-day mortality rate of 12%, CRS continued to dominate the model up to $135,000.00 of perioperative hospitalization charges that could be incurred. At a mortality rate of 8%, CRS dominated the model for this entire range of cost estimates. A graph depicting this two-way sensitivity analysis is shown in Figure 2. A cost-effectiveness acceptability curve was created with probabilistic sensitivity analysis to examine iterations over which CRS would be cost-effective over a willingness-to-pay range varying from 0 to $50,000. At a WTP threshold of $25,000 100% of the 1000 theoretical iterations favored CRS as a cost-effective treatment strategy in Stage IIIA NSCLC (Figure 3).
Figure 2.
Two-way sensitivity analysis varying the probability surgical survival after pulmonary resection in Stage IIIA NSCLC from 88–98% and the cost of 30-day hospitalization charges from $50,000 to $250,000. Willingness to pay was set at a conventional threshold of $50,000. For propensity matched CR and CRS patients, the 30-day mortality rate was 2.2%, and the base cost of 30-day mortality was $55,513. This figure indicates that even with decreases in 30-day surgical survival and associated costs, CRS (denoted in red) dominates the decision model over CR for these clinical variations.
Figure 3.
Cost-effectiveness acceptability curve with a willingness-to-pay threshold varying from 0 to $50,000. 1000 iterations of CR versus CRS for propensity matched Stage IIIA NSCLC patients were run in a Monte Carlo simulation, using survival inputs from the NCDB and Medicare allowable costs. At a willingness-to-pay threshold of $18,000, surgery begins to dominate the model choices. At a willingness-to-pay threshold of $25,000, 100% of model patient simulations favor CRS over CR.
To test the model in different treatment center types, a propensity matched analysis was performed for CR or CRS patients at academic centers. A separate but similar model was created for nonacademic centers. For the academic center model, there were 1,634 matched patient pairs. The incremental cost for CRS patients at academic centers was $14,738 and the incremental effectiveness was 0.81 years. The ICER for CRS at academic centers was $18,144. At nonacademic centers, 3,201 matched patient pairs were identified. The incremental cost for CRS patients at nonacademic centers was $14,780 and the incremental effectiveness was 0.86 years. The ICER for CRS at nonacademic centers was $17,124.
Finally, CRS patients at academic and nonacademic institutions were propensity matched to yield 3,713 matched patient pairs. The incremental effectiveness of CRS at academic centers was 0.12 years with a lower cost when compared to non-academic centers (−$135). The negative ICER for receiving surgery at a non-academic center (−$1,144) indicates that academic centers have a small, but favorable cost-effectiveness profile when compared to non-academic centers.
Comment
This study addresses the relative cost-effectiveness of combined chemotherapy, radiation and surgery in comparison to definitive chemotherapy and radiation. With current Medicare allowable fee estimates, the addition of surgery to chemotherapy and radiation extends the life-expectancy of selected patients with clinical Stage IIIA NSCLC in a cost-effective manner. These results were consistent across both academic and community centers. The ICER for including surgery as part of a treatment plan in Stage IIIA NSCLC varied from $17,000 to $18,000 in our paired comparisons by institution type, and was either well below or similar to other ICERs in thoracic surgery. For example, the ICER for bilateral lung transplantation (versus medical management) is $176,817 [21], lung volume reduction surgery (versus medical management) $140,000 [22], and CABG (versus drug-eluting PCI for left main or multivessel disease) $16,537 [23].
Previously, several single-center studies have demonstrated longer median overall survival with various combinations of neoadjuvant chemoradiation followed by surgical resection in both Stage IIIA and IIIB patients. [8–11, 24, 25] The findings from the larger treatment population of the NCDB are consistent with these studies. [12] To date, four randomized controlled trials have attempted to study the role of surgery in Stage IIIA NSCLC. Of these, two studies did not meet accrual goals [26, 27], while the others demonstrated a survival benefit for patients receiving a lobectomy but not for the overall surgical cohorts. [6, 7] Of note, the perioperative mortality rate for the INT-0139 pneumonectomy group was approximately 25%, while the 30-day mortality after pneumonectomy in our NCDB analysis was 8.6%. [6] In this regard, findings from the NCDB are congruent with previous reports. [28,29] In the European Organization for Research and Treatment of Cancer (EORTC) trial for surgery in Stage IIIA-N2 NSCLC following induction therapy, approximately one half of patients in the surgical arm received a pneumonectomy. [7] This is disproportionate compared to the NCDB where 13% of stage IIIA patients underwent a pneumonectomy. Current recommendations by the National Comprehensive Cancer Network (NCCN) advocate for consideration of pulmonary resection after induction chemotherapy for T1-3, N2 disease if there is no evidence of progression of mediastinal disease after treatment. [30]
A previous cost-effectiveness study using Canadian Health Care cost estimates from the 1990s found that multimodal therapy (including pre- and postoperative chemotherapy, surgical resection, and postoperative radiotherapy) for Stage III NSCLC (per the staging criteria at the time) was cost-effective, with a cost per life-year gained estimate ranging from $3,300 to $15,000. [31] More recent studies have limited cost-effectiveness analyses to comparisons between surgical approaches without addressing the overall treatment plan in local advanced NSCLC patients. [32,33]
External beam radiation therapy may be delivered via 3 dimensional conformal radiotherapy or intensity-modulated radiation therapy (IMRT). Recent work has shown that the national use of IMRT has increased, which is more costly. [34] For the years of treatment evaluated in this study, the 50% estimate of IMRT use is likely higher than the percentage of IMRT actually used. However, this estimate appears to be on trend with current data, and may eventually represent an underestimate of radiation therapy expenditures as IMRT use increases, or as more centers begin using proton therapy.
We believe that the population based NCDB data provides a realistic approximation of practice patterns in the US and provided appropriate survival inputs for our base case analysis. An assumption of this model is that the costs of long-term cancer care (which can include clinical follow-up, treatment of recurrence, and/or palliative care) would be similar between the CR and CRS groups. However, given the greater 5-year survival in the CRS group seen in the NCDB group, higher long-term cancer care costs would be anticipated in this population. Quantifying this amount is unlikely to affect the overall model, as the greatest amount of health care expenditure among all lung cancer patients is in the last year of life. [35]
Previous work by our group has demonstrated a lower 30-day mortality rate and improved overall survival for patients receiving surgery for stage IIIA NSCLC at academic facilities. [36] Specifically, receiving surgical resection at an academic center for Stage IIIA NSCLC was independently associated with decreased thirty-day mortality and improved overall survival. [36] In our propensity matched analysis of academic and nonacademic CRS patients, these conclusions are again supported by a small but significant improvement in survival outcomes and lower cost of CRS therapy at academic centers.
There are some limitations to our analysis to acknowledge. Our base case analysis model is subject to the biases associated with retrospective studies, including selection bias in treatment allocation. Propensity matching cannot account for unmeasured potential confounders. Specific data regarding pathological nodal staging was not available for the majority (93%) of CR patients and could not be included as a matching variable. Therefore, it is possible that patients with bulky N2 disease were more prevalent in the CR arm, thus making the outcomes appear worse.
In continuing to refine the role of surgery in locally advanced NSCLC, it is important to consider quality of life (QoL) outcomes. National databases do not routinely collect QoL measures as these can involve lengthy questionnaires that are costly to administer. Though it is known that patients receiving pneumonectomy have significantly lower QoL outcome measures than those undergoing a lobectomy or bi-lobectomy, prospective comparative information on QoL measures in patients undergoing CR or CRS for Stage IIIA NSCLC is lacking. [37] The model presented above can be further refined with these data.
Supplementary Material
DISCUSSION
106. A DECISION AND COST-EFFECTIVENESS ANALYSIS OF SURGICAL RESECTION IN CLINICAL STAGE IIIA NON-SMALL CELL LUNG CANCER AT ACADEMIC AND NON-ACADEMIC CENTERS. Paper presented by Pamela P. Samson, M.D., St. Louis, Missouri. E-mail: samsonp@wudosis.wustl.edu
Discussion by Scott J. Swanson, M.D., Boston, Massachusetts.
E-mail: sjswanson@partners.org
Dr. S. Swanson (Boston, Massachusetts):
Great paper. I think I understood it. But do you have any sense of how radiation plays into this in terms of the cost piece? If it was just induction chemotherapy, can you predict what you’d see?
106. A DECISION AND COST-EFFECTIVENESS ANALYSIS OF SURGICAL RESECTION IN CLINICAL STAGE IIIA NON-SMALL CELL LUNG CANCER AT ACADEMIC AND NON-ACADEMIC CENTERS. Response by Pamela P. Samson, M.D., St. Louis, Missouri.
DR. SAMSON: Right. So we assumed an induction regimen of chemoradiation, and specifically we used a 50% mix of both 3D conformal radiation therapy and IMRT, which has been gaining in popularity.
So we estimated half the cost with 3D and half the cost with IMRT and came up with an average and put that into the model.
If anything, this probably overestimates the cost because the years of the study, 1998 to 2010, it’s likely that more patients received 3D conformal than IMRT which is more expensive. It does not include proton therapy at this time. But we could certainly do a variation where we have 100% IMRT; although, that’s not still quite yet in clinical practice.
DR. SWANSON: My question was really if you eliminate radiation and just go straight chemo induction, I would assume that makes the cost effectiveness go way up. Because there is some data that shows radiation may not be helpful. And if we’re looking at a cost issue here, you might find that really drives it into a real positive.
DR. SAMSON: Understood. Sorry, I misinterpreted your question. Thank you. You are right, it would increase the cost-effectiveness of surgery - but if they receive radiation therapy adjuvantly, the cost estimates we provide in the model would probably remain quite similar.
106. A DECISION AND COST-EFFECTIVENESS ANALYSIS OF SURGICAL RESECTION IN CLINICAL STAGE IIIA NON-SMALL CELL LUNG CANCER AT ACADEMIC AND NON-ACADEMIC CENTERS. Paper presented by Pamela P. Samson, M.D., St. Louis, Missouri. E-mail: samsonp@wudosis.wustl.edu
Discussion by Joseph B. Shrager, M.D., Stanford, California.
E-mail: shrager@stanford.edu
Dr. J. Shrager (Stanford, California):
I think this is a great effort to shed light on a very important problem. But I’m afraid there could be an apples and oranges problem substantial enough that if you presented this to an oncology group – medical oncologists – that they would point this out to you critically. The problem is that your propensity analysis is totally dependent on what you’re able to propensity match for. And in the National Cancer Database, I gather you don’t have any information on number of N2 nodes, size of N2 nodes, essentially extent of N2 disease – which are probably the pieces of information that really determinehow the patients do, more than any other factors.
So when you calculated your benefit of .84, it may have more to do with the patients who are being selected for surgery than it does to the fact that they’re having surgery.
106. A DECISION AND COST-EFFECTIVENESS ANALYSIS OF SURGICAL RESECTION IN CLINICAL STAGE IIIA NON-SMALL CELL LUNG CANCER AT ACADEMIC AND NON-ACADEMIC CENTERS. Response by Pamela P. Samson, M.D., St. Louis, Missouri.
DR. SAMSON: Right.
DR. SHRAGER: I don’t know. You guys are the great statisticians, so I don’t know how you would try to account for that
DR. SAMSON: Thank you for that point.
Of the definitive chemotherapy and radiation therapy population, only 7% of that propensity-matched population underwent any type of nodal staging. So it’s just not a variable we’re able to match on because then otherwise we wouldn’t have enough patients to study.
Our hope is that in the propensity matching we capture the fittest of the definitive chemoradiation patients when we match on things such as age and comorbidity score in particular. In other words, we are capturing a population of definitive CR patients that would be hypothetically eligible for surgery.
DR. SHRAGER: You can’t get the radiologic reports or the PET scan reports or anything from the National Cancer Data.
DR. SAMSON: Unfortunately, no.
DR. SHRAGER: I’m saying that facetiously.
106. A DECISION AND COST-EFFECTIVENESS ANALYSIS OF SURGICAL RESECTION IN CLINICAL STAGE IIIA NON-SMALL CELL LUNG CANCER AT ACADEMIC AND NON-ACADEMIC CENTERS. Paper presented by Pamela P. Samson, M.D., St. Louis, Missouri. E-mail: samsonp@wudosis.wustl.edu
Discussion by Richard K. Freeman, M.D., M.B.A., Indianapolis, Indiana.
E-mail: richard.freeman@stvincent.org
Dr. R. Freeman (Indianapolis, Indiana):
Great presentation and I congratulate you and your colleagues on the methodology that you used.
Do you have any insight into that about 6% difference between academic and nonacademic? Was it chemotherapy? Was it imaging? Do you have any subset analysis there?
106. A DECISION AND COST-EFFECTIVENESS ANALYSIS OF SURGICAL RESECTION IN CLINICAL STAGE IIIA NON-SMALL CELL LUNG CANCER AT ACADEMIC AND NON-ACADEMIC CENTERS. Response by Pamela P. Samson, M.D., St. Louis, Missouri.
DR. SAMSON: Yes. In a previous study we did with the National Cancer Data Base that is currently under review, we compared the 30-day surgical outcomes and overall mortality of Stage IIIA lung cancer patients at academic and nonacademic centers.
We found that Stage IIIA academic center patients were more likely to receive neoadjuvant chemotherapy than non-academic center patients. Furthermore, receiving surgery at an academic center was independently associated with an decreased 30-day mortality and improved overall survival. At this time, we don’t know which factors specifically lead to improved outcomes at academic centers, but hypothesize that it may be due to neoadjuvant therapy, and higher-patient volumes. Also, if more patients at academic centers are receiving neoadjuvant therapy, this may be selecting out relatively healthier patients that are still fit for surgery after undergoing chemoradiation therapy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Pamela Samson, MD has grant support through NIH Surgical Oncology T32CA009621-25. Varun Puri, MD, MSCI has grant funding through NIH K07CA178120 and K12CA167540-02.
References
- 1.Yabroff KR, Lamont EB, Mariotto A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008;100:630–641. doi: 10.1093/jnci/djn103. [DOI] [PubMed] [Google Scholar]
- 2.Bradley CJ, Yabroff KR, Dahman B, et al. Productivity costs of cancer mortality in the United States: 2000–2020. J Natl Cancer Inst. 2008;100:1763–1770. doi: 10.1093/jnci/djn384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keehan S, Sisko A, Truffer C, et al. Health spending projections through 2017: the baby-boom generation is coming to Medicare. Health Aff (Millwood) 2008;27:w145–155. doi: 10.1377/hlthaff.27.2.w145. [DOI] [PubMed] [Google Scholar]
- 4.Morgensztern D, Waqar S, Subramanian J, et al. Prognostic significance of tumor size in patients with stage III non-small-cell lung cancer: a surveillance, epidemiology, and end results (SEER) survey from 1998 to 2003. J Thorac Oncol. 2012;7:1479–1484. doi: 10.1097/JTO.0b013e318267d032. [DOI] [PubMed] [Google Scholar]
- 5.Groome PA, Bolejack V, Crowley JJ, et al. The IASLC Lung Cancer Staging Project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:694–705. doi: 10.1097/JTO.0b013e31812d05d5. [DOI] [PubMed] [Google Scholar]
- 6.Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009;374:379–386. doi: 10.1016/S0140-6736(09)60737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Meerbeeck JP, Kramer GW, Van Schil PE, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst. 2007;99:442–450. doi: 10.1093/jnci/djk093. [DOI] [PubMed] [Google Scholar]
- 8.Bauman JE, Mulligan MS, Martins RG, et al. Salvage lung resection after definitive radiation (>59 Gy) for non-small cell lung cancer: surgical and oncologic outcomes. Ann Thorac Surg. 2008;86:1632–1638. doi: 10.1016/j.athoracsur.2008.07.042. discussion 1638–1639. [DOI] [PubMed] [Google Scholar]
- 9.Friedel G, Budach W, Dippon J, et al. Phase II trial of a trimodality regimen for stage III non-small-cell lung cancer using chemotherapy as induction treatment with concurrent hyperfractionated chemoradiation with carboplatin and paclitaxel followed by subsequent resection: a single-center study. J Clin Oncol. 2010;28:942–948. doi: 10.1200/JCO.2008.21.7810. [DOI] [PubMed] [Google Scholar]
- 10.D’Angelillo RM, Trodella L, Ciresa M, et al. Multimodality treatment of stage III non-small cell lung cancer: analysis of a phase II trial using preoperative cisplatin and gemcitabine with concurrent radiotherapy. J Thorac Oncol. 2009;4:1517–1523. doi: 10.1097/JTO.0b013e3181b9e860. [DOI] [PubMed] [Google Scholar]
- 11.Vora SA, Daly BD, Blaszkowsky L, et al. High dose radiation therapy and chemotherapy as induction treatment for stage III nonsmall cell lung carcinoma. Cancer. 2000;89:1946–1952. doi: 10.1002/1097-0142(20001101)89:9<1946::aid-cncr10>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 12.Patel A, Crabtree T, Bell J, Guthrie T, et al. National Patterns of Care and Outcomes After Combined Modality Therapy for Stage IIIA Non-Small-Cell Lung Cancer. J Thorac Oncol. 2014;9:612–621. doi: 10.1097/JTO.0000000000000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanni TB, Jr, Grills IS, Kestin LL, Robertson JM. Stereotactic Radiotherapy Reduces Treatment Cost While Improving Overall Survival and Local Control Over Standard Fractionated Radiation Therapy for Medically Inoperable Non-Small-Cell Lung Cancer. Am J Clin Oncol. 2010 doi: 10.1097/COC.0b013e3181ec63ae. [DOI] [PubMed] [Google Scholar]
- 14.Puri V, Crabtree TD, Kymes S, et al. A comparison of surgical intervention and stereotactic body radiation therapy for stage I lung cancer in high-risk patients: a decision analysis. J Thorac Cardiovasc Surg. 2012;143:428–436. doi: 10.1016/j.jtcvs.2011.10.078. [DOI] [PubMed] [Google Scholar]
- 15.Shah A, Hahn SM, Stetson RL, et al. Cost-effectiveness of stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer. Cancer. 2013;119:3123–3132. doi: 10.1002/cncr.28131. [DOI] [PubMed] [Google Scholar]
- 16.Chan J, Rosenfeldt F, Chaudhuri K, Marasco S. Cardiac surgery in patients with a history of malignancy: increased complication rate but similar mortality. Heart Lung Circ. 2012;21:255–259. doi: 10.1016/j.hlc.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Meyers BF, Haddad F, Siegel BA, et al. Cost-effectiveness of routine mediastinoscopy in computed tomography- and positron emission tomography-screened patients with stage I lung cancer. J Thorac Cardiovasc Surg. 2006;131:822–829. doi: 10.1016/j.jtcvs.2005.10.045. discussion 822–829. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson MK. Optimal management when unsuspected N2 nodal disease is identified during thoracotomy for lung cancer: cost-effectiveness analysis. J Thorac Cardiovasc Surg. 2003;126:1935–1942. doi: 10.1016/j.jtcvs.2003.07.031. [DOI] [PubMed] [Google Scholar]
- 19.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. 1. New York: Oxford University Press; 1996. [Google Scholar]
- 20.Stokes ME, Muehlenbein CE, Marciniak MD, et al. Neutropenia-Related Costs in Patients Treated with First-Line Chemotherapy for Advanced Non-Small Cell Lung Cancer. J Manag Care Pharm. 2009;15(8):669–82. doi: 10.18553/jmcp.2009.15.8.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramsey S, Patrick D, Albert R, Larson E, et al. The Cost-Effectiveness of Lung Transplantation. Chest. 1995;108(6):1594–1601. doi: 10.1378/chest.108.6.1594. [DOI] [PubMed] [Google Scholar]
- 22.Ramsey SD, Shroyer AL, Sullivan SD, Wood DE. Updated evaluation of the cost-effectiveness of lung volume reduction surgery. Chest. 2007;131(3):823–32. doi: 10.1378/chest.06-1790. [DOI] [PubMed] [Google Scholar]
- 23.Cohen DJ, Osnabrugge RL, Magnuson EA, Wang K, et al. Cost-effectiveness of pecutaneous coronary intervention with drug-eluting stents versus bypass surgery for patients with 3-vessel or left main coronary arter disease: final results from the Synergy Between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery (SYNTAX) trial. Cicrulation. 2014;130(14):1146–57. doi: 10.1161/CIRCULATIONAHA.114.009985. [DOI] [PubMed] [Google Scholar]
- 24.Caglar HB, Baldini EH, Othus M, et al. Outcomes of Patients with Stage III Nonsmall Cell Lung Cancer Treated With Chemotherapy and Radiation With and Without Surgery. Cancer. 2009:4156–4166. doi: 10.1002/cncr.24492. [DOI] [PubMed] [Google Scholar]
- 25.Uy KL, Darling G, Xu W, et al. Improved results of induction chemoradiation before surgical intervention for selected patients with stage IIIA-N2 non-small cell lung cancer. JTCVS. 2007;134:188–93. doi: 10.1016/j.jtcvs.2007.01.078. [DOI] [PubMed] [Google Scholar]
- 26.Stephens RJ, Girling DJ, Hopwood P, Thatcher N. A randomised controlled trial of preoperative chemotherapy followed, if feasible, by resection versus radiotherapy in patients with inoperable stage T3, N1, M0 or T1-3, N2, M0 non-small cell lung cancer. Lung Cancer. 2005;49:395–400. doi: 10.1016/j.lungcan.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Johnstone DW, Byhardt RW, Ettinger D, Scott CB. Phase III Study Comparing Chemotherapy and Radiotherapy with Preoperative Chemotherapy and Surgical Resection in Patients with Non-Small Cell Lung Cancer with Spread to the Mediastinal Lymph Nodes (N2); Final Report of RTOG 89-01. Int J Radiation Oncology Biol Phys. 2002;54(2):365–369. doi: 10.1016/s0360-3016(02)02943-7. [DOI] [PubMed] [Google Scholar]
- 28.Shapiro M, Swanson SJ, Wright CD, Chin C, et al. Predictors of major morbidity and mortality after pneumonectomy utilizing the Society for Thoracic Surgeons General Thoracic Surgery Database. Ann Thorac Surg. 2010;90(3):927–35. doi: 10.1016/j.athoracsur.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 29.Thomas P, Berbis J, Baste J, Le Pimpec-Barthes F, et al. Pneumonectomy for lung cancer: Contemporary national early morbidity and mortality outcomes. JTCVS. 2015;149(1):73–83. doi: 10.1016/j.jtcvs.2014.09.063. [DOI] [PubMed] [Google Scholar]
- 30.NCCN Clinical Practice Guidelines in Oncology. Non-Small Cell Lung Cancer, Version 1.2014. National Comprehensive Cancer Network, Inc; 10/11/2013. [Google Scholar]
- 31.Evans WK, Will BP, Berthelot JM, Earle CC. Cost of Combined Modality Interventions for Stage III Non-Small-Cell Lung Cancer. J Clin Onc. 1997;15(9):3038–3048. doi: 10.1200/JCO.1997.15.9.3038. [DOI] [PubMed] [Google Scholar]
- 32.Park BJ, Flores RM. Cost comparison of robotic, video-assisted thoracic surgery and thoracotomy approaches to pulmonary lobectomy. Thorac Surg Clin. 2008;18(3):297–300. doi: 10.1016/j.thorsurg.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Swanson SJ, Miller DL, McKenna RJ, et al. Comparing robot-assisted thoracic surgical lobectomy with conventional video-assisted thoracic surgical lobectomy and wedge resection: Results from a multihospital database (Premier) JTCVS. doi: 10.1016/j.jtcvs.2013.09.046. Epub ahead of print. http://dx.doi.org/10.1016/j.jtcvs.2013.09.046. [DOI] [PubMed]
- 34.Shirvani SM, Jiang J, Gomez DR, et al. Intensity modulated radiotherapy for stage III non-small cell lung cancer in the United States: Predictors of use and association with toxicities. Lung Cancer. 2013;82:252–259. doi: 10.1016/j.lungcan.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mariotto AB, Yabroff KR, Shao YW, et al. Projections of the Cost of Cancer Care in the United States: 2010–2020. JNCI. 2011;103(2):117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samson P, Patel A, Robinson C, Morgensztern D, et al. The Role of Surgery in Clinical Stage IIIA Non-Small Cell Lung Cancer (NSCLC): A Decision and Cost-Effectiveness Analysis. Poster session presented at: American College of Surgeons Annual Clinical Congress; 2014 Oct 26–30; San Francisco, CA. [Google Scholar]
- 37.Brunelli A, Pompili C, Koller M. Changes in Quality of Life After Pulmonary Resection. Thorac Surg Cllin. 2012;22:471–485. doi: 10.1016/j.thorsurg.2012.07.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


