Abstract
Bacterial ribosomes stall on polyproline stretches and require the elongation factor P (EF-P) to relieve the arrest. Yet it remains unclear why evolution has favored the development of EF-P, rather than selecting against the occurrence of polyproline stretches in proteins. We have discovered that only a single polyproline stretch is invariant across all domains of life, namely, a proline triplet in ValS, the tRNA synthetase that charges tRNAVal with valine. Here we show that expression of ValS in vivo and in vitro requires EF-P and demonstrate that the proline triplet located in the active site of ValS is important for efficient charging of tRNAVal with valine, preventing formation of mischarged Thr-tRNAVal, as well as for efficient growth of E. coli in vivo. We suggest that the critical role of the proline triplet for ValS activity may explain why bacterial cells co-evolved the EF-P rescue system.
Introduction
Polymerization of amino acids by ribosomes to form polypeptide chains is a fundamental process in all cells. Ribosomes can polymerize most polypeptide chains without difficulty, however distinct amino acid combinations pose serious problems. For example, three or more consecutive proline residues induce translational arrest by preventing peptide-bond formation (Doerfel et al., 2013; Ude et al., 2013; Woolstenhulme et al., 2013). Ribosome stalling results from the slow rate of peptide-bond formation between the peptidyl-Pro-Pro-tRNA located in the P-site and the Pro-tRNA in the A-site (Doerfel et al., 2013). Ribosome stalling in the absence of EF-P has also been observed at diprolyl motifs (XPPY) with the efficiency of stalling dependent on the nature of the amino acid located before (X) and after (Y) the diprolyl motif (PP) (Hersch et al., 2013; Peil et al., 2013; Woolstenhulme et al., 2013). The translational arrest at PPP and XPPY motifs was in all cases relieved by the presence of the translation elongation factor EF-P (Peil et al., 2013), which binds to the stalled ribosomes and stimulates peptide bond formation (Doerfel et al., 2013; Ude et al., 2013). A conserved lysine residue of Escherichia coli EF-P is subject to post-translational modification by YjeA, YjeK and YfcM (EpmA, EpmB and EpmC) (Navarre et al., 2010; Peil et al., 2012; Yanagisawa et al., 2010) and this lysinylation modification is required for the rescue activity of EF-P (Doerfel et al., 2013; Peil et al., 2013; Ude et al., 2013). The equivalent lysine of the orthologue of EF-P in archaea and eukaryotes, IF-5A (a/eIF-5A) is also post-translationally modified, but via hypusinylation rather than lysinylation (Park et al., 2010). In yeast, the post-translational modification of eIF-5A is also critical for rescue of ribosomes stalled on polyproline stretches (Gutierrez et al., 2013). Moreover, the associated archaeal and eukaryotic enzymes, deoxyhypusine synthase (DHS) and deoxyhypusine hydroxylase (DOHH), are evolutionarily unrelated to their bacterial counterparts (Park et al., 2010). Thus, nature has evolved not only specialized translation factors to overcome stalling at polyproline stretches, but also evolved independent sets of modification enzymes to activate these factors. This in itself implies that the benefits of retaining polyproline stretches significantly outweigh the cost of implementing and maintaining the EF-P and a/eIF5A rescue systems.
Results
Conservation of polyproline-stretches
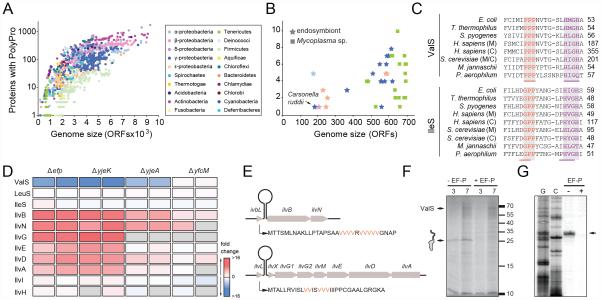
In order to understand which proteins have in fact retained a polyproline stretch (three or more consecutive prolines) throughout evolution, we initially examined the number and conservation of polyproline-containing proteins across 1273 completely sequenced bacterial genomes. As seen in Figure 1A, the number of polyproline-containing proteins varies across the different bacterial phyla, and is generally higher in bacteria with larger genomes, for example, the delta-proteobacterium Sorangium cellulosum, which has the largest sequenced genome to date (9367 protein coding genes (CDS)) (Schneiker et al., 2007), has the most polyproline-containing proteins, i.e. 2406 occurrences in 1779 of the 9367 proteins (19%). By comparison, a typical K12 E. coli strain has ~100 polyproline-containing proteins from a total of ~4100 CDS (2%). Curiously, we identified 19 bacterial genomes ranging in size from 182-2013 CDS encoding only a single polyproline-containing protein (0.05-0.5%) (Fig. 1A, B). These include both free-living bacteria of the Mycoplasma genus, as well as many obligate endosymbiotic bacteria, such as Carsonella ruddii, which co-habit with sap-feeding insects, such as aphids, psyllids and circadas (Fig. 1B). In all cases, the single polyproline-containing CDS was identified as ValS (E. coli nomenclature), the Val-tRNA synthetase that aminoacylates tRNAVal with the amino acid valine. The valS gene, encoding ValS, is an essential gene (Gerdes et al., 2003) and is correspondingly present in all domains of life. The presence and location of the proline triplet in ValS is not only invariant in bacteria, but also in the 205 archaeal and 98 eukaryotic genomes that we analyzed (Fig. 1C). In fact, the proline triplet has a higher conservation than the “HIGH” motif that defines ValS as a class I tRNA synthetase (Arnez and Moras, 1997). No conserved polyproline stretches longer than a triplet were identified. The protein with the next highest conservation of a polyproline stretch was the translational GTPase LepA, which contains a proline triplet in ~65% of the 1273 sequenced genomes and, unlike valS, the lepA gene is not essential for viability (Baba et al., 2006). Given that XPPY motifs other than PPP also cause stalling (albeit at lower levels than PPP), which is relieved by EF-P (Peil et al., 2013), we also analyzed the bacterial genomes for conservation of XPPY motifs. We detected only one diprolyl motif as being correspondingly conserved in all domains of life, namely, the GPP motif found within IleS, the Ile-tRNA synthetase that aminoacylates tRNAIle with the amino acid isoleucine. The presence and location of the GPP motif in IleS is homologous to the PPP motif in ValS, also being located adjacent to the “HIGH” motif (Fig. 1C). We have previously demonstrated that ribosomes stall only weakly at GPP motifs in the absence of EF-P, whereas much stronger stalling is observed at PPP motifs (Peil et al., 2013).
Fig. 1. A conserved proline triplet in ValS confers EF-P dependence for expression.
(A) Scatter-plot illustrating the total number of polyproline-containing proteins relative to genome size [in terms of open reading frame (ORF) number] of species within different bacterial phyla. (B) Zoom of (A) highlighting bacteria with the smallest genomes. (C) Sequence alignment illustrating the conservation of PPP and GPP in Val- and Ile-tRNA synthetases, respectively, across the three domains of life, compared with the highly conserved “HIGH” motif. (D) Heat map representation of changes in expression levels in vivo (blue, down-regulated; red, up-regulated; white, unchanged; grey, not identified or quantified) of selected proteins when comparing Δefp, ΔyjeA, ΔyjeK and ΔyfcM strains against a wildtype E. coli internal standard. Data from two biological replicates taken from (Peil et al., 2013). (E) Schematic illustration of the gene arrangement of the ilvL-BN and ilvL-GMEDA operons, with the corresponding sequence of the leader peptides IvbL and IlvL. Valine residues essential for attenuation are shown in red. (F) Autoradiograph of SDS-PAGE monitoring the in vitro translation at 3 min and 7 min of ValS, in the absence (-EF-P) and presence of EF-P (+EF-P), with full-length (FL) ValS and peptidyl-tRNA (25 kDa) as indicated. (G) Toe-printing of ValS in the absence (-) and presence (+) of active EF-P. G and C sequence lanes are included.
ValS expression is dependent on modified EF-P
To show that active EF-P is in fact required for ValS expression, we analyzed our previous stable isotope labeling and mass spectrometry data that monitored the changes in expression levels of proteins in E. coli strains lacking the efp, yjeK, yjeA and yfcM genes relative to the parental wildtype E. coli strain (Peil et al., 2013). As predicted, ValS was heavily down-regulated when EF-P was absent (Δefp), or EF-P was inactive because of lack of lysinylation (ΔyjeK/ΔyjeA) (Fig. 1D). The lack of YfcM (ΔyfcM), which hydroxylates the lysinylated EF-P (Peil et al., 2012), did not lead to down-regulation of ValS, consistent with previous reports that lysinylated but not hydroxylated EF-P still retains the ability to stimulate peptide-bond formation and thus relieves stalling at polyproline stretches (Doerfel et al., 2013; Peil et al., 2013; Ude et al., 2013). The down-regulation of ValS in the absence of active EF-P is specific, since down-regulation of other tRNA synthetases, such as LeuS or IleS, is not observed (Fig. 1D). The lack of dependence of IleS expression on EF-P is consistent with our previous findings that the GPP motif only induces weak translational stalling compared to PPP (2-fold versus 20-fold, respectively) (Peil et al., 2013).
Notably, we also observed up-regulation of the proteins encoded in the ilvBN and ilvGEDA operons involved in the biogenesis of isoleucine, leucine and valine. This can be explained by transcriptional attenuation of the ilvBN and ilvGEDA operons (Yanofsky, 1987): When amino acids such as valine are limiting, the reduction in Val-tRNAVal causes ribosomes to stall during translation of the upstream valine-rich leader peptides IvbL and IlvL. This in turn prevents formation of a downstream transcription termination structure, leading to induction of expression of the downstream genes in the operon (Yanofsky, 1987) (Fig. 1E). Similarly, a reduction in charged Val-tRNAVal due to the down-regulation of ValS would therefore explain the up-regulation of ilvBN and ilvGEDA operons in the absence of active EF-P (Fig. 1D). In contrast, the absence of active EF-P had little effect on the expression of the ilvI and ilvH, which are not attenuated, indicating that the up-regulation of the ilvBN and ilvGEDA operons is specific. Although the efp gene is not essential in E. coli (Baba et al., 2006; Peil et al., 2013; Peil et al., 2012), the loss of EF-P leads to a reduction in growth rate (Yanagisawa et al., 2010), presumably due to the reduction in levels of important cellular polyproline-containing proteins. Since we have demonstrated that the lack of EF-P leads to a strong down-regulation of ValS (Fig. 1D), we tested and could show that expression of ValS could partially rescue the growth defect due to lack of the efp gene in E. coli (Figure S1).
We also demonstrated that ValS expression is dependent on EF-P in vitro (Fig. 1F): In the absence of EF-P, translation of ValS in vitro leads to accumulation of ~25 kDa band, which correlates with that of the ValS nascent polypeptide chain translated up to the proline triplet (~5 kDa), but still remaining attached to a tRNA (~20 kDa tRNA). As expected, the band for the stalled peptidyl-tRNA is absent when EF-P is included in the in vitro translation reaction (Fig. 1F). Toeprinting confirmed that when EF-P is absent the ribosome stalls at the proline triplet of ValS, specifically with the second proline codon of the proline triplet located in the P-site (Fig. 1G). Collectively, these findings demonstrate that expression of ValS is strictly dependent on the presence of active EF-P in vivo and in vitro.
The proline triplet is located at the active site of ValS
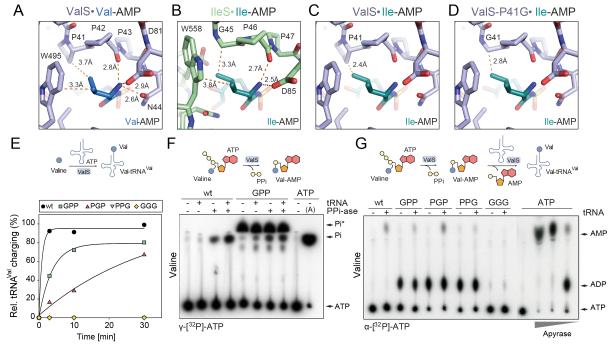
If the EF-P rescue system really evolved to allow the proline triplet in ValS to be retained, this would imply that the proline triplet in ValS is critically important for function, as indicated by its universal conservation. Crystal structures of T. thermophilus ValS reveal that the proline triplet (P41-P43, E. coli numbering) resides in the active site of ValS, namely where valine is activated by ATP to form Val-AMP and then transferred to tRNAVal to form Val-tRNAVal (Fig. 2A) (Fukai et al., 2000; Fukai et al., 2003). The side chain of Pro41 of ValS contacts the γ1-CH3 group of valyl-AMP, whereas Pro42 and Pro43 appear to also contribute to the stable binding of valyl-AMP by positioning the α-CO group of Pro42 to hydrogen bond with the α-NH3 group of valyl-AMP (Fig. 2A) (Fukai et al., 2000; Fukai et al., 2003). In IleS, the presence of Gly45, rather than Pro, allows accommodation of isoleucyl-AMP (Fig. 2B) (Nureki et al., 1998; Silvian et al., 1999), consistent with the high conservation of the GPP motif in Ile-tRNA synthetases (Fig. 1C). In contrast, accommodation of isoleucyl-AMP in ValS is less favorable due to the close distance between Pro41 and the γ1-CH3 group of isoleucyl-AMP (Fig. 2C), which led to the proposed role of Pro41 of ValS in discrimination between valine and isoleucine (Fukai et al., 2000). In a simplified model, one might predict that mutation of P41G in ValS should increase the level of misincorporation of isoleucine (Fig. 2D), however, this to our knowledge has not been tested.
Fig. 2. PPP at the active site of ValS is required for efficient tRNA charging.
(A) Location of PPP in the active site of ValS relative to Val-AMP (PDB1GAX) (Fukai et al., 2000). (B) Active site of IleS with Ile-AMP (1JZQ) (Nakama et al., 2001). (C) ValS from (A) but with superimposed position of Ile-AMP from (B). (D) as in (C) but with in silico Pro41Gly mutation. (E) Charging efficiency of tRNAVal with valine by wildtype (wt) ValS and ValS mutants as a function of time (min). (F,G) Autoradiograph of TLC separation of (F) γ[32P]-Pi from γ[32P]-ATP, and (G) α[32P]-AMP and α[32P]-ADP from α[32P]-ATP, when wildtype ValS (wt), or ValS mutant(s) were incubated with valine, in the absence (-) and presence (+) of pyrophosphatase (PPi-ase) and/or deacylated tRNAVal. ATP at the origin indicates where the samples were loaded onto the TLC plate. In (F), the migration position of Pi is determined by treatment of γ[32P]-ATP with Apyrase (A), and in (G), the migration of AMP and ADP was determined by treatment of α[32P]-ATP with increasing concentrations of Apyrase.
Critical importance of the proline triplet for ValS charging activity
To experimentally validate the role of Pro41 as well as the general functional importance of the proline triplet for ValS activity, we generated the three single ValS Pro-to-Gly mutants (ValS-GPP, -PGP and -PPG) as well as a triple PPP-to-GGG mutant (ValS-GGG). The ability of the ValS mutants to charge tRNAVal with [14C]valine was assessed and compared to wildtype ValS (Fig. 2E). Under our reaction conditions at 37°C, maximal charging of tRNAVal with [14C]valine by wildtype ValS occurred within the first minute (Fig. 2E). In contrast, all ValS mutants were less efficient than wildtype; the ValS-PPG and -GGG mutants were completely devoid of activity, whereas the ValS-PGP and -GPP mutants retained some activity but at lower levels than the wildtype (Fig. 2E).
Charging of tRNAVal by ValS occurs in two steps: Firstly, valine is activated with ATP to form Val-AMP leading to the release of pyrophosphate (Fig. 2F), and secondly, the valine is then transferred to the tRNAVal to form Val-tRNAVal, with a concomitant release of AMP (Fig. 2G). Initially, we employed thin-layer chromatography (TLC) and γ[32P]-ATP to monitor the release of pyrophosphate (γ[32P]-PPi) by wildtype ValS, or the mutant ValS-GPP, in the presence of valine, and in the presence or absence of tRNAVal (Fig. 2F). Pyrophosphate (PPi) release is monitored indirectly by treatment with pyrophosphatase that converts the PPi to inorganic monophosphate (Pi), which is then visualized directly by TLC. As seen in Figure 2F, Pi formation is observed only for wildtype ValS in the presence of the pyrophosphatase and, as expected, is stimulated by the presence of tRNAVal. The migration position of Pi on the TLC was determined using a control reaction of γ[32P]-ATP treated with Apyrase, an ATP diphosphatase that hydrolyzes ATP sequentially to yield AMP and Pi. Surprisingly, the ValS-GPP mutant was observed to produce large quantities of γ[32P]-containing material that migrated at a position similar but distinct to Pi (termed Pi*). Moreover, formation of Pi* by the ValS-GPP mutant was independent of pyrophosphatase treatment or the presence of tRNAVal (Fig. 2F). Pi* was also produced by the ValS-PGP and ValS-PPG mutants in a pyrophosphatase- and tRNAVal-independent fashion, whereas the ValS-GGG mutant was virtually inactive (Figure S2A). Based the retention factor (Rf) of Pi* (Rf 0.69), we can conclude that Pi* is not AMP (Rf 0.83), ADP (Rf 0.17), ATP (Rf 0) or monophosphate Pi (Rf 0.56). Furthermore, Pi* cannot be PPi since PPi is not observable on our TLCs and addition of pyrophosphatase does not influence the migration of Pi* (Fig. 2F). PPi generated by tRNA synthetases has been reported to attack ATP and ADP to generate diadenosine polyphosphates, such as Ap3A and Ap4A via phosphorolysis (Plateau and Blanquet, 1976), however, we can exclude that Pi* is Ap3A or Ap4A since these compounds are resistant to phosphatase treatment whereas Pi* is not (Figure S2B). Further experiments will be required to determine the exact nature of Pi*.
Since the ValS mutants produce Pi*, rather than PPi, we reasoned that the ValS mutants may also catalyze a reaction other than canonical ATP to AMP, as catalyzed by a ValS-tRNA synthetase. To address this, charging assays were performed as before but using α[32P]-ATP instead of γ[32P]-ATP. Each reaction contained the amino acid valine, together with either wildtype ValS, or one of the ValS mutants, and was performed in the absence or presence of tRNAVal (Fig. 2G). As expected, wildtype ValS produced AMP only in the presence of tRNAVal. AMP formation was also observed for the ValS-GPP, PGP and to a lesser extent the PPG mutant, consistent with some degree of charging activity observed in Fig 2E, whereas ValS-GGG was virtually inactive. Strikingly, unlike wildtype ValS, the ValS-GPP, -PGP and -PPG mutants converted large amounts of α[32P]-ATP to α[32P]-ADP (Fig. 2G). The migration position on the TLC of α[32P]-ADP (and α[32P]-AMP) was determined using a control reaction where α[32P]-ATP was treated with increasing concentrations of Apyrase that hydrolyzes sequentially ATP to ADP and then AMP and Pi. Furthermore, the generation of α[32P]-ADP by the ValS-GPP, -PGP and -PPG mutants was independent of the presence of tRNAVal and also occurred when no amino acid was present (Figure S2C). Moreover, the α[32P]-ADP generated by the ValS-GPP, -PGP and -PPG mutants could be converted to α[32P]-AMP by Apyrase (Figure S2D). In summary, these assays suggest that mutations within the conserved proline triplet of ValS not only reduce the efficiency of tRNA charging, but also cause the ValS mutants to non-productively hydrolyze ATP to ADP.
The proline triplet is important for the editing activity of ValS
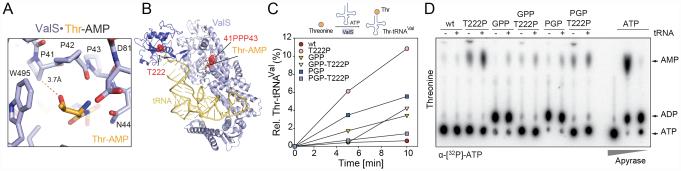
To test the hypothesis that mutation of Pro41 in ValS distinguishes between valine and isoleucine (Fig. 2D), we performed mischarging assays where [14C]isoleucine replaced [14C]valine. No mischarging of tRNAVal with [14C]isoleucine by wildtype ValS, or any of the ValS mutants was observed (data not shown). Furthermore, introduction of T222P mutation that disables the ValS editing function did not promote Ile-tRNAVal formation (data not shown), consistent with Ile not being a substrate for editing by ValS (Tardif et al., 2001). Similarly, isoleucine did not stimulate AMP formation by ValS-GPP (Figure S2E), collectively suggesting that Pro41 is not the only residue in ValS involved in discrimination of valine from isoleucine. On the other hand, threonine, which is isosteric with valine, is readily activated by ValS and transferred to tRNAVal, however, the mischarged Thr-tRNAVal that forms is rapidly deacylated by the editing domain of ValS (Fersht and Kaethner, 1976) (Fig. 3A,B). Indeed, in mischarging assays where [14C]threonine replaced [14C]valine, we observed a low rate of Thr-tRNAVal formation by wildtype ValS, which was enhanced when the editing domain was disabled by the T222P mutation (Doring et al., 2001) (Fig. 3B,C). Formation of Thr-tRNAVal was also enhanced by the ValS-GPP and -PGP mutations. The introduction of the T222P mutation in ValS-GPP did not further enhance mischarging, indicating that the GPP mutation may disable the editing function of ValS (Fig. 3C). Surprisingly, mischarging was suppressed when the T222P was introduced into the ValS-PGP mutant (Fig 3C), suggesting an intricate communication exists between the activation and editing domains of ValS. This interplay was also observed in the TLC analyses measuring the misactivation of threonine, namely, in that the non-productive ADP formation by the ValS-GPP and -PGP mutants was suppressed in the presence of the additional T222P mutation (Fig. 3D). Collectively, the in vitro assays indicate that the conserved proline triplet of ValS is not only important for efficient tRNA charging, but also for communication with the editing domain to ensure efficient deacylation of mischarged Thr-tRNAVal.
Fig. 3. PPP at the active site of ValS is required to prevent Thr-tRNAVal formation.
(A) Scheme for misactivation and mischarging of tRNAVal with threonine and subsequent deacylation by ValS. (B) Overview of ValS bound with tRNA (yellow) and Thr-AMP (orange) (PDB1GAX) (Fukai et al., 2000). PPP and T222 are indicated by red spheres. Inset shows the active site of ValS with PPP relative to Thr-AMP. (C) Mischarging of tRNAVal with threonine by wildtype (wt) ValS and ValS mutants as a function of time (min). (D) Autoradiograph of TLC separation of α[32P]-AMP and α[32P]-ADP from α[32P]-ATP, when wildtype ValS (wt), or ValS mutant(s) were incubated with threonine, in the absence (-) and presence (+) of deacylated tRNAVal. ATP at the origin indicates where the samples were loaded onto the TLC plate and apyrase treatment of ATP provides markers for ADP and AMP positions.
The proline triplet of ValS is important for viability of E. coli
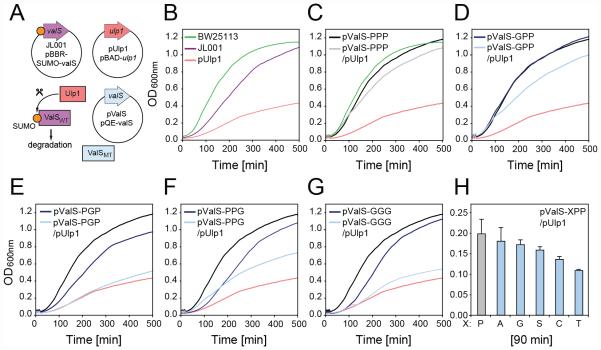
Given the defects of the ValS mutants in vitro, we assessed their functionality in vivo using a genetic complementation system (Fig 4A): The ΔvalS E. coli strain was complemented with a plasmid bearing ValS containing an N-terminal 6xHistidine tag and Small Ubiquitin-like Modifier (SUMO) tag (Hay, 2005) followed by a WFCWS linker. The resulting JL001 strain (Table S1) was viable but exhibited a slightly reduced growth rate, presumably because of the lower expression level of the plasmid encoded ValS (Fig. 4B). Next a plasmid expressing Ulp1 (pUlp1) was introduced into JL001. Ulp1 encodes a SUMO-specific protease that cleaves the SUMO tag from ValS exposing the N-terminal WFCWS sequence motif that, in accordance with the N-end degradation rule, leads to recognition and degradation of the ValS protein by the ClpAP protease (Dougan et al., 2010; Wang et al., 2007) (Fig. 4A). Under the conditions used, Western blotting against the 6xHis tag indicated a rapid loss of ValS protein in the cell within minutes of induction of Ulp1 expression (Figure S3). Consistently, induction of Ulp1 expression also led to strong reduction in growth rate of the JL001 strain (Fig. 4B). In the next step, we repeated the growth experiments with the JL001 strain bearing either pUlp1 (or a control plasmid) together with an additional plasmid bearing an IPTG-inducible copy of either the wildtype ValS (pValS-PPP; Fig. 4C) or one of the ValS mutants (ValS-GPP, -PGP, -PPG, or -GGG; Fig. 4D-G). As expected, the presence of an additional copy of the wildtype ValS (pValS-PPP) could rescue the growth of JL001 following the degradation of the SUMO-ValS that occurs upon induction of Ulp1 expression (Fig. 4C). Similarly, pValS-GPP was also able to rescue growth upon Ulp1 expression (Fig. 4D), albeit not as efficiently as pValS-PPP. By contrast, the pValS-PGP, -PPG and -GGG displayed little or no ability to rescue growth in the absence of wildtype ValS (Fig. 4E-G). Unfortunately, attempts to transform plasmid libraries of pValS-XXX mutants to select for viable mutations of the proline triplet failed, however, given the ability of the ValS-GPP mutant to rescue, we therefore generated all possible pValS-XPP mutants and monitored their ability to rescue growth in the absence of wildtype ValS (Fig. 4H). Of the 20 pValS-XPP mutants tested, six mutants (PPP, GPP, APP, SPP, CPP and TPP) rescue growth in the absence of wildtype ValS, with only APP and SPP being able to sustain growth at levels comparable to PPP and GPP (Fig. 4H).
Fig. 4. The proline triplet of ValS is required for viability of E. coli.
(A) Scheme for ValS complementation system. (B-G) Growth curves (OD600nm) of E. coli strains (B) BW25113, JL001, JL001 + pUlp1, or (C-G) JL001 bearing additional plasmids expressing pUlp1, and/or (C) wildtype ValS (pValS-PPP) or ValS mutants (D) pValS-GPP, (E) pValS-PGP, (F) pValS-PPG or (G) pValS-GGG. (H) Comparison of growth (after 90 min) of JL001 bearing plasmids expressing pUlp1 and pValS/XPP mutants normalized by subtraction of background growth in the presence of pUlp1 alone.
Discussion
We identified only a single polyproline stretch that is invariant across all bacterial genomes, namely a proline triplet present in ValS, the Val-tRNA synthetase (Fig. 1B,C) and demonstrated that efficient expression of ValS in vivo and in vitro requires the presence of EF-P. Since the PPP triplet in ValS is invariant in bacteria, archaea and eukaryotes (Fig. 1C), this leads us to propose that EF-P and a/eIF-5A may have initially (co-)evolved to facilitate primarily the expression of ValS. We cannot however exclude that other polyproline-containing proteins contributed to the evolution of EF-P or that EF-P and a/eIF-5A evolved to facilitate expression of another protein that contained a polyproline-stretch at the time, but which was subsequently lost from some or all of the currently available bacterial genomes.
Regardless of whether EF-P and a/eIF-5A evolved to facilitate expression of ValS or not, our study nevertheless identified a highly conserved PPP motif located in the active site of ValS (Fig. 2A) that is critical for the tRNA charging activity (Fig. 2E) and productive ATPase activity (Fig. 2F,G) of ValS. Although comparisons of ValS and IleS led to the suggestion that Pro41 of ValS is important for the discrimination of valine from isoleucine (Fukai et al., 2000), we find no evidence to support this. Instead, we find that mutations within the PPP motif of ValS enhance formation of mischarged Thr-tRNAVal (Fig. 3C). Moreover, we could also demonstrate that the T222P mutation within the editing site of ValS could suppress the non-productive formation of ADP by the ValS mutants (Fig. 4D) and in the case of ValS-PGP was even able to rescue the editing activity (Fig. 4C). Collectively, these findings suggest an intimate communication between the aminoacylation and editing sites on ValS that warrants further study.
Consistent with our in vitro assays, the ValS-GPP mutant was able to rescue growth and viability of an E. coli strain lacking wildtype valS gene but not as efficiently as ValS-PPP, whereas ValS-PGP, -PPG and -GGG displayed little or no rescue phenotype. Testing of all possible ValS-XPP mutants demonstrated that it is possible to select for alternative motifs, such as APP or SPP, that support viability of E. coli under optimal conditions, however, it remains to be tested whether such mutants are also impaired in tRNA charging activities as observed for the ValS-GPP mutant.
The critical importance of the proline triplet of ValS for tRNA charging and viability of E. coli would provide an explanation for why nature needed to co-evolve EF-P and a/eIF-5A to facilitate expression during translation of the ValS.
Experimental Procedures
Oligonucleotides, plasmids and bacterial strains
Primers, plasmids and strains used in this study are listed in Table S1.
Bioinformatic analysis
To search for ValRS protein sequences and determine the conservation of the PPP motif, 1273 bacterial, 205 archaeal and 98 eukaryotic genomes were searched with a hidden markov model (HMM) profile (Eddy, 1998) with an E value cut-off of e-70 as described previously . The HMM was generated from a MAFFT (Katoh et al., 2005) alignment of ValRS homologs identified in an initial BlastP search (Altschul et al., 1990). As the HMM also hits the close relative Ile-tRNA synthetase, phylogenetic analysis using FastTree (Price et al., 2010) was carried out to extract sequences from the ValRS clade of orthologs. The resulting sequences were aligned and the PPP motif region was inspected for conservation.
tRNAVal charging assays
Charging reactions and assays to monitor aminoadenylate formation were performed as described (Splan et al., 2008).
Growth conditions
Lysogeny broth (LB) was used as complex medium (Bertani, 1951) and modified with NaCl. When indicated, LB was supplemented with 0.4% (w/v) glucose as carbon source to suppress Ulp1 expression before induction with L-arabinose 0.2% (w/v). 1 mM isopropyl-β-D-1-thiogalactopyranoside (IPTG) (Sigma Aldrich) was used to induce expression of pQE70-ValS (pValS-PPP) and GPP, PGP, PPG and GGG variants. Antibiotics were used when necessary with the following concentrations: 100 μg/ml ampicillin sodium salt, 50 μg/ml kanamycin sulfate, 34 μg/ml chloramphenicol, 20 μg/ml gentamycin sulfate.
ValS silencing
E. coli BW2113 was used to delete valS via pRED®/ET® recombination technology. Due to the fact that valS is an essential gene, gene deletion was performed in a strain containing plasmid pBBR1MCS-5-PT7-SUMO-WFCWS-valS encoding SUMO-ValS (ValS*). The resultant strain JL001 was co-transformed with pBAD33 or pBAD33-ulp1, respectively, and pQE70 encoding either wildtype ValS or ValS-GPP, -PGP, -PPG or -GGG mutants. Cells were plated on LB agar containing chloramphenicol and ampicillin for transformant selection as well as IPTG to induce pQE70-ValS expression and glucose to suppress pBAD activity by catabolite repression. Transformants were precultured overnight in LB + glucose + chloramphenicol (pBAD33) + gentamycin sulfate (pBBR1MCS-5) + ampicillin sodium salt (pQE70) + kanamycin sulfate (ΔvalS::kan). A second preculture was started by diluting the overnight culture 1:100. Cells were grown to OD600nm = 0.3-0.7. Subsequently, cells were washed twice in LB + 0.2% (wt/vol) arabinose and adjusted to an OD600nm = 0.1. The main culture was started by a further dilution step to OD600nm = 0.01 in a microtiter plate in a final volume of 200µl LB + 0.2% arabinose + chloramphenicol. Growth was recorded in a Tecan reader (Infinite M200 Pro) by measuring OD600nm every 10min for 8h. In parallel Ulp1 production and ValS degradation was monitored by Western Blot analysis. Cells of the JL001 strain harboring pBAD33-ulp1 and pBBR1MCS-5-PT7-SUMO-WFCWS-valS were harvested at different times after arabinose induction. As control JL001 cells harboring pBAD33 and pBBR1MCS-5-PT7-SUMO-WFCWS-valS were analyzed.
Supplementary Material
Highlights.
The only conserved polyproline stretch is present in ValS
The proline triplet in ValS is critical for tRNAVal charging and editing activities
Mutations within the proline triplet of ValS reduce growth and viability of E. coli
The invariant proline triplet in ValS may explain the co-evolution of EF-P
ACKNOWLEDGMENTS
We would like to thank Ingrid Weitl for technical assistance and Michael Graf for help with purification of the ValS mutants. This research was supported by grants from the Deutsche Forschungsgemeinschaft (WI3285/2-1 to D.N.W. and Exc114/2 to K.J.), the EMBO young investigator grant (D.N.W.), the Estonian Science Foundation (ETF9020 to G.C.A) and IUT 14021 (JR). L.P. and G.A. received support from the European Social Fund program Mobilitas grant MJD144 and MJD99, respectively. A.L.S. is funded by an AXA Research Fund Postdoctoral fellowship and L.P. by the Marie Curie FP7-PEOPLE-2011-IEF Postdoctoral fellowship.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental figures S1-S2 and Tables S1-S3, and can be found with this article online at http://www.sciencedirect.com/science/article/pii/S2211124714007761
REFERENCES
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Arnez JG, Moras D. Structural and functional considerations of the aminoacylation reaction. Trends Biochem Sci. 1997;22:211–216. doi: 10.1016/s0968-0004(97)01052-9. [DOI] [PubMed] [Google Scholar]
- Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol. 2006;2:2006–0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertani G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. Journal of bacteriology. 1951;62:293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfel LK, Wohlgemuth I, Kothe C, Peske F, Urlaub H, Rodnina MV. EF-P is essential for rapid synthesis of proteins containing consecutive proline residues. Science. 2013;339:85–88. doi: 10.1126/science.1229017. [DOI] [PubMed] [Google Scholar]
- Doring V, Mootz HD, Nangle LA, Hendrickson TL, de Crecy-Lagard V, Schimmel P, Marliere P. Enlarging the amino acid set of Escherichia coli by infiltration of the valine coding pathway. Science. 2001;292:501–504. doi: 10.1126/science.1057718. [DOI] [PubMed] [Google Scholar]
- Dougan DA, Truscott KN, Zeth K. The bacterial N-end rule pathway: expect the unexpected. Molecular microbiology. 2010;76:545–558. doi: 10.1111/j.1365-2958.2010.07120.x. [DOI] [PubMed] [Google Scholar]
- Eddy SR. Profile hidden Markov models. Bioinformatics. 1998;14:755–763. doi: 10.1093/bioinformatics/14.9.755. [DOI] [PubMed] [Google Scholar]
- Fersht AR, Kaethner MM. Enzyme hyperspecificity. Rejection of threonine by the valyl-tRNA synthetase by misacylation and hydrolytic editing. Biochemistry. 1976;15:3342–3346. doi: 10.1021/bi00660a026. [DOI] [PubMed] [Google Scholar]
- Fukai S, Nureki O, Sekine S, Shimada A, Tao J, Vassylyev DG, Yokoyama S. Structural basis for double-sieve discrimination of L-valine from L-isoleucine and L-threonine by the complex of tRNA(Val) and valyl-tRNA synthetase. Cell. 2000;103:793–803. doi: 10.1016/s0092-8674(00)00182-3. [DOI] [PubMed] [Google Scholar]
- Fukai S, Nureki O, Sekine S, Shimada A, Vassylyev DG, Yokoyama S. Mechanism of molecular interactions for tRNA(Val) recognition by valyl-tRNA synthetase. RNA. 2003;9:100–111. doi: 10.1261/rna.2760703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes SY, Scholle MD, Campbell JW, Balazsi G, Ravasz E, Daugherty MD, Somera AL, Kyrpides NC, Anderson I, Gelfand MS, et al. Experimental determination and system level analysis of essential genes in Escherichia coli MG1655. J Bacteriol. 2003;185:5673–5684. doi: 10.1128/JB.185.19.5673-5684.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez E, Shin B, Woolstenhulme C, Kim J, Saini P, Buskirk A, Dever T. eIF5A Promotes Translation of Polyproline Motifs. Mol Cell. 2013;51:1–11. doi: 10.1016/j.molcel.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay RT. SUMO: a history of modification. Molecular cell. 2005;18:1–12. doi: 10.1016/j.molcel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Hersch SJ, Wang M, Zou SB, Moon KM, Foster LJ, Ibba M, Navarre WW. Divergent Protein Motifs Direct Elongation Factor P-Mediated Translational Regulation in Salmonella enterica and Escherichia coli. MBio. 2013:4. doi: 10.1128/mBio.00180-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Kuma K, Toh H, Miyata T. MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 2005;33:511–518. doi: 10.1093/nar/gki198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakama T, Nureki O, Yokoyama S. Structural basis for the recognition of isoleucyl-adenylate and an antibiotic, mupirocin, by isoleucyl-tRNA synthetase. J Biol Chem. 2001;276:47387–47393. doi: 10.1074/jbc.M109089200. [DOI] [PubMed] [Google Scholar]
- Navarre WW, Zou SB, Roy H, Xie JL, Savchenko A, Singer A, Edvokimova E, Prost LR, Kumar R, Ibba M, et al. PoxA, yjeK, and elongation factor P coordinately modulate virulence and drug resistance in Salmonella enterica. Mol Cell. 2010;39:209–221. doi: 10.1016/j.molcel.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nureki O, Vassylyev DG, Tateno M, Shimada A, Nakama T, Fukai S, Konno M, Hendrickson TL, Schimmel P, Yokoyama S. Enzyme structure with two catalytic sites for double-sieve selection of substrate. Science. 1998;280:578–582. doi: 10.1126/science.280.5363.578. [DOI] [PubMed] [Google Scholar]
- Park MH, Nishimura K, Zanelli CF, Valentini SR. Functional significance of eIF5A and its hypusine modification in eukaryotes. Amino Acids. 2010;38:491–500. doi: 10.1007/s00726-009-0408-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peil L, Starosta AL, Lassak J, Atkinson GC, Virumae K, Spitzer M, Tenson T, Jung K, Remme J, Wilson DN. Distinct XPPX sequence motifs induce ribosome stalling, which is rescued by the translation elongation factor EF-P. Proc Natl Acad Sci U S A. 2013;110:15265–15270. doi: 10.1073/pnas.1310642110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peil L, Starosta AL, Virumae K, Atkinson GC, Tenson T, Remme J, Wilson DN. Lys34 of translation elongation factor EF-P is hydroxylated by YfcM. Nat Chem Biol. 2012;8:695–697. doi: 10.1038/nchembio.1001. [DOI] [PubMed] [Google Scholar]
- Plateau P, Blanquet S. Synthesis of NpnN' (n = 3 or 4) in vitro and in vivo. In: McLennan AG, editor. In Ap4A and Other Dinucleoside Polyphosphates. CRC Press; Boca Raton: 1976. pp. 81–107. [Google Scholar]
- Price MN, Dehal PS, Arkin AP. FastTree 2--approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiker S, Perlova O, Kaiser O, Gerth K, Alici A, Altmeyer MO, Bartels D, Bekel T, Beyer S, Bode E, et al. Complete genome sequence of the myxobacterium Sorangium cellulosum. Nat Biotechnol. 2007;25:1281–1289. doi: 10.1038/nbt1354. [DOI] [PubMed] [Google Scholar]
- Silvian LF, Wang J, Steitz TA. Insights into editing from an ile-tRNA synthetase structure with tRNAile and mupirocin. Science. 1999;285:1074–1077. [PubMed] [Google Scholar]
- Splan KE, Musier-Forsyth K, Boniecki MT, Martinis SA. In vitro assays for the determination of aminoacyl-tRNA synthetase editing activity. Methods. 2008;44:119–128. doi: 10.1016/j.ymeth.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif KD, Liu M, Vitseva O, Hou YM, Horowitz J. Misacylation and editing by Escherichia coli valyl-tRNA synthetase: evidence for two tRNA binding sites. Biochemistry. 2001;40:8118–8125. doi: 10.1021/bi0103213. [DOI] [PubMed] [Google Scholar]
- Ude S, Lassak J, Starosta AL, Kraxenberger T, Wilson DN, Jung K. Translation elongation factor EF-P alleviates ribosome stalling at polyproline stretches. Science. 2013;339:82–85. doi: 10.1126/science.1228985. [DOI] [PubMed] [Google Scholar]
- Wang KH, Sauer RT, Baker TA. ClpS modulates but is not essential for bacterial N-end rule degradation. Genes & development. 2007;21:403–408. doi: 10.1101/gad.1511907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolstenhulme CJ, Parajuli S, Healey DW, Valverde DP, Petersen EN, Starosta AL, Guydosh NR, Johnson WE, Wilson DN, Buskirk AR. Nascent peptides that block protein synthesis in bacteria. Proc Natl Acad Sci U S A. 2013;110:E878–887. doi: 10.1073/pnas.1219536110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa T, Sumida T, Ishii R, Takemoto C, Yokoyama S. A paralog of lysyl-tRNA synthetase aminoacylates a conserved lysine residue in translation elongation factor P. Nat Struct Mol Biol. 2010;17:1136–1143. doi: 10.1038/nsmb.1889. [DOI] [PubMed] [Google Scholar]
- Yanofsky C. Operon specific control by transcription attenuation. TIG. 1987;3:356–360. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.