Abstract
Background
Paget's disease of bone (PDB) is associated with a germline mutation in Sequestosome1 /p62 (SQSTM1) found in ≤ 16% of sporadic cases worldwide, and in 19-46% of those studied with familial PDB. The P392L is the most prevalent mutation identified to date. This mutation by itself does not confer PDB or define the phenotype of PDB in a given person. Environmental determinants remain elusive, although increasing age of the individual, other gene polymorphisms in the context of SQSTM1 mutations, and measles virus have been implicated. Measles exposure has been unexamined in this context.
Objectives
The goal of this study is to compare the background history and phenotype of patients with PDB carrying the SQSTM1 P392L mutation to those patients without. Focusing on age, ancestry, P329L mutation, family history, measles exposure, distribution of PDB and age of onset, we examined outcomes at 10 years. We postulated that aging may play a role in defining phenotype, and that this may become more visible in a well characterized cohort.
Methods
This is an observational study focused on a cohort of patients with PDB drawn from the New England Registry in whom environmental and family history has been catalogued, linked to radiographic data. Of the 217 persons who were enrolled in the Registry, 42 (19%) responded to a letter inviting them to participate in testing for the presence of the measles antibody, and in genetic testing for the P392L mutation.
Results
The mean age of the cohort in 2001 was 70 years (range 55-79); 27 were men (64%). The measles antibody was found in all cases tested. Nine patients had the P392L mutation (21%), 2 with familial PDB. In these persons, early diagnosis of disease and spinal stenosis marked the male phenotype only. European ancestry was noted in the minority of those with P392L mutation. Most deaths recorded occurred in the 9th decade of life or later.
Conclusions
Spinal stenosis emerges as a prominent phenotype in SQSTM1 P392L + men with aging. In these 42 patients with PDB from the New England Registry, most do not carry the SQSTM1 P392L mutation, and many do not have European ancestry. Exposure to measles was confirmed in the majority.
Keywords: Paget's disease of bone, SQSTM1, observational study
INTRODUCTION
Paget's disease of bone (PDB) is a focal disorder of bone remodeling that may affect one or more bones in an aging skeleton. It is often thought of as a British disease, characterized most definitively by Sir James Paget, identified in the bones buried in old graveyards in Great Britain, and found in other countries where European migration was integral to their populations.
The burden of Paget's disease reflects the abnormal overgrowth of bone, and its consequences to musculoskeletal health through nerve impingement (deafness, spinal stenosis), fracture, deformity and early osteoarthritis leading to joint replacement.1 Because of this progressive physical impairment caused by PDB, it has often been described as a crippling disease. In Europe, it has been defined as one marked by co-morbidities and early mortality.2 A single retrospective epidemiological study in the US was the first to suggest that patients with PDB, particularly women, may have a slightly longer life expectancy than the Caucasian population in general. 3
In the last 10 years, some of the genetic determinants of familial and sporadic cases of Paget's disease have been described, with mutations clustering around the ubiquitin-associated domain of SQSTM1.4 The most prevalent mutation P392L results from a T to C transition in position 1215 in SQSTM1.5 This mutation is carried on two rather conserved haplotypes, presumed European in origin, 6 but its role in onset and skeletal distribution of PDB in an individual remains elusive. Although measles virus has been posited as contributory in PDB in the last century, evidence of the paramyxovirus in pagetic tissue has not been confirmed in all laboratories. 7,8
This study describes ancestry, measles exposure, the presence or absence of the SQSTM1 mutation (P392L) and the musculoskeletal correlates in a remarkably diverse population of people with PDB from the New England Registry for PDB, Boston, MA.
METHODS
Study Population
In 2001, the New England Registry for PDB (NE Registry) was founded in an effort to understand the demographics of this disease in the United States. Enrollment was voluntary. Recruitment depended on responses to information about the study mailed to members of the Paget Foundation (New York, New York); on referrals from physicians in New England; and on patients willingness to participate who were seen at the Massachusetts General Hospital (MGH). Medical record searches through the Research Patient Data Registry at Partners (Boston, MA) were used to identify patients as well, and letters requesting participation were sent to their physicians. Recruitment closed in early 2005 as numbers of interested patients dwindled. We were able to capture 254 persons with confirmed PDB who completed the study questionnaire; in 217 of these imaging was available documenting the skeletal distribution of disease. The Partners Institutional Review Board (Boston, MA) approved the study.
Analyses
In 2004, 42 patients enrolled in the NE Registry responded to a letter inviting them to participate in this study, which involved blood drawn for the genetic analysis (Sequenom) of the SQSTM1 P392L mutation, and the enzyme-linked immunosorbent assay (ELISA) for measles antibody. The primer for the SQSTM1 P392L mutation has been previously described. 9 The patient DNA was isolated and the sequences analyzed at Harvard Partners Center for Genetics and Genomics High Throughput Sequenom Genotyping Facility, Cambridge, MA. The samples were de-identified prior to genetic analysis. Measles antibody testing was performed by the MGH Clinical Laboratory Services (VIDAS Measles IgG assay, BIOMERIEUX SA, France). We compared the SQSTM1 P392L positive patients to the SQSTM1 P392L negative patients. Formal statistics were not pursued because of the small sample size. Living status was documented when that information was available.
RESULTS
Forty-two patients from the NE Registry agreed to have blood drawn for genetic analysis of the SQSTM1 P392L mutation, and for measles antibody testing; 27 were men (64%). The mean age of the cohort at the time of enrollment was 72 (range 30-87 years). This was comparable to the mean age in the NE Registry in general, 73.2 years, but reflected a slightly higher proportion of male participants. Most participants in this study were born in New England towns, with parents or grandparents who immigrated to the US during the early 20th century.
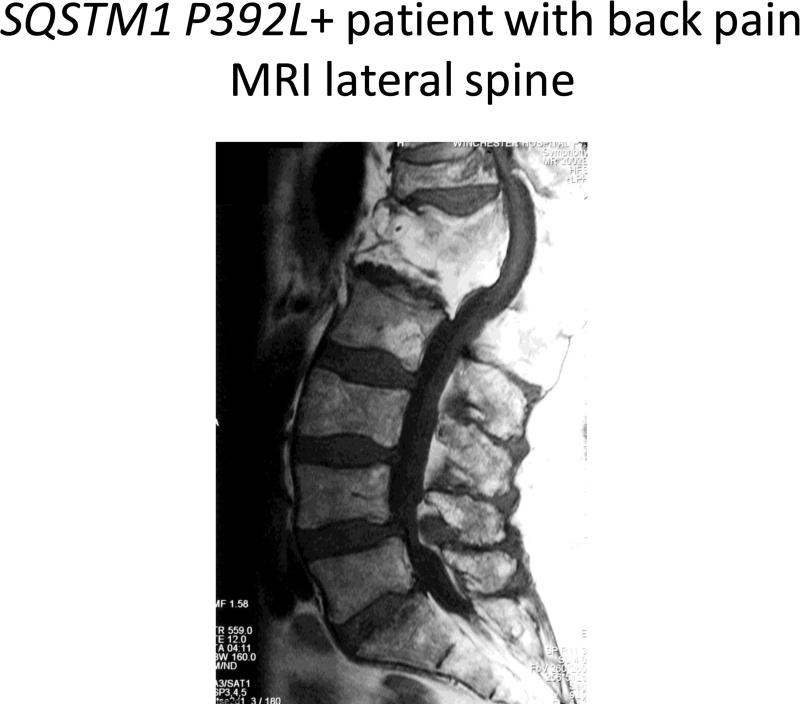
Nine of the 42 patients (21%) tested positive for the SQSTM1 P392L mutation; 7 were men, 2 of whom (28%) had familial PDB. (Table I) The ancestry of the SQSTM1 P392L + group was striking in that 6 of the 9 patients (67%) were from eastern Mediterranean countries, including Greece, Albania, Turkey and Lebanon. Age at diagnosis <50 years of age (67%), polyostotic disease and the evolution of spinal stenosis (56%) appeared more commonly in the men with this mutation. (Image 1) The initial diagnosis of PDB tended to be on the basis of radiographic findings in the SQSTM1 P392L + cohort (55%), rather than on the basis of pain or elevated serum alkaline phosphatase.
| Patient | Gender | DOB | Age (2001) | Heritage | Measles Antibody | Age at Diagnosis (years) | FH PDB* | Diagnosis made by | Pagetic bones | Sites | Living 2011 | Musculoskeletal Morbidity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 1936 | 65 | Portugal | UNK | <40 | Yes | P, X, B | 14 | R/L pelvis, C4, LS spine, T9, sacrum, R/L pelvis, L femur, L/R humerus, L/R scapula, L tibia | Yes | Spinal stenosis, fracture spine |
| 2 | M | 1925 | 76 | Lebanon | Yes | 41-50 | Yes | X | 8 | Skull, R/L pelvis, R femur, L 4th rib T spine, L 4 and L5, sacrum | Yes | Spinal stenosis |
| 3 | M | 1946 | 55 | Italy Scotland | Yes | <40 | No | X | 3 | Sacrum, L 4 and R pelvis | Yes | R THR, gout |
| 4 | M | 1928 | 73 | Greece Turkey | Yes | 41-50 | No | B | 5 | R tibia, L/R pelvis, R/L femur | Yes | Spinal stenosis, fracture tibia, R THR |
| 5 | F | 1928 | 73 | Greece | Yes | 61-70 | No | P-X-B | 2 | Skull, L tibia | Yes | Hearing loss |
| 6 | M | 1922 | 79 | Albania | Yes | 41-50 | No | B | 8 | L/R pelvis, L1 and L3, T11 and T12, R/L femur, R scapula, skull | Yes | Spinal stenosis |
| 7 | F | 1939 | 62 | Portugal | Yes | 61-70 | No | X | 1 | L pelvis | Yes | Crouzon's in grandchild |
| 8 | M | 1927 | 74 | Greece | Yes | 51-60 | No | X | 5 | 5 R/L pelvis, L femur and T spine, LS spine | Yes | Spinal stenosis, fracture L femur |
| 9 | M | 1930 | 71 | Turkey | Yes | 41-50 | No | X | 1 | L tibia | Yes | L TKR |
Family history of PDB in 1st degree relative; P=pain / X= x-ray / B= blood lest. Three samples were improperly delivered to the lab for measles antibody titer. Patients labeled unknown are presumed alive given last visit in medical record, but not confirmed by visit since 2010.
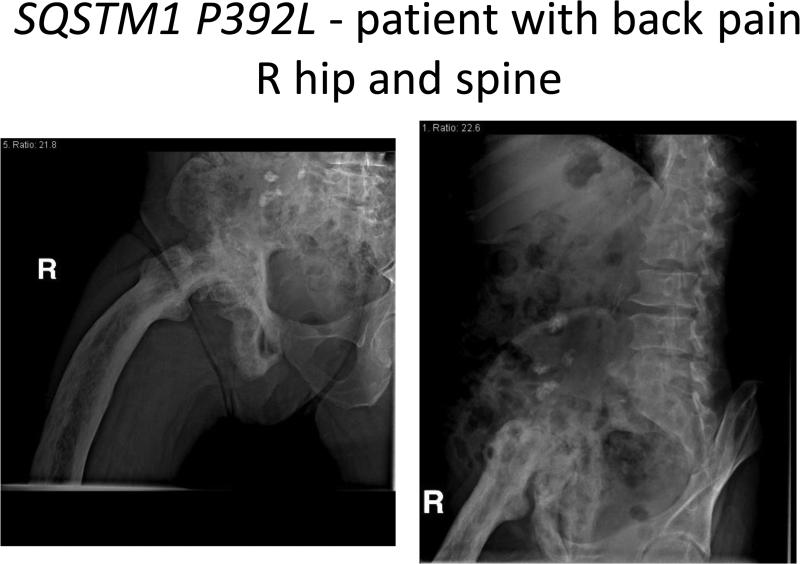
SQSTM1 P392L- Patient with back pain R hip and spine.
In the SQSTM1 P392L negative cohort, 9 (27%) reported a family history of PDB. (Table II) The majority (67%) were from countries in which PDB is readily diagnosed, including Quebec (Canada), Italy, Ireland, Scotland, the United States and France. Diagnosis after age 50 was almost equal to those diagnosed <50 years of age. There was a tendency for less skeletal involvement (2-3 bones average). End-stage degenerative disease of a pagetic joint and fracture, rather than spinal stenosis, characterized the SQSTM1 P392L - cohort. Low back pain was reported in 9 SQSTM1 P392L -persons (27%), a number comparable with that previously reported from the NE Registry.10 Low back pain attributed to sciatica, degenerative disease of the spine, scoliosis were more prevalent than spinal stenosis. (Image 2) Pain alone or in combination with abnormal elevations in serum alkaline phosphatase or radiographic findings led to the diagnosis in 40% of SQSTM1 P392L - cohort.
| Patient | Gender | DOB | Age (2001) | Heritage | Measles Antibody | Age at Diagnosis (years) | FH PDB* | Diagnosis made by | Pagetic bones | Sites | Living (2011) | Musculoskeletal Morbidity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 1918 | 73 | Italy | Yes | 71-80 | Yes | P-X | 4 | LS spine, sacrum, L/R femurs | ↓80 | Spinal stenosis |
| 2 | F | 1933 | 68 | Italy | Yes | 51-60 | Yes | P-X-B | 3 | R/L pelvis, L3 and L5 | Yes | |
| 3 | M | 1935 | 66 | Quebec | Yes | 41-50 | No | X | 2 | Sacrum, R pelvis | ||
| 4 | F | 1926 | 75 | Turkey | Yes | <40 | No | P-B | 2 | Skull, R pelvis | Yes | Endstage DJD R hip, hearing loss |
| 5 | M | 1971 | 30 | USA | Yes | <40 | No | B | 5 | R scapula, R humerus, L3 and 5, sternum, skull | Yes | Gout |
| 6 | M | 1918 | 83 | Russia | Yes | 51-60 | No | X | 2 | Skull, R pelvis | ↓87 | DJD R hip |
| 7 | M | 1950 | 51 | Italy | Yes | 41-50 | No | X | 1 | L pelvis | ||
| 8 | F | 1915 | 86 | Russia Poland | Yes | 61-70 | No | X-B | 2 | Skull, L femur | ↓95 | Fracture L femur |
| 9 | M | 1933 | 68 | Albania | Yes | <40 | No | B | 1 | Skull | unknown | Hearing loss |
| 10 | M | 1932 | 69 | Italy | Yes | 41-50 | No | B | 11 | R/L pelvis, sacrum, R/L femur, LS spine, skull, T spine, L tibia, R humerus, R ribs | Yes | Spinal stenosis |
| 11 | F | 1947 | 54 | Quebec | Yes | 41-50 | No | B | 2 | Skull, R tibia | Yes | |
| 12 | F | 1928 | 73 | Italy | Yes | 41-50 | No | P | 1 | R tibia | unknown | R TKR |
| 13 | M | 1930 | 71 | Ireland | Yes | 41-50 | No | X | 4 | Sacrum, L5, L pelvis, R femur | ↓76 | Low back pain, gout giant cell arteritis |
| 14 | F | 1961 | 40 | USA | Unknown | 41-50 | No | X | 1 | L pelvis | Yes | |
| 15 | M | 1932 | 69 | France | Yes | 41-50 | No | X | 3 | Sacrum, R femur, L tibia | Yes | Fracture L tibia |
| 16 | M | 1920 | 81 | Russia Lithuania | Unknown | 51-60 | No | P-B | 7 | Sacrum, R/L pelvis, scapula, R clavicle, R/L femurs | ↓90 | Fracture femur |
| 17 | M | 1943 | 58 | Africa | Yes | 51-60 | No | P | 1 | L2 | Yes | Low back pain |
| 18 | F | 1928 | 73 | Armenian | Yes | 61-70 | No | P-X | 4 | Sacrum, L3, L femur, R pelvis | Yes | Low back pain |
| 19 | M | 1925 | 76 | USA | Yes | 61-70 | Yes | B | 2 | R pelvis, R femur | ↓86 | Endstage DJD R hip |
| 20 | F | 1924 | 77 | Russia Austria | Yes | 41-50 | Yes | P-X-B | 1 | L pelvis | Yes | THR (bilateral) |
| 21 | M | 1948 | 53 | Quebec, Poland, Czech | Yes | 41-50 | No | X-B | 1 | R pelvis | unknown | Fracture femur |
| 22 | F | 1927 | 74 | Nepal | Yes | 71-80 | No | P-X-B | 3 | R/L pelvis, L3 and L4 | unknown | Fracture spine |
| 23 | M | 1931 | 70 | Ireland Scotland | Yes | 61-70 | Yes | X | 2 | R scapula, L femur | unknown | |
| 24 | F | 1942 | 59 | Italy Ireland | Yes | 51-60 | Yes | P | 2 | C2, L pelvis | Yes | Low back pain/disc herniation with srugery, child with HME |
| 25 | M | 1923 | 78 | Italy | Yes | 41-50 | Yes | X-B | 7 | Skull, L pelvis, R femur, R humerus, C-spine, tibia, fibula | Yes | Hyperparathyroidism, low back pain, neck pain |
| 26 | M | 1933 | 68 | Quebec | Unknown | 61-70 | No | P-X | 3 | Sacrum, R pelvis, R tibia | unknown | Gout |
| 27 | M | 1919 | 82 | Sicily | Yes | 51-60 | Yes | X-B | 2 | Skull, L3 | ↓87 | Low back pain, gout |
| 28 | M | 1918 | 83 | Russia | Yes | 71-80 | No | P | 3 | R tibia, L femur, L 4 | unknown | Fracture L femur |
| 29 | F | 1924 | 77 | Italy | Yes | 51-60 | No | P-X-B | 3 | Skull, L pelvis and L foot | ↓88 | Fracture L hip |
| 30 | M | 1939 | 62 | Italy | Yes | 41-50 | No | X-B | 1 | L femur | Yes | Fracture L femur |
| 31 | M | 1921 | 80 | Sicily | Yes | 61-70 | No | X | 1 | R femur | ↓90 | Low back pain, gout |
| 32 | M | 1914 | 87 | Lithuania | Yes | 61-70 | Yes | B | 3 | L humerus, R pelvis, R femur | ↓96 | Fracture R femur, gout |
| 33 | F | 1935 | 66 | Ireland | Yes | 51-60 | No | X-B | 2 | R/L pelvis | unknown |
Lumbar spine lesions are counted as one, although the distribution of involvement varied; > 3 vertebral bodies = LS Spine w/o further definition FH indicates a first degree relative with PDB
Diagnosis made by x=x-ray, p= pain and b= blood test (elevated serum alkaline phosphatase)
SQSTM1 P392L+ Patient with back pain MRI lateral spine.
All persons tested were positive for the presence of measles antibody. Some causes of death were known, and in these cases cardiovascular complications seemed to prevail, including stroke (age 88), ischemic heart disease, atrial fibrillation and congestive heart failure (ages 80, 87,88, 90, 90, 95, and 96). Cancer was reported in both SQSTM1 P392L + and SQSTM1 P392L - patients, 3 men with prostate cancer, 2 women with breast cancer and 1 woman with renal cancer. Metastatic disease was not recorded in those patients followed through the Partners HealthCare System during the period of observation.
All patients with PDB received multiple cycles of therapy with drugs such as calcitonin, etidronate, tiludronate, pamidronate, alendronate, risedronate and zoledronate throughout the course of their lives.
DISCUSSION
In this study, we describe the diverse ancestry of SQSTM1 P392L + and SQSTM1 P392L - persons, the presence of these mutations in both sporadic and familial forms of PDB in the US, and the occurrence of spinal stenosis as a prevalent phenotype in men carrying the P392L mutation. This study highlights the finding that most people with PDB live well into their 80's and some into their 90's. This longevity is associated with significant physical impairments attributable to PDB that accrue over time, as shown in the accompanying tables. Fracture is a common complication of PDB. There were cases of breast, renal and prostate cancer reported in several patients both SQSTM1 P392L +/− but no skeletal complications of malignancy were noted during years of follow up. 11,12 No one suffered from a pagetic osteosarcoma.
PDB is prevalent in Europe, Canada, the US, Australia and New Zealand. Within these countries, geographic and familial clusters have been reported, particularly in England. 13 The SQSTM1 mutation has been reported in 19% – 46% of familial PDB-cohorts studied in these countries, and in 2.6% - 16% of sporadic cases. 14,15 It is considered rare on the Asian and African continents. In one US study of families with PDB, the SQSTM1 mutation was identified in 20.5% of those with familial PDB, and in 0 in those with sporadic PDB. 16 In a subsequent study of somatic mutations as causal in PDB, 3 patients with sporadic disease were identified as having a germline SQSTM1 P392L mutation.9 Boston, a city of immigrants from the European continent, seemed an ideal site to establish a registry to examine the determinants of PDB, and to search for the prevalence of the most common SQSTM1 P392L mutation, in a cohort of these patients. The NE Registry gave us a unique opportunity to examine the outcomes of these patients.
We found deformity was a consistent finding in PDB, usually present radiographically as a consequence of bone remodeling, and invariably present if there was a fracture through pagetic bone. Twelve fractures occurred in these 42 patients (29%), either as a presenting feature of PDB or as a late complication. All of the patients had received bisphosphonate therapy over the years. As demonstrated in the PRISM trial, 17 treatment may not alter outcome in these fractures occurring in elderly patients with established disease and extant deformity of bone.
There have been different efforts to link the phenotype of patients with PDB with the genetic mutations in SQSTM1, the most convincing being that truncating mutations affecting SQSTM1 may result in more severe disease. 15 The SQSTM1 P392L mutation alone has not been associated with a distinct phenotype. 18 A recent paper studying an international cohort of persons with PDB, tried to assess skeletal extent and severity of PBD as a consequence of certain risk alleles identified in a genome-wide association study. 19 Severity was derived from a composite score of deformity, fracture, osteosarcoma, orthopaedic procedures and hearing loss that were variably documented in this population; along with family history, prior bisphosphonate treatment, and age of onset. Despite discrepancies in data collection and the rather low numbers of bones involved in all patients recruited, there was a striking observation that in the presence of a SQSTM1 mutation, these alleles conveyed a risk for PDB, particularly in men. Without the detailed clinical analysis, spinal stenosis as a distinct outcome was not recognized.
The radiographic evidence of spinal stenosis in the pagetic spine in SQSTM1 P392L + patients, the compression fractures and physical impairments attendant with aging are distinct findings. (Image 1) These were not as prevalent in the low back pain of SQSTM1 P392L – patients, in whom scoliosis imposed by a pagetic hip (Image 2) or disc herniation in a degenerative spine were more common. The American Academy of Orthopaedic Surgeons estimates spinal stenosis occurs in 8-11% of aging Americans, so it is hard to know if the findings are chance alone.
We did not look for other SQSTM1 mutations. Enrollment occurred during 2001, was initiated by a letter, and relied on patients volunteering their time. The report of the findings may reflect bias inherent in a small observational study. Documentation of the last years of their life may prove incomplete, and co-morbidities may have been missed. Some of the challenges in studying PDB are evident in the NE Registry, as the collection of data depends on screening measures to ascertain prevalence in a disease that is largely asymptomatic, and on voluntary enrollment. Fifty-five to 60% of the patients in this study were diagnosed with PDB incidentally, by radiographs or blood tests ordered for other indications. The absence of a national health care registry in the United States means that the prevalence of PDB across New England relies on population estimates from limited screening. 20
The strengths of this study are in the family history, its detail and the correlation of genetic markers with measles virus exposure, which proved universal in those with and those without the SQSTM1 P392L mutation. Measles was prevalent at the turn of the 20th century, and remains prevalent in other parts of the world where PDB is rare, e.g. India and other parts of Asia. Does the measles virus infection play a role in this disorder against a distinct genetic background? This is unknown, as are other environmental determinants. In this small study, other musculoskeletal mutations were identified in the off-spring of two families, Crouzon's syndrome and hereditary multiple exostoses. No patient in the NE Registry has reported a child affected with PDB, implying either a decrease in PDB or diminished expression in this next generation. The declining prevalence of PDB reported in most countries worldwide suggests fewer cases will be diagnosed in the future. 21,22 It is a curious predicament for a disease that is quite ancient. 23
This observational study reports on cases of PDB in the United States with and without the SQSTM1 P392L mutation, documents their remarkable ancestry, confirms the presence of measles exposure and describes what is known of the long-term outcomes in these persons. Debilitating spinal stenosis was a prevalent phenotype in males with the SQSTM1 P392L + mutation, as was early-onset, polyostotic disease. In all persons with PDB, fractures were prevalent. The negative consequences of the disease on musculoskeletal health are well-defined in this cohort, but the possibility that there are positive consequences are suggested by the long life span of these patients; the absence of early dementia that is seen in rare, inherited forms of PDB; and the absence of skeletal complications of malignancy captured in this New England Registry cohort. The role of the SQSTM1 P392L mutation in mediating PDB remains unclear, as the incidence of this disorder of bone seems diminishing in most countries and the majority of patients with PDB test negative for a SQSTM1 germline mutation.
Acknowledgments
Dr. Solomon received salary support for this work from NIH-NIAMS-K24-055989.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
The authors have no conflict of interest.
Contributor Information
Margaret Seton, Harvard Medical School, Director, Metabolic Bone Disease, Brigham & Women's Hospital Rheumatology, 75 Francis St, Boston, MA 02115.
Marc Hansen, Center of Molecular Medicine, University of Connecticut Health Center, 263 Farmington Ave, Farmington, CT 06030.
Daniel H. Solomon, Harvard Medical School, Brigham & Women's Hospital, Rheumatology, Immunology & Allergy, 75 Francis St, Boston, MA 02115.
REFERENCES
- 1.Siris E, Roodman GD. Paget's Disease Section X Chapter 82. In: Favus MJ, editor. Primer on the Metabolic Bone Diseases. ASBMR; Washington, DC: 2003. pp. 495–506. [Google Scholar]
- 2.van Staa TP, Selby P, Leufkens HG, Lyles K, Sprafka JM, Cooper C. Incidence and natural history of Paget's disease of bone in England and Wales. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2002;17:465–71. doi: 10.1359/jbmr.2002.17.3.465. [DOI] [PubMed] [Google Scholar]
- 3.Wermers RA, Tiegs RD, Atkinson EJ, Achenbach SJ, Melton LJ., 3rd. Morbidity and mortality associated with Paget's disease of bone: a population-based study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2008;23:819–25. doi: 10.1359/JBMR.080215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ralston SH, Layfield R. Pathogenesis of paget disease of bone. Calcified Tissue International. 2012;91:97–113. doi: 10.1007/s00223-012-9599-0. [DOI] [PubMed] [Google Scholar]
- 5.Laurin N, Brown JP, Morissette J, Raymond V. Recurrent mutation of the gene encoding sequestosome 1 (SQSTM1/p62) in Paget disease of bone. American Journal of Human Genetics. 2002;70:1582–8. doi: 10.1086/340731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lucas GJ, Hocking LJ, Daroszewska A, et al. Ubiquitin-associated domain mutations of SQSTM1 in Paget s disease of bone: evidence for a founder effect in patients of British descent. Journal of Bone & Mineral Research. 2005;20:227–31. doi: 10.1359/JBMR.041106. [DOI] [PubMed] [Google Scholar]
- 7.Ralston SH, Afzal MA, Helfrich MH, et al. Multicenter blinded analysis of RT-PCR detection methods for paramyxoviruses in relation to Paget s disease of bone. Journal of Bone & Mineral Research. 2007;22:569–77. doi: 10.1359/jbmr.070103. [DOI] [PubMed] [Google Scholar]
- 8.Kurihara N, Zhou H, Reddy SV, et al. Expression of measles virus nucleocapsid protein in osteoclasts induces Paget's disease-like bone lesions in mice. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2006;21:446–55. doi: 10.1359/JBMR.051108. [DOI] [PubMed] [Google Scholar]
- 9.Merchant A, Smielewska M, Patel N, et al. Somatic mutations in SQSTM1 detected in affected tissues from patients with sporadic Paget's disease of bone. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2009;24:484–94. doi: 10.1359/JBMR.081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seton M, Moses AM, Bode RK, Schwartz C. Paget's disease of bone: the skeletal distribution, complications and quality of life as perceived by patients. Bone. 2011;48:281–5. doi: 10.1016/j.bone.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 11.Moscat J, Diaz-Meco MT. p62: a versatile multitasker takes on cancer. Trends Biochem Sci. 2012;37:230–6. doi: 10.1016/j.tibs.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tu SM, Som A, Tu B, Logothetis CJ, Lee MH, Yeung SC. Effect of Paget's disease of bone (osteitis deformans) on the progression of prostate cancer bone metastasis. Br J Cancer. 2012 doi: 10.1038/bjc.2012.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Detheridge FM, Guyer PB, Barker DJ. European distribution of Paget's disease of bone. Br Med J (Clin Res Ed) 1982;285:1005–8. doi: 10.1136/bmj.285.6347.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falchetti A, Di Stefano M, Marini F, et al. Genetic epidemiology of Paget's disease of bone in italy: sequestosome1/p62 gene mutational test and haplotype analysis at 5q35 in a large representative series of sporadic and familial Italian cases of Paget's disease of bone. Calcif Tissue Int. 2009;84:20–37. doi: 10.1007/s00223-008-9192-8. [DOI] [PubMed] [Google Scholar]
- 15.Eekhoff EW, Karperien M, Houtsma D, et al. Familial Paget's disease in The Netherlands: occurrence, identification of new mutations in the sequestosome 1 gene, and their clinical associations. Arthritis and rheumatism. 2004;50:1650–4. doi: 10.1002/art.20224. [DOI] [PubMed] [Google Scholar]
- 16.Rhodes EC, Johnson-Pais TL, Singer FR, et al. Sequestosome 1 (SQSTM1) mutations in Paget's disease of bone from the United States. Calcif Tissue Int. 2008;82:271–7. doi: 10.1007/s00223-008-9114-9. [DOI] [PubMed] [Google Scholar]
- 17.Langston AL, Campbell MK, Fraser WD, et al. Randomized trial of intensive bisphosphonate treatment versus symptomatic management in Paget s disease of bone. Journal of Bone & Mineral Research. 2010;25:20–31. doi: 10.1359/jbmr.090709. [DOI] [PubMed] [Google Scholar]
- 18.Morissette J, Laurin N, Brown JP. Sequestosome 1: mutation frequencies, haplotypes, and phenotypes in familial Paget s disease of bone. Journal of Bone & Mineral Research. 2006;21:P38–44. doi: 10.1359/jbmr.06s207. [DOI] [PubMed] [Google Scholar]
- 19.Albagha OM, Visconti MR, Alonso N, et al. Common susceptibility alleles and SQSTM1 mutations predict disease extent and severity in a multinational study of patients with Paget's disease. J Bone Miner Res. 2013 doi: 10.1002/jbmr.1975. [DOI] [PubMed] [Google Scholar]
- 20.Altman RD, Bloch DA, Hochberg MC, Murphy WA. Prevalence of pelvic Paget's disease of bone in the United States. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2000;15:461–5. doi: 10.1359/jbmr.2000.15.3.461. [DOI] [PubMed] [Google Scholar]
- 21.Rendina D, Gennari L, De Filippo G, et al. Evidence for increased clinical severity of familial and sporadic Paget's disease of bone in Campania, southern Italy. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2006;21:1828–35. doi: 10.1359/jbmr.060822. [DOI] [PubMed] [Google Scholar]
- 22.Cundy HR, Gamble G, Wattie D, Rutland M, Cundy T. Paget's disease of bone in New Zealand: continued decline in disease severity. Calcified Tissue International. 2004;75:358–64. doi: 10.1007/s00223-004-0281-z. [DOI] [PubMed] [Google Scholar]
- 23.Mays S. Archaeological skeletons support a northwest European origin for Paget's disease of bone. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010;25:1839–41. doi: 10.1002/jbmr.64. [DOI] [PubMed] [Google Scholar]