Abstract
Objective
To determine the feasibility and efficacy of using the Gynecologic Cancer Lymphedema Questionnaire (GCLQ) as a symptom scale for lymphedema of the lower extremity (LLE).
Methods
Twenty-eight gynecologic cancer survivors with documented LLE and 30 without a history or presence of lymphedema completed the GCLQ and provided feedback about their satisfaction with and feasibility of using the GCLQ at their oncology follow-ups. The study survey took approximately 5–10 minutes to complete, and it was easily understood by the majority of the sample.
Results
Participants had a mean age of 59.6 years (range, 28–80 years). Twenty-eight women (48%) had LLE and 30 (52%) had no history or presence of LLE (confirmed by limb volume [LV] measurements at assessment). Type of cancer history included: endometrial, 38 (66%); cervical, 13 (22%); and vulvar, 7 (12%). GCLQ scores differed significantly by lymphedema diagnosis; LLE patients had higher scores (P<0.01). The large area under the curve (AUC) of 0.95 (95% CI: 0.90–1.000) suggests that the GCLQ can distinguish between patients with and without LLE. Although all 28 (100%) of the LLE patients were aware of their LLE diagnosis, only 23 (82%) underwent treatment. The GCLQ was easily understood by most (55/58, 95%); and overall, patients showed a high willingness (56/58, 96%) to complete the questionnaire at future appointments. Twenty-five (88%) of the LLE patients found the GCLQ to be helpful in identifying symptoms of lymphedema.
Conclusions
The GCLQ effectively distinguished between gynecologic cancer survivors with and those without LLE, with good sensitivity and specificity. The patients, particularly those with LLE, showed high confidence in the GCLQ’s ability to detect LLE symptoms.
Keywords: gynecologic cancer, lymphedema, lower extremity, assessment tool, Gynecologic Cancer Lymphedema Questionnaire
INTRODUCTION
In the western world, cancer and its subsequent treatment is recognized as a leading cause of secondary lymphedema. As a result, many cancer survivors are living with disfigurement, discomfort, and disruption of activities due to limb swelling [1,2].
Lymphedema, by definition, is a chronic, progressive condition in which protein-rich fluid accumulates in the superficial tissues of the body. Lymphedema can be characterized as either primary or secondary in nature [3], as is the case in the cancer setting. Lymphedema can be categorized into four stages. In stage 0 (or Ia), the condition is considered sub-clinical; swelling is not present. In stage I, edema is mild; fluid accumulates throughout the day but resolves overnight. In stage II, the lymphedema is always present but varies in severity. Stage III disease is characterized by persistent, moderate-to-severe edema of the involved limb(s) [4].
To date, the preponderance of research on lymphedema within the field of oncology has focused on the development of lymphedema of the upper extremity in patients treated for breast cancer [5–8]. These data have aided in the investigation of mechanisms for diagnosis and strategies for treatment of lymphedema, and have altered the clinical care of breast cancer patients with the incorporation of sentinel lymph node dissection [9,10]. Despite these promising strides in breast cancer, lymphedema at other sites remains under-recognized and underreported. Furthermore, investigations on the impact of lymphedema of the lower extremity (LLE) on gynecologic cancer patients are limited [11–15].
Armer and colleagues developed a lymphedema symptom measurement tool, the Lymphedema Breast Cancer Questionnaire (LBCQ), for the assessment of upper extremity lymphedema in breast cancer survivors [16,17]. Self-reported lymphedema symptoms (i.e., heaviness, swelling, and numbness) have proven helpful in detecting lymphedema in breast cancer patients at an early stage. Research suggests that untreated symptoms of lymphedema may contribute to poorer quality of life (QOL) [16–18]. Specialists identified swelling (P=0.001), heaviness (P=0.003), tightness (P=0.007), and skin problems (P<0.001) as the presenting problem in a higher proportion of LLE patients in comparison to those with upper extremity lymphedema [19].
The Gynecologic Cancer Lymphedema Questionnaire (GCLQ) was initially adapted by Dr. Suzy Lockwood from the LBCQ in order to identify LLE symptoms in gynecologic cancer survivors (unpublished data). Currently, limb volume measurements are typically used for the evaluation of lymphedema. Other methods include: imaging modalities, and tonometry and bio-electrical impedance [4].
Assessment of lymphedema patient self-reported symptoms could be easily incorporated into the clinical care setting as a simple, feasible, time-efficient screening/triaging method to identify women with or at a high risk of developing lymphedema. The objective of this pilot study was to determine whether the GCLQ could detect symptoms of lymphedema of the lower extremity and differentiate between gynecologic cancer survivors with documented LLE and gynecologic cancer survivors without LLE. The feasibility and satisfaction of using the GCLQ as a brief assessment tool was also assessed.
METHODS
This was an Institutional Review Board (IRB)-approved quality improvement pilot study to determine the efficacy and feasibility of using a modified lymphedema symptom assessment tool in gynecologic cancer survivors. Informed consent was obtained from all participants at their oncology follow-ups. The GCLQ and satisfaction/feasibility survey was a one-time assessment completed by all participants.
Participants
The study criteria included: 1) women 21 years of age or older, 2) history of primary diagnosis of gynecologic cancer (cervical, uterine, or vulvar only), 3) history of a gynecologic cancer surgery, including lymph node removal, 4) no active cancer treatment, 5) fluent in English, and 6) able and willing to provide consent. Gynecologic cancer survivors with lymphedema had documented LLE confirmed by the medical chart, treating physician, and/or active lymphedema treatment. A comparison group of gynecologic cancer survivors without lymphedema were required to have no history or presence of LLE confirmed by limb volume measurements at the time of study assessment.
Procedure
Medical records of the Gynecology Service at Memorial Sloan-Kettering Cancer Center (MSKCC) were screened daily to identify patients who met the study criteria. Women were approached at their oncology follow-up, given a description of the study, and invited to participate. Sixty-six potential participants were identified; of these, 3 were deemed ineligible based on study criteria after further discussion with research staff. Out of the 63 potential participants, 5 declined (8% refusal rate). Based on eligibility criteria, 58 consecutive women were enrolled on study (28 with documented LLE and 30 without a history or presence of LLE).
All women completed the one-time self-report study survey consisting of the GCLQ (Appendix 1) and items addressing participants’ satisfaction with and the feasibility of using the GCLQ as a brief assessment tool. The study survey took approximately 5–10 minutes to complete. Additionally, women without any history or diagnosis of LLE underwent limb volume assessment to ensure they did not have LLE prior to completing the GCLQ survey. Both limbs were measured by a trained nurse or research study member. Using a centimeter tape, circumferential measurements were made in the supine position starting from the heel and then proceeding at 10-cm intervals to the inguinal crease. The measurements were recorded and entered into a computer program that calculated the individual’s limb volume and excess volume. [1]. The limb volume measurements took approximately 10–15 minutes to complete.
Study Survey
The GCLQ is a modification of the validated LBCQ [16,17]. Psychometric properties of the LBCQ include an internal consistency reliability of 0.785 for all items. The GCLQ, initially modified by Dr. Suzy Lockwood of Texas Christian University, was piloted with patients experiencing LLE and showed good construct and face validity (unpublished data). For the current study, we further modified Dr. Lockwood’s GCLQ into a brief, 20-symptom assessment tool with 4 supplemental items to determine a patient’s awareness of their lymphedema diagnosis and their utilization of lymphedema-specific treatment. We used this modified GCLQ survey in a pilot study of gynecologic cancer survivors at MSKCC to test its efficacy and feasibility. For the purposes of this pilot study, responses were dichotomized. The GCLQ self-report lymphedema questionnaire assessed symptoms associated with lymphedema measured as present within the past 4 weeks. Each item was scored 0=No or 1=Yes. The total score is the summation of patient self-reported symptom scores combined from the following 7 symptom clusters (Appendix 1): heaviness (item 14); swelling (general) (items 8, 9, 20); swelling (limb) (items 18, 19); infection-related (items 10 [redness], 11 [blistering], 13 [increased temperature in leg); aching (item 17); numbness (items 7, 12, 15, 16); and physical functioning (items 1–6). The internal consistency reliability of the GCLQ total score was 0.95. Participants answered 7 questions addressing their satisfaction with and feasibility of using the GCLQ as a screening tool. Participants were queried as to whether the GCLQ was confusing as an instrument. Possible responses were: 1) not at all confusing; 2) somewhat confusing; 3) very confusing; or 4) extremely confusing. Participants were also encouraged to offer ideas about any questions or items that could be added to the GCLQ to help identify lymphedema of the lower extremity and to ensure that no symptoms associated with LLE were overlooked since the GCLQ was developed from a lymphedema measure validated for upper extremity lymphedema patients.
Statistical Analysis
This study primarily examined the proportion of consecutive patients drawn from the Gynecology Service at MSKCC who were truly identified as having lymphedema by the GCLQ assessment tool. The proportion of patients with diagnosed LLE (i.e., true proportion of LLE) versus the proportion of responders who were identified to have LLE via the GCLQ (i.e., patients’ responses) was compared via the McNemar’s test. The study was designed for a sample size of 50. A sample of 50 provided 80% power to detect a difference of 9% in the above proportions assuming 10% discordant pairs and Type I error of 10%. Our initial target accrual goal was 50, and the study was designed and powered for a sample size of 50; however, we had the opportunity to enroll 58 women on the study. Fisher’s exact tests were used to test for group differences (LLE vs. no LLE) in the number of symptoms endorsed in each of the 7 symptom cluster subscales, while difference in the median GCLQ total score was assessed via a Wilcoxon rank sum test. Based on the sensitivity and specificity values calculated at each observed GCLQ total score value, the receiver operating characteristic (ROC) curve was plotted and the four best (i.e., that yielded values of both sensitivity and specificity greater than 60%) potential clinical cutoff scores were chosen such that a GCLQ total score equal to or higher than the given score would be considered a positive test for lymphedema. Diagnostic accuracy statistics were calculated for each of these cutoff scores, including positive predictive value (PPV), negative predictive value (NPV) and Cohen’s kappa [20]. The area under the ROC curve (AUC) and 95% confidence interval was calculated for the GCLQ total score and each symptom cluster subscale in comparison to the identification of documented LLE and/or active lymphedema treatment in the medical chart confirmed by the treating physician to determine the usefulness of the GCLQ scale (and symptom cluster) in assessing LLE symptoms. Descriptive statistics were also performed for the exploratory items addressing patients’ satisfaction with and feasibility of using the GCLQ as a brief assessment tool to identify LLE, in addition to an individual’s history of lymphedema treatment. The internal consistency reliability coefficient (Cronbach’s alpha) for the GCLQ total score was calculated using the items’ tetrachoric correlation matrix, obtained using Mplus software version 4.21 [21]. Because all items are dichotomous, this method is more appropriate than the more common method using Pearson correlations [22]. ROC curves and AUCs were obtained using SPSS Version 15 [23]. All other analyses were performed in the SAS software version 9.2 [24].
RESULTS
Demographic and medical information
Table 1 presents the demographic and medical information for the sample participants. Twenty-eight patients (48%) had LLE and 30 (52%) had no history or presence of LLE as confirmed by limb volume (LV) measurements at assessment. Cancer by disease site included the following: endometrial, 38 (66%); cervical, 13 (22%); and vulvar, 7 (12%). Of the women diagnosed with LLE, 16 (53%) had a history of endometrial cancer, 5 (18%) had a history of cervical cancer, and 7 (25%) had a history of vulvar cancer. Seventeen (30%) of the patients had been diagnosed at least 5 years before participating in this study; 16 (28%) had been diagnosed 3–5 years before; 23 (40%) had been diagnosed 1–3 years before; and 2 (3%) had been diagnosed less than 1 year before participating in this study. Although the mean ages of the LLE and no history or presence of LLE cohorts differed (61.36 and 57.93 years, respectively), there was no statistical difference (P=0.331). Similarly, those with LLE were further from diagnosis (mean, 58.18 months) than those with no history or presence of LLE (mean, 46.00 months); however, there was no statistical difference (P=0.253). The type of diseases (cervical, endometrial, vulvar) differed statistically (P=0.013) across the LLE groups; of note, there were no vulvar cancer patients in the no history or presence of LLE cohort.
Table 1.
Demographic and Medical Characteristics of the Sample (n=58)
| Demographic and Medical Characteristics | * LLE (n=28; 48%) | No LLE (n=30; 52%) | Total Sample (n=58) | *** p-value | ||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |||
| Type of Cancer | Endometrial cancer | 16 | 57% | 22 | 73% | 38 | 66% | P=0.013 |
| Cervical cancer | 5 | 18% | 8 | 27% | 13 | 22% | ||
| Vulvar cancer | 7 | 25% | -- | -- | 7 | 12% | ||
| Time since diagnosis | Mean; **SD | 58.18; (50.64) | 46.00; (34.51) | 51.88; (43.11) | P=0.253 | |||
| At least 5 years ago | 9 | 32% | 8 | 30% | 17 | 27% | P=0.674 | |
| 3–5 years ago | 7 | 25% | 9 | 28% | 16 | 30% | ||
| 1–3 years ago | 12 | 43% | 11 | 40% | 23 | 37% | ||
| Less than 1 year ago | -- | -- | 2 | 3% | 2 | 7% | ||
| Age | Mean; SD | 61.36; (9.60) | 57.93; (12.12) | 59.59; (11.02) | P=0.331 | |||
| 28–35 years | --- | --- | 2 | 3% | 2 | 7% | P=0.709 | |
| 36–50 years | 5 | 18% | 5 | 17% | 10 | 17% | ||
| 51–65 years | 13 | 46% | 15 | 48% | 28 | 50% | ||
| 66–80 years | 10 | 36% | 8 | 31% | 18 | 27% | ||
| Marital Status | Single | 3 | 11% | 9 | 21% | 12 | 30% | P=0.288 |
| Married | 17 | 61% | 14 | 53% | 31 | 47% | ||
| Separated or divorced | 3 | 10.7% | 5 | 14% | 8 | 17% | ||
| Widowed | 5 | 18% | 2 | 12% | 7 | 7% | ||
| Race/Ethnicity | White Non-Hispanic | 22 | 79% | 21 | 74% | 43 | 70% | P=.653 |
| White Hispanic | 2 | 7% | 1 | 5% | 3 | 3% | ||
| Black Non-Hispanic | 2 | 7% | 5 | 12% | 7 | 17% | ||
| Asian Pacific Islander | -- | -- | 1 | 2% | 1 | 3% | ||
| Unknown | 2 | 7% | 2 | 7% | 4 | 7% | ||
LLE, lower extremity lymphedema;
SD, standard deviation. The p-values for differences by lymphedema status for continuous age and continuous months since diagnosis are based on independent sample t-tests.
The p-values for all other variables are based on Fisher’s exact tests.
Primary Outcomes
Gynecologic Cancer Lymphedema Questionnaire (GCLQ)
The primary outcome is the patient GCLQ self-reported lymphedema symptoms measured as “present within the past 4 weeks”. A comparison was made between the GCLQ scores in gynecologic cancer survivors with documented LLE in comparison to those without any history or presence of lymphedema (confirmed by LV measurements prior to group assignment). Table 2 presents the participants’ responses to GCLQ items by group.
Table 2.
Frequencies for the Gynecologic Cancer Lymphedema Questionnaire (GCLQ) Items
| GCLQ Item # | Symptom cluster | LLE (n=28) | No LLE (n=30) | GCLQ Lower Extremity Lymphedema Symptom Items |
|---|---|---|---|---|
| n (%) | n (%) | |||
| 8 | SW | 28 (100%) | 7 (23%) | Experienced swelling |
| 15 | N | 20 (71%) | 7 (23%) | Experienced numbness |
| 12 | N | 19 (68%) | 1 (3%) | Experienced firmness/tightness |
| 14 | H | 19 (68%) | 3 (10%) | Experienced heaviness |
| 7 | N | 18 (64%) | 4 (13%) | Experienced tenderness |
| 17 | A | 17 (61%) | 8 (27%) | Experienced aching |
| 9 | SW | 17 (61%) | 4 (13%) | Experienced swelling with pitting |
| 16 | N | 16 (57%) | 4 (13%) | Experienced stiffness |
| 19 | LSW | 11 (39%) | ------- | Experienced groin swelling |
| 10 | INF | 11 (39%) | 1 (3%) | Experienced redness |
| 3 | PF | 11 (39%) | ------- | Limited movement of your ankle |
| 20 | SW | 10 (36%) | ------- | Experienced pockets of fluid |
| 13 | INF | 10 (36%) | ------- | Experienced increased temperature in the leg |
| 6 | PF | 10 (36%) | 3 (10%) | Leg or foot feels weak |
| 5 | PF | 9 (32%) | 1 (3%) | Limited movement of your toes |
| 4 | PF | 8 (29%) | 2 (7%) | Limited movement of your foot |
| 2 | PF | 6 (21%) | 2 (7%) | Limited movement of your knee |
| 18 | LSW | 4 (14%) | ------- | Experienced hip swelling |
| 1 | PF | 3 (11%) | 2 (7%) | Limited movement of your hip |
| 11 | INF | 2 (7%) | ------- | Experienced blistering |
LLE-lymphedema of the lower extremity; SW-swelling [general]; N-neuropathy; H-heaviness; A-ache; LSW-swelling [limb]; INF-infection; PF-physical function
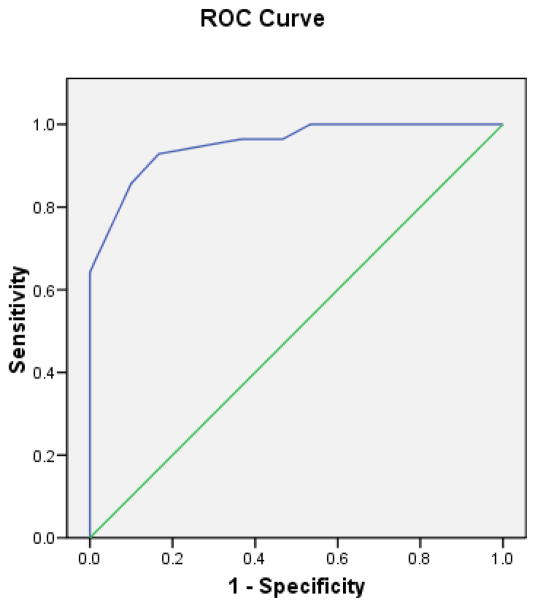
The internal consistency reliability of the GCLQ total score was 0.95. Table 3 presents the distributions of scores on the GCLQ total score and on the symptom cluster subscales. The GCLQ scores differed significantly by lymphedema diagnosis; lymphedema patients had higher scores (all significance test P values <0.01). The ROC curve for the GCLQ total score is presented in Figure 1, and the AUCs for the total and symptom cluster subscale scores are listed in Table 4. The large AUC of 0.95 (95% Confidence Interval: 0.90–1.000) suggests that the GCLQ overall effectively distinguished between patients with and those without lymphedema. Two subscales (Swelling-General and Numbness) also had AUCs larger than 0.90.
Table 3.
Distributions of Total and Symptoms Cluster Scores on The Gynecologic Cancer Lymphedema Questionnaire (GCLQ) By Gynecologic Cancer Survivors with and without a Diagnosis of Lower Extremity Lymphedema (LLE)
|
|
|||
|---|---|---|---|
| Score (%) | 0 | 1 | |
|
|
|||
| Heaviness* (Item 14) | LLE No (n=30) | 90 | 10 |
| LLE Yes (n=28) | 32.1 | 67.9 | |
|
|
|||||
|---|---|---|---|---|---|
| Score (%) | 0 | 1 | 2 | 3 | |
|
|
|||||
| Swelling General * (Items 8, 9, 20) | LLE No (n=30) | 76.67 | 10 | 13.33 | 0 |
| LLE Yes (n=28) | 0 | 28.57 | 46.43 | 25 | |
|
|
||||
|---|---|---|---|---|
| Score (%) | 0 | 1 | 2 | |
|
|
||||
| Swelling Limb * (Items 18, 19) | LLE No (n=30) | 100 | 0 | 0 |
| LLE Yes (n=28) | 57.14 | 32.14 | 10.71 | |
|
|
||||
|---|---|---|---|---|
| Score (%) | 0 | 1 | 2 | |
|
|
||||
| Infection-related * (Items 10, 11, 13) | LLE No (n=30) | 96.67 | 3.33 | 0 |
| LLE Yes (n=28) | 46.43 | 25 | 28.57 | |
|
|
|||
|---|---|---|---|
| Score (%) | 0 | 1 | |
|
|
|||
| Aching* (Item 17) | LLE No (n=30) | 73.33 | 26.67 |
| LLE Yes (n=28) | 39.29 | 60.71 | |
|
|
||||||
|---|---|---|---|---|---|---|
| Score (%) | 0 | 1 | 2 | 3 | 4 | |
|
|
||||||
| Numbness* (Items 7, 12, 15, 16) | LLE No (n=30) | 60 | 26.67 | 13.33 | 0 | 0 |
| LLE Yes (n=28) | 7.14 | 7.14 | 28.57 | 32.14 | 25 | |
|
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Score (%) | 0 | 1 | 2 | 3 | 4 | 5 | 6 | |
|
|
||||||||
| Physical Functioning* (Items 1–6) | LLE No (n=30) | 73.33 | 20 | 6.67 | 0 | 0 | 0 | 0 |
| LLE Yes (n=28) | 42.86 | 10.71 | 17.86 | 10.71 | 7.14 | 3.57 | 7.14 | |
|
|
||||
|---|---|---|---|---|
| Mean | (SE) | Median | ||
|
|
||||
| GCLQ Total Symptom Score† (All Items) | LLE No (n=30) | 1.63 | (0.33) | 1 |
| LLE Yes (n=28) | 8.89 | (0.81) | 9.5 | |
Physical Functioning distribution differed significantly between LLE vs. no LLE (P<0.05). All other symptom cluster scores were significantly different (P<0.001). P values are from Fisher’s exact tests for the Heaviness and Aching clusters, and from exact chi-square tests for the remaining symptom clusters.
GCLQ Total Symptom Scores were significantly different between LLE and no LLE, Wilcoxon rank-sum test (P<0.001).
Figure 1.
Receiver Operating Characteristic (ROC) Curve for GCLQ Total Score Compared to Lymphedema Diagnosis Gold-Standard
Table 4.
Areas Under the Receiver Operating Characteristic (ROC) Curves for GCLQ Total and Symptom Cluster Scores Against the Gold-Standard of a Lymphedema Diagnosis
| Scale | Area Under Curve (AUC) | Asymptotic 95% Confidence Interval
|
|
|---|---|---|---|
| Lower Bound | Upper Bound | ||
| Heaviness (Item 14) | 0.789 | 0.666 | 0.912 |
| Swelling General (Items 8, 9, 20) | 0.917 | 0.844 | 0.989 |
| Swelling Limb (Items 18, 19) | 0.714 | 0.577 | 0.851 |
| Infection-related (Items 10, 11, 13) | 0.756 | 0.626 | 0.886 |
| Aching (Item 17) | 0.670 | 0.529 | 0.811 |
| Numbness (Items 7, 12, 15, 16) | 0.912 | 0.833 | 0.991 |
| Physical Functioning (Items 1–6) | 0.705 | 0.567 | 0.843 |
| GCLQ Total Symptom Score (All Items) | 0.952 | 0.903 | 1.000 |
While the large AUC demonstrates the overall utility of the GCLQ at identifying lymphedema patients across all potential clinical cutoff scores, more detailed analysis at specific cutoff scores is required to evaluate the practical utility of the GCLQ in aiding clinical decision making. To this end, the sensitivity and specificity was calculated at each observed GCLQ total score, such that patients with scores equal to or greater than a given score were considered to have a positive test for lymphedema. Four of these potential cutoff scores yielded sensitivity and specificity both greater than 60%, thus showing promise as optimal GCLQ clinical cutoffs for identifying lymphedema. Diagnostic accuracy statistics were calculated for each of these four cutoff scores (greater than or equal to 3, 4, 5, or 6) and are presented in Table 5. The two middle cutoffs (4 and 5) both had good sensitivity, specificity, PPV, and NPV (Table 5). Both also had high overall accuracy percents, AUCs, and kappas (measure of diagnostic agreement). These two cutoff scores (≥4 and ≥5) distinguished between patients with and without lymphedema in this sample with similar accuracy, while both performed better than the other two promising scores (≥3 and ≥6). A range of four potential clinical cutoff scores are presented to facilitate comparisons with cutpoints in future studies.
Table 5.
Diagnostic Accuracy Statistics for Plausible Clinical Cut-Off Scores on the GCLQ Total Score
| Potential Clinical Cut-Off Score* | N | Identified LLE Positive by GCLQ Cut-Off Score (%) | True LLE Positive (%) | Sensitivity (%) | Specificity (%) | Positive Predictive Value (%) | Negative Predictive Value (%) | Kappa | McNemar P value |
|---|---|---|---|---|---|---|---|---|---|
| >=3 | 58 | 65.52 | 48.28 | 96.43 | 63.33 | 71.05 | 95.00 | 0.59 | 0.004 |
| >=4 | 58 | 53.45 | 48.28 | 92.86 | 83.33 | 83.87 | 92.59 | 0.759 | 0.26 |
| >=5 | 58 | 46.55 | 48.28 | 85.71 | 90.00 | 88.89 | 87.09 | 0.758 | 0.71 |
| >=6 | 58 | 31.03 | 48.28 | 64.29 | 100.00 | 100.00 | 75.00 | 0.65 | 0.001 |
GCLQ Supplemental Items
The supplemental items of the GCLQ examined women’s lymphedema awareness, history, and treatment methods. Of the 28 gynecologic cancer survivors with LLE, 100% were aware of their diagnosis; however, only 23 (82%) had been or were being treated for their condition. For those women who participated in some form of treatment, the most common modalities consisted of the following: compression garment, 21 (75%); directed exercise, 14 (50%); and specialized lymphedema massage, 12 (43%). Table 6 provides greater detail. Participants reported that their main source of information or training about lymphedema was from a lymphedema specialist (16, 57%) or from physical therapy consults (13, 46%).
Table 6.
GCLQ Supplemental Lymphedema History Items (N=28)
| Positive Endorsement | N (%) | Type of Lymphedema Treatment or Intervention |
|---|---|---|
| Are you undergoing or have you undergone lymphedema treatment | 23 (82%) | |
| Indicate the treatment recommendation | 21 (75%) | Compression garment |
| 14 (50%) | Directed exercise | |
| 12 (43%) | Specialized lymphedema massage | |
| 9 (32%) | Manual lymphatic drainage | |
| 6 (21%) | Skin care instruction | |
| 6 (21%) | Multi-limb bandaging | |
| Have you received any of the following information or training | ||
| 16 (57%) | Lymphedema specialist | |
| 13 (46%) | Physical Therapy consult | |
| 4 (14%) | Nurse Education |
GCLQ Satisfaction and Feasibility Survey
Fifty-five patients (95%) indicated that the GCLQ was not at all confusing; the other 3 patients (5%) indicated it was only “somewhat confusing”. In addition, 56 (97%) of the women (were willing to complete the survey at follow-up appointments. Of the remaining 2 women, one indicated a preference to complete the survey at some follow-ups and the other indicated a preference to not complete the survey at future visits. As the current standard practice of detecting lymphedema requires some form of limb measurement, participants were asked if they would undergo limb measurement at future follow-up appointments. Fifty-six (97%) expressed a willingness to undergo limb measurements at their appointments.
When queried “Do you think the GCLQ survey would be able to detect lymphedema symptoms?”, 45 (77%) indicated the GCLQ was somewhat to extremely helpful in the detection of lymphedema symptoms. A stronger confidence in the instrument’s ability to capture LLE symptoms was found within the LLE group; 25 (88%) found the GCLQ to be helpful in identifying symptoms of lymphedema.
Ten women (17%) (9 with LLE and 1 without LLE) provided additional feedback regarding ideas about any questions or items that could be added to the GCLQ to help identify lymphedema. Suggestions included the following: advice (i.e., don’t wait too long before your leg bothers you [n=1]); recommendations for items addressing diet and exercise (n=2) or function items (tripping, interference with sleep [n=3]); and specification of the type of ache or swelling that could occur (n=1). Study participants were also offered the opportunity to write in any additional comments or suggestions for the research team. Seven women (12%) out of 58 provided written comments. Topics included: the need for treatment resources (n=2); information about symptoms or risk (n=2); recognition of the importance of investigating this “frustrating” condition (n=2); and a suggestion to measure limbs before and after surgery (n=1).
DISCUSSION
The GCLQ distinguished between gynecologic cancer survivors with and without lymphedema, with good sensitivity and specificity. Lymphedema patients had higher scores (P<0.01). Two subscales (Swelling-General and Numbness) also had AUCs larger than 0.90. Symptoms of lymphedema, which have been identified as important to the experience of LLE in the literature [16,17], were supported by the findings of this pilot study. The literature also suggests that untreated symptoms (i.e., heaviness, swelling, and numbness) of lymphedema may contribute to poorer QOL [16–18]. A mechanism to detect, prevent, and successfully treat lymphedema symptoms is paramount.
The GCLQ was deemed a feasible tool to detect symptoms of lymphedema of the lower extremity in the clinical care setting, and was easily understood and considered user-friendly by the majority of the sample. This may have contributed to participants’ willingness to complete the survey (56, 97%) as part of their follow-up care. Interestingly, women were also enthusiastic about the idea of routine limb measurements (56, 97%) despite the greater time commitment needed for this procedure. The confidence in the GCLQ’s ability to detect LLE symptoms was high overall (45, 77%) but even higher within the group with LLE (25, 88%), further supporting the integrity of this instrument. It is important to note that although high accuracy of the GCLQ was reported, LLE patients may have been more inclined to endorse LLE symptoms due to their awareness and knowledge of lymphedema. Similarly, the gynecologic cancer survivors with no history or presence of LLE symptoms may have been less likely to report symptoms.
Qualitative items included within the design of this pilot study also allowed participants to share their thoughts and feelings about the GCLQ and the study. Of the 10 women (17%) who offered additional feedback about potential items that could be added to the GCLQ, themes addressing function as well as information about LLE and its symptoms emerged. In light of this fact, we recommend that a formal assessment of physical function/disability be included in lymphedema investigations. Seven women provided additional suggestions that reflected their frustration with this condition and positive endorsements of research in this area (i.e., “excellent study, about time”). Clearly, patients and medical professionals recognize the need for more evidence-based medicine in this understudied aspect of cancer survivorship.
Future Directions
The GCLQ detected lymphedema symptoms in gynecologic cancer survivors with LLE in comparison to those without LLE. The lymphedema symptoms identified as problematic (i.e., swelling, heaviness, and numbness) by our sample were consistent with the literature. Overall, our study participants, particularly those living with LLE, reported a high confidence in the GCLQ’s ability to detect LLE symptoms. The findings also revealed that although women are aware of their condition, not all are actively seeking treatment—a concerning fact since lymphedema is a chronic and progressive condition. As cited by the International Society of Lymphology, lymphedema treatment “should be directed at preventing, reversing or ameliorating the specific lymphatic defect and restoring function and quality of life” [4]. Identifying lymphedema as early as possible has been noted by specialists caring for these patients, as unmanaged lymphedema can progress, causing difficulty in management as well as quality of life disruption [19]. Although many women receive information from lymphedema specialists or physical therapists, this is usually done after symptoms have been noted. It is imperative that this issue be addressed with screening modalities and educational programs before the onset of symptoms. Nurses can take a leadership role to address this important survivorship issue in the clinical care setting through these screening modalities and educational programs.
Self-reported symptoms have been shown to be helpful in detecting upper lymphedema in breast cancer populations at an early stage. As such, it is recommended that objective limb measurements be conducted in conjunction with subjective symptom assessments in future study designs. Assessment of lymphedema self-reported symptoms could be easily incorporated into the clinical care setting as a simple, feasible, time-efficient screening/triaging method to identify women with or at a high risk of developing lymphedema for further evaluation (i.e., limb measurement) and early intervention.
Because of these findings, we plan to investigate the association between symptoms of LLE and predicting the development or identifying early signs of LLE (as demonstrated in breast cancer populations experiencing upper extremity lymphedema) using the GCLQ. The predictive value of self-reported lymphedema symptoms (GCLQ) in detecting early onset lymphedema will be formally tested within a large national cooperative group study.
Appendix 1. Gynecologic Cancer Lymphedema Questionnaire (GCLQ)
The following questions regarding your experiences with movement, use and sleep in the past 4 weeks.
| 1. | Do you have limited movement of your hip? | Yes □ | No □ |
| 2. | Do you have limited movement of your knee? | Yes □ | No □ |
| 3. | Do you have limited movement of your ankle? | Yes □ | No □ |
| 4. | Do you have limited movement of your foot? | Yes □ | No □ |
| 5. | Do you have limited movement of your toes? | Yes □ | No □ |
| 6. | Does your leg or foot feel weak? | Yes □ | No □ |
The following questions relate to symptoms you might experience on your foot, leg, hip, groin or your lower body in the past 4 weeks. Please check one answer per line.
| 7. | Have you experienced tenderness? | Yes □ | No □ |
| 8. | Have you experienced swelling? | Yes □ | No □ |
| 9. | Have you experienced swelling with pitting? (Pitting is when you press firmly on your skin and the dent stays long enough to feel it when you slide the pad of your finger across it.) | Yes □ | No □ |
| 10. | Have you experienced redness? | Yes □ | No □ |
| 11. | Have you experienced blistering? | Yes □ | No □ |
| 12. | Have you experienced firmness/tightness? | Yes □ | No □ |
| 13. | Have you experienced increased temperature in your leg? | Yes □ | No □ |
| 14. | Have you experienced heaviness? | Yes □ | No □ |
| 15. | Have you experienced numbness? | Yes □ | No □ |
| 16. | Have you experienced stiffness? | Yes □ | No □ |
| 17. | Have you experienced aching? | Yes □ | No □ |
| 18. | Have you experienced hip swelling? | Yes □ | No □ |
| 19. | Have you experienced groin swelling? (genital, labia/vulvar) | Yes □ | No □ |
| 20. | Have you experienced pockets of fluid? | Yes □ | No □ |
Footnotes
- Jeanne Carter, PhD: no conflicts of interest to declare
- Leigh Raviv, BA: no conflicts of interest to declare
- Kathleen Appollo, RN: no conflicts of interest to declare
- Alexia Iasonos, PhD: no conflicts of interest to declare
- Raymond E. Baser, MS: no conflicts of interest to declare
- Richard R. Barakat, MD: no conflicts of interest to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lymphoedema Framework. International consensus. London: MEP Ltd; 2006. Best Practice for the Management of Lymphoedema. [Google Scholar]
- 2.International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema. Consensus document of the International Society of Lymphology. Lymphology. 2003;36:84–91. [PubMed] [Google Scholar]
- 3.Badger C, Preston N, Seers K, Mortimer P. Physical therapies for reducing and controlling lymphoedema of the limbs. Cochrane Database Syst Rev. 2004;(4):CD003141. doi: 10.1002/14651858.CD003141.pub2. [DOI] [PubMed] [Google Scholar]
- 4.International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema. 2009 Consensus Document of the International Society of Lymphology. Lymphology. 2009;42:51–60. [PubMed] [Google Scholar]
- 5.Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer. 2001;92:1368–77. doi: 10.1002/1097-0142(20010915)92:6<1368::aid-cncr1459>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 6.Ganz PA. The quality of life after breast cancer--solving the problem of lymphedema. N Engl J Med. 1999;340:383–5. doi: 10.1056/NEJM199902043400511. [DOI] [PubMed] [Google Scholar]
- 7.Passik SD, Newman M, Brennan M, Tunkel R. Predictors of psychological distress, sexual dysfunction and physical functioning among women with upper-extremity lymphedema related to breast cancer. Psycho-oncology. 1995;4:255–63. [Google Scholar]
- 8.Passik SD, McDonald MV. Psychosocial aspects of upper extremity lymphedema in women treated for breast carcinoma. Cancer. 1998;83(12 Suppl American):2817–20. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2817::aid-cncr32>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 9.Burak WE, Hollenbeck ST, Zervos EE, Hock KL, Kemp LC, Young DC. Sentinel lymph node biopsy results in less postoperative morbidity compared with axillary lymph node dissection for breast cancer. Am J Surg. 2002;183:23–7. doi: 10.1016/s0002-9610(01)00848-0. [DOI] [PubMed] [Google Scholar]
- 10.Schrenk P, Rieger R, Shamiyeh A, Wayand W. Morbidity following sentinel lymph node biopsy versus axillary lymph node dissection for patients with breast carcinoma. Cancer. 2000;88:608–14. doi: 10.1002/(sici)1097-0142(20000201)88:3<608::aid-cncr17>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 11.Janda M, Obermair A, Cella D, Crandon AJ, Trimmel M. Vulvar cancer patients’ quality of life: a qualitative assessment. Int J Gynecol Cancer. 2004;14:875–81. doi: 10.1111/j.1048-891X.2004.14524.x. [DOI] [PubMed] [Google Scholar]
- 12.Pereira de Godoy JM, Braile DM, de Fatima Godoy M, Longo O., Jr Quality of life and peripheral lymphedema. Lymphology. 2002;35:72–5. [PubMed] [Google Scholar]
- 13.Ryan M, Stainton MC, Jaconelli C, Watts S, MacKenzie P, Mansberg T. The experience of lower limb lymphedema for women after treatment for gynecologic cancer. Oncol Nurs Forum. 2003;30:417–23. doi: 10.1188/03.ONF.417-423. [DOI] [PubMed] [Google Scholar]
- 14.Beesley V, Janda M, Eakin E, Obermair A, Battistutta D. Lymphedema after gynecological cancer treatment: prevalence, correlates, and supportive care needs. Cancer. 2007;109:2607–14. doi: 10.1002/cncr.22684. [DOI] [PubMed] [Google Scholar]
- 15.Bergmark K, Avall-Lundqvist E, Dickman PW, Henningsohn L, Steineck G. Lymphedema and bladder-emptying difficulties after radical hysterectomy for early cervical cancer and among population controls. Int J Gynecol Cancer. 2006;16:1130–9. doi: 10.1111/j.1525-1438.2006.00601.x. [DOI] [PubMed] [Google Scholar]
- 16.Armer JM, Radina ME, Porock D, Culbertson SD. Predicting breast cancer-related lymphedema using self-reported problems. Nurs Res. 2003;52:370–8. doi: 10.1097/00006199-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Armer JM. The problem of post-breast cancer lymphedema: impact and measurement issues. Cancer Inves. 2005;1:76–83. [PubMed] [Google Scholar]
- 18.Ridner SH. Quality of life and a symptom cluster associated with breast cancer treatment-related lymphedema. Support Care Cancer. 2005;13:904–115. doi: 10.1007/s00520-005-0810-y. [DOI] [PubMed] [Google Scholar]
- 19.Langbecker D, Hayes SC, Newman B, Janda M. Treatment for upper limb and lower limb lymphedema by professionals specializing in lymphedema care. Eur J Cancer Care. 2008;17:557–64. doi: 10.1111/j.1365-2354.2007.00878.x. [DOI] [PubMed] [Google Scholar]
- 20.Fleiss JL. Statistical Methods for Rates and Proportions. 2. Somerset, NJ: John Wiley & Sons, Inc; 1981. [Google Scholar]
- 21.Muthen LK, Muthen BO. Mplus user’s guide version 4. Los Angeles: Muthen & Muthen; 2006. [Google Scholar]
- 22.Zumbo BD, Gadermann AM, Zeisser C. Ordinal versions of coefficients alpha and theta for Likert rating scales. J Modern Applied Statistical Method. 2007;6:21–9. [Google Scholar]
- 23.SPSS for Windows, Version 15. Chicago: SPSS Inc; 2007. [Google Scholar]
- 24.SAS Institute Inc. SAS Software Version 9.2. Cary, NC: SAS Institute Inc; 2008. [Google Scholar]