Abstract
The subventricular zone (SVZ) has been implicated in the origination, development, and biological behavior of gliomas. Tumor-SVZ contact is also postulated to be a poor prognostic factor in glioblastomas. We aimed to evaluate the prognostic consequence of the anatomical involvement of low-grade gliomas with the SVZ. To that end, we reviewed 143 patients with diffuse astrocytomas, and tumor lesions were manually delineated on magnetic resonance images. We initially investigated the prognostic role of SVZ contact in all patients. Additionally, we investigated the influence of the anatomical proximity of the tumor lesion centroids to the SVZ in the SVZ-involved patient cohorts, as well as location within the SVZ. We found SVZ contact with tumors to be a significant prognostic factor of overall survival in all patients with diffuse astrocytomas (p = 0.027). In the SVZ-involved cohort, a shorter distance from the tumor centroid to the SVZ (≤30 mm) correlated with shorter overall survival (p = 0.022) on univariate analysis. However, there was no significant difference in overall survival with respect to the SVZ region involved with the tumor (p = 0.930). Multivariate analysis showed that a shorter distance between the tumor centroid and the SVZ (p = 0.039) was significantly associated with poor overall survival in SVZ-involved patients. Hence, this study helps establish the prognostic role of the anatomical interaction of tumors with the SVZ in low-grade astrocytomas.
Introduction
The tumorigenesis of gliomas is still not completely understood. Glial cells were once believed to be the only type of cells possessing proliferative capability in the adult brain, and gliomas were thought to originate from the neoplastic transformation of these fully differentiated glia [1,2]. Subsequently, investigators proposed the cancer stem cell (CSC) theory [3,4], which suggested that several primary brain tumors, including gliomas, are derived from the malignant transformation of multipotent neural stem cells (NSC) [1,5,6]. The brain’s subventricular zone (SVZ), which contains a large amount of NSCs, was postulated to be the region that is involved in the formation of gliomas.
Previous clinical studies investigated the relationship between gliomas and the SVZ. Tumors that are in contact with the SVZ exhibit unique biological characteristics; they are most likely to present multifocal lesions on magnetic resonance (MR) images at diagnosis [7] and to be associated with shorter overall survival (OS) [8–11]. Radiotherapy co-targeted to the SVZ improves the progression-free survival (PFS) of patients compared to radiotherapy targeted at the tumor alone [12]. Additionally, increasing the mean radiotherapy dose to the ipsilateral SVZ was associated with significantly improved OS [13,14].
Previous studies that investigated the association between the SVZ and gliomas mainly focused on glioblastoma, whereas low-grade gliomas were generally overlooked. The current clinical study enrolled a cohort of patients with diffuse astrocytomas (WHO grade II). The locations of tumor lesions were examined in relation to the SVZ using MR images to assess direct contact. In cases where tumor-SVZ contact was evident, the distance from the tumor centroid to the SVZ was measured, and the anatomical region of the SVZ that was involved with the tumor was determined. The prognostic roles of clinical characteristics and radiological features were evaluated using multivariate survival analyses.
Materials and Methods
Patients
We conducted a retrospective review of patients who were newly diagnosed with primary diffuse astrocytoma (WHO grade II) histopathologically and were treated at the Glioma Treatment Center of Beijing Tiantan Hospital between 2006 and 2010. One hundred and forty-three patients who met the following criteria were included: pathologically confirmed astrocytoma (WHO grade II), preoperative T2-weighted MR images of the brain, treated with surgical resection, and no prior craniotomy or stereotactic biopsy. Nineteen patients were lost to follow-up and thus excluded. The extent of surgical resection was determined by comparing the preoperative and postoperative MR images. The gross total resection (GTR) was defined by removing all the abnormalities on T2 weighted sequences. Meanwhile, <GTR was defined as any resection that failed to achieve GTR. Clinical variables including age, sex, and preoperative Karnofsky Performance Status Scale (KPS) score were derived from medical documents. Age was dichotomized into <40 years and ≥40 years. Furthermore, the KPS score was dichotomized as <80 and ≥80.
Magnetic resonance imaging
T2-weighted images were acquired using standard pulse sequences on a Magnetom Trio 3T scanner (Siemens AG, Erlangen, Germany). The T2-weighted image parameters included TR = 5800 ms, TE = 110 ms, flip angle = 150°, field of view = 240 × 188 mm2, and voxel size = 0.6 × 0.6 × 5 mm3.
Ethics statement
All research activities in this study were approved by the Ethics Committee of the Beijing Tiantan Hospital, and written informed consent was obtained from all patients.
Neuroimaging analysis
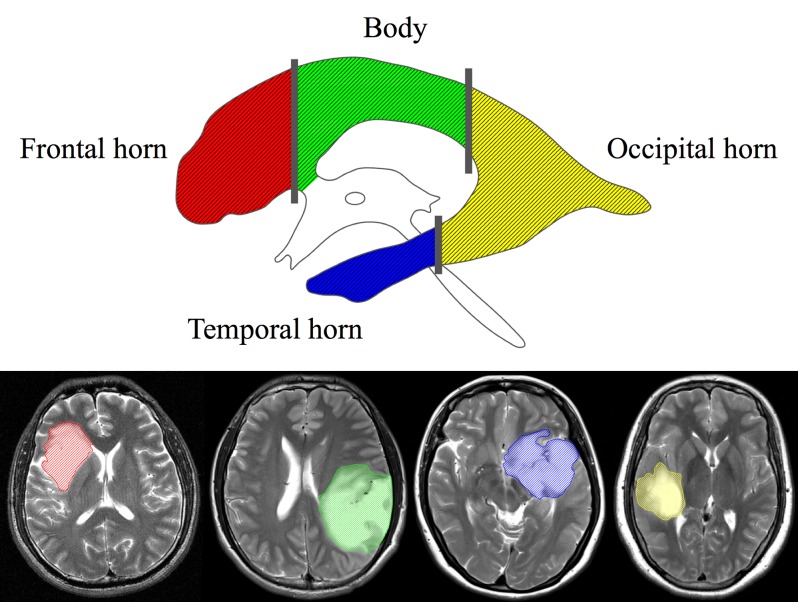
Manual segmentation is still the reference method for image-based lesion analysis, despite the rapid development of automated lesion segmentation [15]. Tumor lesions and the lateral ventricles were manually identified by two neurosurgeons on T2-weighted images using the MRIcron software (http://www.mccauslandcenter.sc.edu/mricro/) (Fig 1). All images were then reevaluated by an independent neuroradiologist who determined the tumor and ventricle masks that were further used in the analysis. SVZ involvement was defined as the overlapping of the identified tumor region with the ventricular region, indicating contact. The centroid of a tumor (i.e., the geometric center of the tumor) was defined as the location of the mean coordinates of all voxels contained in the masked region in three orthogonal directions, and were calculated using the Matlab software (R2012a, MathWorks). We further calculated the shortest distance from the edge of the tumor to the ventricles (TS) as well as the shortest distance from the tumor centroid to the edge of the lateral ventricles (CS). The SVZ was divided into four regions along the rostrocaudal axis as described previously by A. L. Rhoton [16]: 1) frontal horn, 2) body, 3) occipital horn, and 4) temporal horn (Fig 2). The number of cases of tumor involvement for each region of the SVZ was determined.
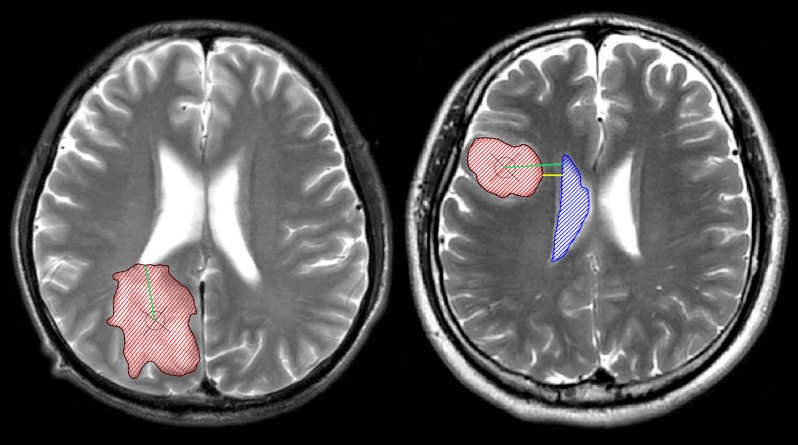
Fig 1. The anatomical relationship between tumors and the subventricular zone (SVZ).
The left panel shows a tumor contacting the SVZ; the right panel shows a tumor that is not involved with the SVZ. The manually defined tumor lesions and ventricles are shown as red and blue masks, respectively. The centroid of the tumor is marked with a cross within a circle. The green lines represent the shortest distances from the tumor centroid to the lateral ventricles (CS), while the yellow line represents the shortest distance between the tumor edge and the lateral ventricles (TS). In this study, 143 patients with diffuse astrocytomas were separately grouped by CS/TS, and variables such as survival, age, tumor volume, extent of resection, tumor location, and others were compared among these groups.
Fig 2. Subregions of the subventricular zone (SVZ).
The four subregions are each shown in a different color (upper panel). Examples of tumors involved in each region are depicted with the corresponding color (lower panel).
Survival analysis
The current study used PFS and OS as the main outcome determinants. Images were assessed by experienced radiological specialists, and progression was confirmed when observed unequivocally on radiography or when clinical symptoms deteriorated; progression was defined by development of new lesions, an increase of enhancement, or an increase in lesion size on T2 or fluid attenuation inversion recovery imaging that was not attributable to a radiation effect. PFS was calculated from the date of the primary surgical resection to disease progression, or to the last recorded date of follow-up without progression. OS was defined as the duration from the date of the primary surgery to the date of death or last contact. If a patient died without experiencing progression, PFS information was censored at the date of death during analyses. The PFS and OS of patient cohorts were determined by Kaplan-Meier analysis that was performed based on a log-rank test. In the group of SVZ-involved tumors, a Cox proportional hazards model was applied to evaluate the prognostic role of clinical factors including age, sex, preoperative KPS, tumor volume, CS, the region of the SVZ with tumor involvement, extent of surgery, radiation, and chemotherapy. Factors with a probability value (p) <0.05 on univariate analyses were subjected to multivariate Cox regression analyses. In cases where tumors contacted multiple regions of the SVZ, the region with the greatest area of involvement was considered for survival analysis.
Results
Patient population and tumor characteristics
A total of 143 patients with supratentorial diffuse astrocytomas were systematically reviewed. Detailed patient characteristics are shown in Table 1. There were 115 patients (80%) with tumors involving the SVZ. Specifically, 58 tumors involved the frontal horn, 49 the body, 38 the occipital horn, and 54 the temporal horn. The shortest distance between the tumor centroid and ventricles was 53.2 ± 88.1 mm (mean ± standard deviation) for tumors involving the SVZ. There were 28 tumors (20%) that did not involve the SVZ; the shortest distance between the tumor centroid and the ventricles in these was 44.3 ± 67.7 mm and the shortest distance from the tumor edge to the ventricles was 13.6 ± 11.0 mm. Among the 143 patients, 35 experienced tumor progression and 31 died. The median follow-up time was 1736 days for those patients still alive during follow-up.
Table 1. Clinical characteristics.
| Variables | SVZ involved | SVZ non-involved | p *,b | ||
|---|---|---|---|---|---|
| CS ≤30 mm | CS >30 mm | p *,a | |||
| Number (%) | 53 (46) | 62 (54) | 28 | ||
| Age <40 years (%) | 31 (58) | 43 (69) | 0.225 | 18 (64) | 0.995 |
| Sex (Male, %) | 37 (70) | 40 (65) | 0.547 | 21 (75) | 0.411 |
| KPS <80 | 6 (11) | 9 (15) | 0.612 | 2 (7) | 0.387 |
| Volume <60 cm3 | 27 (51) | 27 (44) | 0.428 | 22(79) | 0.003 |
| Tumor locationc | |||||
| Side (Left,%) | 32 (60) | 29 (47) | 0.145 | 17 (61) | 0.465 |
| Frontal lobed (%) | 42 (51) | 44 (37) | 0.308 | 22 (63) | 0.676 |
| Temporal lobe (%) | 21 (26) | 38 (32) | 0.020 | 4 (11) | <0.001 |
| Others (%) | 19 (23) | 36 (31) | 0.017 | 9 (26) | 0.134 |
| Regions contacting SVZ | |||||
| Frontal horn (%) | 26 (30) | 32 (29) | 0.785 | ||
| Temporal horn (%) | 18 (21) | 36 (32) | 0.010 | ||
| Body (%) | 28 (32) | 21 (19) | 0.040 | ||
| Occipital horn (%) | 15 (17) | 23 (20) | 0.318 | ||
| Extent of resection | |||||
| GTR (%) | 15 (28) | 17 (27) | 0.916 | 19 (68) | <0.001 |
| Radiation therapy (%) | 35 (66) | 51 (82) | 0.046 | 20 (71) | 0.716 |
| Chemotherapy (%) | 11 (21) | 11 (18) | 0.682 | 4 (14) | 0.551 |
CS, the shortest distance between the tumor centroid and the edge of the lateral ventricles; GTR, gross total resection; KPS, Karnofsky Performance Status scale; SVZ, subventricular zone.
*, Chi-square test.
a, Comparison between SVZ-involved tumors with different CS. For “Regions contacting SVZ”, the chi-square test was performed for each subgroup versus the other three subgroups combined.
b, Comparison between SVZ-involved tumors and non-involved tumors.
c, Some tumors involve more than one lobe; hence, the total count is greater than 115.
d, Frontal tumors may involve both the frontal horn and the body of the SVZ. This makes the total number of frontal tumors greater than if counting those that only involve the frontal horn.
We performed additional t-tests to compare the distance between the tumor centroid and the SVZ for tumors involving the SVZ vs. those not involving the SVZ (p = 0.617). The results revealed no significant difference between the groups, despite the fact that a larger mean distance is observed in tumors involving the SVZ. In addition, we compared the volumes of tumors involving the body and temporal horn of the SVZ by using the t-test; the result indicated no significant difference between the two groups (p = 0.215).
Univariate analysis
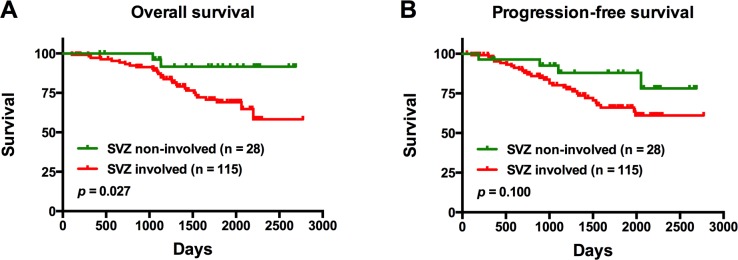
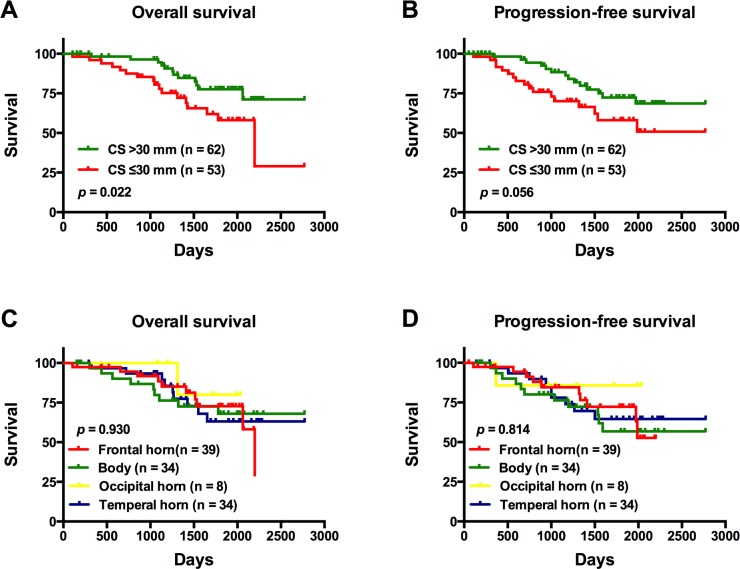
On univariate analysis, patients with tumors involving the SVZ were found to have a significantly shorter OS compared to those with tumors not involving the SVZ (p = 0.027) (Fig 3A). Notably, there was no significant difference in PFS between the same groups of patients (p = 0.100) (Fig 3B). In patients with SVZ involvement, those with tumors exhibiting a CS ≤30 mm from the SVZ were found to have a significantly shorter OS compared to patients with tumor centroids located further away from the SVZ (CS >30 mm; p = 0.022) (Fig 4A); there was no significant difference in PFS between these same patient groups (p = 0.056) (Fig 4B). In the same set of patients, there was no significant difference in OS with respect to the region of the SVZ where the tumor was involved (p = 0.930) (Fig 4C); similarly; the SVZ region of tumor involvement was not a significant factor for PFS (p = 0.814) (Fig 4D).
Fig 3. Overall survival and progression-free survival outcomes of patients with tumors contacting the subventricular zone (SVZ), and those with tumors not involved in the SVZ.
There were 143 patients enrolled in this study; 35 patients experienced progression and 31 died.
Fig 4. The overall survival (OS) and progression-free survival (PFS) of patients with tumors involving the subventricular zone (SVZ).
(A) OS and (B) PFS Kaplan-Meier curves of patients according to the distance between the tumor centroid and the SVZ. (C) OS and (D) PFS Kaplan-Meier curves of patients with tumors according to the SVZ region of involvement.
Other clinical factors found to be significant predictors of a shorter OS included age ≥40 years (p = 0.047), tumor volume ≥60 cm3 (p = 0.030), and an extent of surgical resection <GTR (p = 0.012). Moreover, age ≥40 years (p = 0.024), tumor volume ≥60 cm3 (p = 0.043), and extent of surgical resection <GTR (p = 0.018) were significant predictors of a shorter PFS (Table 2).
Table 2. Univariate analysis of survival outcomes in patients with SVZ-involved tumors.
| Characteristic | PFS | OS | ||||
|---|---|---|---|---|---|---|
| p | HR | 95% CI | p | HR | 95% CI | |
| Age ≥40 years | 0.024 | 2.259 | 1.116–4.574 | 0.047 | 2.093 | 1.009–4.338 |
| Sex (Male) | 0.718 | 1.149 | 0.541–2.441 | 0.781 | 1.118 | 0.508–2.462 |
| KPS <80 | 0.375 | 1.543 | 0.592–4.024 | 0.351 | 1.584 | 0.603–4.160 |
| Volume ≥60 cm3 | 0.043 | 2.145 | 1.025–4.492 | 0.030 | 2.349 | 1.085–5.084 |
| CS ≤30 mm | 0.061 | 1.977 | 0.970–4.027 | 0.026 | 2.353 | 1.108–4.919 |
| Regions contacting SVZ | ||||||
| Frontal horn/others | 0.850 | 1.070 | 0.528–2.171 | 0.700 | 1.156 | 0.554–2.409 |
| <GTR | 0.018 | 4.227 | 1.284–13.909 | 0.012 | 6.278 | 1.491–26.431 |
| Radiation | 0.504 | 0.747 | 0.318–1.756 | 0.333 | 0.652 | 0.275–1.548 |
| Chemotherapy | 0.752 | 1.139 | 0.508–2.553 | 0.618 | 1.232 | 0.544–2.789 |
CI, confidence interval; CS, the shortest distance from the tumor centroid to the edge of the lateral ventricles; GTR, gross total resection; HR, hazard ratio; KPS, Karnofsky Performance Status scale; OS, overall survival; PFS, progression-free survival; SVZ, subventricular zone.
Multivariate analysis
In all patients with tumors involving the SVZ, significant survival factors as determined by multivariate analysis are shown in Table 3. Age ≥40 years (p = 0.042), tumor volume ≥60 cm3 (p = 0.029), CS ≤30 mm (p = 0.039), and extent of surgical resection <GTR (p = 0.025) were significantly associated with a shorter OS. However, only age ≥40 years (p = 0.014) and extent of surgical resection <GTR (p = 0.013) were identified as worse prognostic factors for PFS.
Table 3. Multivariate analysis of survival outcomes in patients with SVZ-involved tumors.
| Predictor | p | HR | 95% CI |
|---|---|---|---|
| PFS | |||
| Age ≥40 years | 0.014 | 2.430 | 1.199–4.927 |
| Volume ≥60 cm3 | 0.076 | 1.998 | 0.930–4.293 |
| <GTR | 0.013 | 4.526 | 1.373–14.915 |
| OS | |||
| Age ≥40 years | 0.042 | 2.192 | 1.030–4.665 |
| Volume ≥60 cm3 | 0.029 | 2.461 | 1.094–5.531 |
| CS ≤30 mm | 0.039 | 2.260 | 1.042–4.904 |
| <GTR | 0.025 | 5.273 | 1.235–22.511 |
CI, confidence interval; CS, the shortest distance from the tumor centroid to the edge of the lateral ventricles; GTR, gross total resection; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; SVZ, subventricular zone.
Discussion
The SVZ has been the focus of increasing attention by neurooncologists because of its key role in tumorigenesis. Using quantitative neuroimaging assessment of 143 low-grade astrocytomas, we demonstrated that SVZ tumor involvement was associated with worse patient prognosis. In particular, the distance from the tumor centroid to the SVZ was found to be a significant prognostic factor in astrocytomas that involved the SVZ. To our knowledge, this is the first study to investigate the prognostic role of the SVZ in low-grade gliomas.
The SVZ was revealed to be closely associated with the origination, development, and biological behavior of gliomas [1,7,9], although the specific mechanism of glioma tumorigenesis is still unclear. Previous studies using animal models revealed that the administration of a carcinogen induced the formation of tumors that arose preferentially in the SVZ [17,18]. In humans, glioblastoma was also found to be preferentially located in this particular region [19]. Gliomas that were not located in the SVZ are postulated to have originated there and migrated to other regions of the brain [20]. Eighty percent of diffuse astrocytomas were found to involve the SVZ in the current study; this proportion was consistent with that of a previous report on low-grade gliomas [21]. The high rate of SVZ involvement indicates a close association between glioma and the SVZ.
As we found the prognostic role of SVZ involvement to be similar to that described in several previous studies of glioblastoma [8–10], and considering that patients with SVZ involvement comprised the majority of our cohort, we explored the prognostic role of the association of low-grade gliomas with specific anatomical regions of the SVZ. First, we examined whether or not the distance between the tumor centroid and the SVZ influenced prognosis. The centroid of the lesion was used to represent the location of the tumor in previous studies [8,22]. During tumor growth, the location of the tumor centroid remains virtually unchanged [22], which is why it was deemed a suitable point from which to measure the tumor’s proximity to the SVZ. We found that CS was associated with prognosis in patients with tumors contacting the SVZ. Patients with a shorter CS (≤30 mm) were found to have worse prognoses than patients with a longer CS (>30 mm). This result appears to support the current hypothesis that tumors located closer to the SVZ are more likely to be affected by both the NSCs and the SVZ microenvironment.
NSCs appear to preferentially migrate towards tumors [23,24]. Non-tumorigenic multipotent stem cells, which are thought to increase the biological aggressiveness of glioma-initiating cells, have been found in gliomas [25]. Such stem cells are possibly recruited from the SVZ; therefore, tumors closer to the SVZ are likely to have greater malignant potential. Additionally, the microenvironment of the SVZ was found to be significantly different from other regions of the adult brain in that it supports the growth and reproduction of NSCs [26,27]. Therefore, exposure to this particular microenvironment is likely to alter the biological features of CSCs [28,29]. These two aspects may together explain our findings that the proximity of tumors to the SVZ predicts a poor prognosis.
We additionally examined whether tumors originating from different regions of the SVZ have distinct biological characteristics. NSCs are a group of inhomogeneous cells [30–34]. Different regions of the brain ventricles harbor NSCs that may differentiate into various types of cells during brain development [30,34–36]. Importantly, previous studies suggested that biological characteristics of tumors were associated with the location from which their nonmalignant predecessors originated [37,38]. Our study addressed this issue by examining the effect on prognosis of the location of the tumor with respect to the SVZ. Of the 115 tumors that contacted the SVZ, 50% involved the frontal horn, while the smallest proportion (33%) contacted the occipital horn. On univariate analysis, the location of involvement within the SVZ was not a significant survival factor. This finding can be attributed to the fact that Rhoton’s segmentation of the SVZ is based on anatomy and may not represent the heterogeneity of NSCs. A new classification of the SVZ that relies on cellular characteristics is expected to be utilized in future studies.
There are several limitations in this study. The geometric centers of the analyzed tumors described the anatomical location of tumor, but are not necessarily the tumors’ original locations. Although a mathematical model that simulates tumor growth was previously investigated [39], the algorithm requires further validation on the histological level. It remains impractical to identify the location of the tumor origin using only MR imaging. Additionally, current MR technology can hardly delineate the pathological boundary of a tumor lesion. In order to reduce inaccuracies, all tumor boundaries were manually defined by two experienced neurosurgeons and then reevaluated by an independent senior neuroradiologist. This manual segmentation of tumor lesions has been widely performed during volumetric analysis under similar image resolutions. Future studies are expected to provide direct evidence for glioma tumorigenesis in the SVZ.
It is also worth noting that the dentate gyrus within the hippocampus is another niche besides the SVZ that is associated with neurogenesis [1]. Temporal tumors involving this region may have a distinct biology; however, the dentate gyrus is adjacent to the temporal horn of the lateral ventricles, and it is difficult to distinguish which of these two regions a tumor is involved with using MR imaging. Future studies ought to clarify the variations in the niches harboring NSCs at the cytological level. Finally, the proportion of male subjects in our cohort was high compared to females; however, there were no significant difference between the groups according to sex, and this did not affect the results of our survival analysis.
Conclusions
Our study demonstrated a prognostic consequence for SVZ involvement in low-grade astrocytomas in that SVZ involvement with tumors was associated with worse patient prognosis. Specifically, a shorter distance from the tumor centroid to the SVZ (≤30 mm) was found to be a significant prognostic factor for astrocytomas that contact the SVZ. These findings may serve as a reference for identifying high-risk patients who require more aggressive treatments.
Supporting Information
(XLSX)
Acknowledgments
We would like to thank Yuling Yang for tissue sample collection and clinical data retrieval.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Funding for this work was provided by the National 973 Program (No.2015CB755500) [Tao Jiang], http://www.973.gov.cn; and the Research Special Fund for Public Welfare industry of health (No. 201402008) [Wenbin Ma], http://www.shenjingzhongliu.org.
References
- 1. Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med. 2005; 353: 811–822. [DOI] [PubMed] [Google Scholar]
- 2.Holland EC. Progenitor cells and glioma formation. Curr Opin Neurol. 2001; 14: 683–688. [DOI] [PubMed] [Google Scholar]
- 3.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004; 64: 7011–7021. [DOI] [PubMed] [Google Scholar]
- 4.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004; 432: 396–401. [DOI] [PubMed] [Google Scholar]
- 5.Alcantara Llaguno S, Chen J, Kwon CH, Jackson EL, Li Y, Burns DK, et al. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell. 2009; 15: 45–56. 10.1016/j.ccr.2008.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu Y, Guignard F, Zhao D, Liu L, Burns DK, Mason RP, et al. Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma. Cancer Cell. 2005; 8: 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim DA, Cha S, Mayo MC, Chen MH, Keles E, VandenBerg S, et al. Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro Oncol. 2007; 9: 424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young GS, Macklin EA, Setayesh K, Lawson JD, Wen PY, Norden AD, et al. Longitudinal MRI evidence for decreased survival among periventricular glioblastoma. J Neurooncol. 2011; 104: 261–269. 10.1007/s11060-010-0477-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jafri NF, Clarke JL, Weinberg V, Barani IJ, Cha S. Relationship of glioblastoma multiforme to the subventricular zone is associated with survival. Neuro Oncol. 2013; 15: 91–96. 10.1093/neuonc/nos268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adeberg S, Bostel T, Konig L, Welzel T, Debus J, Combs SE. A comparison of long-term survivors and short-term survivors with glioblastoma, subventricular zone involvement: a predictive factor for survival? Radiat Oncol. 2014; 9: 95 10.1186/1748-717X-9-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaichana KL, Pendleton C, Chambless L, Camara-Quintana J, Nathan JK, Hassam-Malani L, et al. Multi-institutional validation of a preoperative scoring system which predicts survival for patients with glioblastoma. J Clin Neurosci. 2013; 20: 1422–1426. 10.1016/j.jocn.2013.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evers P, Lee PP, DeMarco J, Agazaryan N, Sayre JW, Selch M, et al. Irradiation of the potential cancer stem cell niches in the adult brain improves progression-free survival of patients with malignant glioma. BMC Cancer. 2010; 10: 384 10.1186/1471-2407-10-384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L, Guerrero-Cazares H, Ye X, Ford E, McNutt T, Kleinberg L, et al. Increased subventricular zone radiation dose correlates with survival in glioblastoma patients after gross total resection. Int J Radiat Oncol Biol Phys. 2013; 86: 616–622. 10.1016/j.ijrobp.2013.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta T, Nair V, Paul SN, Kannan S, Moiyadi A, Epari S, et al. Can irradiation of potential cancer stem-cell niche in the subventricular zone influence survival in patients with newly diagnosed glioblastoma? J Neurooncol. 2012; 109: 195–203. 10.1007/s11060-012-0887-3 [DOI] [PubMed] [Google Scholar]
- 15.Pallud J, Taillandier L, Capelle L, Fontaine D, Peyre M, Ducray F, et al. Quantitative morphological magnetic resonance imaging follow-up of low-grade glioma: a plea for systematic measurement of growth rates. Neurosurgery. 2012; 71: 729–739; discussion 739–740. [DOI] [PubMed] [Google Scholar]
- 16.Rhoton AL Jr. The lateral and third ventricles. Neurosurgery. 2002; 51: S207–271. [PubMed] [Google Scholar]
- 17.Copeland DD, Vogel FS, Bigner DD. The induction of intractranial neoplasms by the inoculation of avian sarcoma virus in perinatal and adult rats. J Neuropathol Exp Neurol. 1975; 34: 340–358. [DOI] [PubMed] [Google Scholar]
- 18.Copeland DD, Bigner DD. The role of the subependymal plate in avian sarcoma virus brain tumor induction: comparison of incipient tumors in neonatal and adult rats. Acta Neuropathol. 1977; 38: 1–6. [DOI] [PubMed] [Google Scholar]
- 19.Ellingson BM, Cloughesy TF, Pope WB, Zaw TM, Phillips H, Lalezari S, et al. Anatomic localization of O6-methylguanine DNA methyltransferase (MGMT) promoter methylated and unmethylated tumors: a radiographic study in 358 de novo human glioblastomas. Neuroimage. 2012; 59: 908–916. 10.1016/j.neuroimage.2011.09.076 [DOI] [PubMed] [Google Scholar]
- 20.Vick NA, Lin MJ, Bigner DD. The role of the subependymal plate in glial tumorigenesis. Acta Neuropathol. 1977; 40: 63–71. [DOI] [PubMed] [Google Scholar]
- 21.Barami K, Sloan AE, Rojiani A, Schell MJ, Staller A, Brem S. Relationship of gliomas to the ventricular walls. J Clin Neurosci. 2009; 16: 195–201. 10.1016/j.jocn.2008.03.006 [DOI] [PubMed] [Google Scholar]
- 22.Bohman LE, Swanson KR, Moore JL, Rockne R, Mandigo C, Hankinson T, et al. Magnetic resonance imaging characteristics of glioblastoma multiforme: implications for understanding glioma ontogeny. Neurosurgery. 2010; 67: 1319–1327; discussion 1327–1318. 10.1227/NEU.0b013e3181f556ab [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeon JY, An JH, Kim SU, Park HG, Lee MA. Migration of human neural stem cells toward an intracranial glioma. Exp Mol Med. 2008; 40: 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci U S A. 2000; 97: 12846–12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bourkoula E, Mangoni D, Ius T, Pucer A, Isola M, Musiello D, et al. Glioma-associated stem cells: a novel class of tumor-supporting cells able to predict prognosis of human low-grade gliomas. Stem Cells. 2014; 32: 1239–1253. 10.1002/stem.1605 [DOI] [PubMed] [Google Scholar]
- 26.Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004; 41: 683–686. [DOI] [PubMed] [Google Scholar]
- 27.Ferron SR, Charalambous M, Radford E, McEwen K, Wildner H, Hind E, et al. Postnatal loss of Dlk1 imprinting in stem cells and niche astrocytes regulates neurogenesis. Nature. 2011; 475: 381–385. 10.1038/nature10229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Filatova A, Acker T, Garvalov BK. The cancer stem cell niche(s): the crosstalk between glioma stem cells and their microenvironment. Biochim Biophys Acta. 2013; 1830: 2496–2508. 10.1016/j.bbagen.2012.10.008 [DOI] [PubMed] [Google Scholar]
- 29.Goffart N, Kroonen J, Rogister B. Glioblastoma-initiating cells: relationship with neural stem cells and the micro-environment. Cancers (Basel). 2013; 5: 1049–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alvarez-Buylla A, Kohwi M, Nguyen TM, Merkle FT. The heterogeneity of adult neural stem cells and the emerging complexity of their niche. Cold Spring Harb Symp Quant Biol. 2008; 73: 357–365. 10.1101/sqb.2008.73.019 [DOI] [PubMed] [Google Scholar]
- 31.Suslov ON, Kukekov VG, Ignatova TN, Steindler DA. Neural stem cell heterogeneity demonstrated by molecular phenotyping of clonal neurospheres. Proc Natl Acad Sci U S A. 2002; 99: 14506–14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giachino C, Basak O, Lugert S, Knuckles P, Obernier K, Fiorelli R, et al. Molecular diversity subdivides the adult forebrain neural stem cell population. Stem Cells. 2014; 32: 70–84. 10.1002/stem.1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nabilsi NH, Deleyrolle LP, Darst RP, Riva A, Reynolds BA, Kladde MP. Multiplex mapping of chromatin accessibility and DNA methylation within targeted single molecules identifies epigenetic heterogeneity in neural stem cells and glioblastoma. Genome Res. 2014; 24: 329–339. 10.1101/gr.161737.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007; 317: 381–384. [DOI] [PubMed] [Google Scholar]
- 35.Young KM, Fogarty M, Kessaris N, Richardson WD. Subventricular zone stem cells are heterogeneous with respect to their embryonic origins and neurogenic fates in the adult olfactory bulb. J Neurosci. 2007; 27: 8286–8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelsch W, Mosley CP, Lin CW, Lois C. Distinct mammalian precursors are committed to generate neurons with defined dendritic projection patterns. PLoS Biol. 2007; 5: e300 10.1371/journal.pbio.0060091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma MK, Mansur DB, Reifenberger G, Perry A, Leonard JR, Aldape KD, et al. Distinct genetic signatures among pilocytic astrocytomas relate to their brain region origin. Cancer Res. 2007; 67: 890–900. [DOI] [PubMed] [Google Scholar]
- 38.Gibson P, Tong Y, Robinson G, Thompson MC, Currle DS, Eden C, et al. Subtypes of medulloblastoma have distinct developmental origins. Nature. 2010; 468: 1095–1099. 10.1038/nature09587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harpold HL, Alvord EC Jr., Swanson KR. The evolution of mathematical modeling of glioma proliferation and invasion. J Neuropathol Exp Neurol. 2007; 66: 1–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.