ABSTRACT
This study aimed to compare the efficacy and safety of HAI fluoropyrimidine (FUDR)/capecitabine or single capecitabine as first-line treatment for elderly patients with unresectable colorectal liver metastases (CLMs). Fifty-one elderly patients with liver-only CLMs were eligible for enrollment. Patients were divided into HAI FUDR /capecitabine group and single capecitabine group randomly. The primary endpoint was median survival time (MST), defined as the time from the date of catheter implantation to the date of death or the date of the last follow-up. The secondary endpoint was objective antitumor response and adverse events. The HAI pump was implanted before chemotherapy. All patients received a 3-week cycle of oral capecitabin. In Group A, the RR and DCR were both 95.8%. In Group B, the RR and DCR were 48.1% and 81.5%, respectively. There was significant difference between the RRs of the 2 groups (P < 0.001). But there was no significant difference between the DCRs of the 2 groups (P = 0.053). There was a statistical difference between the MSTs of the 2 groups (18.5 vs.13 months, P = 0.0312). HAI FUDR combined with oral capecitabine as the first-line treatment for elderly patients with CLMs has promising efficacy and safety.
KEYWORDS: Capecitabine, colorectal liver metastases, floxuridine, hepatic arterial infusion
Introduction
Colorectal cancer (CRC) occupies the fourth place of diagnosed malignant disease over the world.1 The liver is the most common site of CRC metastasis, with 15% of patients presenting with liver metastases as diagnosis and almost 60% developing liver metastases during the disease course.2
Colorectal liver metastases (CLMs) are the major reason for the failure to treatment.3 Though hepatic resection has been proposed as the standard protocol for curing CLMs, 75–85% of patients with liver-only metastases are considered unresectable.4,5 Hence in patients with CLMs, the choices of treatment strategy vary according to factors such as the urgency of operation on the primary lesion, liver function, patient's performance condition, as well as the operation conditions of the institute.6 Especially in the absence of symptoms, primary tumor resection is not recommended.7
Previous studies have suggested that the combination of hepatic arterial infusion (HAI) and systematic chemotherapy can obviously reduce the tumor burden of CRC and thus increase the resection rate.8-10 And this combination is better than systematic chemotherapy alone.11,12
The hepatic extraction rate with HAI of floxuridine (FUDR) is 95% in patients with unresectable CLMs. HAI FUDR can increase the possibility of tumor response and might improve liver function, thus producing minimal systemic toxicity.13,14 From Oct. 2010, HAI FUDR has been applied to treat patients with CLMs in our department.
Capecitabine (Xeloda; Hoffmann-La Roche Inc., Nutley, NJ) is an oral fluoropyrimidine with similar efficacy to bolus 5-fluorouracil/folinic acid as the first-line treatment for metastatic CRC. The efficacy and safety of capecitabine as single-agent therapy has been established based on comparisons with bolus 5-fluorouracil/folinic acid regimens. Besides, the oral administration is more convenient.15,16
In this article we compare the efficacy and safety of HAI FUDR/capecitabine or single capecitabine in patients with CLMs. We aim to provide evidences for the application of HAI/capecitabine treating patients with CLMs clinically.
Results
Patient baseline characteristics
Between June 2011 and May 2014, we treated 51 consecutively eligible patients with CLMs were enrolled in our department. Patients were divided into HAI/capecitabine group and single capecitabine group based on their wishes. Of these patients, 24 were in Group A (HAI + oral capecitabine) and 27 in Group B (oral capecitabine alone). There were no statistical differences among the clinicopathological characteristics of the 2 groups (Table 1). No patients were lost to follow-up.
Table 1.
Baseline of patients' characteristics (n = 51).
| Characteristics | Total (n = 51) | Group A (n = 24) | Group B (n = 27) | P |
|---|---|---|---|---|
| Age (year) | 0.781 | |||
| Median | 78 | 78 | 77.5 | |
| Range | 75–82 | 75–80 | 75–82 | |
| Sex | 0.778 | |||
| Male | 33 | 15 | 18 | |
| Female | 18 | 9 | 9 | |
| Performance status | 0.540 | |||
| 0 | 16 | 9 | 7 | |
| 1 | 26 | 12 | 14 | |
| 2 | 9 | 3 | 6 | |
| Time to liver metastases | 0.546 | |||
| Synchronous | 21 | 9 | 7 | |
| Metachronous | 30 | 15 | 20 | |
| Site of primary cancer | 0.404 | |||
| Colon | 28 | 10 | 15 | |
| Rectum | 23 | 14 | 12 | |
| Lobulor involvement | 0.264 | |||
| Bilobar | 27 | 15 | 12 | |
| Unilobar | 24 | 9 | 15 | |
| Number of lesions | 0.555 | |||
| ≤.5 | 19 | 7 | 12 | |
| > 5 | 32 | 17 | 15 | |
| Baseline of CEA | 0.405 | |||
| > 200 ng/ml | 22 | 12 | 10 | |
| < 200 ng/ml | 29 | 12 | 17 |
Abbreviations: CEA, carcinoembryonic antigen; LDH, lactate dehydrogenase
Group A: hepatic arterial infusion of fluorouracil + capecitabine; Group B: capecitabine alone
Response
In Group A, there were 6 complete responses (CRs), 17 partial responses (PRs), no stable disease (SD) and 1 progressive disease (PD), the response rate (RR) and disease control rate (DCR) were both 95.8%. In Group B, there were no CR, 13 PRs, 9 SDs and 5 PDs, the RR and DCR were 48.1% and 81.5%, respectively. There was significant difference between the RRs of the 2 groups (P < 0.001). But there was no significant difference between the DCRs of the 2 groups (P = 0.053). See Table 2.
Table 2.
Objective response evaluated by CT scan [n (%)].
| Group A (n = 24) | Group B (n = 27) | P | |
|---|---|---|---|
| Complete response | 6 (25.0) | 0 (0) | |
| Partial response | 17 (70.8) | 13 (48.1) | |
| Stable disease | 0 (0) | 9 (37.5) | |
| Progressive disease | 1 (0.42) | 5 (18.5) | |
| Response rate | 95.8% | 48.1% | < 0.001 |
| Disease control rate | 95.8% | 81.5% | 0.053 |
Group A: hepatic arterial infusion of fluorouracil + capecitabine; Group B: capecitabine alone
Survival
Group A: n = 24, 8 alive, 16 died, median survival time 18.5 months, follow-up 8-42 months. Group B: n = 27, 7 alive, 20 died, median survival time (MST) 13 months, follow-up 4-26 months. There was a statistical difference between the MSTs of the 2 groups (P = 0.0312). See Fig. 2.
Figure 2.
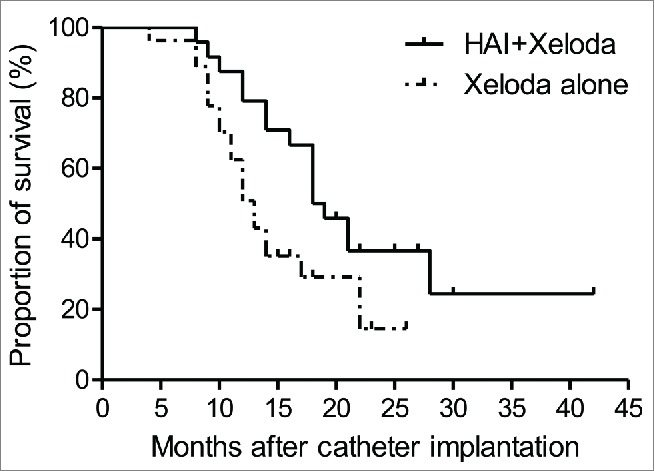
Survival curves for patients in Capecitabine and Capecitabine/HAI groups. HAI/Capecitabine group: n = 24, 8 live, 16 died, median survival time 18.5 months, follow-up 8–42 momths; Capecitabine group: n = 27, 7 live, 20 died, median survival time 13 months, follow-up 4–26 momths (P=0.0312).
Adverse events
Grade 3 adverse events are evaluated according to National Cancer Institute Common Toxicity Criteria version 3. In Group A, 1 patient experienced neutropenia, 3 experienced alanine aminotransferase/aspartate transaminase (ALT/AST) elevation, 4 experienced nausea and vomiting, 4 experienced abdominal pain, 3 experienced diarrhea, 2 experienced gastric ulceration, 1 experienced paresthesia, 1 experienced fatigue, 3 experienced hand and foot syndrome. In Group B, 1 patient experienced neutropenia, 1 experienced ALT/AST elevation, 2 experienced nausea and vomiting, 3 experienced abdominal pain, 1 experienced diarrhea, 1 experienced paresthesia, 1 experienced fatigue, 4 experienced hand and foot syndrome. No patient had catheter occlusion during the follow-up period. See Table 3.
Table 3.
Grade 3 adverse events.
| Grade 3 adverse event | Group A (n = 24) | Group B (n = 27) |
|---|---|---|
| Hematologic | ||
| Neutropenia | 1 (4.2) | 1 (3.7) |
| Hepatic | ||
| ALT/AST | 3 (12.5) | 1 (3.7) |
| Gastrointestinal | ||
| Nausea and vomiting | 4 (16.7) | 2 (7.4) |
| Abdominal pain | 4 (16.7) | 3 (11.1) |
| Diarrhea | 3 (12.5) | 1 (3.7) |
| Ulcer | 2 (8.3) | 0 (0) |
| Neurologic | ||
| Paresthesia | 1 (4.2) | 1 (3.7) |
| Constitutional | ||
| Fatigue | 1 (4.2) | 1 (3.7) |
| Hand and foot syndrome | 3 (12.5) | 4 (14.8) |
Adverse events are evaluated according to National Cancer Institute Common Toxicity Criteria version 3
Group A: hepatic arterial infusion of fluorouracil + capecitabine; Group B: capecitabine alone
Discussion
The theoretical basis of HAI therapy is that the preferential blood supply from the hepatic artery to CLMs allows for delivery of agents through a HAI catheter for eligible patients with liver-only or liver-dominant disease. During the past 5 years, our group has treat hundreds of patients with CLMs, most of who received a hepatic response and thus might be treated with subsequent surgical therapy. The images of a typical patient are showed in Fig 1.
Figure 1.
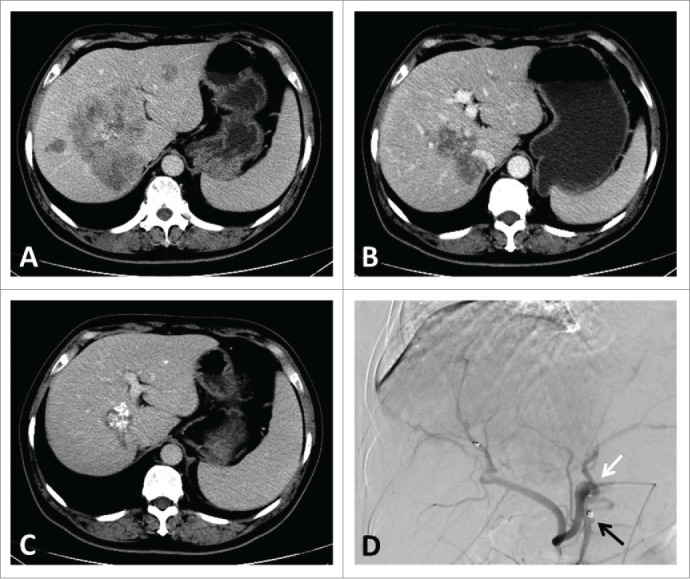
A typical patient's images of CT scan and angiography through infusion catheter. CT image showing (A). liver metastases involving all vessels at baseline; (B). 3 months after HAI pump implantation; (C). 1 year after HAI pump implantation. Angiography showing (D). the infusion catheter with side-hole (white arrow) is fixed into the gastroduodenal artery with metallic coils (black arrow).
The treatment strategy for patients with unresectable CLMs depends on the symptoms and resectability of the primary tumor. All the criteria for these patients should include 1) true unresectable disease, signifying massive tumor extent which results in inability to achieve a margin-negative resection; and 2) relative unresectable disease such as multiple hepatic metastases and synchronous disease.17 The enrolled patients in the present study were all bore relative unresectable disease, so they were possible to receive other therapy modalities including HAI.
These hepatic metastases are discovered synchronously in 15%–25% of patients,18,19 while 20%–25% of patients metachronously develop hepatic tumors.20 So the rate of synchronous over metachronous CLMs is consistent with that in our study.
HAI chemotherapy as the first-line treatment for patients with unresectable CLMs is not common. Notably, the current evidence does not support the use of HAI FUDR alone for treatment of patients with unresectable CLMs.21 While being combined with systematic chemotherapy, HAI therapy not only improves the RR, but also increases the resectability.22
Two meta-analyses observed a survival advantage (27% relative risk reduction)23 and a obviously higher RR of HAI (41% vs. 14%) 24 comparing with systemic therapy alone. Besides, CALGB 9481 trial compared the administration of HAI pump vs. systematic chemotherapy. Results suggested that HAI FUDR had a significant longer overall survival (24.4 months vs. 20.0 months) and higher RR (47% vs. 24%). These results confirmed the advantage of HAI to systematic chemotherapy when same agents were used.12 The mode of this trial is similar with ours, in which we also applied the same agent, capecitabine, in both 2 groups. And likewise we observed that difference between the RRs of the 2 groups were statistical significant. In the present study, the RR and DCR of HAI/capecitabine were both 95.8%, which is much higher than other trials. The high response rate seen in this study may be due to the use of HAI FUDR.
Besides and more importantly, the results from the major endpoint was even more promising, with a significant longer overall survival in HAI/capecitabine was observed. In a clinical trial, the median survival in the group receiving combined therapy was 68.4 months compared to 58.8 months for those receiving systemic therapy alone.11 We believe that the increased part of overall survival is contributed to HAI FUDR mostly.
Hence, our results provide evidences for the combination of HAI FUDR and chemotherapeutics for patients with CLMs. Furthermore, HAI therapy not only helps improve the RR of chemotherapy, but also increases the magnitude of tumor shrinkage, which can improve the resectability.25,26
Generally speaking, the adverse events were tolerable. Adverse events were possibly related to extrahepatic perfusion of FUDR, although the main branch vessels from the arteries associated with the stomach or duodenum were routinely embolized before catheter implantation. Gastrointestinal and hepatotoxicity are main side effects of HAI therapy. It seemed that the occurrence of adverse events was inevitable to some extent but fortunately, these adverse events were reversible in all patients and no patients permanently discontinued HAI therapy. As for hand and foot syndrome, it was supposed to be due to the administration of capecitabine. Fortunately, technical (related to the catheter, port, or pump) adverse events did not occur.
All the enrolled subjects were elderly patients who preferred a safer and more convenient regimen with slighter adverse events, which was the reason that we chose capecitabine. Actually, most of these elderly patients could not endure combined regimens containing oxaliplatin and irinotecan. On the other hand, oxaliplatin and irinotecan have been investigated thoroughly in clinical trials focusing on chemotherapy for colorectal liver metastases. However, oral chemotherapeutic drugs were investigated much less. Moreover, most of these elderly patients elder than 75 y old could not endure or do not wish to receive combined regimens containing oxaliplatin and irinotecan. Elderly Chinese patients tend to receive oral chemotherapy. Hence, we set the arm of capecitabine for elderly patients. Generally speaking, capecitabine leads to slighter or less adverse events. In present China, only a small portion of patients with colorectal cancer are able to afford the expenses of molecular targeted agents such as cetuximab and bevacizumab.
According to the follow-up data, patients were administered completely on their own. There were only 47 patients enrolled and fortunately all were compliant with oral capecitabine. There were some adverse events occurred, but no patient ended the therapy during the treatment. In future studies with larger sample sizes, it is not possible to reach a 100%-compliance.
The purpose of this study is mainly to confirm the advantage of hepatic arterial infusion and the results do achieve the expectation. The dihydropyrimidine dehydrogenase enzyme is responsible for the detoxifying metabolism of fluoropyrimidines, a class of drugs that includes FUDR and capecitabine.21,27 Capecitabine leads to a systemic anti-tumor effect but HAI FUDR aims to provide a locally durable accumulation of anti-tumor response via the pump. The mechanism of the 2 treatment modalities were different.
This is a prospective study and has limitations because of its small sample and lack a control group with similarly unresectable CLMs, which was initially treated with modern systemic chemotherapy alone.
Patients and Methods
Patient enrollment
This prospective, non-randomized study was approved by the ethics committees of the participating medical institutes and conducted according to the principles of the Declaration of Helsinki and Good Clinical Practice Guidelines. All patients gave their written informed consent before enrollment.
Patients were potentially eligible if they 1) had liver metastases considered technically unresectable 2) had no detectable extrahepatic metastatic lesions; 3) had not undergone prior treatment; 4) were not receiving concurrent treatment that interfered with the study evaluation; 5) had hematologic and chemistry parameters in acceptable ranges including: absolute neutrophil count ≥ 1.2 × 109/L, direct bilirubin ≤ 1.5 times the instituteal upper normal limit (UNL), AST ≤ 2.5 times the UNL; the creatinine below the UNL or the creatinine clearance of higher than 60 mL/min/1.73 m2 if the creatinine was above the UNL 28,29; 6) were at least 18 y and not pregnant or breastfeeding women; 7) had a Eastern Cooperative Oncology Group (ECOG) performance score of 0 to 1; and 8) were having inadequate calorie and fluid intake.
The primary endpoint was MST, defined as the time from the date of catheter implantation to the date of death or the date of the last follow-up. The secondary endpoint was objective antitumor response and adverse events.
Pretreatment evaluation
Pretreatment evaluation included a complete history, physical examination, and laboratory studies including complete blood cell, AST, total bilirubin, alkaline phosphatase, lactate dehydrogenase and carcinoembryonic antigen were obtained within 1 week before therapy. Computed tomography (CT) scans of the chest, abdomen, and pelvis were obtained within 6 weeks before therapy. Hepatic computed tomography angiogram (CTA) including visualization of the celiac and superior mesenteric arteries was also obtained before HAI pump implantation.
Data of sex, age, time of liver metastases, location of primary cancer, number of metastatic lesions, baseline level of tumor markers, adverse events related to treatment, survival time from time of catheter implantation were collected. The treatment protocol was determined by the multi-disciplinary treatment group composed of an oncologist, an interventional therapy specialist, a radiologist and a surgeon.
HAI pump implantation
The operation of HAI pump implantation was performed under the monitoring of CTA in a standard digital subtraction angiography operation room. After local injection of anesthetic, the Seldinger technique was applied to gain access to the right femoral artery. Arteriography for the celiac trunk and superior mesenteric artery was performed to reveal the hepatic arterial anatomy.
In patients with multiple hepatic arteries, all the hepatic arteries except the largest one were embolized to redistribute the hepatic arterial flow to enable the use of a single indwelling catheter to infuse chemotherapeutic agents to the entire liver.30 The gastroduodenal artery, right gastric artery (if necessary, left gastric artery or dorsal pancreatic artery) were embolized using metallic coils (Tornade, Cook, Bloomington, IL, USA) to prevent extrahepatic drug distribution and gastroduodenal injury caused by the chemotherapeutic agents. To avoid dislodgment of the catheter tip and hepatic arterial occlusion, the infusion catheter (Celsite, B. Braun, Chasseneuil, France) with side-hole was fixed into the gastroduodenal artery with metallic coils (n = 36) or inserted into the peripheral branch of the hepatic artery (n = 6) as described by Tanaka et al.31 The position of the side hole was sited at the common hepatic artery to ensure that the chemotherapeutic agents infuse the entire liver from the side-hole. The proximal end of the catheter was connected to the injection port and the device was implanted in a subcutaneous pocket in the right inner thigh. After the administration of chemotherapeutic agents, the implanted port and indwelling catheter system were flushed and filled with 2 mL of heparin solution (1,000 IU/mL).
Once the catheter was in place, intraoperative assessment of perfusion was required by infusion of 5 mL of FUDR via the catheter or port. Postoperatively, a technetium sulfur colloid radionuclide liver scan was required to assess liver perfusion. HAI were kept performed synchronous with capecitabine until disease progression or patients' refusal.
Chemotherapy
All patients received a 3-week cycle of oral capecitabin. The day patients received HAI implantation was defined as day 0. Both of the HAI therapy and oral capecitabine were initiated on day 1, lasting on day 1–14. Floxuridine was delivered in a 14-day infusion at 1.5 mg·kg−1·d−1 divided by flow rate. Dexamethasone at 1 mg/m2/day divided by flow rate was always placed in the pump with floxuridine heparin and saline. Oral capecitabine was administered at 1,000 mg/m2 twice daily for 2 weeks. The first dose of capecitabine was given in the evening of day 1 and the last dose on the morning of day 15. The next cycle began on day 22.
If serious technical catheter-related problems, hepatic disease progression or severe toxicity did not occur, HAI were kept performed until disease progression or patients' refusal.
If a grade 1 or 2 adverse event related to FUDR occurred, HAI FUDR was held until clinical resolution. The FUDR dose was reduced if moderate chemical hepatitis or gastroduodenitis was noted. Dose reduction was based on the abnormal liver function tests in the preceding treatment cycle. FUDR administration was permanently discontinued if severe chemical hepatitis or biliary stricture was observed.
Patients who developed abdominal pain prompted workup received gastrointestinal endoscopy. If an ulcer or gastroduodenitis was observed, HAI therapy was held for 1 month and the dose of FUDR was reduced in subsequent cycles of HAI therapy. Resection of the primary colorectal tumor was indicated if obstruction or significant bleeding occurred.
Disease assessment and follow-up
Disease assessments (CT scan) were conducted within 28 d before starting study treatment and repeated after every 2 cycles according to the RECIST criteria 32 by investigators. Confirmation of response of therapy was required after at least 4 weeks. After completion of study treatment, patients were followed every 3 months until 5 y postregistration or death. Patients lost to follow-up were censored.
Statistical analysis
All statistical analyses were performed using SPSS 13.0 software (SPSS Inc., USA). Categorical variables were compared using χ2 test. Continuous variables by compared by Wilcoxon's rank-sum test. The differences between the 2 groups were compared by χ2 test. The survival curve was estimated using the Kaplan–Meier method. Survival curves were compared using the log-rank test. A P value less than 0.05 was considered statistically significant.
Conclusions
Our data show that initial HAI FUDR combined with oral capecitabine as the first-line treatment for patients with CLMs. The high RR, acceptable MST and tolerable adverse events are the advantage of the combination comparing with capecitabine. Future research should focus on the alteration of the agents in the combination. A multi-center randomized study is warranted to confirm the efficacy and safety of HAI therapy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No.81201741, 81301960 and 81272017).
References
- 1.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA: Cancer J Clin 2014; 64:104-17; PMID:24639052; http://dx.doi.org/ 10.3322/caac.21220 [DOI] [PubMed] [Google Scholar]
- 2.Sheth KR, Clary BM. Management of hepatic metastases from colorectal cancer. Clin Colon Rectal Surg 2005; 18:215-23; PMID:20011304; http://dx.doi.org/ 10.1055/s-2005-916282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruo L, Gougoutas C, Paty PB, Guillem JG, Cohen AM, Wong WD. Elective bowel resection for incurable stage IV colorectal cancer: prognostic variables for asymptomatic patients. J Am Coll Surg 2003; 196:722-8; PMID:12742204; http://dx.doi.org/ 10.1016/S1072-7515(03)00136-4 [DOI] [PubMed] [Google Scholar]
- 4.Poston GJ, Figueras J, Giuliante F, Nuzzo G, Sobrero AF, Gigot JF, Nuzzo G, Sobrero AF, Gigot JF, Nordlinger B, et al. Urgent need for a new staging system in advanced colorectal cancer. J Clin Oncol 2008; 26:4828-33; PMID:18711170; http://dx.doi.org/ 10.1200/JCO.2008.17.6453 [DOI] [PubMed] [Google Scholar]
- 5.Fusai G, Davidson BR. Management of colorectal liver metastases. Colorectal Dis 2003; 5:2-23; PMID:12780921; http://dx.doi.org/ 10.1046/j.1463-1318.2003.00410.x [DOI] [PubMed] [Google Scholar]
- 6.Iguchi T, Arai Y, Inaba Y, Yamaura H, Sato Y, Miyazaki M, Shimamoto H. Hepatic arterial infusion chemotherapy through a port-catheter system as preoperative initial therapy in patients with advanced liver dysfunction due to synchronous and unresectable liver metastases from colorectal cancer. Cardiovasc Inter Radiol 2008; 31:86-90; PMID:17926088; http://dx.doi.org/ 10.1007/s00270-007-9189-0 [DOI] [PubMed] [Google Scholar]
- 7.Poultsides GA, Servais EL, Saltz LB, Patil S, Kemeny NE, Guillem JG, Weiser M, Temple LK, Wong WD, Paty PB. Outcome of primary tumor in patients with synchronous stage IV colorectal cancer receiving combination chemotherapy without surgery as initial treatment. J Clin Oncol 2009; 27:3379-84; PMID:19487380; http://dx.doi.org/ 10.1200/JCO.2008.20.9817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kemeny N, Jarnagin W, Paty P, Gonen M, Schwartz L, Morse M, Leonard G, D'Angelica M, DeMatteo R, Blumgart L, et al. Phase I trial of systemic oxaliplatin combination chemotherapy with hepatic arterial infusion in patients with unresectable liver metastases from colorectal cancer. J Clin Oncol: Off J Am Soc Clin Oncol 2005; 23:4888-96; PMID:16009951; http://dx.doi.org/ 10.1200/JCO.2005.07.100 [DOI] [PubMed] [Google Scholar]
- 9.Martin RC, Joshi J, Robbins K, Tomalty D, Bosnjakovik P, Derner M, Padr R, Rocek M, Scupchenko A, Tatum C. Hepatic intra-arterial injection of drug-eluting bead, irinotecan (DEBIRI) in unresectable colorectal liver metastases refractory to systemic chemotherapy: results of multi-institutional study. Ann Surg Oncol 2011; 18:192-8; PMID:20740319; http://dx.doi.org/ 10.1245/s10434-010-1288-5 [DOI] [PubMed] [Google Scholar]
- 10.Tsimberidou AM, Fu S, Ng C, Lim JA, Wen S, Hong D, Wheler J, Bedikian AY, Eng C, Wallace M, et al. A phase 1 study of hepatic arterial infusion of oxaliplatin in combination with systemic 5-fluorouracil, leucovorin, and bevacizumab in patients with advanced solid tumors metastatic to the liver. Cancer 2010; 116:4086-94; PMID:20564148; http://dx.doi.org/ 10.1002/cncr.25277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kemeny NE, Gonen M. Hepatic arterial infusion after liver resection. N Engl J Med 2005; 352:734-5; PMID:15716576; http://dx.doi.org/ 10.1056/NEJM200502173520723 [DOI] [PubMed] [Google Scholar]
- 12.Kemeny NE, Niedzwiecki D, Hollis DR, Lenz HJ, Warren RS, Naughton MJ, Weeks JC, Sigurdson ER, Herndon JE 2nd, Zhang C, et al. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). J Clin Oncol 2006; 24:1395-403; PMID:16505413; http://dx.doi.org/ 10.1200/JCO.2005.03.8166 [DOI] [PubMed] [Google Scholar]
- 13.Meta-Analysis Group in C, Piedbois P, Buyse M, Kemeny N, Rougier P, Carlson R, Allen-Mersh T, O'Connell M, Chang A, Sondak V, et al. Reappraisal of hepatic arterial infusion in the treatment of nonresectable liver metastases from colorectal cancer. J Natl Cancer Inst 1996; 88:252-8; PMID:8614003; http://dx.doi.org/ 10.1093/jnci/88.5.252 [DOI] [PubMed] [Google Scholar]
- 14.Collins JM. Pharmacologic rationale for regional drug delivery. J Clin Oncol 1984; 2:498-504; PMID:6547166 [DOI] [PubMed] [Google Scholar]
- 15.Van Cutsem E, Findlay M, Osterwalder B, Kocha W, Dalley D, Pazdur R, Cassidy J, Dirix L, Twelves C, Allman D, et al. Capecitabine, an oral fluoropyrimidine carbamate with substantial activity in advanced colorectal cancer: Results of a randomized phase II study. J Clin Oncol 2000; 18:1337-45; PMID:10715306 [DOI] [PubMed] [Google Scholar]
- 16.Twelves C. Capecitabine as first-line treatment in colorectal cancer: pooled data from two large, phase III trials. Eur J Cancer 2002; 38:15-20; PMID:11841931; http://dx.doi.org/ 10.1016/S0959-8049(01)00415-4 [DOI] [PubMed] [Google Scholar]
- 17.Kemeny NE, Melendez FD, Capanu M, Paty PB, Fong Y, Schwartz LH, Jarnagin WR, Patel D, D'Angelica M. Conversion to resectability using hepatic artery infusion plus systemic chemotherapy for the treatment of unresectable liver metastases from colorectal carcinoma. J Clin Oncol 2009; 27:3465-71; PMID:19470932; http://dx.doi.org/ 10.1200/JCO.2008.20.1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cady B, Monson DO, Swinton NW. Survival of patients after colonic resection for carcinoma with simultaneous liver metastases. Surg Gynecol Obstet 1970; 131:697-700; PMID:5458530 [PubMed] [Google Scholar]
- 19.Jatzko G, Wette V, Muller M, Lisborg P, Klimpfinger M, Denk H. Simultaneous resection of colorectal carcinoma and synchronous liver metastases in a district hospital. Int J Colorectal Dis 1991; 6:111-4; PMID:1875119; http://dx.doi.org/ 10.1007/BF00300206 [DOI] [PubMed] [Google Scholar]
- 20.Altendorf-Hofmann A, Scheele J. A critical review of the major indicators of prognosis after resection of hepatic metastases from colorectal carcinoma. Surg Oncol Clin N Am 2003; 12:165-92, xi; PMID:12735137; http://dx.doi.org/ 10.1016/S1055-3207(02)00091-1 [DOI] [PubMed] [Google Scholar]
- 21.Caudle KE, Thorn CF, Klein TE, Swen JJ, McLeod HL, Diasio RB, Schwab M. Clinical Pharmacogenetics Implementation Consortium guidelines for dihydropyrimidine dehydrogenase genotype and fluoropyrimidine dosing. Clin Pharmacol Ther 2013; 94:640-5; PMID:23988873; http://dx.doi.org/ 10.1038/clpt.2013.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li C, Gu Y, Zhao M, Yuan Y, Wang F, Wang Z, Li W, Luo H, Chen C, Chen G, et al. Phase I trial of hepatic arterial infusion (HAI) of floxuridine with modified oxaliplatin, 5-fluorouracil and leucovorin (m-FOLFOX6) in Chinese patients with unresectable liver metastases from colorectal cancer. Cancer Chemother Pharmacol 2014; 74:1079-87; PMID:25217393; http://dx.doi.org/ 10.1007/s00280-014-2585-7 [DOI] [PubMed] [Google Scholar]
- 23.Cancer MAGi . Reappraisal of hepatic arterial infusion in the treatment of nonresectable liver metastases from colorectal cancer. J Natl Cancer Inst 1996; 88:252-8; PMID:8614003; http://dx.doi.org/ 10.1093/jnci/88.5.252 [DOI] [PubMed] [Google Scholar]
- 24.Harmantas A, Rotstein LE, Langer B. Regional versus systemic chemotherapy in the treatment of colorectal carcinoma metastatic to the liver. Is there a survival difference? Meta analysis of the published literature. Cancer Disc 1996; 78:1639-45; PMID:8859174 [DOI] [PubMed] [Google Scholar]
- 25.Bouchahda M, Levi F, Adam R, Rougier P. Modern insights into hepatic arterial infusion for liver metastases from colorectal cancer. Eur J Cancer 2011; 47:2681-90; PMID:21783358; http://dx.doi.org/ 10.1016/j.ejca.2011.06.037 [DOI] [PubMed] [Google Scholar]
- 26.Folprecht G, Grothey A, Alberts S, Raab HR, Kohne CH. Neoadjuvant treatment of unresectable colorectal liver metastases: correlation between tumour response and resection rates. Ann Oncol 2005; 16:1311-9; PMID:15870084; http://dx.doi.org/ 10.1093/annonc/mdi246 [DOI] [PubMed] [Google Scholar]
- 27.Amstutz U, Froehlich TK, Largiader CR. Dihydropyrimidine dehydrogenase gene as a major predictor of severe 5-fluorouracil toxicity. Pharmacogenomics 2011; 12:1321-36; PMID:21919607; http://dx.doi.org/ 10.2217/pgs.11.72 [DOI] [PubMed] [Google Scholar]
- 28.Kemeny NE, Gonen M. Hepatic arterial infusion after liver resection. N Engl J Med 2005; 352:734-5; PMID:15716576; http://dx.doi.org/ 10.1056/NEJM200502173520723 [DOI] [PubMed] [Google Scholar]
- 29.Kemeny NE, Niedzwiecki D, Hollis DR, Lenz HJ, Warren RS, Naughton MJ, Weeks JC, Sigurdson ER, Herndon JE 2nd, Zhang C, et al. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). J Clin Oncol 2006; 24:1395-403; PMID:16505413; http://dx.doi.org/ 10.1200/JCO.2005.03.8166 [DOI] [PubMed] [Google Scholar]
- 30.Herrmann KA, Waggershauser T, Sittek H, Reiser MF. Liver intraarterial chemotherapy: use of the femoral artery for percutaneous implantation of catheter-port systems. Radiology 2000; 215:294-9; PMID:10751501; http://dx.doi.org/ 10.1148/radiology.215.1.r00ap14294 [DOI] [PubMed] [Google Scholar]
- 31.Tanaka T, Arai Y, Inaba Y, Matsueda K, Aramaki T, Takeuchi Y, Kichikawa K.. Radiologic placement of side-hole catheter with tip fixation for hepatic arterial infusion chemotherapy. J Vasc Inter Radiol: JVIR 2003; 14:63-8; PMID:12525587; http://dx.doi.org/ 10.1097/01.RVI.0000052292.26939.59 [DOI] [PubMed] [Google Scholar]
- 32.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45:228-47; PMID:19097774; http://dx.doi.org/ 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]


