Abstract
Recently published paper by Haller and coworkers in Journal of Clinical Investigation reported that a combination of low‐dose anti‐thymocyte globulin (ATG) and pegylated granulocyte‐colony stimulating factor (G‐CSF) may preserve β‐cell function in patients with established type 1 diabetes.
Past Challenges for the Prevention of Type 1 Diabetes
Type 1 diabetes is considered to be an autoimmune disease characterized by the selective destruction of the insulin‐producing islet β‐cells in the pancreas resulting from the environmental triggers on genetically disease‐susceptible individuals1. The hallmark of autoimmune type 1 diabetes is T cell‐mediated destruction of β‐cells, which results from an imbalance in the activity between disease promoting autoreactive effector T cells and protective elements, which is now known as regulatory T cells (Tregs). Clinical diagnosis of type 1 diabetes by hyperglycemia is made when the residual functional β‐cell mass reaches a critical threshold, which is usually suggested to be 20–30% of normal, and insulin therapy is started. However, the β‐cell loss continues even after the onset of disease until the majority of insulin‐producing cells are no longer functional, resulting in a lifelong insulin‐dependent state. Preserving the functional β‐cell mass after the onset of diabetes is important to maintain quality of life for patients with type 1 diabetes by stable blood control, which leads to the prevention of acute complications, such as severe hypoglycemia or diabetic ketoacidosis and chronic diabetic vascular complications; that is, retinopathy, neuropathy, nephropathy and atherosclerosis.
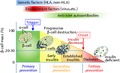
Therefore, in order to prevent the progressive destruction of β‐cells in patients with type 1 diabetes and high‐risk individuals, immune intervention studies were initiated as early as the late 1970s2. Prevention of type 1 diabetes is carried out at three stages: (i) before the development of autoimmunity to islet autoantigens (primary prevention); (ii) after the development of humoral or metabolic markers of high risk of progression to diabetes (secondary prevention); and (iii) in the attempt to maintain residual β‐cells after the onset of diabetes (tertiary prevention; Figure1). To date, several primary prevention trials have been carried out in newborns at high risk for type 1 diabetes, especially those with first‐degree relatives with high‐risk human leukocyte antigen haplotypes, including the avoidance of early exposure to cow's milk protein (Trial to Reduce IDDM in the Genetically at Risk), the complete avoidance of bovine insulin (Finnish Dietary Intervention Trial for the Prevention of Type 1 Diabetes), the delayed introduction of gluten (BABYDIET), or the omega‐3 fatty acid supplementation with docosahexaenoic acid (The Nutritional Intervention to Prevent Type 1 Diabetes). Unfortunately, so far, none of these trials have shown a beneficial effect on autoimmunity or the development of diabetes. The multinational Trial to Reduce IDDM in the Genetically at Risk and the Primary Oral Insulin Trial are currently under way.
Figure 1.

Schematic representation of natural history of type 1 diabetes and three stages of prevention trials. Type 1 diabetes is a multifactorial autoimmune disease, and a strong genetic component and environmental factors have been implicated in the pathogenesis of type 1 diabetes both as triggers and potentiators of β‐cell destruction. Anti‐islet autoantibodies develop after the initiation of islet autoimmunity, and are used as a predictive and diagnostic marker. Prevention of type 1 diabetes is classified according to their timing relative to clinical onset into primary prevention (before the development of autoimmunity to islet autoantigens), secondary prevention (after the development of islet autoimmunity) and tertiary prevention (after the onset of type 1 diabetes). HLA, human leukocyte antigen.
Secondary prevention trials are targeted to first‐degree relatives of type 1 diabetes patients with anti‐islet autoantibodies with additional metabolic testing by an intravenous glucose tolerance test. Most of the secondary prevention trials are based on data from the animal models for type 1 diabetes, especially the non‐obese diabetic mouse. Notable secondary prevention trials include the Deutsche Nicotinamide Intervention Study and the European Nicotinamide Diabetes Intervention Trial, which trialed nicotinamide as a prevention therapy, the Diabetes Prevention Trial‐Type 1 Diabetes using either parenteral or oral insulin, and the Type 1 Diabetes Prediction and Prevention Project testing intranasal insulin administration.
Currently, the type 1 diabetes TrialNet, an international study group carrying out studies of the prevention and early treatment of type 1 diabetes, is carrying out some secondary prevention trials using oral insulin, teplizumab (anti‐CD3 antibody) or abatacept (soluble fusion protein, which links the extracellular domain of human cytotoxic T lymphocyte‐associated antigen 4). Other ongoing secondary prevention studies include the vaccination of GAD65 with aluminum or peptide derived from heat shock protein 60. All of those are trials that showed beneficial effects in the tertiary prevention trials, which can be evaluated within a much shorter time frame compared with secondary prevention trials.
New Approach by the Combined Intervention
Prevention trials can be divided into two main classes. The first concept of altering autoimmunity is non‐antigen‐specific treatment using drug regimens to silence and/or modulate the immune response. The second concept of prevention trials aims to induce antigen‐specific tolerance by antigen‐based treatment in order to induce expansion/activation of Tregs or anergy/deletion of pathogenic T cells (effector T cells).
A recently published study by Haller et al.3 reported that a combination of low‐dose anti‐thymocyte globulin (ATG) and pegylated granulocyte‐colony stimulating factor (G‐CSF) might preserve β‐cell function in patients with established type 1 diabetes. ATG is used in organ transplantation, and preclinical studies have shown that ATG treatment can induce disease remission in non‐obese diabetic mice. However, the Study of Thymoglobulin to Arrest Type 1 Diabetes (START) trial, a randomized, placebo‐controlled, clinical trial in patients with recent‐onset type 1 diabetes, showed that over a course of 4 days treatment of ATG alone did not result in preservation of β‐cell function at 1 year after treatment4. It has been reported that treatment with G‐CSF can prevent spontaneous diabetes and destructive insulitis in non‐obese diabetic mice5. This phase IIa tertiary prevention trial of type 1 diabetes by a combination of ATG and G‐CSF showed the maintenance of β‐cell function at baseline levels in 56% of treated patients after 1 year follow up, whereas in the placebo group it was in just one of nine patients. Of note, older age at diagnosis and lower baseline insulin dose were predictive factors of responders. As for the adverse events, cytokine release syndrome and serum sickness syndrome occurred during ATG infusion. However, low‐dose ATG administration was able to minimize the adverse events including infection, and none of subjects developed primary cytomegalovirus (CMV) or Epstein–Barr virus infection, or clinical CMV reactivation.
In the previous tertiary prevention trials, neither ATG nor G‐CSF proved beneficial effects when used alone. Although the START trial, which used a threefold higher dose of ATG monotherapy (6.5 mg/kg), induced T cell depletion with marked reduction of Tregs, low‐dose ATG/G‐CSF combination therapy can preserve Tregs with a reduction of pathogenic effector T cells, which work closely together and might lead to the maintenance of β‐cell function. To date, only a few clinical trials of a combination therapy have been carried out in the type 1 diabetes prevention trials, and all of which failed to show benefit6, 7, 8. Therefore, the results of ATG/G‐CSF combination therapy are very important, even though the sample size was very small.
Future Directions on the Type 1 Diabetes Prevention Trials
There are several unmet needs addressed for better prevention trials in human type 1 diabetes. First, compared with typical type 1 diabetes prevention studies, which enrol patients with short duration (less than 1 year), Haller et al.3 enrolled patients with duration of 4 months to 2 years. They reported that the efficacy of ATG/G‐CSF combination therapy was observed in patients with both less than 1‐year and greater than 1‐year duration. These results suggest that ATG/G‐CSF combination therapy might have a wider therapeutic window. Furthermore, the eligible patients in the study by Haller et al.3 were aged 12–45 years (mean 23.5 years), acute‐onset patients with type 1 diabetes and had a minimum stimulated C‐peptide response of 0.3 ng/mL (0.1 nmol/L) during a mixed‐meal tolerance test, which is lower than the C‐peptide threshold more commonly used in type 1 diabetes intervention trials (0.6 ng/mL). Therefore, it is worth to testing the efficacy of this combination therapy in child‐onset patients with type 1 diabetes or other types of diabetes, such as fulminant type 1 diabetes or latent autoimmune diabetes in adults.
Disclosure
The author declares no conflict of interest.
References
- 1. Kawasaki E, Gill RG, Eisenbarth GS. Type I diabetes mellitus In: Eisenbarth GS. (ed). Endocrine and Organ Specific Autoimmunity. Landes Bioscience, Austin, TX, 1999; 149–182. [Google Scholar]
- 2. Skyler JS. Prevention and reversal of type 1 diabetes – past challenges and future opportunities. Diabetes Care 2015; 38: 997–1007. [DOI] [PubMed] [Google Scholar]
- 3. Haller MJ, Gitelman SE, Gottlieb PA, et al Anti‐thymocyte globulin/G‐CSF treatment preserves β cell function in patients with established type 1 diabetes. J Clin Invest. 2015; 125: 448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gitelman SE, Gottlieb PA, Rigby MR, et al Antithymocyte globulin treatment for patients with recent‐onset type 1 diabetes: 12‐month results of a randomised, placebo‐controlled, phase 2 trial. Lancet Diabetes Endocrinol 2013; 1: 306–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kared H, Masson A, Adle‐Biassette H, et al Treatment with granulocyte colony‐stimulating factor prevents diabetes in NOD mice by recruiting plasmacytoid dendritic cells and functional CD4+CD25+ regulatory T‐cells. Diabetes 2005; 54: 78–84. [DOI] [PubMed] [Google Scholar]
- 6. Gottlieb PA, Quinlan S, Krause‐Steinrauf H, et al Failure to preserve β‐cell function with mycophenolate mofetil and daclizumab combined therapy in patients with new‐ onset type 1 diabetes. Diabetes Care 2010; 33: 826–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Long SA, Rieck M, Sanda S, et al Rapamycin/IL‐2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently impairs β‐cell function. Diabetes 2012; 61: 2340–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Griffin KJ, Thompson PA, Gottschalk M, et al Combination therapy with sitagliptin and lansoprazole in patients with recent‐onset type 1 diabetes (REPAIR‐T1D): 12‐month results of a multicentre, randomised, placebo‐controlled, phase 2 trial. Lancet Diabetes Endocrinol 2014; 2: 710–718. [DOI] [PMC free article] [PubMed] [Google Scholar]


