Abstract
Aims/Introduction
Recent studies have shown that periodontitis can contribute to adipose tissue inflammation and subsequent systemic insulin resistance in the obese rat model. However, the related inflammatory mechanism is not yet clear. The present study aims to investigate the effects of periodontitis on the function of pancreatic β‐cells with pro‐inflammatory cytokines‐related immune mechanism in a mouse model.
Materials and Methods
C57BL/6‐db/db and inbred C57BL/6 mice were chosen here to establish a mouse model with periodontitis, which was induced by ligatures for 8 weeks. Glucose‐stimulated insulin secretion was introduced to evaluate the function of pancreatic islets and β‐cells. Serum levels of pro‐inflammatory cytokines and Klotho were also measured, and the correlation between immunostimulation and Klotho level was deeply investigated in vitro.
Results
Pancreatic β‐cell failure, with insulin resistance, was observed in db/db mice, while periodontitis could aggravate β‐cell dysfunction‐related features. Serum levels of interleukin (IL)‐12 and Klotho showed a negatively synergistic change, whereas the expression of Klotho was also inhibited under IL‐12 treatment in MIN6 β‐cells or isolated islets. Furthermore, IL‐12‐induced immune stimulation and also decreased insulin secretion were proven to be reversed by Klotho overexpression.
Conclusions
Periodontitis aggravated pancreatic β‐cell failure in diabetic mice. Further in vitro studies showed IL‐12 regulation on Klotho, while Klotho also acted as an inhibitor on IL‐12, indicating the potential of Klotho for preserving pancreatic β‐cell function in diabetes.
Keywords: Klotho, Pancreatic β‐cell, Periodontitis
Introduction
Diabetes is a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action or both. Physiologically, the pancreatic β‐cells constantly synthesize insulin, the principal hormone that regulates uptake of glucose from the blood into most cells including skeletal muscle cells and adipocytes1. Type 2 diabetes mellitus, the most common form in the human population2, is a complex pathophysiological spectrum including insulin resistance and β‐cell failure. Significant β‐cell failure is now believed to take place at an early stage in the disease progression; that is, β‐cell function declines sharply before and after diagnosis of type 2 diabetes mellitus3. Maintaining recommended targets of blood glucose control is difficult for many patients with type 2 diabetes mellitus because of the progressive loss of β‐cell function. Thus, one of the goals in the treatment of type 2 diabetes mellitus is to preserve functional β‐cells in pancreatic islets.
The mouse Klotho (also called α‐Klotho) gene, predominantly expressed in the kidney and the brain choroid plexus4, has been reported to function as a cofactor for activation of fibroblast growth factor receptor 1c (FGFR1c) by fibroblast growth factor 23 (FGF23) in the regulation of calcium, phosphate and vitamin D metabolism5. It is noted that Klotho−/− mutant mice show pancreatic islet atrophy, decreases in insulin content and messenger ribonucleic acid (mRNA) levels in pancreatic islets, and decreases in serum insulin levels6. Most recently, it was reported that Klotho mRNA and proteins are expressed in the mouse pancreatic islets, and that silencing of Klotho impaired glucose‐stimulated insulin secretion in MIN6 β‐cells7. Furthermore, it was concluded that β‐cell‐specific expression of Klotho protects β‐cell function and attenuates the development of diabetes in db/db mice8.
Chronic periodontitis (CP), one of the most widespread oral inflammatory diseases, is characterized by inflammatory breakdown of connective tissue attachment and alveolar bone9. Plenty of studies suggest that periodontitis is associated with insulin resistance, and inflammatory processes as common pathways are involved in the pathogenesis of periodontitis, obesity and type 2 diabetes mellitus10, 11, 12. Recently, it was reported that experimental periodontitis can induce initial stages of adipose tissue inflammation, and acted as a contributing factor to exacerbate pro‐inflammatory phenotype of adipose tissue and promote the development of insulin resistance in the obese rat model13. However, the role of periodontitis in regulating pancreatic β‐cell function, involving the level change of pro‐inflammatory cytokines and Klotho, remains unknown.
In the present study, periodontitis model was established in db/db mice, a commonly used type 2 diabetes mouse model. Pancreatic β‐cell function, including insulin secretion, Klotho expression and related immune response, was mainly investigated. The purpose of the present study is: (i) to explore whether chronic low‐grade inflammation induced by experimental periodontitis can impact pancreatic β‐cell function; and (ii) to find the key points during pancreatic β‐cell failure, involving pro‐inflammatory cytokines and Klotho; and (iii) to provide evidence to support a novel role for Klotho in preserving functional β‐cells in pancreatic islets.
Materials and Methods
Animal studies
Male C57BL/6‐db/db mice and inbred C57BL/6 mice aged 8 weeks were purchased from the Laboratory Animal Center of Nanjing University. All mice were housed in cages at room temperature (25 ± 1°C), and were provided with laboratory chow (Purina) and tap water ad libitum throughout the experiment. Every 16 db/db or C57BL/6 mice were randomly divided into two groups of eight, respectively: (i) db/db mice with periodontitis (combination group, DCP[+]); (ii) db/db mice without periodontitis (diabetic group, DCP[−]); (iii) C57BL/6 mice with periodontitis (periodontitis group, CCP[+]); and (iv) C57BL/6 mice without periodontitis (control group, CCP[−]). In the combination and periodontitis groups, when the mice were aged 12 weeks, 3/0 silk ligatures soaked with periodontal pathogens were placed subgingivally around the bilateral maxillary first and second molars for 8 weeks, with the control group free of this ligation14. The mice in all groups were killed under general anesthesia at the end for tissue studies.
Analysis of diabetic periodontitis model
The periodontal status was assessed by two standard periodontal parameters, gingival index (GI)15 and probing pocket depth (PD). The GI, defined as the distance from the cementum enamel to the gingival margin bone loss defect, is measured by the distance from the metal wire to the base of the defect, whereas PD is defined as the distance from the gingival margin into the bottom of the pocket16. GI and PD measurements were recorded at six points around each tooth with a manual periodontal probe (Hu‐Friedy, Chicago, IL, USA), and the average values were calculated and subjected to statistical analysis. In addition, blood glucose was also measured weekly from the tail vein blood using a Reli OnUltima glucose reader (Solartek Products, Alameda, CA, USA) after fasting for 12 h, and serum insulin level as described later. Accordingly, insulin resistance was calculated using a homeostasis model assessment for insulin resistance17.
Enzyme‐linked immunosorbent assay analysis
Whole blood was allowed to stand at room temperature for 2 h and then centrifuged at 4°C for 15 min. Serum was collected to detect the concentrations of tumor necrosis factor‐α, interleukin (IL)‐6, IL‐1β, and IL‐12 by enzyme‐linked immunosorbent assay according to the manufacturer's instructions. Cytokines concentrations in the respective samples were determined on the basis of standard curves prepared using recombinant cytokines of known concentrations. Serum Klotho level was also measured using an enzyme‐linked immunosorbent assay according to the kit (Immuno‐Biological Laboratories Co, Gunma, Japan), consisting of a solid‐phase sandwich enzyme‐linked immunosorbent assay (ELISA) according using two kinds of highly specific antibodies18.
Mouse pancreatic islet isolation
Mouse pancreatic islets were isolated from C57BL/6 mice with a modified protocol as described previously19. In brief, collagenase‐P was injected into the common bile duct of a mouse. The pancreas was then excised and digested at 37°C. The islets were first purified with premixed Histopaque gradient, and then purified by handpicking the separated islets with low‐retention pipette tips under a dissecting microscope. When viewed under the microscope, spherical and golden‐brown particles (darker color) with a diameter of 50–250 μm were considered as islets7. Isolated islets were cultured with RPMI1640 with 10% fetal bovine serum overnight for further experiments.
Glucose‐stimulated insulin secretion
Glucose‐stimulated insulin secretion (GSIS) test for islets was executed as described before, with minor changes20. After overnight incubation of isolated islets in 10% fetal bovine serum RPMI1640 (Invitrogen, Shanghai, China), islets were starved with Krebs–Ringer bicarbonate buffer (125 mmol/L NaCl, 4.74 mmol/L KCl, 1 mmol/L CaCl2, 1.2 mmol/L KH2PO4, 1.2 mmol/L MgSO4, 5 mmol/L NaHCO3 and 25 mmol/L HEPES, pH 7.4) supplemented with 0.1% bovine serum albumin and 2.8 mmol/L glucose for 1 h. Then, islets were washed with phosphate‐buffered saline and incubated with Krebs–Ringer bicarbonate buffer supplemented with 3.3 or 16.7 mmol/L glucose (Invitrogen) for 1 h. Media were sampled and insulin measured using an insulin ELISA kit according to the manufacturer's instruction (ALPCO Diagnostics, Windham, NH, USA).
MIN6 cell culture
Pancreatic insulinoma MIN6 β‐cells were kindly provided by Dr Miyazaki and Dr Steiner21. MIN6 cells were cultured and maintained in Dulbecco's modified Eagle's medium (DMEM, Invitrogen) containing 25 mmol/L glucose, 10% fetal bovine serum, 1% penicillin/streptomycin, 2 mmol/L glutamine and 100 μmol/L β‐mercaptoethanol. MIN6 β‐cells of less than 20 passages were used in this experiment. For the GSIS test, where indicated, cells were washed with phosphate‐buffered saline and pre‐incubated in Krebs–Ringer bicarbonate buffer supplemented with 0.5% bovine serum albumin and 1.1 mmol/L glucose for 10 min. Then, cells were stimulated with 2 or 20 mmol/L glucose for 20 min. Media were sampled and insulin measured as described above.
Adenoviral vector production and transfection
As previously described, an adeno‐associated virus carrying mouse Klotho full‐length complementary deoxyribonucleic acid was constructed, with adeno‐associated virus carrying green fluorescent protein as the control22. Plasmid deoxyribonucleic acid including pAAV‐mKL, a recombinant adeno‐associated virus (rAAV) vector carrying the open reading frame(ORF) of the membrane form Klotho protein, and pAAV‐GFP with green fluorescent protein gene was purified with a QIAGEN Maxikit (Valencia, CA, USA). MIN6 cells were transfected with plasmid deoxyribonucleic acid using Optifect reagent (Invitrogen) according to the manufacturer's protocol, followed by 72‐h incubation in DMEM with 10% fetal bovine serum at 37°C in a CO2 incubator. For isolated islets, after incubation with adenovirus‐containing media for 1 h, new media with serum were added to the plate; then, the islets were washed and placed into culture for in vitro studies.
RNA isolation and reverse transcription polymerase chain reaction
Total RNA was purified from isolated mouse pancreatic islets and MIN6 cells using TRIzol reagent, followed by a QIAGEN RNeasy minikit. RNA (~500 ng) was reverse transcript using SuperScript III reverse transcriptase with OligodT20 (Invitrogen) in the presence of 10 μL deoxynucleotide triphosphate for 1 h at 50°C. The resulting complementary deoxyribonucleic acid were used as templates for polymerase chain reaction with oligonucleotide primers to amplify the mRNAs of Klotho (F: 5′‐GGGTCACTGGGTCAATC T‐3′; R: 5′‐GCAAAGTAGCCACAAAGG‐3′), inducible nitric oxide synthase (iNOS) (F: 5′‐CCTGGTACGGGCATTGCT‐3′, R: 5′‐GCTCATGCGGCCTCCTT‐3′) and β‐actin (F: 5′‐AGGTCATCACTATTGGCAACGA‐3′; R: 5′‐CACTTCATGATGGAATTGAATGTAGTT‐3′). Real‐time polymerase chain reaction was carried out on a Bio‐Rad CFX96™ Real‐Time polymerase chain reaction Detection System (Hercules, CA, USA), and the results of Klotho or iNOS mRNA levels were normalized against β‐actin mRNA in each sample through three independent experiments.
Western blotting analysis
Western blot analysis was carried out as described previously23. Briefly, MIN6 cells or isolated islets were lysed with radioimmunoprecipitation assay buffer containing the protease inhibitor cocktail. Protein concentration was measured with the Pierce BCA assay (Rockford, IL, USA). Lysates (40 μg protein/well) under the reducing condition were directly subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (4 ~ 20% Tris‐HCL precast gel) followed by western blotting with the anti‐Klotho or anti‐iNOS antibody. The same blot was re‐probed with antibody against β‐actin after stripping the blot. Bands were detected by Electrochemiluminescence (ECL) detection reagents (Amersham Biosciences, Piscataway, NJ, USA).
Luciferase assay and nitrite determination
Nuclear factor‐κB (NF‐κB) luciferase activity was measured with luciferase assay and normalized with β‐gal activity, by using reporter genes plasmid cytomegalovirus vector (pCMV)‐β‐galactosidase and Endothelial cellleukocyte adhesion molecule luciferase reporter plasmid (ELAM)‐NF‐κB‐luciferase. After a 24‐h transfection, cells were stimulated for 24 h with 2.5 ng/mL IL‐12 (PeproTech, Rocky Hill, NJ, USA). Then, cells were lysed, and luciferase activity was measured with a Promega kit (Promega, Madison, WI, USA) and a luminometer. β‐Galactosidase activity was determined by the calorimetric method to normalize transfection efficiency as described earlier24. Measurement of nitrite accumulation into the culture medium was used to determine NO production. At the indicated time‐points, the culture medium was collected and nitrite was measured by the Griess reaction25. Data shown are means of three independent experiments.
Statistical analysis
Data are presented as the mean ± standard deviation. The significance of differences among groups was evaluated using analysis of variance with Fisher post‐hoc least significant difference test. A P‐value of <0.05 was considered to be statistically significant.
Results
Periodontitis aggravated islets damage with potential insulin resistance
During periodontitis induced by ligatures for 8 weeks, GI, based on the clinical characteristics of the different grades of gingival inflammation16 and PD were scored and recorded to evaluate the periodontal status. As shown in Figure 1, these two parameters both increased significantly in mice with periodontitis (periodontitis and combination group) compared with mice without periodontitis (control and diabetic group; Figure 1a). Fasting blood glucose and plasma insulin both showed higher level in db/db mice compared with C57BL/6 mice, whereas increased glucose level and decreased insulin level were detected in the combination group compared with the diabetic group (Figure 1b), with the strong increase of homeostasis model assessment for insulin resistance values only shown in diabetic mice compared with controls (Figure 1c). After inducing periodontitis, mice in all groups were killed under general anesthesia at 20 weeks for islets isolation, and partial islets were subjected to a GSIS test. The results showed that low glucose stimulation increased the insulin secretion in the diabetic group compared with the control, whereas high glucose resulted in decreased trends, indicating badly damaged islets (Figure 1d). Furthermore, both low and high glucose stimulation led to decreased secretion of insulin in the combination group compared with the diabetic group.
Figure 1.
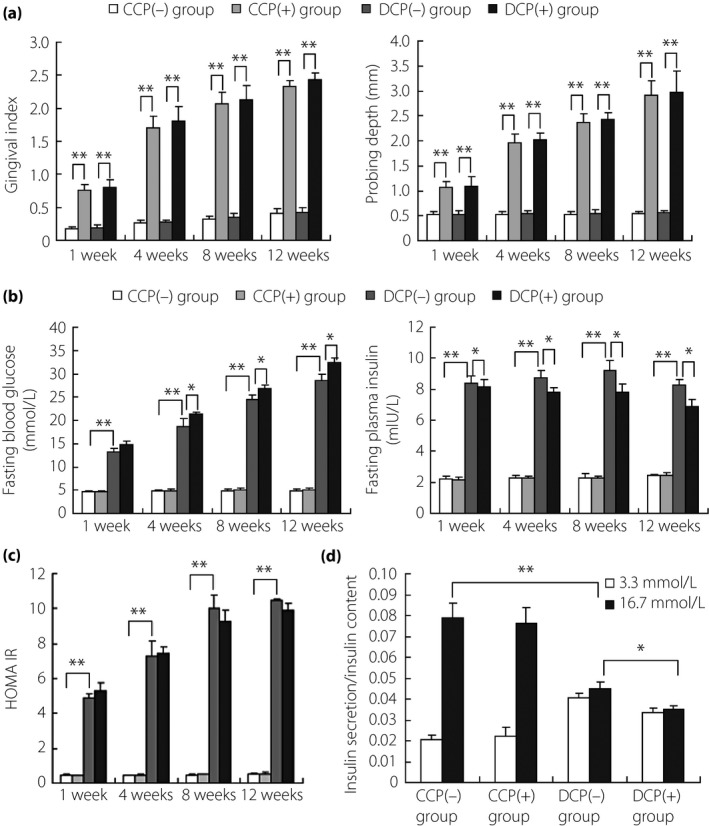
Impaired function of pancreatic β‐cells was aggravated under the introduction of periodontitis in diabetic mice. Every 16 db/db or C57BL/6 mice were randomly divided into two groups of eight, respectively: (i) db/db mice with periodontitis (combination group, DCP[+]); (ii) db/db mice without periodontitis (diabetic group, DCP[−]); (iii) C57BL/6 mice with periodontitis (periodontitis group, CCP[+]); and (iv) C57BL/6 mice without periodontitis (control group, CCP[−]). (a) Increased gingival index and probing depth in periodontitis‐introduced mice compared with controls (CCP[+] vs CCP[−], DCP[+] vs DCP[−]). (b) Introduction of periodontitis led to increased glucose level and decreased insulin level in diabetic mice (DCP[+] vs DCP[−]), whereas the effect was not obvious in normal mice (CCP[+] vs CCP[−]). (c) Homeostasis model assessment for insulin resistance scores showed a strong increase in diabetic status (DCP[−] vs CCP[−]), with no obvious change observed in periodontitis‐induced mice (CCP[+] vs CCP[−]; DCP[+] vs DCP[−]). (d) Glucose‐stimulated insulin secretion test for pancreatic islets showed that low glucose (3.3 mmol/L) stimulation increased the insulin secretion of diabetic mice compared with normal mice (DCP[−] vs CCP[−]), whereas high glucose (16.7 mmol/L) resulted in decreased secretion of insulin, indicating impaired function of pancreatic β‐cells. In addition, low and high glucose stimulation both inhibited the insulin secretion under periodontitis introduction in diabetic mice (DCP[+] vs DCP[−]), whereas the inhibition was not obvious in normal mice (CCP[+] vs CCP[−]). *P < 0.05, **P < 0.01.
Periodontitis induced increased IL‐12 level and decreased Klotho level in diabetic mice
Recent studies have identified inflammation as an underlying component of diabetes. Tumor necrosis factor‐α, IL‐6, IL‐1β and IL‐12 are common pro‐inflammatory cytokines; these cytokines are increased in serum, vitreous, retinas or islets from diabetic patients or rats26, 27. In the present study, all of these cytokines were activated in the diabetic group compared with the control (Figure 2a–d), indicating general pro‐inflammatory response. However, these cytokines were not evaluated under periodontitis treatment in normal mice, whereas only particularly increased IL‐12 was detected in diabetic mice with periodontitis, showing the correlation between IL‐12 and periodontitis‐aggravated islets dysfunction. Furthermore, serum Klotho level decreased synchronously with increased IL‐12 (Figure 2e), indicating the possible modulatory function of IL‐12 on Klotho.
Figure 2.
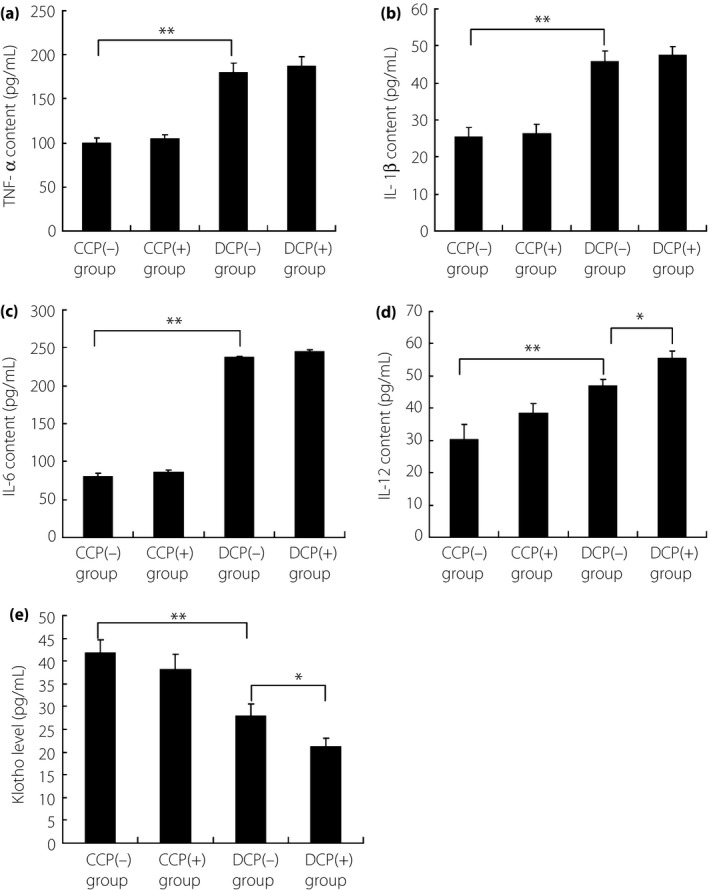
Detection for the levels of pro‐inflammatory cytokines, including (a) tumor necrosis factor (TNF)‐α, (b) interleukin (IL)‐1β, (c) IL‐6 and (d) IL‐12, and (e) also serum Klotho level. TNF‐α, IL‐1β, IL‐6 and IL‐12 were all activated in diabetic mice without periodontitis compared with normal mice. Furthermore, IL‐12 level was further induced under periodontitis introduction in diabetic mice, whereas the increase was not obvious in normal mice with periodontitis. The level change of IL‐12 was consistent with the impaired pancreatic function. Besides, serum Klotho level was also decreased in diabetic mice without periodontitis compared with normal mice, and this inhibition was aggravated under periodontitis introduction in diabetic mice, but not obvious in normal mice. *P < 0.05, **P < 0.01. CCP(−), C57BL/6 mice without periodontitis; CCP(+), C57BL/6 mice with periodontitis; DCP(−), db/db mice without periodontitis; DCP(+), db/db mice with periodontitis.
Klotho expression was inhibited by IL‐12 stimulation in MIN6 β‐cells and isolated islets
In order to investigate the relationship between IL‐12 and Klotho, MIN6 β‐cells and isolated mice islets were both introduced and performed under IL‐12 treatment for 24 h. Results showed that β‐cell‐specific expression of Klotho was significantly repressed at both mRNA and protein levels by IL‐12 (Figure 3). As former studies have proven that Klotho can improve β‐cell function and attenuate the development of type 2 diabetes20, it can be speculated that periodontitis aggravated islets damage, mainly β‐cell dysfunction, through the negative modulation of IL‐12 on Klotho expression. Furthermore, mounting evidence suggests that Klotho can protect against endothelial dysfunction and regulate the production of nitric oxide28, whereas induction of iNOS and activation of NF‐κB by IL‐12 has also been reported in microglial cells29. Here, nitric oxide production and insulin secretion were chosen to evaluate the effect of IL‐12 treatment, and also of Klotho function.
Figure 3.
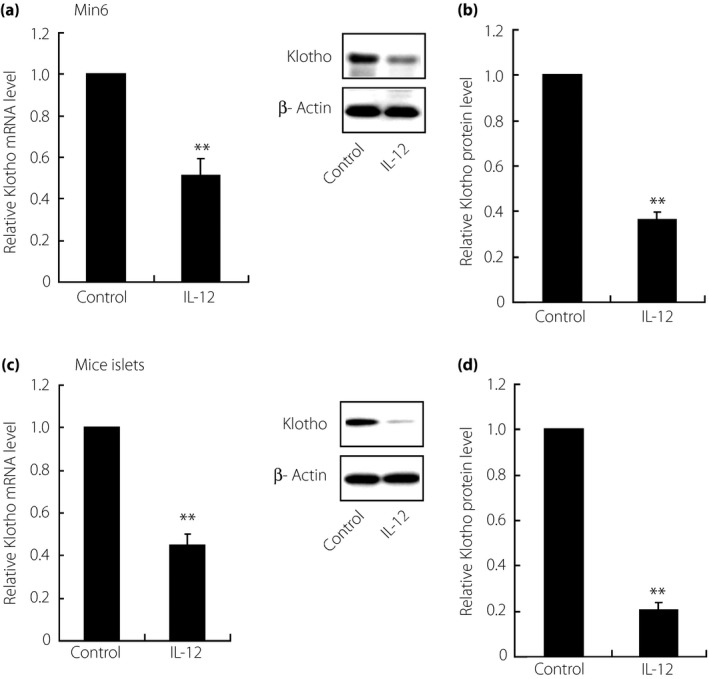
Inhibited expression of Klotho under interleukin (IL)‐12 treatment in MIN6 β‐cells and primary isolated mice islets. After treatment with IL‐12 (2.5 ng/mL) for 24 h, the expression of Klotho was detected at both (a, c) messenger ribonucleic acid (mRNA) and (b, d) protein levels through reverse transcription polymerase chain reaction and western blot analysis, respectively, with results showing significantly downregulated Klotho in both MIN6 β‐cells (Min6) and isolated mice islets. Data shown here were derived from at least three independent experiments. **P < 0.01.
Klotho overexpression could reverse both immunostimulation and decreased insulin secretion induced by IL‐12
To investigate the function of Klotho under IL‐12 treatment, Klotho overexpression was executed in both MIN6 β‐cells and isolated islets through adenoviral transfection. Then, IL‐12 induced immunostimulation about NO production and insulin secretion changes were observed in Klotho‐overexpressed subjects. As shown in Figure 4, NF‐κB was activated under IL‐12 treatment, with upregulated iNOS expression and also NO production in MIN6 β‐cells. Interestingly, Klotho overexpression would reverse IL‐12‐induced abundance of nitric oxide, indicating the key role of Klotho in IL‐12‐induced pathways. In addition, a GSIS test was also carried out in Klotho overexpressed subjects, with results showing that Klotho overexpression could also recover impaired insulin secretion by IL‐12 in both MIN6 β‐cells and isolated islets (Figure 5). Taken together, it can be speculated that islets damage was aggravated through the modulation of IL‐12 on Klotho, involving functional β‐cells in pancreatic islets, in the diabetic mouse model with periodontitis.
Figure 4.
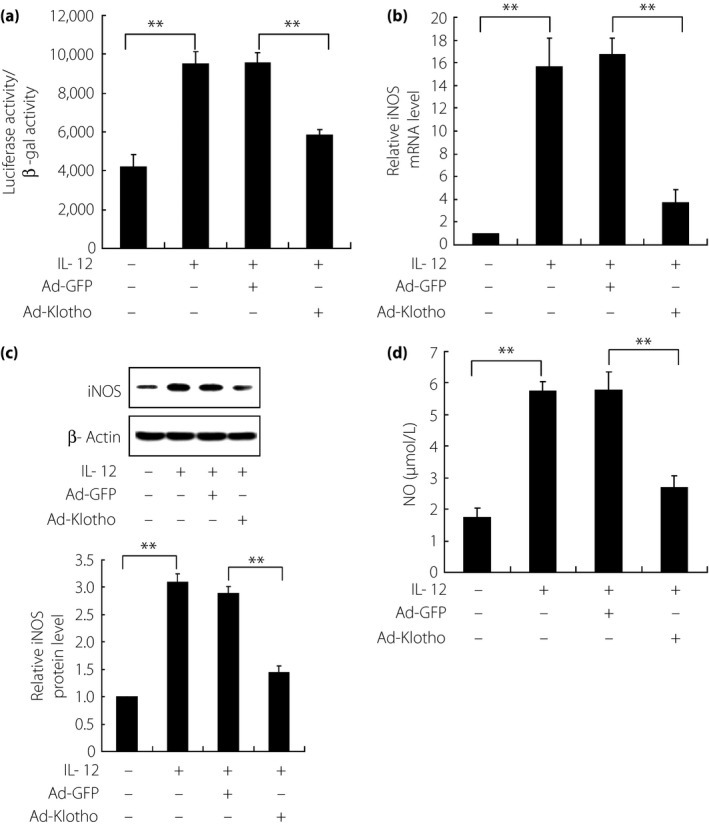
Reversed immune profiles by Klotho overexpression in interleukin (IL)‐12‐treated MIN6 β‐cells. (a) Nuclear factor‐κB activity, (b, c) iNOS expression and (d) nitric oxide (NO) content were all increased under IL‐12 treatment in normal cells (adeno‐associated virus carrying mouse Klotho full‐length complementary deoxyribonucleic acid [Ad‐GFP]), whereas these effects were reversed in Klotho overexpressed cells (adeno‐associated virus carrying green fluorescent protein [Ad‐Klotho]). The expression of iNOS was detected at both (b) messenger ribonucleic acid (mRNA) and (c) protein levels through reverse transcription polymerase chain reaction and western blot analysis, respectively. Three independently repetitive experiments were carried out for data expression. iNOS: inducible nitric oxide synthase. **P < 0.01.
Figure 5.
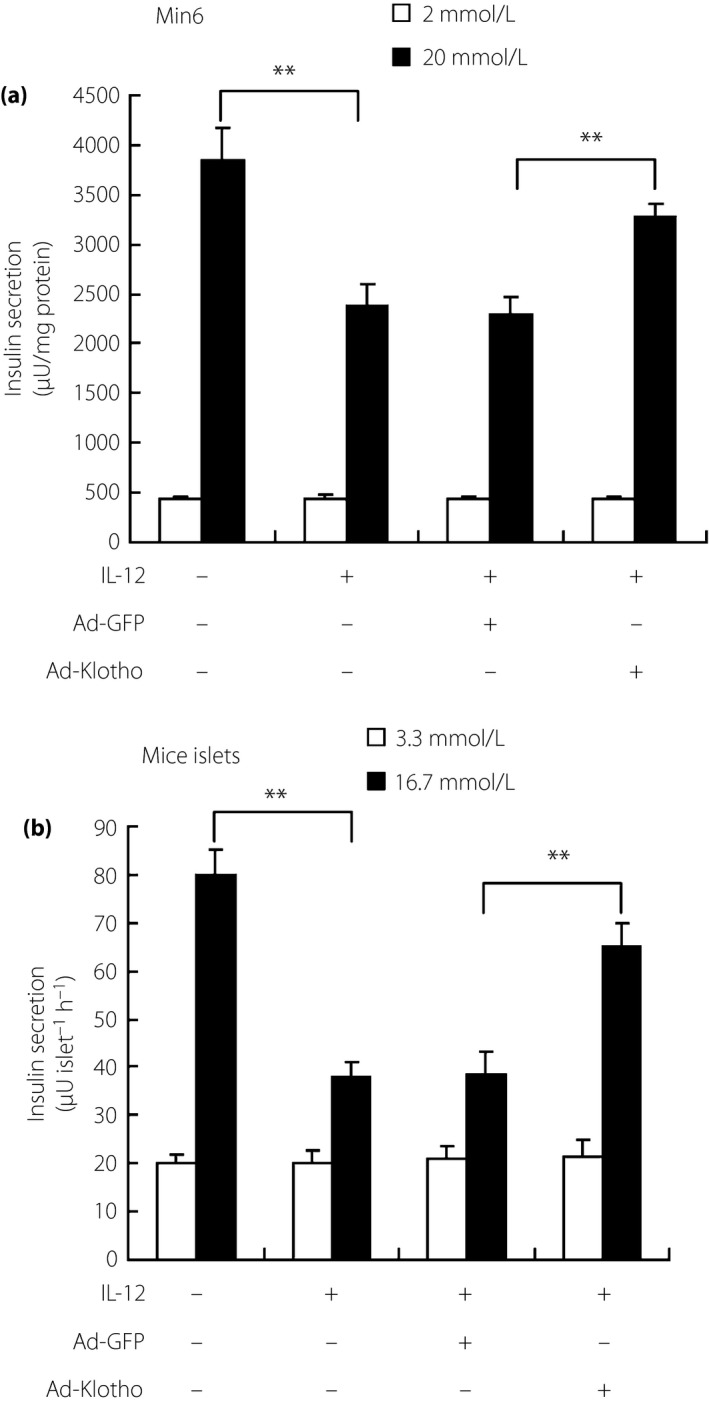
Klotho overexpression improved the impaired function of (a) MIN6 β‐cells and (b) primary isolated mice islets under interleukin (IL)‐12 treatment. Glucose‐stimulated insulin secretion was detected in Klotho‐overexpressed MIN6 β‐cells or mice islets after IL‐12 treatment. Results showed that the impaired function of insulin secretion, induced by IL‐12 treatment, could be recovered through Klotho overexpression in both MIN6 β‐cells and mice islets. **P < 0.01. Ad‐GFP, adeno‐associated virus carrying mouse Klotho full‐length complementary deoxyribonucleic acid; Ad‐Klotho, adeno‐associated virus carrying green fluorescent protein.
Discussion
The present study showed that periodontitis could aggravate pancreatic β‐cell failure in a diabetic mouse model, and further showed the functional role of Klotho, as an inhibitor on IL‐12, in preserving functional β‐cells in pancreatic islets. Previous studies have confirmed the direct effect of periodontitis on adipose tissue inflammation and also subsequent systemic insulin resistance in an obese rat model13. Thus, this effect was investigated here in a diabetic mouse model, mainly focusing on the influence on insulin secretion. As expected, diabetic mice with periodontitis showed potential insulin resistance with more serious dysfunction in insulin secretion under glucose stimulation, indicating aggravated pancreatic β‐cell failure.
Previous study has indicated that some pro‐inflammatory cytokines cannot only work as a network to regulate inflammation, but also influence insulin sensitivity and glucose metabolism30. Therefore, serum levels of common pro‐inflammatory cytokines, like tumor necrosis factor‐α, IL‐6, IL‐1β and IL‐12, were detected here. Fortunately, only particularly increased IL‐12 was observed synchronously with periodontitis‐aggravated islets dysfunction. Klotho, an anti‐aging gene, can be repressed in pancreatic islets of db/db mice though its normal expression is slight in pancreas20, 28. Thus, serum Klotho level was also measured here with results showing decreased Klotho content along with increased IL‐12. Although the correlation between IL‐12 and Klotho has not been explored yet, these results showed the important role of this correlation in periodontitis aggravated dysfunction of islets.
Former evidence suggests that IL‐12 can functionally impair GSIS in INS‐1 cells, one homogeneous β‐cell line, and human donor islets, suggesting a direct role of IL‐12 in mediating β‐cell pathology27. Therefore, the direct effects of IL‐12 on mice islets and β‐cells were observed, and the corresponding change of Klotho expression was also investigated. After IL‐12 treatment, mice islets and β‐cells both showed decreased function in insulin secretion, whereas Klotho expression was also proven to be negatively regulated by IL‐12. It was the first time that the functional negative regulation of IL‐12 on Klotho was explored. Furthermore, it can be speculated that periodontitis‐aggravated dysfunction of islets was as a result of the inhibition of Klotho under upregulated IL‐12, given the fact that β‐cell‐specific expression of Klotho can attenuate the development of diabetes in db/db mice20.
For further verification of the functional role of Klotho, Klotho overexpression was carried out to examine its influence on IL‐12 stimulation, including IL‐12‐induced NO production29. Interestingly, IL‐12‐stimulating results could be significantly reversed by Klotho overexpression. In addition, impaired β‐cell function in insulin secretion was also recovered to some extent under Klotho overexpression, providing more evidence for the function of Klotho in preserving β‐cell function.
IL‐12, as an important cytokine in early inflammatory responses, has been implicated in immune‐mediated pathogenesis of pancreatic islets in diabetes27. However, the mechanism of IL‐12‐mediated β‐cell function is not clear. In the present study, IL‐12 was chosen for acting as an important mediator in periodontitis‐aggravated dysfunction of islets in diabetic mice. Subsequently, the negative regulation of IL‐12 on Klotho was shown, and the function of Klotho as an inhibitor in IL‐12 stimulation was also shown, indicating the potential role of Klotho to be one candidate for the improvement of insulin resistance in diabetes. Further studies will be required to determine the functional role of Klotho in vivo, as well as its application in clinical therapy.
Disclosure
The authors declare no conflict of interest.
J Diabetes Investig 2016; 7: 303–311
References
- 1. Ferrannini E, Mari A. Beta cell function and its relation to insulin action in humans: a critical appraisal. Diabetologia 2004; 47: 943–956. [DOI] [PubMed] [Google Scholar]
- 2. Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature 2001; 414: 782–787. [DOI] [PubMed] [Google Scholar]
- 3. Leahy JL, Hirsch IB, Peterson KA, et al Targeting beta‐cell function early in the course of therapy for type 2 diabetes mellitus. J Clin Endocrinol Metab 2010; 95: 4206–4216. [DOI] [PubMed] [Google Scholar]
- 4. Kuro‐o M, Matsumura Y, Aizawa H, et al Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997; 390: 45–51. [DOI] [PubMed] [Google Scholar]
- 5. Kurosu H, Kuro OM. The Klotho gene family as a regulator of endocrine fibroblast growth factors. Mol Cell Endocrinol 2009; 299: 72–78. [DOI] [PubMed] [Google Scholar]
- 6. Utsugi T, Ohno T, Ohyama Y, et al Decreased insulin production and increased insulin sensitivity in the klotho mutant mouse, a novel animal model for human aging. Metabolism 2000; 49: 1118–1123. [DOI] [PubMed] [Google Scholar]
- 7. Lin Y, Sun Z. Antiaging gene Klotho enhances glucose‐induced insulin secretion by up‐regulating plasma membrane levels of TRPV2 in MIN6 beta‐cells. Endocrinology 2012; 153: 3029–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin Y, Sun Z. In vivo pancreatic beta‐cell‐specific expression of antiaging gene Klotho: a novel approach for preserving beta‐cells in type 2 diabetes. Diabetes 2015; 64: 1444–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet 2005; 366: 1809–1820. [DOI] [PubMed] [Google Scholar]
- 10. Saxlin T, Ylostalo P, Suominen‐Taipale L, et al Association between periodontal infection and obesity: results of the Health 2000 Survey. J Clin Periodontol 2011; 38: 236–242. [DOI] [PubMed] [Google Scholar]
- 11. Lalla E, Papapanou PN. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol 2011; 7: 738–748. [DOI] [PubMed] [Google Scholar]
- 12. Preshaw PM, Alba AL, Herrera D, et al Periodontitis and diabetes: a two‐way relationship. Diabetologia 2012; 55: 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Su Y, Wang D, Xuan D, et al Periodontitis as a novel contributor of adipose tissue inflammation promotes insulin resistance in a rat model. J Periodontol 2013; 84: 1617–1626. [DOI] [PubMed] [Google Scholar]
- 14. Kimura S, Nagai A, Onitsuka T, et al Induction of experimental periodontitis in mice with Porphyromonas gingivalis‐adhered ligatures. J Periodontol 2000; 71: 1167–1173. [DOI] [PubMed] [Google Scholar]
- 15. Loe H. The gingival index, the plaque index and the retention index systems. J Periodontol 1967; 38(Suppl): 610–616. [DOI] [PubMed] [Google Scholar]
- 16. Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand 1963; 21: 533–551. [DOI] [PubMed] [Google Scholar]
- 17. Matthews DR, Hosker JP, Rudenski AS, et al Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 18. Yamazaki Y, Imura A, Urakawa I, et al Establishment of sandwich ELISA for soluble alpha‐Klotho measurement: age‐dependent change of soluble alpha‐Klotho levels in healthy subjects. Biochem Biophys Res Commun 2010; 398: 513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carter JD, Dula SB, Corbin KL, et al A practical guide to rodent islet isolation and assessment. Biol Proced Online 2009; 11: 3–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin Y, Sun Z. In vivo pancreatic beta cell‐specific expression of anti‐aging gene klotho, a novel approach for preserving beta cells in type II diabetes. Diabetes 2015; 64: 1444–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miyazaki J, Araki K, Yamato E, et al Establishment of a pancreatic beta cell line that retains glucose‐inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology 1990; 127: 126–132. [DOI] [PubMed] [Google Scholar]
- 22. Wang Y, Sun Z. Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension 2009; 54: 810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin Y, Sun Z. Thyroid hormone potentiates insulin signaling and attenuates hyperglycemia and insulin resistance in a mouse model of type 2 diabetes. Br J Pharmacol 2011; 162: 597–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Muzio M, Ni J, Feng P, et al IRAK (Pelle) family member IRAK‐2 and MyD88 as proximal mediators of IL‐1 signaling. Science 1997; 278: 1612–1615. [DOI] [PubMed] [Google Scholar]
- 25. Green LC, Wagner DA, Glogowski J, et al Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 1982; 126: 131–138. [DOI] [PubMed] [Google Scholar]
- 26. Yu Z, Gong C, Lu B, et al Dendrobium chrysotoxum Lindl. alleviates diabetic retinopathy by preventing retinal inflammation and tight junction protein decrease. J Diabetes Res 2015; 2015: 518317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Taylor‐Fishwick DA, Weaver JR, Grzesik W, et al Production and function of IL‐12 in islets and beta cells. Diabetologia 2013; 56: 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang Y, Sun Z. Current understanding of klotho. Ageing Res Rev 2009; 8: 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pahan K, Sheikh FG, Liu X, et al Induction of nitric‐oxide synthase and activation of NF‐kappaB by interleukin‐12 p40 in microglial cells. J Biol Chem 2001; 276: 7899–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Balistreri CR, Caruso C, Candore G. The role of adipose tissue and adipokines in obesity‐related inflammatory diseases. Mediators Inflamm 2010; 2010: 802078. [DOI] [PMC free article] [PubMed] [Google Scholar]


