Abstract
Aims/introduction
The aims of the present study were to investigate the performance of a novel sandwich enzyme‐linked immunosorbent assay (ELISA) for measuring glucagon (1–29) with monoclonal antibodies against both the C‐ and N‐terminal regions of glucagon (1–29), and to analyze the differences in plasma levels and responses of glucagon (1–29) to oral glucose loading in normal glucose tolerance (NGT) subjects and patients with type 2 diabetes mellitus.
Materials and Methods
The cross‐reactivity against proglucagon fragments using the ELISA kit and two types of conventional radioimmunoassay (RIA) kits was evaluated. A 75‐g oral glucose tolerance test was carried out with NGT subjects and patients with type 2 diabetes mellitus, and the glucagon (1–29) concentration was measured using three types of kit.
Results
The ELISA kit clearly had the lowest cross‐reactivity against miniglucagon (19–29) and glicentin (1–61). The oral glucose tolerance test was carried out with 30 NGT and 17 patients with type 2 diabetes mellitus. The glucagon (1–29) levels measured by the ELISA kit after glucose loading were significantly higher at all time‐points in the type 2 diabetes mellitus group than in the NGT group. However, the glucagon (1–29) levels measured by one RIA kit were significantly higher in the NGT group, and those measured with the other RIA kit were approximately the same among the groups.
Conclusions
The novel sandwich ELISA accurately determines plasma glucagon (1–29) concentrations with much less cross‐reactivity against other proglucagon fragments than conventional RIA kits.
Keywords: Glucagon (1–29), Glucagon‐like peptides, Type 2 diabetes mellitus
Introduction
Proglucagon, a straight‐chain peptide composed of 160 amino acids, is synthesized by intestinal L‐cells, pancreatic islet α‐cells, gastric α‐cells and in certain neurons in the nucleus of the solitary tract in the brain stem1, 2, 3. In pancreatic α‐cells, glucagon (1–29) is produced through processing of proglucagon by prohormone convertase 2 (Figure 1a)4. In the gastrointestinal tract, in contrast, glucagon‐like peptide‐1 (GLP‐1), glucagon‐like peptide‐2 (GLP‐2), glicentin and oxyntomodulin are derived from proglucagon processed by prohormone convertase 1 (Figure 1b)5, 6.
Figure 1.
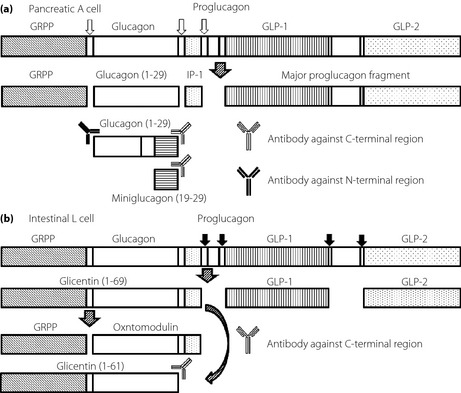
A schematic representation of the differential processing of proglucagon as it occurs in (a) the pancreatic α‐cell and (b) the intestinal L cell in humans. GLP‐1, glucagon‐like peptide‐1; GLP‐2, glucagon‐like peptide‐2; GRPP, glicentin‐related pancreatic peptide; IP‐1, intervening peptide 1; Prohormone convertase 1, black arrow; Prohormone convertase 2, white arrow.
In healthy subjects, food intake results in increased insulin secretion from the islets of Langerhans, and this has been known to suppress glucagon secretion from the pancreatic α‐cells7. In contrast, the paradoxical rise of plasma levels of glucagon has been reported to be one of the causes of postprandial blood glucose increase in patients with long‐standing diabetes7, 8, 9, 10, 11.
Proglucagon is processed to various proglucagon fragments including glucagon (1–29)12. So far, pancreatic glucagon is conventionally measured with radioimmunoassay (RIA) kits that use polyclonal antibodies against the glucagon C‐terminal region. However, the existence of proglucagon fragments with the same C‐terminal region as glucagon (1–29) has been reported13, 14, 15, 16, 17, 18. Such RIA kits might therefore not accurately measure plasma glucagon (1–29) because of cross‐reactivity of the antibodies with oxyntomodulin, glicentin, miniglucagon (19–29), GLP‐1, GLP‐2 and gastric inhibitory polypeptide (GIP)19.
Recently, a quantitative assay kit (E‐M; Mercodia Glucagon enzyme‐linked immunosorbent assay [ELISA], Uppsala, Sweden) known as sandwich ELISA using monoclonal antibodies against both of the C‐ and N‐terminal regions of glucagon has been developed20. This kit reportedly measures glucagon concentrations with much lower cross‐reactivity against proglucagon fragments other than glucagon (1–29), and with higher specificity and reliability than previous assay methods20.
However, few investigators have evaluated the performance of this ELISA kit in measuring glucagon (1–29)20. We therefore decided to evaluate the kit performance. In the present study, we compared the cross‐reactivity against proglucagon fragments associated with two conventional RIA kits and the novel ELISA kit. We also analyzed differences in measured plasma levels and responses of glucagon to oral glucose loading in normal glucose tolerance (NGT) subjects and patients with type 2 diabetes mellitus.
Materials and methods
Participants
The present study was carried out with 30 healthy NGT subjects (NGT group) and 17 patients with type 2 diabetes mellitus undergoing dietary and exercise therapy, but not receiving any oral antidiabetic agents (type 2 diabetes mellitus group). The criteria for normal glucose tolerance in a 75‐g oral glucose tolerance test (OGTT), set according to the diagnostic criteria of Japan Diabetes Society and World Health Organization, was plasma glucose concentration of less than 110 mg/dL in the fasting state and less than 140 mg/dL 2 h after glucose loading. Diagnosis of type 2 diabetes mellitus was made in accordance with type 2 diabetes mellitus diagnostic criteria of the Japan Diabetes Society. This study was approved by the ethics committee of Hyogo College of Medicine Hospital (No. 1661), and was registered with the University Hospital Medical Information Network Center (UMIN 000015235). This study was carried out in accordance with the Declaration of Helsinki. All participants gave informed consent, and signed the informed consent form.
Methods
The OGTT was carried out using a glucose formulation, Trelan G® (Ajinomoto Pharma Co., Ltd, Tokyo, Japan). The participants were fasted for 12 h before Trelan G® administration, and the entire quantity was taken within 5 min. Bodyweight and height in the participants were measured at the time of the OGTT. Blood was collected before, and 15, 30, 60 and 120 min after initiation of the OGTT, and plasma glucose, immunoreactive insulin (IRI), and glucagon (1–29) were measured. Plasma glucose was measured with the hexokinase ultraviolet method using the BioMajesty JCA‐BM9000 series (Japan Electron Optics laboratory, Tokyo, Japan). IRI was measured with chemiluminescence enzyme immunoassay using Lumipulse Presto II (Fujirebio Inc., Tokyo, Japan). In preparation for glucagon (1–29) measurement, plasma was separated using BD P800 tubes (BD Diagnostics, Franklin Lakes, NJ, USA) containing K2 ethylenediaminetetraacetic acid, protease, esterase and dipeptidyl peptidase‐4 inhibitors. Glucagon (1–29) in the separated plasma was measured with three types of glucagon measurement kits in accordance with the manufacturers' recommendations.
The glucagon (1–29) concentration was measured by RIA using two types of kit: EURIA‐Glucagon (R‐E; EuroDiagnostica, Malmö, Sweden) and Glucagon RIA Kit (R‐M; Millipore, Billerica, MA, USA). The E‐M sandwich ELISA kit (E‐M; Mercodia Glucagon ELISA, Uppsala, Sweden) was also used. The basic performance of the kits as presented in the instruction manuals is shown below. The coefficient of variation (CV) for intra‐assay variation was 3.3–5.1% for the E‐M, 4.5–8.1% for the R‐E and 4.0–6.8% for the R‐M21, 22, 23. The CV for inter‐assay variation was 7.3–9.4% for the E‐M, 3.9–8.3% for the R‐E and 7.3–13.5% for the R‐M21, 22, 23. Recovery rates were 96–101% for the E‐M, 97.6% for the R‐E and 96–98% for the R‐M21, 22, 23. Dilution linearity was 81–96% for the E‐M (but not indicated for the R‐E or R‐M)21, 22, 23. The blood plasma used was not extracted for any kit. Miniglucagon (19–29), glicentin (1–69), glicentin (1–61), oxyntomodulin, GLP‐1 (1–37), GLP‐1 (7–36 amide), GLP‐2 and GIP were measured with these kits to determine cross‐reactivity. Oxyntomodulin, GLP‐1 (1–36) and glucagon (1–29) were obtained from Biological Industries (Kibbutz Beit Haemek, Israel), Anygen (Jeollanam‐do, Korea) and Phoenix Pharmaceuticals (Burlingame, CA, USA), respectively. Synthetic glicentin (1–61) peptide and synthetic glicentin (1–69) peptide were synthetized by BEX (Tokyo, Japan), and their authenticity was confirmed using high‐performance liquid chromatography and mass spectrometry. All other peptides were purchased from Peptide Institute, Inc. (Osaka, Japan).
Kit cross‐reactivity was evaluated by comparing the EC50 values for the peptides analyzed. More specifically, EC50 values were calculated by measuring different concentrations of the peptides, in assay buffer, over the range of linearity, with cross‐reactivity evaluated by calculating the ratio of individual EC50 values to the EC50 value of glucagon. The basic performance of this study involved dual measurements, and reproducibility was confirmed by means of three independent assays.
Statistical Analysis
The results were presented as means ± standard deviations (SD) unless otherwise stated. Fisher's exact test was used to test the differences in sex between the NGT group and the type 2 diabetes mellitus group. Welch's t‐test was used to evaluate the differences between the backgrounds of the NGT group and the type 2 diabetes mellitus group. Two‐way repeated‐measures anova was used to test the time‐courses of changes in glucagon (1–29) concentration in the NGT group and the type 2 diabetes mellitus group, measured by the OGTT, and Dunnett's test was used to test the changes from baseline. When the principal effects were found to be significant, and/or interactions were found, intergroup comparison was carried out using Welch's t‐test. StatView software (version 5.0; SAS Institute Inc.; Cary, NC, USA) was used for all analyses.
Results
First, we verified reproducibility by four measurements each with low‐, medium‐ and high‐concentration pooled plasma, and, with respect to intra‐assay variation and inter‐assay variation for E‐M, respectively, the coefficients of variation were 2.5–4.7%, and 11.5% or lower. Next, low, medium and high concentrations of glucagon were added to four samples with different endogenous glucagon concentrations, and the yields were evaluated, and found to be 94.4–119.3%. When the dilution linearity of the E‐M kit was evaluated by preparing a twofold dilution series using a calibrator 0 included in the E‐M kit, for two samples with different endogenous glucagon concentrations, dilution linearity was confirmed, in that the curves were almost straight lines (r > 0.998), and passed through the origin. In addition, the sensitivity of the E‐M kit was evaluated by measuring glucagon standard substance over a 5‐day period. The finding was that the coefficient of variation between 1.35 and 128 pmol/L, which is the standard concentration range included in the E‐M kit, was 8.5%, differing from the theoretical value by no more than 5.1%.
The cross‐reactivity curves are shown in Figure 2a–c. The cross‐reactivities against glucagon‐related peptides using the three assay kits are summarized in Table 1. The cross‐reactivities against miniglucagon (19–29) were 23.1% and over 100%, respectively in the R‐E and R‐M, whereas no cross‐reactivity was found in the E‐M. The cross‐reactivities against glicentin (1–69) were 0.004%, 0.01% and 17.3%, respectively, in the R‐E, R‐M and E‐M. The cross‐reactivities against glicentin (1–61) were over 100%, over 100% and 22.7%, respectively, in the R‐E, R‐M and E‐M. The cross‐reactivities against oxyntomodulin were 0.7%, 0.9% and 10.2%, respectively, in the R‐E, R‐M and E‐M. No cross‐reactivity against GLP‐1 (1–36), GLP‐1 (7–36 amide), GLP‐2 and GIP were found in any of the kits. These experiments were repeated two more times, and the results were approximately the same (data not shown).
Figure 2.
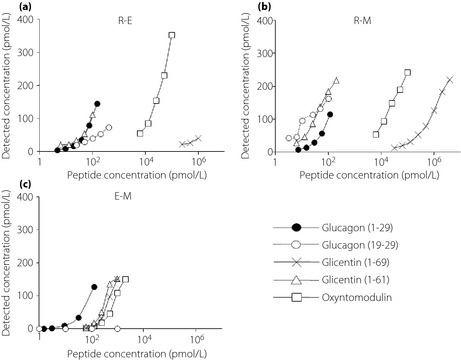
Cross‐reactivity curves for proglucagon fragments: glucagon (1–29), filled circles with a solid line; minigluagon (19–29), open circles with a dashed line; glicentin (1–69), cross marks with a solid line; glicentin (1–61), open triangles with a solid line; oxyntomodulin, open squares with a solid line. (a) Evaluated by R‐E (EURIA‐Glucagon, EuroDiagnostica AB, Malmö, Sweden). (b) Evaluated by R‐M (Glucagon RIA Kit; Millipore, Billerica, MA, USA). (c) Evaluated by E‐M (Mercodia Glucagon ELISA, Mercodia AB, Uppsala, Sweden).
Table 1.
Cross‐reactivities of proglucagon fragments in respective glucagon assay kits
| Glucagon (1–29) (%) | Glucagon (19–29) (%) | Glicentin (1–69) (%) | Glicentin (1–61) (%) | Oxyntomodulin (%) | GLP‐1 (1–36) | GLP‐1 (7–36) | GLP‐2 | GIP | |
|---|---|---|---|---|---|---|---|---|---|
| E‐M | 100 | ND | 17.3 | 22.7 | 10.2 | ND | ND | ND | ND |
| R‐E | 100 | 23.1 | 0.004 | >100 | 0.7 | ND | ND | ND | ND |
| R‐M | 100 | >100 | 0.01 | >100 | 0.9 | ND | ND | ND | ND |
GIP, gastric inhibitory polypeptide; GLP, glucagon‐like peptide; ND, not detectable.
The characteristics of the participants are shown in Table 2. The present study included 30 NGT subjects and 17 patients with type 2 diabetes mellitus. The mean age was significantly higher in the type 2 diabetes mellitus group than in the NGT group (62.7 ± 11.0 vs 24.0 ± 1.7, P < 0.01). Body mass index (BMI) was significantly higher in the type 2 diabetes mellitus group than in the NGT group (24.8 ± 3.6 vs 21.4 ± 2.6, P < 0.01). The duration of diabetes was 5.5 ± 5.1 years, and glycated hemoglobin (National Glycohemoglobin Standardization Program) was 6.6 ± 0.6% in the type 2 diabetes mellitus group.
Table 2.
Characteristics of participants
| Variables | Healthy participants | Diabetes patients | P‐value |
|---|---|---|---|
| n (female: male) | 30 (13:17) | 17 (5:12) | NS |
| Age (years) | 24.0 ± 1.7 | 62.7 ± 11.0 | <0.01 |
| BMI (kg/m²) | 21.4 ± 2.6 | 24.8 ± 3.6 | <0.01 |
| HOMA‐R | 1.6 ± 0.7 | 2.2 ± 1.7 | NS |
| HOMA‐β | 81.0 ± 32.5 | 41.4 ± 27.8 | <0.01 |
| IGI | 1.2 ± 0.7 | 0.3 ± 0.3 | <0.01 |
Data are expressed as means ± standard deviations. BMI, body mass index; HOMA‐β, homeostasis model assessment of β‐cells; HOMA‐R, homeostasis model assessment ratio; IGI, insulinogenic index; NS, not significant.
Plasma glucose levels were significantly higher in the type 2 diabetes mellitus group at all time‐points than in the NGT group (P < 0.01 for all time‐points; Figure 3a). In addition, IRI levels were significantly lower in the type 2 diabetes mellitus group at 15 and 30 min after glucose loading (P < 0.01 for 15 and 30 min; Figure 3b).
Figure 3.
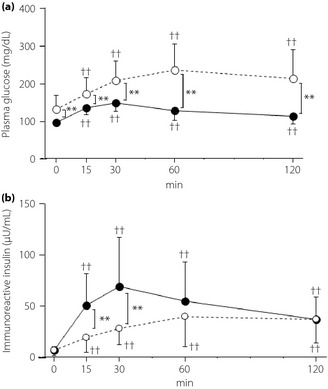
Changes of (a) plasma glucose and (b) immunoreactive insulin after oral glucose tolerance test in healthy participants with normal glucose tolerance (solid line) and patients with type 2 diabetes mellitus (dashed line). **P < 0.01 normal glucose tolerance vs type 2 diabetes mellitus, ††P < 0.01 vs 0 min.
Changes of plasma glucagon (1–29) levels are shown in Figure 4a–c. R‐E showed decreases in glucagon (1–29) level in the NGT group after glucose loading (Figure 4a). R‐M showed no significant changes in glucagon (1–29) levels after glucose loading in either the NGT or type 2 diabetes mellitus group (Figure 4b). Glucagon (1–29) levels measured by the E‐M decreased over 60 min after glucose loading in the NGT group, but increased over 30 min after loading in the type 2 diabetes mellitus group (Figure 4c). Glucagon (1–29) levels determined by the R‐E after glucose loading were significantly higher in the NGT group than the type 2 diabetes mellitus group, whereas with the E‐M glucagon (1–29), levels were significantly higher at all time‐points in the type 2 diabetes mellitus group (Figure 4a,c). In addition, the glucagon (1–29) levels measured by the R‐M were approximately the same among the groups (Figure 4b).
Figure 4.
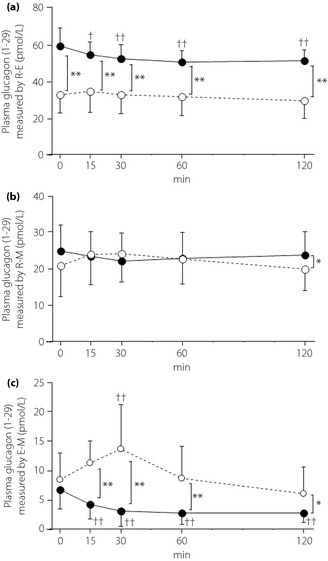
Changes of plasma glucagon (1–29) measured by (a, b) conventional radioimmunoassay kits and (c) a sandwich enzyme‐linked immunoassay kit after oral glucose tolerance test in healthy participants with normal glucose tolerance (solid line) and patients with type 2 diabetes mellitus (dashed line). (a) Evaluated by R‐E (EURIA‐Glucagon, EuroDiagnostica AB, Malmö, Sweden). (b) Evaluated by R‐M (Glucagon RIA Kit; Millipore, Billerica, MA, USA). (c) Evaluated by E‐M (Mercodia Glucagon ELISA, Mercodia AB, Uppsala, Sweden). *P < 0.05, **P < 0.01 normal glucose tolerance vs type 2 diabetes mellitus, †P < 0.05, ††P < 0.01 vs 0 min.
Discussion
In the present study, the cross‐reactivity against proglucagon fragments associated with three glucagon assay kits was evaluated to compare kit specificities.
In this research, the cross‐reactivities against glucagon (1–29) and other proglucagon fragments in each measurement kit were investigated. The cross‐reactivities of the E‐M kit against glicentin (1–61) and miniglucagon (19–29) were lower than those of the R‐E and R‐M kits, whereas its cross‐reactivities against glicentin (1–69) and oxyntomodulin were higher than those of the other kits.
The kits were also used to determine parameters such as glucagon (1–29) secretion over time in NGT subjects and patients with type 2 diabetes mellitus after OGTT in the study. It has been reported that glucagon (1–29) concentration decreases after glucose loading in healthy volunteers24, 25, 26. The results of measurement by R‐E and E‐M showed decreases in glucagon (1–29) levels with time‐course after glucose loading in the NGT group, but no such changes were shown by R‐M. In contrast to the participants with normal glucose tolerance, it has been reported that plasma glucagon concentration paradoxically rises after meals in patients with longstanding diabetes, and this has been supposed to be one of the causes of postprandial hyperglycemia in patients with diabetes mellitus7, 8, 9, 10, 11. In addition, it has been reported that intrinsic insulin secretion from pancreatic β‐cells inhibits glucagon secretion from pancreatic α‐cells27, 28. In the present study, glucagon (1–29) secretion in patients with type 2 diabetes mellitus was elevated 30 min after glucose loading according to E‐M measurements, but R‐E and R‐M measurements showed no such change. Finally, E‐M glucagon (1–29) levels were significantly higher in the type 2 diabetes mellitus group than the NGT group, but R‐E levels were higher in the NGT group at all‐time points, and R‐M levels were higher in the NGT group at 120 min after glucose loading.
Differences in cross‐reactivity could partially explain the variation in the glucagon (1–29) measurements among the kits. The principle underlying ELISA indicates this procedure to be specific for glucagon (1–29), and cross‐reactivity investigations show the E‐M to have lower cross‐reactivity against miniglucagon (19–29) and glicentin (1–61). The aforementioned explain why the E‐M produced the lowest glucagon (1–29) measurements. Although cross‐reactivity against glicentin (1–69) and oxyntomodulin was highest for the E‐M, these cross‐reactivities likely minimally affect glucagon (1–29) measurements20. Another study found that glicentin (1–69) increases and oxyntomodulin is unchanged after glucose loading in NGT people29, 30. In the present study, glucagon (1–29) secretion decreased after glucose loading in the NGT group, which suggests that glicentin (1–69) and oxyntomodulin minimally affect glucagon (1–29) measurements. It has been reported that glucagon is primarily metabolized in the kidneys, and in the present study the mean age of participants in the type 2 diabetes mellitus group was older than that of those in the NGT group, and it is possible that aging‐related physiological decreases in renal function affect glucagon (1–29) measurement31. Hormone plasma concentrations reflect the balance between secretion and clearance, and it is therefore possible that, for evaluating the precision of glucagon (1–29) measurement kits, more detailed evaluation of the secretion and clearance of glicentin (1–69), and oxyntomodulin and other proglucagon fragments, which probably affect glucagon (1–29) measurement, is required.
The more accurate glucagon (1–29) measurements obtained with the E‐M show a paradoxical rise in glucagon (1–29) after glucose loading in patients with mild type 2 diabetes mellitus treated solely with dietary and exercise therapy. Functional abnormalities of α‐cells must be accurately characterized to properly determine the pathological mechanism of type 2 diabetes mellitus and develop treatment strategies. Glucagon secretion should thus be closely analyzed in patients with impaired glucose tolerance or mild to severe type 2 diabetes mellitus. The sandwich ELISA kit used in the present study is well suited for such analyses.
The present study had several limitations. It was primarily concerned with evaluating the usefulness of E‐M for glucagon (1–29) measurement, and such aspects of the basic performance of R‐E and R‐M, such as the CV of intra‐assay variation, were therefore not investigated. With respect to the basic performance of R‐E and R‐M, although no major differences have been found between the presented reports and the manufacturer's published data32, 33, 34, 35, 36, further investigation of this area is considered to be necessary. Only blood glucose, insulin, and glucagon (1–29) were measured. It has been reported that GIP promotes glucagon secretion37, 38, and GLP‐1 inhibits it39. The additional measurement of incretin hormones that affect glucagon secretion, such as GLP‐1 and GIP, would provide more data for consideration. Age and BMI differed significantly between the NGT and type 2 diabetes mellitus groups. The small sample size prevents us from properly evaluating the effects of age and weight on glucagon secretion. Further work using a larger sample size might be required. Finally, glicentin (1–69), oxyntomodulin and other proglucagon fragments were not measured in the study, but should be measured to further substantiate our conclusion that the E‐M produces more accurate measurements than the other kits studied.
In conclusion, a recently developed sandwich ELISA kit that uses monoclonal antibodies against both the C‐ and N‐terminal regions of glucagon more accurately measured plasma glucagon (1–29) concentrations with much less cross‐reactivity against other proglucagon fragments than conventional RIA based on polyclonal antibodies.
Disclosure
J‐I Miyagawa received research grants from Cosmic Corporation. A Kushida and T Inagaki are full‐time employees of Cosmic Corporation. T Matsuo, Y Kusunoki, M Miuchi, T Ikawa, T Akagami, M Tokuda, T Katsuno and M Namba declare no conflict of interest.
Acknowledgments
We thank all the volunteers who participated in this study. This study was funded by Cosmic Corporation.
J Diabetes Investig 2016; 7: 324–331
Clinical Trial Registry
University Hospital Medical Information
Network Center
UMIN000015235
References
- 1. Holst JJ. Enteroglucagon. Annu Rev Physiol 1997; 59: 257–271. [DOI] [PubMed] [Google Scholar]
- 2. Larsen PJ, Tang‐Christensen M, Holst JJ, et al Distribution of glucagon‐like peptide‐1 and other preproglucagon‐derived peptides in the rat hypothalamus and brainstem. Neuroscience 1997; 77: 257–270. [DOI] [PubMed] [Google Scholar]
- 3. Munõz BL, Rufener C, Srikant CB, et al Immunocytochemical evidence for glucagon‐containing cells in the human stomach. Horm Metab Res 1977; 9: 37–39. [DOI] [PubMed] [Google Scholar]
- 4. Rouillé Y, Westermark G, Martin SK, et al Proglucagon is processed to glucagon by prohormone convertase PC2 in alpha TC1‐6 cells. Proc Natl Acad Sci U S A 1994; 91: 3242–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dhanvantari S, Seidah NG, Brubaker PL. Role of prohormone convertases in the tissue‐specific processing of proglucagon. Mol Endocrinol 1996; 10: 342–355. [DOI] [PubMed] [Google Scholar]
- 6. Tucker JD, Dhanvantari S, Brubaker PL. Proglucagon processing in islet and intestinal cell lines. Regul Pept 1996; 62: 29–35. [DOI] [PubMed] [Google Scholar]
- 7. Yabe D, Kuroe A, Watanabe K, et al Early phase glucagon and insulin secretory abnormalities, but not incretin secretion, are similarly responsible for hyperglycemia after ingestion of nutrients. J Diabetes Complications 2015; 29: 413–421. [DOI] [PubMed] [Google Scholar]
- 8. Henkel E, Menschikowski M, Koehler C, et al Impact of glucagon response on postprandial hyperglycemia in men with impaired glucose tolerance and type 2 diabetes mellitus. Metabolism 2005; 54: 1168–1173. [DOI] [PubMed] [Google Scholar]
- 9. Kozawa J, Okita K, Iwahashi H, et al Early postprandial glucagon surge affects postprandial glucose levels in obese and non‐obese patients with type 2 diabetes. Endocr J 2013; 60: 813–818. [DOI] [PubMed] [Google Scholar]
- 10. Müller WA, Faloona GR, Aguilar‐Parada E, et al Abnormal alpha‐cell function in diabetes. Response to carbohydrate and protein ingestion. N Engl J Med 1970; 283: 109–115. [DOI] [PubMed] [Google Scholar]
- 11. Kawai K, Murayama Y, Okuda Y, et al Postprandial glucose, insulin and glucagon responses to meals with different nutrient compositions in non‐insulin‐dependent diabetes mellitus. Endocrinol Jpn 1987; 34: 745–753. [DOI] [PubMed] [Google Scholar]
- 12. Bataille D, Dalle S. The forgotten members of the glucagon family. Diabetes Res Clin Pract 2014; 106: 1–10. [DOI] [PubMed] [Google Scholar]
- 13. Bataille D. Pro‐protein convertases in intermediary metabolism: islet hormones, brain/gut hormones and integrated physiology. J Mol Med 2007; 85: 673–684. [DOI] [PubMed] [Google Scholar]
- 14. Blache P, Kervran A, Le‐Nguyen D, et al Miniglucagon production from glucagon: an extracellular processing of a hormone used as a prohormone. Biochimie 1994; 76: 295–299. [DOI] [PubMed] [Google Scholar]
- 15. Blache P, Kervran A, Dufour M, et al Glucagon (19‐29), a Ca2 + pump inhibitory peptide, is processed from glucagon in the rat liver plasma membrane by a thiol endopeptidase. J Biol Chem 1990; 265: 21514–21519. [PubMed] [Google Scholar]
- 16. Thim L, Moody AJ. The primary structure of porcine glicentin (proglucagon). Regul Pept 1981; 2: 139–150. [DOI] [PubMed] [Google Scholar]
- 17. Baldissera FG, Holst JJ. Glicentin 1‐61 probably represents a major fraction of glucagon‐related peptides in plasma of anaesthetized uraemic pigs. Diabetologia 1986; 29: 462–467. [DOI] [PubMed] [Google Scholar]
- 18. Holst JJ. Evidence that glicentin contains the entire sequence of glucagon. Biochem J 1980; 187: 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bak MJ, Albrechtsen NW, Pedersen J, et al Specificity and sensitivity of commercially available assays for glucagon and oxyntomodulin measurement in humans. Eur J Endocrinol 2014; 170: 529–538. [DOI] [PubMed] [Google Scholar]
- 20. Wewer Albrechtsen NJ, Hartmann B, Veedfald S, et al Hyperglucagonaemia analysed by glucagon sandwich ELISA: nonspecific interference or truly elevated levels? Diabetologia 2014; 57: 1919–1926. [DOI] [PubMed] [Google Scholar]
- 21. Mercodia AB. (Internal company date). Available from: http://www.mercodia.se/ Accessed May 13, 2014.
- 22. EuroDiagnostica . (Internal company date). Available from: http://www.eurodiagnostica.com/ Accessed May 13, 2014.
- 23. Merck Millipore Corp. (Internal company date). Available from: http://www.merckmillipore.com/ Accessed May 13, 2014.
- 24. Knop FK, Vilsbøll T, Larsen S, et al Glucagon suppression during OGTT worsens while suppression during IVGTT sustains alongside development of glucose intolerance in patients with chronic pancreatitis. Regul Pept 2010; 164: 144–150. [DOI] [PubMed] [Google Scholar]
- 25. Bagger JI, Knop FK, Lund A, et al Glucagon responses to increasing oral loads of glucose and corresponding isoglycaemic intravenous glucose infusions in patients with type 2 diabetes and healthy individuals. Diabetologia 2014; 57: 1720–1725. [DOI] [PubMed] [Google Scholar]
- 26. Harada N, Hamasaki A, Yamane S, et al Plasma gastric inhibitory polypeptide and glucagon‐like peptide‐1 levels after glucose loading are associated with different factors in Japanese subjects. J Diabetes Investig 2011; 2: 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Unger RH, Dobbs RE, Orci L. Insulin, glucagon, and somatostatin secretion in the regulation of metabolism. Annu Rev Physiol 1978; 40: 307–343. [DOI] [PubMed] [Google Scholar]
- 28. Koh DS, Cho JH, Chen L. Paracrine interactions within islets of Langerhans. J Mol Neurosci 2012; 48: 429–440. [DOI] [PubMed] [Google Scholar]
- 29. Naito H, Ohneda A, Kojima R, et al Plasma glicentin in diabetic and gastrectomized patients. Regul Pept 1999; 79: 55–61. [DOI] [PubMed] [Google Scholar]
- 30. Laferrère B, Swerdlow N, Bawa B, et al Rise of oxyntomodulin in response to oral glucose after gastric bypass surgery in patients with type 2 diabetes. J Clin Endocrinol Metab 2010; 95: 4072–4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Trebbien R, Klarskov L, Olesen M, et al Neutral endopeptidase 24.11 is important for the degradation of both endogenous and exogenous glucagon in anesthetized pigs. Am J Physiol Endocrinol Metab 2004; 287: 431–438. [DOI] [PubMed] [Google Scholar]
- 32. Robertson MD, Livesey G, Morgan LM, et al The influence of the colon on postprandial glucagon‐like peptide 1 (7‐36) amide concentration in man. J Endocrinol 1999; 161: 25–31. [DOI] [PubMed] [Google Scholar]
- 33. Kärvestedt L, Andersson G, Efendic S, et al A rapid increase in beta‐cell function by multiple insulin injections in type 2 diabetic patients is not further enhanced by prolonging treatment. J Intern Med 2002; 251: 307–316. [DOI] [PubMed] [Google Scholar]
- 34. de Galan BE, Tack CJ, Lenders JW, et al Theophylline improves hypoglycemia unawareness in type 1 diabetes. Diabetes 2002; 51: 790–796. [DOI] [PubMed] [Google Scholar]
- 35. Marina AL, Utzschneider KM, Wright LA, et al Colesevelam improves oral but not intravenous glucose tolerance by a mechanism independent of insulin sensitivity and β‐cell function. Diabetes Care 2012; 35: 1119–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Calanna S, Urbano F, Piro S, et al Elevated plasma glucose‐dependent insulinotropic polypeptide associates with hyperinsulinemia in metabolic syndrome. Eur J Endocrinol 2012; 166: 917–922. [DOI] [PubMed] [Google Scholar]
- 37. Meier JJ, Deacon CF, Schmidt WE, et al Suppression of glucagon secretion is lower after oral glucose administration than during intravenous glucose administration in human subjects. Diabetologia 2007; 50: 806–813. [DOI] [PubMed] [Google Scholar]
- 38. Christensen M, Vedtofte L, Holst JJ, et al Glucose‐dependent insulinotropic polypeptide: a bifunctional glucose‐dependent regulator of glucagon and insulin secretion in humans. Diabetes 2011; 60: 3103–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seino Y, Fukushima M, Yabe D. GIP and GLP‐1, the two incretin hormones: Similarities and differences. J Diabetes Investig 2010; 1: 8–23. [DOI] [PMC free article] [PubMed] [Google Scholar]


