Abstract
Aims/Introduction
To determine the efficacy and safety of ipragliflozin in combination with metformin in Asian patients with type 2 diabetes mellitus.
Materials and Methods
This phase 3, multicenter, placebo‐controlled, double‐blind, parallel‐group study was carried out at 18 sites in Korea and 12 sites in Taiwan. After an 8‐week washout period for patients using drugs other than metformin and a 2‐week run‐in period, patients were randomized to either 50 mg ipragliflozin or a placebo for 24 weeks while continuing metformin. Efficacy outcomes included the changes in hemoglobin A1c, fasting plasma glucose (FPG) and bodyweight from baseline to the end of treatment (with last observation carried forward). Safety outcomes included treatment‐emergent adverse events.
Results
Between November 2011 and January 2013, 171 patients were randomized to and administered ipragliflozin (n = 87) or a placebo (n = 83). The mean changes (standard deviation) in hemoglobin A1c were −0.94% (0.75%) and −0.47% (0.81%) in the ipragliflozin and placebo groups, respectively (between‐group difference −0.46%, P < 0.001). The changes in fasting plasma glucose and bodyweight were also significantly greater in the ipragliflozin group, with between‐group differences of −14.1 mg/dL and −1.24 kg, respectively (both P < 0.001). The most common treatment‐emergent adverse events (ipragliflozin vs placebo) were upper respiratory tract infection (9.2% vs 12.0%) and urinary tract infection (6.9% vs 2.4%).
Conclusions
These results show that ipragliflozin is effective and well tolerated when used in combination with metformin in Asian patients with type 2 diabetes mellitus.
Keywords: Asia, Ipragliflozin, Type 2 diabetes mellitus
Introduction
Recent estimates of the International Diabetes Federation indicate that type 2 diabetes mellitus affects approximately 138 million people in the Western Pacific region alone, increasing to 202 million people by 20351. Furthermore, approximately one‐third of all patients with type 2 diabetes mellitus are in the Western Pacific region. The rising incidence of type 2 diabetes mellitus is accompanied by increasing trends towards obesity and overweight2, which are especially apparent in Asian countries.
The pathophysiology of type 2 diabetes mellitus differs slightly between Asian patients and other patient populations in terms of greater insulin resistance, and reduced insulin secretion in Asian patients2, 3. Additionally, Asian patients tend to develop diabetes at a lower body mass index relative to Western populations2.
Current treatment recommendations for type 2 diabetes mellitus highlight the importance of diet and exercise as initial therapy, followed by the addition of a glucose‐lowering drug, commonly metformin4, 5. Other available drug classes for initial or second‐line therapy, including sulfonylureas and incretin modulators, improve glycemic control by enhancing insulin secretion. However, increased insulin resistance and a reduced insulin secretion capacity might limit the efficacy of these drugs. Therefore, other classes of drugs that lower blood glucose in an insulin‐independent manner are required.
Sodium‐glucose cotransporter 2 (SGLT2) is a glucose transporter that is primarily expressed in proximal tubules, where it is responsible for approximately 90% of the glucose reabsorption in the kidney6. Some studies have suggested that SGLT2 expression is increased in diabetic rats7 and in human exfoliated proximal tubular epithelial cells obtained from urine samples of patients with type 2 diabetes mellitus8. Several SGLT2 inhibitors are currently under development or have been approved in some countries for the treatment of type 2 diabetes mellitus; these lower blood glucose levels by preventing glucose reabsorption in the kidney in an insulin‐independent manner9, 10, 11.
Ipragliflozin, a SGLT2 inhibitor, was first approved in Japan for the treatment of type 2 diabetes mellitus based on the results of clinical trials in Japanese patients with type 2 diabetes mellitus when used as both monotherapy12, 13 and in dual combination with other glucose‐lowering agents, including metformin14. Because SGLT2 inhibitors improve glycemic control in an insulin‐independent manner, they are expected to be useful in Asian patients with type 2 diabetes mellitus, especially considering the reduced insulin secretory capacity in Asians2, 3. Until now, no studies have focused on ipragliflozin in non‐Japanese Asian patients. Therefore, the present study aimed to examine the efficacy and safety of ipragliflozin in combination with metformin in Taiwanese and Korean patients with type 2 diabetes mellitus.
Materials and methods
Patients
Korean or Taiwanese patients who met the following criteria were eligible for the present study: age ≥20 years, diagnosis of type 2 diabetes mellitus ≥12 weeks before enrolment, stable diet and exercise regimen for ≥8 weeks, hemoglobin A1c (HbA1c) between 7.0 and 10.0%, and body mass index between 20.0 and 45.0 kg/m2. Patients using metformin were also eligible provided metformin was administered at a stable dose of ≥1,500 mg/day (or ≥1,000 mg/day if safety concerns prohibited higher doses) for ≥8 weeks. The exclusion criteria are summarized in the Supporting Information. All patients provided written informed consent.
Study design and treatments
The present phase 3, multicenter, placebo‐controlled, double‐blind, parallel‐group study was carried out at 18 sites in Korea and 12 sites in Taiwan. The study consisted of a single‐blind, 2‐week placebo run‐in period (visit 1), a 24‐week double‐blind treatment period (visits 2–9) and a 4‐week follow‐up period (visits 9–10). Visits 2–9 were scheduled at weeks 0, 2, 4 and every 4 weeks thereafter; visit 9 (week 24) was defined as the end of treatment. An additional visit was scheduled for patients who withdrew before visit 9. Patients using hypoglycemic drugs other than metformin entered an 8‐week washout period before visit 1.
Patients who met the eligibility criteria at visit 1 were randomized at visit 2 to receive 50 mg ipragliflozin or a placebo in a 1:1 ratio with stratification for study site and HbA1c at visit 1 (<8.0% or ≥8.0%) using an interactive web randomization system with treatment codes prepared by an independent assignment manager. The study drugs were identical in size, color and appearance, and were provided in identical packaging. To maintain blinding, the patients and investigators were blinded to the treatment codes. The treatment code was broken once the study database was locked.
During the study, patients were prohibited from using antidiabetic drugs other than the study drug and metformin, provided metformin was continued at the same dose. Patients were also prohibited from continuous systemic administration of corticosteroids, immunosuppressants or loop diuretics for chronic diseases; however, these drugs were allowed for topical or temporary use. Changes to diet and exercise therapy were also prohibited.
Treatment compliance was assessed in terms of the number of prescribed and returned tablets, the number of tablets lost, and patient diaries at each visit.
The study protocol, case report forms and patient information sheets were approved by institutional review boards at each participating site. The study was carried out in accordance with Good Clinical Practice, International Conference on Harmonization Technical Requirements for Registration of Pharmaceuticals for Human Use, as well as applicable local laws and regulations.
Efficacy and safety outcomes
The primary efficacy outcome was HbA1c (National Glycohemoglobin Standardization Program units), which was measured every 4 weeks. Secondary efficacy outcomes included proportions of patients with HbA1c <7.0% or <6.5% at each visit, fasting plasma glucose (FPG; measured at each visit), bodyweight (measured at each visit), waist circumference (visits 2 and 9), and fasting serum insulin (FSI; visits 2, 6 and 9). For all efficacy outcomes, the value measured at visit 2 was used as the baseline value.
The main safety outcomes were adverse events (AEs), vital signs, hematology, serum chemistry and urinalysis, which were assessed at visits 2–10. Other safety outcomes included urinalysis for bacteria and sediment (visits 2, 5, 7 and 9), bone turnover markers (visits 2, 6, 9 and 10) and bone metabolism markers (visits 2, 9 and 10). All protocol‐defined clinical laboratory assessments were carried out at a central laboratory (Mitsubishi Chemical Medience Corporation, Tokyo, Japan) using routine methods. AEs were reported in terms of system organ class and preferred term using the MedDRA version 14.0, and classified in terms of severity, seriousness and causal relationship to the study drug. AEs that occurred after the first dose of the study drug in the treatment period were defined as treatment‐emergent adverse events (TEAEs). Serious AEs were any untoward medical events that resulted in death, were life‐threatening, resulted in persistent or significant disability/incapacity, resulted in a congenital anomaly or birth defect, required inpatient hospitalization or prolonged hospitalization and other medically important events. AEs were also graded as mild (no disruption of normal daily activities), moderate (affected normal daily activities) or severe (inability to carry out daily activities). To maintain blinding, investigators and patients were not to carry out urinary glucose tests, unless deemed essential for safety reasons.
Statistical analysis
A total of 40 patients per country were planned for enrolment to satisfy local regulations and the recommendation of the respective health authorities. From a statistical viewpoint, enrolment of at least 80 participants per group provided over 90% power to show the statistical difference with a two‐sided significance level of 0.05, based on the results of a Japanese dose‐finding study12.
Primary and secondary efficacy variables were evaluated in the full analysis set, comprising all patients who received at least one dose of the study drug in the treatment period, and in whom the efficacy variable was measured at least once after starting administration of the study drug. Safety variables were assessed in the safety analysis set, which consisted of all patients who received at least one dose of the study drug during the treatment period.
For efficacy and safety variables, missing values were imputed using the last observation carried forward method. Statistical analyses were carried out by combining data for both countries and for each country individually. Baseline characteristics are summarized as the mean (standard deviation) for continuous variables, or the n (%) for categorical variables. Two‐sample t‐tests and Fisher's exact test were used to compare continuous and categorical variables, respectively, between the groups.
The change in HbA1c from baseline to the end of treatment (with last observation carried forward) was compared between the groups using analysis of covariance (ancova) with the baseline value as a covariate, and treatment group and country as fixed effects. The mean difference between the ipragliflozin and placebo groups (between‐group difference) was also calculated using the ancova model. Sensitivity analyses were carried out using the per‐protocol set, by ancova with interactions among the included variables, and by ancova for each country individually. The changes in FPG, FSI, bodyweight and waist circumference were assessed as described for the change in HbA1c. AEs are summarized descriptively as the n (%) of patients in each group.
Results
Patient disposition and characteristics
Between November 2011 and January 2013, 238 patients provided informed consent. Of 171 who were randomized at visit 2, 87 received ipragliflozin and 83 received a placebo in the treatment period (Figure S1). These patients were included in the full analysis set and safety analysis set. One patient assigned to the placebo group discontinued before administration of the study drug and was not included in the full analysis set or safety analysis set. Overall, 74 and 69 patients treated with ipragliflozin and placebo, respectively, completed the study. As shown in Table S1, the characteristics of both groups were comparable, except for a slight imbalance in the proportion of patients with an estimated glomerular filtration rate of <90 mL/min/1.73 m2. The mean (standard deviation) duration of exposure to the study drug was 155 (39) and 149 (47) days in the ipragliflozin and placebo groups, respectively. Treatment compliance was high, with mean compliance rates of 97% in both groups.
Efficacy
Glycemic Control
As shown in Figure 1a, HbA1c decreased significantly over time to a greater extent in the ipragliflozin group than in the placebo group. The mean (standard deviation) change from baseline to the end of treatment was −0.94% (0.75%) and −0.47% (0.81%) in the ipragliflozin and placebo groups, respectively, with a between‐group difference of −0.46% (95% confidence interval [CI] −0.66 to −0.27%; P < 0.001). Similar differences were also observed in subgroup analyses after stratifying patients by sex, age and baseline HbA1c (data not shown). The proportion of patients with HbA1c <7.0% increased from 11.5% (10/87) at baseline to 69.4% (59/85) at the end of treatment in the ipragliflozin group, and from 3.6% (3/83) to 44.6% (37/83) in the placebo group. The proportion of patients with HbA1c <6.5% increased from 1.1% (1/87) at baseline to 25.9% (22/85) at the end of treatment in the ipragliflozin group, and from 0% (0/83) to 9.6% (8/83) in the placebo group.
Figure 1.
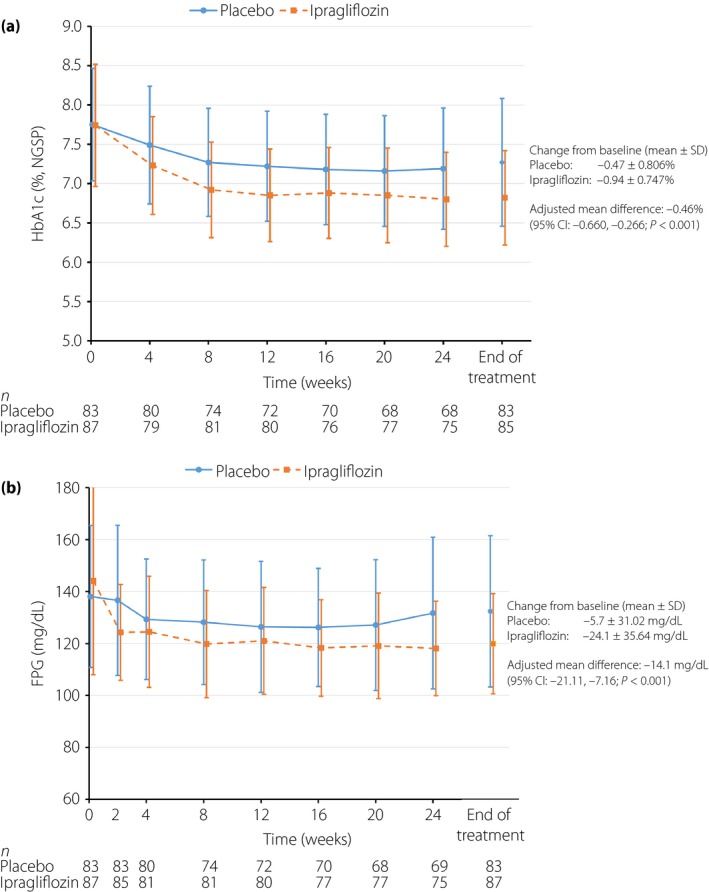
Time‐courses of (a) hemoglobin A1c (HbA1c) and (b) fasting plasma glucose (FPG) measurements. Values are mean (standard deviation [SD]). CI, confidence interval; NGSP, National Glycohemoglobin Standardization Program.
Figure 1b shows the time‐course of FPG levels in both groups. FPG decreased over time in both groups, and remained lower in the ipragliflozin group than in the placebo group from week 2 until the end of treatment. The mean change in FPG from baseline to the end of treatment was significantly greater in the ipragliflozin group than in the placebo group (24.1 vs −5.7 mg/dL; between‐group difference −14.1 mg/dL [95% CI −21.1 to −7.2 mg/dL; P < 0.001]).
Other Secondary Efficacy and Laboratory Outcomes
Bodyweight decreased over time in both groups (Figure S2a), but the mean change from baseline to the end of treatment was significantly greater in the ipragliflozin group than in the placebo group (−2.93 vs −1.70 kg), with a between‐group difference of −1.24 kg (95% CI −1.92 to −0.56 kg; P < 0.001). By the end of treatment, 33.3% (29/87) and 18.1% (15/83) of patients in the ipragliflozin and placebo groups, respectively, had a weight reduction of ≥5%.
Consistent with the change in bodyweight, the reduction in waist circumference (Figure S2b) was significantly greater in the ipragliflozin group (−1.72 vs −0.85 cm; between‐group difference −0.91 cm; 95% CI −1.74 to −0.08 cm; P = 0.032). Although FSI decreased in the ipragliflozin group and increased in the placebo group (change in FSI with ipragliflozin vs placebo −0.99 [9.91] vs 0.22 [6.34] μU/mL; P = 0.321), the difference was not significant. The changes in homeostasis model assessment of insulin resistance (−0.78 [3.373] vs −0.05 [2.793]; P = 0.111) were similar between the ipragliflozin and placebo groups. Plasma triglyceride levels decreased significantly in the ipragliflozin group, but increased in the placebo group (−24.6 [91.9] vs 10.7 [79.6] mg/dL; P = 0.008). The increase in high‐density lipoprotein cholesterol was slightly greater in the ipragliflozin group, while the increase in total cholesterol and decrease in low‐density lipoprotein (LDL) cholesterol were slightly greater in the placebo group than in the ipragliflozin group (Table 1). The change in free fatty acids was similar in both groups. The decrease in estimated glomerular filtration rate at the end of treatment was greater in the placebo group (−18.28 [49.27] mL/min/1.73 m2] than in the ipragliflozin group (−9.86 [40.29] mL/min/1.73 m2), although the difference between groups did not reach statistical significance.
Table 1.
Clinical and laboratory parameters (safety analysis set)
| Variable | Change† in the placebo group (n = 83) | Change† in the ipragliflozin group (n = 87) | P‐value¶ |
|---|---|---|---|
| Triglycerides (mg/dL) | 10.7 (79.6) | −24.6 (91.9) | 0.008§ |
| FFA (mEq/L) | −0.084 (0.322) | −0.020 (0.304) | 0.183 |
| Total cholesterol (mg/dL) | 5.3 (27.2) | 2.1 (27.5) | 0.445 |
| HDL cholesterol (mg/dL) | 0.9 (7.0) | 2.6 (7.0) | 0.118 |
| LDL cholesterol (mg/dL) | −2.0 (27.2) | −0.8 (29.9) | 0.798 |
| eGFR (mL/min/1.73 m2) | −18.28 (49.27) | −9.86 (40.29) | 0.224 |
| SBP (mmHg) | −1.3 (12.9) | −6.8 (14.1) | 0.009§ |
| DBP (mmHg) | −1.0 (8.0) | −3.7 (9.4) | 0.047‡ |
| Pulse rate (/min) | −1.9 (9.9) | −2.1 (8.7) | 0.889 |
Values are means (standard deviation). †Change from baseline to the end of treatment. ‡P < 0.05 versus placebo; §P < 0.01 versus placebo; ¶t‐test. DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FFA, free fatty acids; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; SBP, systolic blood pressure.
Systolic and diastolic blood pressures decreased to a greater extent in the ipragliflozin group (systolic/diastolic blood pressure −6.8 [14.1]/−3.7 [9.4] mmHg) than in the placebo group (−1.3 [12.9]/−1.0 [8.0] mmHg; Table 1).
Safety
TEAEs occurred in just over one‐half of the patients, and drug‐related TEAEs occurred in approximately one‐fifth of the patients in each group (Table 2). All of the TEAEs and drug‐related TEAEs were classified as mild or moderate in severity. Two patients in the ipragliflozin group and one in the placebo group discontinued treatment because of TEAEs. Meanwhile, serious TEAEs occurred in 2.3% (2/87) and 6.0% (5/83) of patients in the ipragliflozin and placebo groups, respectively. For one patient in the placebo group, the serious TEAEs were considered to be study drug‐related. The serious TEAEs in the ipragliflozin group were synovial cyst and knee arthroplasty in one patient each, while those in the placebo group were concussion, spinal disorder and contusion in one patient; hydronephrosis, ureteric stenosis and acute pyelonephritis in one patient (all considered study drug‐related); and compression fracture, colonic polyp and increased alanine aminotransferase in one patient each.
Table 2.
Treatment‐emergent adverse events (safety analysis set)
| Placebo | Ipragliflozin | |
|---|---|---|
| n | 83 | 87 |
| All TEAEs | 51 (61.4) | 50 (57.5) |
| No. TEAEs | 131 | 130 |
| TEAEs by severity | ||
| Mild | 41 (49.4) | 40 (46.0) |
| Moderate | 10 (12.0) | 10 (11.5) |
| Severe | 0 (0) | 0 (0) |
| Serious TEAEs | 5 (6.0) | 2 (2.3) |
| TEAEs leading to study discontinuation | 1 (1.2) | 2 (2.3) |
| Drug‐related TEAEs | 17 (20.5) | 17 (19.5) |
| No. drug‐related TEAEs | 34 | 29 |
| Drug‐related TEAEs by severity | ||
| Mild | 13 (15.7) | 15 (17.2) |
| Moderate | 4 (4.8) | 2 (2.3) |
| Severe | 0 (0) | 0 (0) |
| Serious drug‐related TEAEs | 1 (1.2) | 0 (0) |
| Drug‐related TEAEs leading to study discontinuation | 1 (1.2) | 2 (2.3) |
| TEAEs in ≥ 3% of patients in either group | ||
| Upper respiratory tract infection | 10 (12.0) | 8 (9.2) |
| Urinary tract infection | 2 (2.4) | 6 (6.9) |
| Proteinuria | 3 (3.6) | 3 (3.4) |
| Nasopharyngitis | 3 (3.6) | 2 (2.3) |
| Dysuria | 3 (3.6) | 2 (2.3) |
| Dizziness | 2 (2.4) | 3 (3.4) |
| Weight decreased | 1 (1.2) | 3 (3.4) |
| Osteoarthritis | 3 (3.6) | 1 (1.1) |
| Bilirubin conjugated increased | 3 (3.6) | 0 (0) |
| Nocturia | 3 (3.6) | 0 (0) |
| Constipation | 0 (0) | 3 (3.4) |
Values are n (%) of patients. TEAEs, treatment‐emergent adverse events.
The most common TEAEs in the ipragliflozin group were upper respiratory tract infection (9.2%; 8/87) and urinary tract infection (6.9%; 6/87). Other TEAEs occurred in <4 patients in the ipragliflozin group. Upper respiratory tract infection and urinary tract infection occurred in 12.0% (10/83) and 2.4% (2/83) of patients, respectively, in the placebo group. The most common drug‐related TEAEs in the ipragliflozin group were urinary tract infection (6.9%; 6/87) and decreased weight (3.4%; 3/87). The corresponding rates of these TEAEs in the placebo group were 2.4% (2/83) and 1.2% (1/83). There were no episodes of hypoglycemia or genital infection in either group. Polyuria occurred in one patient (1.1%) in the ipragliflozin group, and pollakiuria occurred in four patients (4.8%) in the placebo group.
Table 3 summarizes the changes in variables related to hematology, renal function, liver function and electrolytes. There were no apparent differences in these laboratory variables, with the exception of slightly greater increases in the red blood cell count, hemoglobin, hematocrit, urine osmolality, blood urea nitrogen, blood urea nitrogen/creatinine ratio, magnesium, urine potassium:creatinine ratio and urine phosphorus:creatinine ratio in the ipragliflozin group than in the placebo group. There were no apparent differences in bone markers, liver function markers or other laboratory variables.
Table 3.
Hematology and other laboratory variables (safety analysis set)
| Variable | Change† in the placebo group (n = 83) | Change† in the ipragliflozin group (n = 87) | P‐value†† |
|---|---|---|---|
| BUN (mg/dL) | 0.7 (3.7) | 2.9 (4.2) | <0.001¶ |
| Cre (mg/dL) | 0.050 (0.100) | 0.031 (0.114) | 0.251 |
| BUN:Cre ratio | −1.57 (12.00) | 3.81 (9.54) | 0.001¶ |
| RBC count (×104/μL) | −8.7 (20.9) | 14.9 (19.5) | <0.001¶ |
| Hemoglobin (g/dL) | −0.24 (0.77) | 0.37 (0.61) | <0.001¶ |
| Hematocrit (%) | −0.60 (2.01) | 1.45 (1.84) | <0.001¶ |
| Na (mEq/L) | 0.0 (1.9) | 0.0 (2.2) | 0.940 |
| K (mEq/L) | 0.05 (0.34) | 0.07 (0.32) | 0.663 |
| Cl (mEq/L) | −0.2 (2.7) | 0.3 (3.3) | 0.284 |
| Ca (mg/dL) | −0.10 (0.41) | −0.09 (0.52) | 0.860 |
| Mg (mg/dL) | 0.03 (0.14) | 0.14 (0.19) | <0.001¶ |
| P (mg/dL) | −0.01 (0.48) | 0.12 (0.51) | 0.094 |
| Urine NAG:Cre ratio (U/g Cre) | 0.32 (4.78) | 0.85 (5.96) | 0.526 |
| Urine albumin:Cre ratio (mg/g Cre) | 33.08 (282.92) | 4.90 (153.16) | 0.418 |
| Urine osmolality (mOsm/L) | −13.0 (255.2) | 72.6 (255.2) | 0.030‡ |
| Urine Na/Cre ratio (mEq/g Cre) | 16.0 (105.0) | 26.5 (94.4) | 0.491 |
| Urine K/Cre ratio (mEq/g Cre) | 4.55 (35.28) | 21.09 (38.42) | 0.004§ |
| Urine Cl/Cre ratio (mEq/g Cre) | 3.0 (110.6) | 21.9 (106.0) | 0.256 |
| Urine Ca/Cre ratio (mg/g Cre) | 8.98 (88.14) | 25.74 (95.74) | 0.237 |
| Urine Mg/Cre ratio (mEq/g Cre) | 5.12 (35.42) | 10.94 (33.26) | 0.271 |
| Urine P/Cre ratio (mEq/g Cre) | 59.82 (247.54) | 197.82 (293.39) | 0.001¶ |
| AST (U/L) | −3.1 (9.1) | −4.8 (8.2) | 0.224 |
| ALT (U/L) | −8.9 (44.1) | −9.3 (17.8) | 0.929 |
| Total bilirubin (mg/dL) | 0.00 (0.21) | 0.03 (0.19) | 0.368 |
| Direct bilirubin (mg/dL) | 0.00 (0.10) | 0.00 (0.09) | 0.821 |
| LDH (U/L) | −1.3 (25.5) | −8.0 (34.2) | 0.154 |
| ALP (U/L) | −9.7 (60.4) | −7.4 (36.5) | 0.765 |
| γ‐GTP (U/L) | −7.9 (53.6) | −4.7 (29.1) | 0.630 |
| CTx (pmol/L) | 0.457 (1.130) (n = 81) | 0.823 (1.413) (n = 83) | 0.069 |
| BAP (μg/L) | −0.58 (2.64) (n = 81) | −0.61 (2.60) (n = 83) | 0.940 |
| Intact PTH (pg/mL) | 5.6 (12.1) (n = 79) | 6.2 (13.3) (n = 81) | 0.779 |
| Urine NTx | 2.68 (916.43) (n = 81) | 3.61 (21.00) (n = 83) | 0.753 |
Values are means (SD). †Change from baseline to the end of treatment. ‡P < 0.05 versus placebo; §P < 0.01 versus placebo; ¶P ≤ 0.001 versus placebo; ††t‐test. ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; BAP, bone‐specific alkaline phosphatase; BUN, blood urea nitrogen; Cre, creatinine; CTx, cross‐linked C‐terminal telopeptide of type I collagen; γ‐GTP, γ‐glutamyl transpeptidase; LDH, lactate dehydrogenase; NAG, β‐N‐acetyl‐D‐glucosaminidase; NTx, cross‐linked N‐terminal telopeptide of type I collagen; PTH, parathyroid hormone; RBC, red blood cells.
Discussion
The present phase 3 study was carried out to examine the efficacy and safety of ipragliflozin in combination with metformin in Asian patients with type 2 diabetes mellitus inadequately controlled with metformin. In this study, ipragliflozin significantly improved glycemic control in terms of both HbA1c and FPG, and led to reductions in bodyweight and waist circumference, and improvements in other clinical/laboratory variables compared with the placebo. Ipragliflozin was well tolerated without being associated with any previously undocumented TEAEs, and its safety profile was comparable with that of the placebo, with only urinary tract infection being slightly more common in the ipragliflozin group than in the placebo group.
Many existing treatment options for type 2 diabetes mellitus are ultimately reliant on insulin. Therefore, they might have limited glucose‐lowering effects in some Asian patients, especially those with increased insulin resistance and/or insulin secretory defects, highlighting the need for alternative classes of drugs that lower glucose in an insulin‐independent manner. SGLT2 inhibitors, such as ipragliflozin, lower plasma glucose levels by reducing renal glucose reuptake, thus enhancing urinary glucose excretion.
The present results compare favorably with those of earlier studies in Asian patients in which ipragliflozin achieved significant reductions in HbA1c and FPG when used as monotherapy13. The results are also consistent with those for dapagliflozin in Asian patients15, 16, confirming the glucose‐lowering effects of SGLT2 inhibitors in Asian patients. The present study was carried out in a similar manner to the ILLUMINATE study in Japanese patients treated with metformin14. In the ILLUMINATE study, the mean changes in HbA1c in the ipragliflozin and placebo groups were −0.87% and 0.38%, respectively, corresponding to a between‐group difference of −1.30% (95% CI −1.501 to −1.095%; P < 0.001). In the present study, the mean change in HbA1c in the ipragliflozin group was −0.94%, compared with −0.47% in the placebo group, resulting in a between‐group difference of −0.46% (95% CI −0.66 to −0.27%; P < 0.001). Although the reason for the larger reduction in HbA1c in the placebo group in the present study is unclear, the further reduction in HbA1c in the ipragliflozin group is clinically important, and ensured that greater proportions of patients achieved a HbA1c of <7.0% or <6.5% at the end of treatment.
In the present study, we also observed small but favorable changes in bodyweight, waist circumference, blood pressure and triglycerides in the ipragliflozin group compared with the placebo group, whereas the changes in LDL cholesterol and total cholesterol were smaller in the ipragliflozin group than in the placebo group. Similar beneficial changes in blood pressure and triglycerides were also observed in the ILLUMINATE study14, which suggest that the glucose‐lowering effects of ipragliflozin are associated with beneficial changes in other clinically relevant parameters. Of note, the reduction in blood pressure seems to represent a class‐effect of SGLT2 inhibitors17.
There were slight increases in the red blood cell count and hematocrit in the ipragliflozin group in the present study, as in the ILLUMINATE study14. These changes were probably as a result of an increase in urine output or water loss, although this could not be assessed in this study because urine volume was not measured. However, these changes were not clinically significant in this study. We also observed slight increases in urinary potassium and phosphorus levels in the ipragliflozin group, although the cause and clinical relevance of these changes remain unknown. Intriguingly, there were no episodes of hypoglycemia, pollakiuria or genital infection, while the rates of nasopharyngitis, polyuria and urinary tract infection were quite low. Of these, only urinary tract infection occurred in more patients in the ipragliflozin group than in the placebo group. Skin disorders have also been reported in some Japanese patients using SGLT2 inhibitors, prompting the Japanese Diabetes Society to issue recommendations regarding the use of SGLT2 inhibitors18. In the present study, skin and subcutaneous tissue disorders included alopecia, which occurred in 0.0 and 2.3% of patients in the placebo and ipragliflozin groups, respectively, and pruritis, which occurred in 2.4 and 2.3% of patients, respectively.
The present results also warrant comparison with the results of an 18‐week study in which Asian patients on metformin alone or metformin plus sulfonylurea were randomized to 100 mg canagliflozin, 300 mg canagliflozin or a placebo19. The changes in HbA1c from baseline to the end of treatment were −0.97, −1.06 and −0.47% for 100 mg canagliflozin, 300 mg canagliflozin and the placebo, respectively, corresponding to placebo‐subtracted changes of −0.51 and −0.59% for 100 mg and 300 mg canagliflozin, respectively. The placebo‐subtracted changes in bodyweight were −1.5 and −1.6 kg for 100 and 300 mg canagliflozin, respectively. The reduction in bodyweight was slightly greater during the first 3 weeks of the study than from week 3 to week 18. These changes in HbA1c and bodyweight were similar to those observed in the present study. The authors also observed reductions in blood pressure and triglycerides, and increases in high‐density lipoprotein cholesterol and LDL cholesterol. Such increases in LDL cholesterol were not observed in the present study. Urinary tract infections occurred in 3.1, 2.6 and 4.9% of patients in the 100 mg canagliflozin, 300 mg canagliflozin and placebo groups, respectively, while osmotic diuresis‐related AEs occurred in 0.9, 0.9 and 1.3% of patients, respectively.
Some limitations of the present study warrant mention, including the treatment duration of 24 weeks, which might be too short to examine the longer‐term effects on glycemic control. Another possible limitation was that approximately two‐thirds of patients had HbA1c levels of <8.0% at baseline, which possibly limited the extent of the reductions in glucose levels. Finally, the study was carried out in two Asian countries (Taiwan and Korea). Therefore, the results might not be generalizable to patients in other Asian countries or Asian patients living in Western countries. Nevertheless, the overall results of the present study are broadly consistent with those of the ILLUMINATE study14, and support the use of ipragliflozin in combination with metformin for treating Asian patients with type 2 diabetes mellitus.
In conclusion, the results of this study of Taiwanese and Korean patients support those of an earlier study of Japanese patients14, and confirm the efficacy and safety of ipragliflozin in Asian patients with type 2 diabetes mellitus and inadequate glycemic control with metformin alone.
Disclosure
The authors declare no conflict of interest.
Supporting information
Figure S1 | Patient disposition. *This patient did not undergo administration of placebo after randomization, and was excluded from the full analysis and safety analysis sets.
Figure S2 | Time‐courses of the changes in (a) bodyweight and (b) waist circumference measurements. Values are mean (standard deviation).
Table S1 | Patient characteristics at visit 1 (full analysis set).
Data S1 | Exclusion criteria.
Acknowledgments
This study was sponsored by Astellas Pharma Inc., Japan. Medical writing and editorial support was funded by Astellas, and provided by Dr Nicholas D Smith, Dr Keyra Martinez Dunn and by ELMCOM™. SK and SY are employees of Astellas Pharma Inc. LMC and BSC have received research support from Astellas, and both have acted as consultants to Astellas. KWM and CHL have received research support from Astellas.
J Diabetes Investig 2016; 7: 366–373
Clinical Trial Registry
ClinicalTrials.gov NCT01505426
References
- 1. International Diabetes Federation . IDF Diabetes Atlas, 6th edn Brussels: International Diabetes Federation, 2013. [Google Scholar]
- 2. Chan JC, Malik V, Jia W, et al Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 2009; 301: 2129–2140. [DOI] [PubMed] [Google Scholar]
- 3. Abdullah N, Attia J, Oldmeadow C, et al The architecture of risk for type 2 diabetes: understanding Asia in the context of global findings. Int J Endocrinol 2014; 2014: 593982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. International Diabetes Federation Global Guidelines Task Force . Global Guideline for Type 2 Diabetes. Brussels: International Diabetes Federation, 2011. [Google Scholar]
- 5. Inzucchi SE, Bergenstal RM, Buse JB, et al Management of hyperglycemia in type 2 diabetes: a patient‐centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35: 1364–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wright EM. Renal Na+‐glucose cotransporters. Am J Physiol Renal Physiol 2001; 280: F10–F18. [DOI] [PubMed] [Google Scholar]
- 7. Marks J, Carvou NJ, Debnam ES, et al Diabetes increases facilitative glucose uptake and GLUT2 expression at the rat proximal tubule brush border membrane. J Physiol 2003; 553: 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rahmoune H, Thompson PW, Ward JM, et al Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non‐insulin‐dependent diabetes. Diabetes 2005; 54: 3427–3434. [DOI] [PubMed] [Google Scholar]
- 9. Elkinson S, Scott LJ. Canagliflozin: first global approval. Drugs 2013; 73: 979–988. [DOI] [PubMed] [Google Scholar]
- 10. Poole RM, Dungo RT. Ipragliflozin: first global approval. Drugs 2014; 74: 611–617. [DOI] [PubMed] [Google Scholar]
- 11. Traynor K. Dapagliflozin approved for type 2 diabetes. Am J Health Syst Pharm 2014; 71: 263. [DOI] [PubMed] [Google Scholar]
- 12. Kashiwagi A, Kazuta K, Yoshida S, et al Randomized, placebo‐controlled, double‐blind glycemic control trial of a novel SGLT2 inhibitor ipragliflozin in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig 2013; 5: 382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kashiwagi A, Kazuta K, Takinami Y, et al Ipragliflozin improves glycemic control in Japanese patients with type 2 diabetes mellitus: the BRIGHTEN study. Diabetol Int 2015: 6: 8–18. [Google Scholar]
- 14. Kashiwagi A, Kazuta K, Goto K, et al Ipragliflozin in combination with metformin for the treatment of Japanese patients with type 2 diabetes: ILLUMINATE, a randomised, double‐blind, placebo‐controlled study. Diabetes Obes Metab 2015; 17: 304–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaku K, Inoue S, Matsuoka O, et al Efficacy and safety of dapagliflozin as a monotherapy for type 2 diabetes mellitus in Japanese patients with inadequate glycaemic control: a phase II multicentre, randomized, double‐blind, placebo‐controlled trial. Diabetes Obes Metab 2013; 15: 432–440. [DOI] [PubMed] [Google Scholar]
- 16. Ji L, Ma J, Li H, et al Dapagliflozin as monotherapy in drug‐naive Asian patients with type 2 diabetes mellitus: a randomized, blinded, prospective phase III study. Clin Ther 2014; 36: 84.e109–100.e109. [DOI] [PubMed] [Google Scholar]
- 17. Baker WL, Smyth LR, Riche DM, et al Effects of sodium‐glucose co‐transporter 2 inhibitors on blood pressure: a systematic review and meta‐analysis. J Am Soc Hypertens 2014; 8: 262.e269–275.e269. [DOI] [PubMed] [Google Scholar]
- 18. Japan Diabetes Society Committee on the Proper Use of SGLT2 inhibitors. [Recommendations on the proper use of SGLT2 inhibitors], 2014. Available at: www.jds.or.jp/modules/important/index.php?page=article&storyid=48. Last accessed May 15, 2015.
- 19. Ji L, Han P, Liu Y, et al Canagliflozin in Asian patients with type 2 diabetes on metformin alone or metformin in combination with sulphonylurea. Diabetes Obes Metab 2015; 17: 23–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Patient disposition. *This patient did not undergo administration of placebo after randomization, and was excluded from the full analysis and safety analysis sets.
Figure S2 | Time‐courses of the changes in (a) bodyweight and (b) waist circumference measurements. Values are mean (standard deviation).
Table S1 | Patient characteristics at visit 1 (full analysis set).
Data S1 | Exclusion criteria.


