Abstract
Aims/Introduction
We carried out an observational cohort study to examine the relationship between the efficacy of oral antidiabetic drugs and clinical features in type 2 diabetics.
Materials and Methods
We analyzed the CoDiC® database of the Japan Diabetes Data Management Study Group across 67 institutions in Japan. In a total of 3,698 drug‐naïve patients who were initiated with metformin, dipeptidyl peptidase‐4 inhibitor (DPP‐4i) or sulfonylurea (SU) from 2007 to 2012, we evaluated body mass index (BMI) and hemoglobin A1c (HbA1c). The patients were stratified according to their clinical features, and matched using a propensity score to adjust for baseline factors.
Results
HbA1c was reduced with all drugs, with the largest effect elicited by DPP‐4i and the smallest by SU (P = 0.00). HbA1c increased with SU after 6 months in the patients stratified by an age‐of‐onset of <50 years (P = 0.00). BMI increased with SU in the patients stratified by a BMI of <25 (P = 0.00), and decreased with metformin in the patients with a BMI >25 (P = 0.00). The reduction in HbA1c was larger in patients with HbA1c of ≥8%, compared with that in patients with HbA1c of <8% (P = 0.00). HbA1c during the study period was higher in patients who were added to or swapped with other drug(s), than in patients continued on the original drug (P = 0.00).
Conclusions
The effect on bodyweight and glycemic control differed among metformin, DPP‐4i and SU, and the difference was associated with clinical features.
Keywords: Oral antidiabetic drug, Propensity score‐matched cohort study, Type 2 diabetes mellitus
Introduction
The majority of patients with type 2 diabetes mellitus require oral antidiabetic drugs (OADs) in addition to lifestyle intervention1, 2. Many studies have focused on the effectiveness and safety of medications for type 2 diabetes, with the overall benefits of OADs assessed in several recent systematic reviews and meta‐analyses3, 4, 5. These latter studies, which analyzed data mainly obtained from randomized control trials (RCTs), showed similar drug efficacy and reduction in hemoglobin A1c (HbA1c) levels by an average of 1 percentage point (1%) across most of the diabetes medications. In contrast, our impression based on clinical practice is that efficacy might differ among OADs, and that such differences could be associated with specific clinical features of the patients.
Evidence‐based medicine is classified according to “grades of evidence” built into the research design6. The highest grade is reserved for research involving “at least one properly randomized controlled trial,” whereas observational studies fall within the intermediate grades7. Indeed, a recent review showed that findings from RCTs and observational studies were remarkably similar across several clinical topics, but that observational studies showed less variability in point estimates (i.e., less heterogeneity of results) than RCTs on the same topic, indicating that evidence from both study types can and should be used to find the optimal treatment regimen8, 9. In contrast, results obtained from cohort studies are liable to be more affected by biases and confounding baseline factors that might influence treatment selection. One approach to reduce or eliminate the effect of treatment selection bias and confounding effects is the use of propensity score matching10, 11, and indeed, such methods were recently used successfully to evaluate the efficacy of treatments for diabetes12, 13.
To more robustly examine the relationship between the efficacy of three widely used OADs, metformin (Met), dipeptidyl peptidase‐4 inhibitor (DPP‐4i) and sulfonylurea (SU)5, 14, and the patients’ clinical features, we used the CoDiC® database collected from multiple institutions across Japan15, 16, 17, 18, analyzed cohort study results that were stratified according to a specific clinical feature and then matched by the propensity score‐matching method.
Materials and Methods
Study design and participants
Data were extracted from the CoDiC® database to incorporate patient records from 67 clinics or general/university‐affiliated hospitals across Japan15, 16, 17, 18. The data were obtained in primary care settings for patients diagnosed with type 2 diabetes, which was classified based on criteria in the ‘Report of the Committee of Japan Diabetes Society (JDS) on the Classification and Diagnostic Criteria of Diabetes Mellitus’19. Treatment goals recommended by the JDS were achieving HbA1c <6.5% (JDS value, later described), with fasting and postprandial plasma glucose (PPPG) levels of <130 mg/dL and <180 mg/dL, respectively20. In total, we reviewed 3,698 drug‐naïve patients who were initiated with Met, SU or DPP‐4i from May 2007 to July 2012. The clinical data were collected in the Central Analytical Center established by the Japan Diabetes Clinical Data Management Study Group (JDDM) on CD‐R storage disks in October 2012, and then analyzed using Microsoft Access® and Excel® software (Microsoft Corporation, Redmond, WA, USA). The JDDM ethics committee approved the study protocol, and informed consent was obtained from patients at each institution participating in the study, based on the requirements stated in the Guidelines for Epidemiology Study in Japan21.
Outcomes noted and analyzed were the prescription of OADs, and the comparison of drug effects on body mass index (BMI) and glycemic control (changes in HbA1c levels, mean decline in HbA1c and achievement ratio of HbA1c <7.0% and HbA1c <7.5% 12 months after the initiation of drug). The mean decline in BMI and HbA1c were calculated by subtracting each value at 3, 6, 9 and 12 months after the initiation of drug from the equivalent values at the initiation time.
Laboratory methods
HbA1c levels were measured using high‐performance liquid chromatography in each clinic or hospital. The levels were standardized in each institution according to the criteria recommended by the JDS committee22 and presented as HbA1c (JDS value) levels, with the normal range defined as 4.3–5.8%. This range is comparable with the 4.0–6.0% and 4.5–6.2% quoted by the American Diabetes Association criteria23 and UK Prospective Diabetes Study (UKPDS) criteria24, respectively. Recently, the JDS committee recommended that HbA1c (%) is estimated as a National Glycohemoglobin Standardization Program (NGSP) equivalent value (%), calculated by the formula HbA1c (%) = HbA1c (JDS; %) + 0.4%25, and in the present study, HbA1c values are presented as a National Glycohemoglobin Standardization Program value calculated by the same formula. Other variables collected were determined by standard methods, including BMI, blood pressure (BP), PPPG, low‐density lipoprotein (LDL) cholesterol and other biochemical markers.
Statistical analysis
Statistical analyses were carried out using the spss version 20 software package (IBM, Armonk, NY, USA). Clinical and biochemical characteristics were compared among the patients by using Student's t‐test and one‐way analysis of variance (anova), and then by Tukey's honest significant difference for continuous variables and the Fisher's exact test for categorical outcomes, as appropriate. To reduce the effect of treatment selection bias and potential confounding effects, we carried out adjustments for differences in the clinical characteristics at the time of OAD initiation by propensity score matching10, 12. Patients were stringently selected based on this score calculated using the Greedy 5‐to‐1 digit‐matching algorithm for the characteristics. The data are presented as mean ± standard deviation.
Results
Clinical characteristics in patients
In the 3,943 drug‐naive patients, Met, DPP‐4i, and SU were prescribed in 1793, 877 and 1273 patients, respectively, from May 2007 to July 2012. Table 1 (left) lists the patients’ clinical characteristics immediately before initiation of the drug. The patients on Met had a lower age and age‐of‐onset, as well as a shorter disease duration than those on DPP‐4i or SU (P = 0.00). BMI was higher in the patients taking Met, compared with patients taking DPP‐4i or SU (P = 0.00). HbA1c level was higher in the patients taking SU compared with patients taking Met or DPP‐4i (P = 0.00).
Table 1.
Clinical characteristics in the patients at the start of treatment with an initial oral hypoglycemic agent
| Variable | All cases | Cases matched by propensity score | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Met (n = 1,793) | DPP4i (n = 877) | SU (n = 1,273) | Total (n = 3,943) | P‐value | Met (n = 441) | DPP4i (n = 441) | SU (n = 441) | Total (n = 1,323) | P‐value | |
| Age (years) | 57.1 ± 11.2 | 63.1 ± 11.0 | 62.7 ± 11.1 | 60.3 ± 11.5 | 0.00 | 61.9 ± 10.0 | 62.3 ± 10.8 | 63.7 ± 10.5 | 62.6 ± 10.2 | 0.02 |
| Male (%) | 1,124 (62.6) | 508 (57.9) | 821 (64.4) | 2,453 (62.2) | 0.00 | 272 (61.6) | 267 (60.5) | 279 (63.2) | 818 (61.8) | 0.45 |
| Age‐of‐onset (years) | 51.5 ± 11.3 | 56.7 ± 11.1 | 54.3 ± 12.0 | 53.3 ± 11.6 | 0.00 | 54.5 ± 10.0 | 55.1 ± 11.0 | 55.6 ± 11.7 | 55.1 ± 10.9 | 0.30 |
| Diabetes duration (years) | 5.31 ± 5.69 | 6.61 ± 6.71 | 7.42 ± 8.11 | 6.28 ± 6.85 | 0.00 | 7.63 ± 7.21 | 7.12 ± 5.93 | 7.99 ± 7.48 | 7.49 ± 6.91 | 0.15 |
| Body mass index† | 26.2 ± 4.7 | 23.6 ± 3.6 | 23.6 ± 3.4 | 24.8 ± 4.3 | 0.00 | 24.0 ± 3.4 | 24.0 ± 3.5 | 23.5 ± 3.5 | 23.8 ± 3.5 | 0.03 |
| Systolic BP (mmHg) | 130 ± 16 | 130 ± 16 | 131 ± 17 | 130 ± 16 | 0.38 | 129 ± 15 | 131 ± 16 | 130 ± 16 | 130 ± 16 | 0.22 |
| Diastolic BP (mmHg) | 77.8 ± 11.0 | 78.2 ± 11.3 | 76.9 ± 10.8 | 76.9 ± 11.0 | 0.00 | 75.9 ± 10.2 | 76.8 ± 11.6 | 75.2 ± 10.7 | 76.0 ± 10.9 | 0.09 |
| PPPG (mg/dL) | 179 ± 65 | 163 ± 54 | 194 ± 75 | 180 ± 67 | 0.00 | 175 ± 65 | 166 ± 56 | 176 ± 65 | 172 ± 61 | 0.06 |
| HbA1c (%) | 7.73 ± 1.17 | 7.37 ± 1.00 | 8.07 ± 1.42 | 7.76 ± 1.28 | 0.00 | 7.45 ± 0.96 | 7.52 ± 1.05 | 7.46 ± 1.08 | 7.49 ± 1.03 | 0.68 |
| Total cholesterol (mg/dL) | 200 ± 34 | 196 ± 34 | 203 ± 37 | 201 ± 36 | 0.01 | 201 ± 32 | 196 ± 34 | 194 ± 33 | 196 ± 33 | 0.13 |
| LDL cholesterol (mg/dL) | 118 ± 30 | 108 ± 27 | 117 ± 33 | 116 ± 31 | 0.00 | 113 ± 31 | 110 ± 30 | 112 ± 29 | 111 ± 30 | 0.52 |
| TG (mg/dL) | 194 ± 143 | 152 ± 121 | 168 ± 159 | 176 ± 145 | 0.00 | 188 ± 154 | 159 ± 123 | 147 ± 93 | 164 ± 126 | 0.00 |
BP, blood pressure; DPP4i, dipeptidyl‐peptidase 4 inhibitor; LDL, low‐density lipoprotein; Met, metformin; PPPG, post‐prandial plasma glucose; SU, sulfonylurea; TG triglyceride. Data are mean ± standard deviation. P‐value: variables are compared among the patient groups by one‐way analysis of variance (anova). †The body‐mass index is the weight in kilograms divided by the square of the height in meters.
In 1,323 cases matched by propensity scoring for the following characteristics: age, age‐of‐onset, duration of diabetes, BMI, systolic and diastolic BP, PPPG, HbA1c, total cholesterol, LDL cholesterol and triglycerides (TG; Table 1 right), there were no differences in most variables, although age, BMI and TG levels showed a slight, but, significant difference among patients treated with Met, DPP‐4i and SU.
Changes in BMI and HbA1c
Figure 1a–c show the changes in BMI and HbA1c over the study period, as well as the achievement rate for HbA1c goals, respectively, in all studied patients. Met was prescribed in the cases with higher BMI, in preference to DPP‐4i or SU, and while the mean BMI decreased on Met, it increased on SU from the earliest phase, and gradually increased on DPP‐4i (Figure 1a). SU was prescribed in the cases with the highest HbA1c level, and DPP‐4i was prescribed in the cases with the lowest level (Figure 1b). Accordingly, the largest reduction in HbA1c was seen with SU. In the patients matched by propensity score, BMI changed as well in all studied patients (Figure 1d). As shown in Figure 1e, HbA1c levels were not different among drugs at the time of initiation, but subsequently, the reduction in HbA1c was largest in DPP‐4i‐treated patients and lower in those taking SU. The achievement rates for HbA1c <7% and <7.5% were highest with DPP‐4i treatment, and lowest with SU in all studied patients and in the patients matched by propensity score (Figure 1c,f, respectively).
Figure 1.
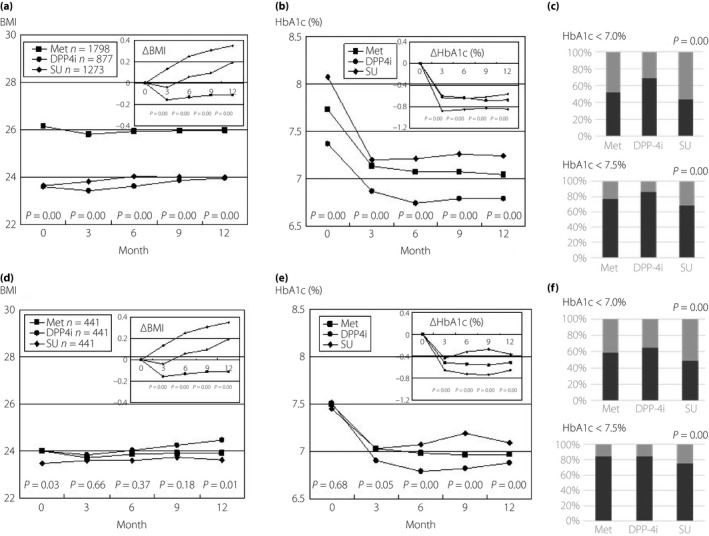
Changes in body mass index (BMI), hemoglobin A1c (HbA1c) and the HbA1c target rate after the start of metformin (Met), dipeptidyl peptidase‐4 inhibitor (DPP‐4i) and sulfonylurea (SU) treatment. The changes in (a) BMI, (b) HbA1c, and (c) the achievement rate of HbA1c <7.0% (black column) and <7.5% (gray column) in all patients are shown. The figures for the mean decline in BMI, ΔBMI and the mean decline in HbA1c, ΔHbA1c, which were calculated by subtracting the each value 3, 6, 9 and 12 months after the initiation of drug from the value at the initiation time, are inserted in each figure. The changes in (d) BMI, (e) HbA1c and (f) the achievement rate of HbA1c <7.0% (black column) and <7.5% (gray column) in the patients selected by means of propensity score matching for the following characteristics: age, age‐of‐onset, duration of diabetes, body mass index, systolic and diastolic blood pressure, post‐prandial plasma glucose, hemoglobin A1c, total cholesterol, lowdensity lipoprotein cholesterol and triglyceride are shown. Statistical analyses were carried out using one‐way analysis of variance (anova), a Tukey's honest significant difference and the Fisher's exact test.
We next measured changes in BMI or HbA1c in the patients stratified by age‐of‐onset, duration of this disease, BMI and HbA1c levels at the start of drug therapy, and then matched these patients by propensity scores for the characteristics described earlier. First, the changes in HbA1c were examined in the patients stratified by age‐of‐onset and then matched using a propensity score. There were 132 patients with an age‐of‐onset <50 years in each drug group, 151 patients with an age‐of‐onset ≥50 years and <60 years, and 140 patients with an age‐of‐onset ≥60 years, and there were no differences in age, age‐of‐onset, duration of diabetes, BMI, HbA1c level, BP, total cholesterol, LDL cholesterol and TG at the start of the drug in each stratified patient group (data not shown). In the patients with an age‐of‐onset <50 years, HbA1c reduced to the same extent on Met, DPP‐4i and SU at 3 months after initiation; however, HbA1c increased on SU after 6 months, but remained stable on Met or DPP‐4i (Figure 2a). In the patients with an age‐of‐onset ≥50 and <60 years, or ≥60 years, the reduction in HbA1c was also slightly, but significantly, smaller on SU, compared with that on Met or DPP‐4i (Figure 2b,c, respectively). Also, the changes in HbA1c were examined in the patients stratified by duration of this disease and then matched using a propensity score. There were 182 patients with a duration <5 years in each drug group, and 239 patients with a duration ≥5 years, and there were no differences in age, age‐of‐onset, duration of diabetes, BMI, HbA1c level, BP, total cholesterol, LDL cholesterol and TG at the start of the drug in each stratified patient group (data not shown). Age‐of‐onset was older in the patients with a duration <5 years than in the patients with a duration ≥5 years (57.5 ± 11.0 years and 52.4 ± 10.2 years, respectively P = 0.00), and conversely, age at the start of OAD was younger in the former patients than in the latter patients (59.6 ± 11.0 years and 64.2 ± 9.7 years, respectively, P = 0.00). In the patients with a duration <5 years, HbA1c reduced to almost the same extent on Met, DPP‐4i and SU during the period, and the mean HbA1c levels reached <7% (Figure 2d). In the patients with a duration ≥5 years, the reduction in HbA1c was largest in the DPP‐4i‐treated patients and smallest in SU‐treated patients. The mean level reached <7% in the patients taking DPP‐4i, but did not reach <7% in the patients taking Met and SU (Figure 2e).
Figure 2.
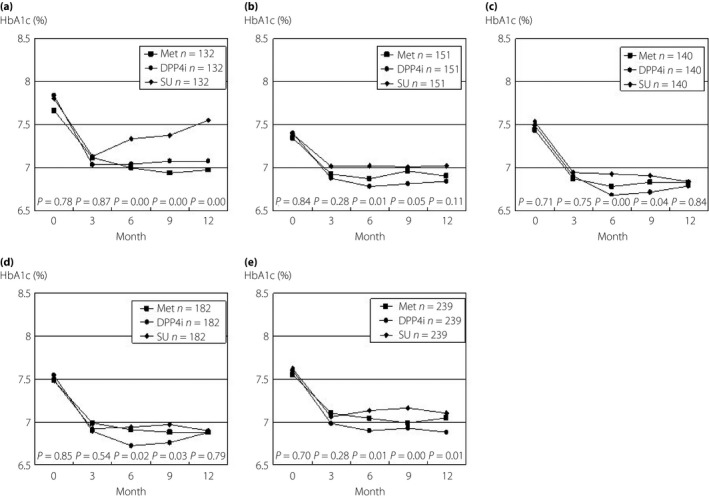
The changes in hemoglobin A1c (HbA1c) in the patients stratified by age‐of‐onset and duration of diabetes mellitus. The patients who had been stratified were matched by the propensity score for the following characteristics: age, age‐of‐onset, duration of diabetes, body mass index, systolic and diastolic blood pressure, post‐prandial plasma glucose, hemoglobin A1c, total cholesterol, lowdensity lipoprotein cholesterol and triglyceride. The changes in HbA1c for patients with an age‐of‐onset of (a) <50 years, (b) ≥50 years and <60 years, and (c) the patients aged more than 60 years are shown. The changes for patients with a duration (d) <5 years and (e) ≥5 years are shown. Statistical analyses were carried out using one‐way analysis of variance (anova), and then by Tukey's honest significant difference.
Second, the changes in BMI and HbA1c level were examined in the patients stratified by BMI at the start of drug therapy and then matched using a propensity score. There were 271 patients with a BMI ≥18.5 and <25 in each drug group, 127 patients with a BMI ≥25 and <30, and 16 patients with a BMI ≥30. There were no differences in the clinical variables described earlier in each stratified patient group (data not shown). In the ≥18.5 to <25 BMI group, BMI did not change with Met, increased gradually on DPP‐4i, and promptly increased on SU (Table 2). In the patients with a BMI ≥25 and <30, BMI promptly and markedly decreased with Met treatment, but not with DPP‐4i or SU. HbA1c was higher in the patients with a BMI ≥25 and <30 than in those with a BMI ≥18.5 and <25 (7.63 ± 1.01% vs 7.42 ± 0.92%, P = 0.00). In the patients with a BMI ≥25 and <30, the reduction in HbA1c was slightly, but significantly, smaller with SU than with Met or DPP‐4i (P = 0.02). The patients with a BMI >30 were not analyzed because of the small sample size.
Table 2.
Changes in body mass index in the patients stratified by body mass index at the start of drug therapy
| Duration (months) | Changes in BMI | ||||||
|---|---|---|---|---|---|---|---|
| Met (n = 271) | P‐value | DPP‐4i (n = 271) | P‐value | SU (n = 271) | P‐value | anova (P‐value) | |
| BMI ≥18.5 and <25 | |||||||
| Initiation time | 22.3 ± 1.7 | 22.4 ± 1.6 | 22.1 ± 1.7 | 0.19 | |||
| 3 | 22.2 ± 1.7 | 0.02 | 22.4 ± 1.6 | 0.39 | 22.4 ± 1.8 | 0.00 | 0.44 |
| 6 | 22.2 ± 1.7 | 0.75 | 22.5 ± 1.6 | 0.04 | 22.4 ± 1.8 | 0.00 | 0.30 |
| 9 | 22.3 ± 1.7 | 0.42 | 22.6 ± 1.6 | 0.00 | 22.4 ± 1.8 | 0.00 | 0.14 |
| 12 | 22.3 ± 1.8 | 0.63 | 22.8 ± 1.6 | 0.00 | 22.6 ± 1.9 | 0.00 | 0.02 |
| Met (n = 127) | P‐value | DPP‐4i (n = 127) | P‐value | SU (n = 127) | P‐value | anova (P‐value) | |
|---|---|---|---|---|---|---|---|
| BMI ≥25.0 and <30 | |||||||
| Initiation time | 27.0 ± 1.3 | 26.9 ± 1.3 | 26.8 ± 1.3 | 0.52 | |||
| 3 | 26.6 ± 1.9 | 0.04 | 26.8 ± 1.8 | 0.25 | 26.7 ± 1.4 | 0.68 | 0.73 |
| 6 | 26.7 ± 1.5 | 0.00 | 26.9 ± 1.7 | 0.77 | 26.9 ± 1.6 | 0.84 | 0.71 |
| 9 | 26.8 ± 1.6 | 0.02 | 26.8 ± 1.9 | 0.87 | 26.9 ± 1.6 | 0.94 | 0.92 |
| 12 | 26.6 ± 1.5 | 0.00 | 27.1 ± 1.7 | 0.02 | 26.8 ± 1.6 | 0.37 | 0.41 |
Data are mean ± standard deviation. P value: variables are compared with the value at initiation time by Student's t‐test. anova (P‐value): body mass indexes (BMI) are compared among the patients treated with metformin (Met), dipeptidyl‐peptidase 4 inhibitor (DPP‐4i) or sulfonylurea (SU) by one‐way analysis of variance. BMI is the weight in kilograms divided by the square of the height in meters.
Third, the changes in HbA1c were examined in the patients stratified by HbA1c level at the start of the drug and then matched using a propensity score. There were 188 patients with HbA1c ≥7% and <8% in each drug group, and 61 patients with an HbA1c ≥8% and <9%. There were no differences in the clinical variables described earlier in each stratified patient group (data not shown). In the lower HbA1c patients, HbA1c reduced significantly on Met, DPP‐4i and SU, but the reduction was larger with Met and DPP‐4i compared with the reduction with SU (Table 3a). In the higher HbA1c patients, the reduction did not differ among Met, DPP‐4i and SU. Finally, as shown in Table 3b, the mean declines in HbA1c with all drugs were larger in the higher HbA1c patients than in those with a lower HbA1c level at the start of the drug (P = 0.00), and the declines were largest in DPP‐4i‐treated patients and smallest in SU‐treated patients in the lower HbA1c group, although the declines did not differ among the drugs in the higher HbA1c patients.
Table 3.
(a) Changes and (b) Mean decline in hemoglobin A1c in the patients stratified by hemoglobin A1c at the start of drug therapy
| (a) | |||||||
|---|---|---|---|---|---|---|---|
| Duration (months) | Changes in HbA1c | ||||||
| Met (n = 188) | P‐value | DPP‐4i (n = 188) | P‐value | SU (n = 188) | P‐value | anova (P‐value) | |
| HbA1c ≥7% and <8% | |||||||
| Initiation time | 7.39 ± 0.24 | 7.44 ± 0.27 | 7.44 ± 0.29 | 0.12 | |||
| 3 | 6.90 ± 0.43 | 0.00 | 6.86 ± 0.50 | 0.00 | 7.04 ± 0.55 | 0.00 | 0.00 |
| 6 | 6.85 ± 0.41 | 0.00 | 6.79 ± 0.48 | 0.00 | 7.07 ± 0.69 | 0.00 | 0.00 |
| 9 | 6.84 ± 0.42 | 0.00 | 6.82 ± 0.53 | 0.00 | 7.15 ± 0.58 | 0.00 | 0.00 |
| 12 | 6.90 ± 0.50 | 0.00 | 6.81 ± 0.49 | 0.00 | 7.08 ± 0.64 | 0.00 | 0.00 |
| Met (n = 61) | P‐value | DPP‐4i (n = 61) | P‐value | SU (n = 61) | P‐value | anova (P‐value) | |
|---|---|---|---|---|---|---|---|
| HbA1c ≥8 and <9% | |||||||
| Initiation time | 8.32 ± 0.27 | 8.36 ± 0.28 | 8.37 ± 0.28 | 0.66 | |||
| 3 | 7.56 ± 0.62 | 0.00 | 7.44 ± 0.54 | 0.00 | 7.54 ± 0.67 | 0.00 | 0.39 |
| 6 | 7.41 ± 0.69 | 0.00 | 7.31 ± 0.93 | 0.00 | 7.49 ± 0.85 | 0.00 | 0.56 |
| 9 | 7.40 ± 0.79 | 0.00 | 7.29 ± 0.66 | 0.00 | 7.49 ± 0.96 | 0.00 | 0.49 |
| 12 | 7.27 ± 0.88 | 0.00 | 7.30 ± 0.53 | 0.00 | 7.40 ± 0.87 | 0.00 | 0.87 |
| (b) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Stratification of HbA1c (%) | ≥7 and <8 | ≥8 and <9 | ||||||
| Duration (months) | Met (n = 188) | DPP‐4i (n = 188) | SU (n = 188) | P‐value | Met (n = 61) | DPP‐4i (n = 61) | SU (n = 61) | P‐value |
| 3 | −0.47 ± 0.41 | −0.58 ± 0.46 | −0.41 ± 0.61 | 0.01 | −0.76 ± 0.59 | −0.93 ± 0.55 | −0.84 ± 0.74 | 0.39 |
| 6 | −0.55 ± 0.42 | −0.65 ± 0.46 | −0.37 ± 0.74 | 0.00 | −0.91 ± 0.67 | −1.07 ± 0.87 | −0.90 ± 0.94 | 0.56 |
| 9 | −0.54 ± 0.44 | −0.59 ± 0.51 | −0.29 ± 0.66 | 0.00 | −0.91 ± 0.78 | −1.08 ± 0.67 | −0.88 ± 1.03 | 0.49 |
| 12 | −0.48 ± 0.55 | −0.62 ± 0.49 | −0.35 ± 0.95 | 0.00 | −1.06 ± 0.84 | −1.05 ± 0.58 | −0.98 ± 0.95 | 0.87 |
(a): Data are mean ± standard deviation. P‐value: variables are compared with the value at initiation time by Student's t‐test. anova (P‐value): hemoglobin A1c (HbA1c) levels are compared among the patients treated with metformin (Met), dipeptidyl‐peptidase 4 inhibitor (DPP‐4i) or sulfonylurea (SU) by one‐way analysis of variance.
(b): Data are mean ± standard deviation. P‐value: mean decline in HbA1c is compared among the patients treated with metformin (Met), dipeptidyl‐peptidase 4 inhibitor (DPP‐4i) or sulfonylurea (SU) by one‐way analysis of variance (anova). Mean decline in HbA1c is calculated by subtracting each value at 3, 6, 9 and 12 months after initiation of the drug from the value at the initiation time.
Changes in the prescription of drugs and HbA1c
Figure 3a shows changes in the prescription of Met, DPP‐4i or SU over 12 months. The rate of patients whose drug regimes were altered (added to or changed to other drugs) did not differ among the patients during the 12 months after initiation (P = 0.33 at 3 months, P = 0.96 at 6 months and P = 0.96 at 12 months). In the patients originally started with Met, DPP4i or SU, the regimes were altered in 27.0%, 29.5% and 30.7%, respectively, during the period, with add‐ons mainly of SU or DPP4i, SU or Met, and Met or pioglitazone, respectively. Just 6%, 3.6% and 2.6%, respectively, of the patients had been changed to another drug. In the patients who continued on the original drug, HbA1c levels achieved less than 7% after 3 months and the levels were lowest in the patients originally started with Met (Figure 3b). In the patients who were added to or swapped with other drug(s), the HbA1c levels at the time started on the original drug and during the study period were higher (P = 0.00), compared with levels in the patients continued on the original drug, and the levels did not achieve <7% (Figure 3b,c).
Figure 3.
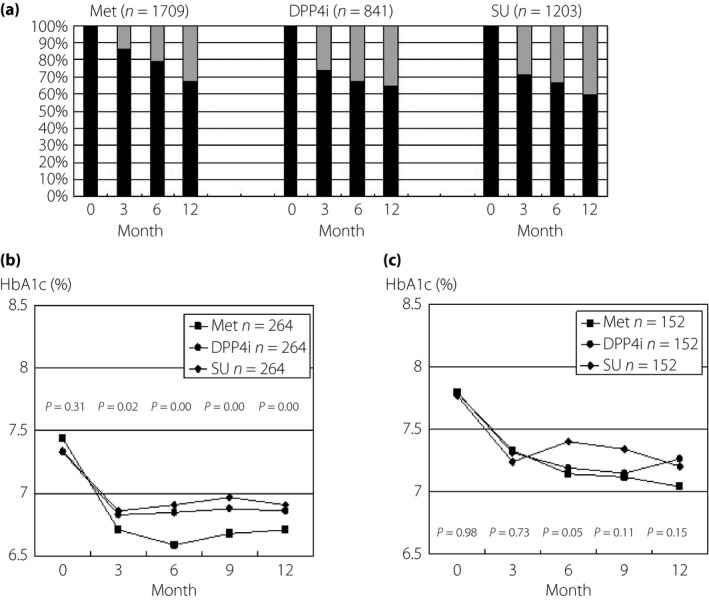
The changes in the prescription of metformin (Met), dipeptidyl peptidase‐4 inhibitor (DPP‐4i) and sulfonylurea (SU), and the corresponding changes in hemoglobin A1c (HbA1c) in the patients who continued to be treated with the original drug or who were treated with additions or changing to drug(s) during 12 months. (a) The rate of patient numbers who continued to be treated with the original drug (black column) and were added to or swapped in with other drug(s) (gray column). The changes in HbA1c in the patients who (a) continued to be treated with original drug and (b) were added of other drug(s) are shown. Statistical analyses were carried out by one‐way analysis of variance (anova), and then by Tukey's honest significant difference.
Discussion
Herein, results from a cohort study of patients treated with an OAD were stratified based on clinical features and then matched by a propensity score method to adjust for baseline factors. The analyses showed that the efficacy on bodyweight and glycemic control differed among Met, DPP‐4i and SU, and the differences were related to the clinical features, although the analyses using results in all enrolled patients showed that the findings were consistent with previous reports documenting that most diabetes medications had similar efficacy and reduced HbA1c levels by an average of 1 percentage point3, 4, 5 (Figure 1).
Met is prescribed to many overweight patients based on published recommendations and reports. The Treatment Guide for Diabetes edited by the Japan Diabetes Society 2007 recommends that biguanides are particularly effective in cases of type 2 diabetes accompanied by overweight or obesity in which insulin resistance is high26. The UK Prospective Diabetes Study documented that in obese patients, Met produced comparable reductions in fasting plasma glucose and HbA1c concentrations, but did not induce weight gain27. However, this drug has the prominent effect of lowering blood glucose both in normal weight and overweight patients, supporting ‘A Consensus Algorithm for the Initiation and Adjustment of Therapy A consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes’14. However, the effect of Met on bodyweight remains controversial, with some authors describing no effects on bodyweight28, whereas others reported a Met‐induced weight loss29. Our present study found that Met had a neutral effect in normal‐weight patients, but induced a reduction in bodyweight in overweight or obese patients, suggesting that the discrepancy in previous findings might reflect differences in BMI at the start of drug therapy. An important issue in SUs therapy is weight gain, which is problematic in a group of patients frequently already overweight30. However, our observations suggest that although SU massively increases bodyweight in the normal weight patients, this drug does not induce weight gain in the overweight patients. It was reported that DPP‐4i's were generally weight neutral31, and less effective in decreasing bodyweight than Met5. Herein, we found that the efficacy of DPP‐4i was also related to BMI, in that bodyweight gradually increased in normal weight patients, but was neutral in overweight patients. These findings that the effect of OADs on bodyweight is associated with BMI at the initiating time is very interesting both for clinical practice and in terms of the underlying pathophysiology.
As previously reported, SUs are the second choice in OADs (after Met), most likely because they are inexpensive and quite efficient32. SUs were prescribed in our patients with higher HbA1c levels, compared with the cases on Met and DPP‐4i. However, the glucose‐lowering effect of SUs was related to age‐of‐onset, duration of this disease and HbA1c levels at the initiation time, and the overall effect of SU was inferior to that of Met and DPP‐4i, especially in patients diagnosed in earlier life and with long duration of this disease at the start of therapy. A RCT study reported that compared with Met, DPP‐4i were associated with a smaller decline in HbA1c and a lower chance of reaching the HbA1c goal of <7%, suggesting the inferiority of DPP‐4i to Met as monotherapy5. The glucose‐lowering effects of DPP‐4i and Met were similar in our patients, and superior to those of SU, especially in patients diagnosed in earlier life and with long duration of this disease, suggesting that DPP‐4i is not inferior to Met and is superior to SU.
Why SU showed decreased durability of glycemic control in the patients diagnosed at an earlier age and low efficacy of glycemic control in the patients with long duration of this disease is unclear, but is unlikely to be due to poor medication adherence, as there was no decrease in the durability of glycemic control in patients taking Met and DPP‐4i. The differential effect among drugs could also not be explained by baseline characteristics, because the analyzed patients were vigorously matched using a propensity score method. A previous RCT study showed that glycemic durability was lowest in the patients (aged 30–75 years) taking glyburide compared with those taking Met or rosiglitazone33. In another study, the efficacy of treatment regimens was compared among Met monotherapy, and the combination of Met and rosiglitazone, showing durable glycemic control in children and adolescents with recent‐onset type 2 diabetes, and showing that the rate of treatment failure with Met monotherapy was higher in this cohort than in similar cohorts of adults treated with Met34. Type 2 diabetes mellitus is a progressive disease in which poor glycemic control is exacerbated over time and pancreatic β‐cell function declines2, 35. β‐Cell deterioration determines the onset and rate of progression of secondary dietary failure in type 2 diabetes mellitus2. Efficacy of SU might decline easier along β‐cell deterioration that has progressed for a long time, compared with patients taking Met or DPP‐4i. Further analysis is required to determine whether the apparent decrease in efficacy of glycemic control with SU in patients diagnosed in earlier life and with long duration of this disease, compared with those taking Met or DPP‐4i, reflects biological differences, pathophysiological differences, or both.
Although starting insulin therapy is recommended in type 2 diabetes patients if HbA1c levels do not reach 7.0%36 or 7.5%14, despite oral antidiabetic drugs, insulin therapy is at times initiated with HbA1c levels of >8.5% in clinical practice15. In our clinical practice, we found that the HbA1c level did not lower to <7% in such patients after 1 year, and the chance of reaching this HbA1c goal was very low despite adding other drug(s) to the regime. Together with published recommendations and reports, we recommend that the initiation of insulin therapy should be considered in some drug‐naïve patients with HbA1c levels >8%.
In the ‘Position Statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD),’ all treatment decisions should be made in conjunction with the patient, focusing on his/her preferences, needs, and values37. Evidence both from RCTs and from well‐designed observational studies can and should be used to find the optimal treatment regimen8, 9. The data both from our present observational study and from the RCTs3, 4, 5 potentially apply to all clinical situations of diabetes care. Thus, doctors and patients should be aware of these findings, before prescribing an OAD as the initial drug therapy in addition to lifestyle intervention.
The cohort study presented here had some limitations. The possibility of unmeasured confounding factors affecting the effect of studied drugs could not be ruled out. Therefore, the propensity score‐matching method used in the present study might not have adequately matched the clinical characteristics of the patient groups. Also, because we did not analyze the patients whose data input had stopped during the studied period, the possibility of selection bias cannot be completely excluded. We also did not take account of complications, including micro‐ and macrovascular diseases and accidental diseases, and such factors could affect the choice of OAD and motivation to treatment of both patients and providers. Finally, the present study had insufficient standardization of treatment regimens, drug dosage and glycemic goals, although drug therapy was initiated according to JDS guidelines for the management of diabetes20. Because the analyzed patients were stratified and vigorously matched based on propensity scoring, the patient numbers in each studied group were limited, and larger studies are required to draw more rigorous conclusions.
In conclusion, the present cohort study using the stratification of patients based on clinical features and propensity‐score matching method to adjust for baseline factors found that the effect on bodyweight and glycemic control differs among Met, DPP‐4i, and SU, in association with the studied clinical features. These findings strengthen the proposal that physicians should choose an OAD according to the patient's clinical features, and that some patients lose the opportunity to receive proper ODA therapy to achieve optimal glycemic control.
Disclosure
The authors declare no conflict of interest.
Acknowledgment
This study was supported by a grant from the Japan Diabetes Foundation.
The following members of JDDM participated in this study: Dr Nobuyuki Abe, Dr Keiko Arai, Dr Azuma Kanatsuka, Dr Hiroshi Fujiya, Dr Yoshihide Fukumoto, Dr Koichi Hirao, Dr Fumihiko Dake, Dr Tomohiro Iizumi, Dr Masaaki Ito, Dr Koichi Iwasaki, Dr Akira Kanamori, Dr Sumio Kato, Dr Masakazu Kato, Dr Koichi Kawai, Dr Akira Kawara, Dr Kenichi Kimura, Dr Kazumasa Chikamori, Dr Kotaro Iemitsu, Dr Shigetake Kou, Dr Mikihiko Kudo, Dr Yoshio Kurihara, Dr Gendai Lee, Dr Akira Tsuruoka, Dr Naoki Manda, Dr Kiyokazu Matoba, Dr Hiroshi Hayashi, Dr Masae Minami, Dr Nobuichi Kuribayashi, Dr Kazuhiro Miyazawa, Dr Yasuko Chiba, Dr Takeshi Osonoi, Dr Shin Nakamura, Dr Hideo Sasaki, Dr Katsutoshi Komori, Dr Mariko Oishi, Dr Akira Okada, Dr Fuminobu Okuguchi, Dr Morifumi Yanagisawa, Dr Hidekatsu Sugimoto, Dr Hiromichi Sugiyama, Dr Masahiko Takai, Dr Masato Takaki, Dr Hiroshi Takamura, Dr Hiroshi Takeda, Dr Hiroshi Takeda, Dr Kokichi Tanaka, Dr Takashi Miwa, Dr Osamu Tomonaga, Dr Madoka Taguchi, Dr Katsuya Yamazaki, Dr Takako Wada, Dr Noriharu Yagi, Dr Kuniko Yamaoka and Dr Atsuyoshi Yuhara.
J Diabetes Investig 2016; 7: 386–395
References
- 1. Turner RC, Cull CA, Frighi V, et al Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA 1999; 281: 2005–2012. [DOI] [PubMed] [Google Scholar]
- 2. Levy J, Atkinson AB, Bell PM, et al Beta‐cell deterioration determines the onset and rate of progression of secondary dietary failure in type 2 diabetes mellitus: the 10‐year follow‐up of the Belfast Diet Study. Diabet Med 1998; 15: 290–296. [DOI] [PubMed] [Google Scholar]
- 3. Sherifali D, Nerenberg CK, Pullenayegum E, et al The effect of oral antidiabetic agents on A1C levels. Diabetes Care 2010; 33: 1859–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bennett WL, Maruthur NM, Singh S, et al Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2‐drug combinations. Ann Intern Med 2011; 154: 602–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karagiannis T, Paschos P, Paletas K, et al Dipeptidyl peptidase‐4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta‐analysis. BMJ 2012; 344: 1–15. [DOI] [PubMed] [Google Scholar]
- 6. Evidence‐Based Medicine Working Group . Evidence‐based medicine: a new approach to teaching the practice of medicine. JAMA 1992; 268: 2420–2425. [DOI] [PubMed] [Google Scholar]
- 7. Preventive Services Task Force . Guide to clinical preventive services: report of the U.S. Preventive Services Task Force. 2nd ed Baltimore: Williams & Wilkins, 1996. [Google Scholar]
- 8. Concato J, Shah N, Holwitz RI. Randomized, controlled trials, observational studies, and the hierarch designs. N Engl J Med 2000; 342: 1887–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. MacKee M, Britton A, Black N, et al Interpreting the evidence: choosing between randomized and non‐randomized studies. BMJ 1999; 319: 312–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Austin CP. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011; 46: 399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Parsons LS. Reducing bias in a propensity score matched‐pair sample using Greedy matching techniques In: Proceedings of the Twenty‐Sixth Annual SAS Users Groups International Conference, Long Beach, Calif., April 22‐25, 2001, Cary, N.C: SAS Institute, 2001. [Google Scholar]
- 12. Sato D, Sato Y, Masuda S, et al Effects of a sitagliptin safety on prescription behavior for oral antihyperglycemic drugs: a propensity score‐matched cohort study of prescription receipt data in Japan. Drug Saf 2013; 36: 605–615. [DOI] [PubMed] [Google Scholar]
- 13. Kanatsuka1 A, Sato Y, Kawai K, et al Evaluation of insulin regimens as an effective option for glycemic control in patients with type 2 diabetes: a propensity score‐matched cohort study across Japan (JDDM31). J Diabetes Investig 2014; 5: 539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nathan DM, Buse JB, Davidson MB, et al Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy a consensus statement from the American diabetes association and the European association for the study of diabetes. Diabetes Care 2006; 29: 1963–1972. [DOI] [PubMed] [Google Scholar]
- 15. Kanatsuka A, Kawai K, Hirao K, et al The initiation of insulin therapy in type 2 diabetic patients treated with oral anti‐diabetic drugs: an observational study in multiple institutes across Japan (JDDM 27). Diabetol Int 2012; 3: 164–173. [Google Scholar]
- 16. Kobayashi M, Yamazaki K, Hirao K et al. The status of diabetes control and antidiabetic drug therapy in Japan‐A cross‐sectional survey of 17,000 patients with diabetes mellitus (JDDM1). Diabetes Res Clin Pract 2006; 73: 198–204. [DOI] [PubMed] [Google Scholar]
- 17. Kanatsuka A, Kawai K, Hirao Ket al. Actual usage and clinical effectiveness of insulin preparations in patients with Type 1 diabetes mellitus in Japan: CoDiC®‐based analysis of clinical data obtained at multiple institutions (JDDM 3). Diabetes Res Clin Pract 2006; 72: 277–283. [DOI] [PubMed] [Google Scholar]
- 18. Yokoyama H, Kawai K, Oishi MJ, et al. . Familial predisposition to cardiovascular risk and disease contributes to cardiovascular risk and disease interacting with other cardiovascular risk factors in diabetes‐Implication for common soil. Atherosclerosis 2008; 201: 332–338. [DOI] [PubMed] [Google Scholar]
- 19. Committee of Japan Diabetes Society for the Diagnostic Criteria of Diabetes Mellitus . Report of the committee of Japan diabetes society on the classification and diagnosis of diabetes mellitus. J Japan Diab Soc 1999; 42: 395–404 (Japanese). [Google Scholar]
- 20. Guideline Committee of the Japan Diabetes Society . Japan diabetes society guideline for the management of diabetes based on scientific evidences. Nankodo Tokyo: Japan Diabetes Society; 2010. (Japanese). [Google Scholar]
- 21. The Guideline for Epidemiology Study in Japan . The Ministry of Health, Labor and Welfare. June 30, 2003 (Japanese).
- 22. Tominaga M, Makino E, Yoshino G, et al Report of the committee on standardization of laboratory testing related to diabetes mellitus: the seventh national survey on Hemoglobin A1c. J Japan Diab Soc 2003; 46: 961–965 (Japanese). [Google Scholar]
- 23. American Diabetes Association . Clinical practice recomm‐endations 1995. Diabetes Care 1995; 18(suppl 1): S1–S596. [PubMed] [Google Scholar]
- 24. UKPDS Group . UK Prospective Diabetes Study XI: biochemical risk factors in type 2 diabetic patients at diagnosis compared with age‐matched normal subjects. Diabet Med 1994; 11: 534–544. [PubMed] [Google Scholar]
- 25. Kashiwagi A, Kasuga M, Araki E, et al International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig 2012; 3: 39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Treatment Guide for Diabetes Editorial Committee, the Japan Diabetes Society . The Treatment Guide for Diabetes 2007. Bunkodo Tokyo 2007.
- 27. United Kingdom Prospective Diabetes Study Group . United Kingdom prospective diabetes study (UKPDS) 13: relative efficacy of randomly allocated diet, sulphonylurea, insulin, or metformin in patients with newly diagnosed non‐insulin dependent diabetes followed for three years. BMJ 1995; 310: 83–88. [PMC free article] [PubMed] [Google Scholar]
- 28. Pernicova I, Korbonits M. Metformin‐mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol 2014; 10: 143–156. [DOI] [PubMed] [Google Scholar]
- 29. Cignarelli A, Giorgino F, Vettor R. Pharmacologic agents for type 2 diabetes therapy and regulation of adipogenesis. Arch Physiol Biochem 2013; 119: 139–150. [DOI] [PubMed] [Google Scholar]
- 30. Inzucchi S. Oral anti‐hyperglycemic therapy for type2 diabetes: scientific review. JAMA 2002; 287: 360–372. [DOI] [PubMed] [Google Scholar]
- 31. Schweizer A, Couturier A, Foley JE, et al Comparison between vildagliptin and metformin to sustain reductions in HbA1c over 1 year in drug‐naive patients with Type 2 diabetes. Diabet Med 2007; 24: 955–961. [DOI] [PubMed] [Google Scholar]
- 32. Morsink LM, Smits MM, Diamant M. Advances in pharmacologic therapies for type2 diabetes. Curr Atheroscler Rep 2013; 15: 302. [DOI] [PubMed] [Google Scholar]
- 33. Kahn SE, Haffner SM, Heise MA, et al Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006; 355: 2427–2443. [DOI] [PubMed] [Google Scholar]
- 34. Zeitler P, Hirst K, Pyle L, et al A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 2012; 366: 2247–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. UK Prospective Diabetes Study (UKPDS) Group . UK Prospective Diabetes Study 16: overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes 1995; 44: 1249–1258. [PubMed] [Google Scholar]
- 36. Hirssch IB, Bergenstal RM, Parkin CG, et al A real‐world approach to insulin therapy in primary care practice. Clin Diabetes 2005; 23: 78–86. [Google Scholar]
- 37. Inzucchi S, Bergenstal RM, Buse JB, et al Management of Hyperglycemia in Type 2 Diabetes: a Patient‐Centered Approach Position Statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35: 1364–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]


