Abstract
Aims/Introduction
The usefulness of markers of carotid plaque, such as sum (PS) and maximum (P‐max) of the plaque thickness, in combination with intima‐media thickness in the common carotid artery (CIMT) for the detection of obstructive coronary artery disease (CAD) was investigated in patients with type 2 diabetes without known CAD.
Materials and Methods
B‐mode ultrasonographic scanning of the carotid artery and multislice computed tomography coronary angiography were carried out in 332 asymptomatic patients with type 2 diabetes.
Results
For the presence of obstructive CAD when incorporating PS or P‐max to standard risk factors in a multiple logistic regression model, the classification ability in PS and P‐max increased greatly (area under the curve [AUC] 0.827 vs 0.720 [net reclassification index {NRI} = 0.652, P < 0.01] and AUC 0.820 vs 0.720 [NRI = 0.775, P < 0.01], respectively), and it in CIMT increased slightly (AUC 0.740 vs 0.720, NRI = 0.230, P = 0.041). Furthermore, the classification abilities for a model with interaction terms between PS* or P‐max* and CIMT were statistically larger than those for a model without interaction terms (AUC 0.833 vs 0.827 [NRI = 0.411, P < 0.01] and 0.823 vs 0.820 [NRI = 0.269, P < 0.05], respectively). Partitioning showed the patients in the values of the PS <2.6 mm and CIMT <0.725 mm (100%), or in P‐max <2.1 mm and CIMT <0.725 mm (95.4%), did not have obstructive CAD, whereas those in the values of PS 2.6 mm, presence of hyperlipidemia and CIMT 0.675 mm (84%) or those in the value of P‐max 2.1 mm and body mass index 24 (91.7%) had obstructive CAD.
Conclusions
Although the P‐max and PS in the carotid artery were useful as detectors of CAD, combining them with CIMT provided a much superior first‐line screening method in detecting CAD in asymptomatic patients with diabetes.
Keywords: Coronary artery disease, Maximal plaque thickness, Plaque score
Introduction
Diabetes mellitus is a major risk factor for coronary artery disease (CAD), and it is associated with a two‐ to fourfold increase of CAD when compared with the risk in the general population1. Patients suffering from diabetes mellitus are often asymptomatic regardless of the presence or progression of CAD. Simple, non‐invasive, inexpensive, radiation‐free tests for screening to identify patients with diabetes as being in either high‐ or low‐risk groups for CVD are required.
Although intima‐media thickness (IMT) has been reported to be an important surrogate marker for the prediction of future CAD and stroke in the general population2, 3, recent meta‐analysis has shown that the addition of IMT does not significantly improve the ability to predict future cardiovascular events when compared with the Framingham Risk Score, indicating IMT is not important in clinical use4. However, when carotid plaque such as the presence of plaque (>1.5 mm) was used, the prediction power of future cardiovascular events significantly improved3, showing the importance of the evaluation of carotid plaque for future myocardial infarction.
In a recent study involving patients with diabetes, it was reported that the usefulness of maximum IMT thickness including plaque (max‐IMT) has been used in both the detection of obstructive (50%) CAD5, 6 and in identifying severe CAD (revascularization)7. However, participants in those studies were limited to patients with diabetes with the presence of plaque of the carotid artery (max‐IMT >1.1 mm)5, 6 or to those being screened by the exercise electrocardiogram test7. In addition to this, we believe it might not be appropriate to combine the plaque and IMT, and to treat them as being in a single category (such as max‐IMT), as the thickening of IMT and formation of plaque reflect biologically and genetically different phenomena in the atherosclerotic process8. Because of these factors, we believe that the separate characterization of plaque and IMT could provide better information in predicting CAD. Furthermore, we witnessed a significant interaction between carotid plaque and common carotid artery IMT (CIMT). Herein, we report that the power to detect CAD was even further increased by using both markers in combination.
Materials and methods
The present study, carried out at Shin‐Koga Hospital, Kurume city, Fukuoka, Japan, included 332 eligible outpatients with type 2 diabetes mellitus that were undergoing multislice computed tomography (MSCT) coronary angiography and B‐mode ultrasonography of the carotid artery for the evaluation of CAD. The exclusion criteria were: (i) the presence of typical angina pectoris; (ii) a history of myocardial infarction; and (iii) a history of percutaneous coronary intervention and surgery for coronary artery bypass grafting. Among 633 diabetic patients, 301 patients with diabetes with known CAD were excluded from this study. The remaining 332 patients were included in the study. Half of the patients had some atypical symptoms including chest discomfort.
Hypertension was defined as blood pressure >140/90 mmHg or treatments with antihypertensive medication. Hyperlipidemia was defined as low‐density lipoprotein cholesterol >140 mg/dL or triglyceride >150 mg/dL or use of lipid‐lowering medication.
Two trained ultrasonographers, blinded to the information obtained from the MSCT coronary angiography, carried out B‐mode ultrasonographic scanning of the carotid artery using an echotomographic system (Toshiba AplioXG, Tochigi, Japan) with an electrical linear transducer (7.5–10 MHz). The IMT measurement was obtained from the near and far wall of both the left and right common carotid artery within 2 cm proximal of the carotid bulb. Four parts of the thickest IMT were averaged to obtain the mean IMT (CIMT). Plaques were defined as local prominence (>1.1 mm) in the IMT. Plaques were measured in the carotid bifurcation as well as in the internal carotid artery and common carotid artery. The PS was calculated by summing the maximum plaque thickness (P‐max) measured on the near and far wall of four divisions from both sides of the carotid wall9 (Figure S1), and the P‐max and the number of plaque (PN) were also measured. The coefficients of interassay variation of the CIMT and plaque score were 5.5% and 6.4%, respectively. The intra‐assay coefficient for the variation of CIMT and PS has been reported previously10.
Coronary MSCT angiography
All examinations were carried out with a 64‐detector scanner with a 4‐cm detector length (Lightspeed VCT; GE Healthcare, Milwaukee, WI, USA). The coronary arteries were divided into 17 segments, according to the modified American Heart Association11. The presence of coronary plaques was evaluated visually using axial images and curved multiplanar reconstructions. Plaques were classified as obstructive (luminal stenosis 50% of the coronary diameter) and non‐obstructive (luminal stenosis <50%). The extent and the severity of CAD for each patient was evaluated, both by measuring the number of obstructive segments and by Gensini score12.
Statistical analysis
Continuous variables were expressed as the mean ± standard deviation, while categorical variables were expressed as percentages. In comparing percentages, we used the χ2‐test.
In multiple logistic regression analysis for the presence of obstructive CAD, the area under the receiver operating characteristic (ROC) curves (AUCs) for sets of standard risk factors was estimated. First, we assessed the AUC for the standard risk factors (RFs) such as age, sex, body mass index, duration of diabetes, smoking, presence of hypertension, presence of hyperlipidemia and glycated hemoglobin (HbA1c). Next, we evaluated the RFs with the addition of the markers of carotid plaque; PN, P‐max and PS. In addition, we added the interaction terms between CIMT and each maker of carotid plaque into the corresponding model and then evaluated the AUC. The classification abilities were then compared with the net reclassification index (NRI) and integral discrimination index (IDI)13.
To make use of each risk factor in practice, partitioning, in which the best splitting was determined by the largest likelihood ratio χ2 statistics (G2), was carried out for the presence or absence of obstructive CAD in: (i) CIMT and PS; and (ii) CIMT and P‐max, together with the RFs. The statistical software JMP 11 (SAS Institute Inc, Cary, NC, USA) was used to analyze the data.
Results
In Table 1, we summarized the characteristics of 332 patients with diabetes both with and without obstructive CAD. Of the 332 patients included in the study, 219 (66%) were men, aged 64 ± 11 years. The patients in our study with obstructive CAD were predominantly male and of a significantly advanced age, they had suffered a longer duration of diabetes, had higher proportions of smoking, higher proportions of hypertension and hyperlipidemia, higher systolic blood pressure, and higher proportions of retinopathy and increased HbA1c. Their carotid ultrasonography, also, showed a significantly higher frequency of carotid plaque, and elevated levels of CIMT, PN, P‐max and PS when compared with those without obstructive CAD (Table 1).
Table 1.
Characteristics of patients with diabetes
| All | CAD(−) | CAD(+) | P‐value | |
|---|---|---|---|---|
| n | 332 | 133 | 199 | |
| Sex (male/female) | 219/113 | 78/55 | 141/58 | 0.021 |
| Age (years) | 64 ± 11 | 61.6 ± 11.5 | 65.6 ± 9.6 | <0.001 |
| BMI (kg/m2) | 24.2 ± 4 | 24.5 ± 4.5 | 24.0 ± 3.7 | 0.2781 |
| Duration of diabetes (years) | 10.2 ± 9 | 7.9 ± 7.8 | 11.7 ± 9.4 | <0.001 |
| Smoking (%) | 197/331 (59.5) | 69/133 (51.9) | 128/198 (64.6) | 0.020 |
| Systolic blood pressure (mmHg) | 139 ± 21 | 134 ± 19 | 142 ± 22 | 0.001 |
| Diastolic blood pressure (mmHg) | 77 ± 13 | 78 ± 13 | 77 ± 14 | 0.694 |
| Hypertension (%) | 234/332 (70.5) | 79/133 (59.4) | 155/199 (77.9) | <0.001 |
| Hyperlipidemia(%) | 208/332 (62.7) | 74/133 (55.6) | 134/199 (67.3) | 0.031 |
| Family history of CAD (%) | 36/276 (13.0) | 19/111(17.1) | 17/165 (10.3) | 0.147 |
| Therapy, diet/oral hypoglycemic agents/insulin (%) | 59/160/110 (18/49/33) | 33/64/34 (25/49/26) | 26/96/76 (13/49/38) | |
| Retinopathy (%) | 111/330 (33.6) | 33/132 (25.0) | 78/198 (39.4) | 0.007 |
| Nephropathy (%) | 102/331 (30.8) | 36/132 (27.3) | 66/199 (33.2) | 0.255 |
| Neuropathy (%) | 150/330 (45.5) | 55/132 (41.7) | 95/198 (48.0) | 0.259 |
| HbA1c | 8.0 ± 1.9 | 7.8 ± 1.9 | 8.3 ± 1.9 | 0.026 |
| TC (mg/dL) | 200 ± 40 | 197 ± 38 | 201 ± 41 | 0.320 |
| LDL‐C (mg/dL) | 116 ± 33 | 112 ± 31 | 119 ± 34 | 0.057 |
| HDL‐C (mg/dL) | 53 ± 15 | 53 ± 15 | 52 ± 15 | 0.464 |
| TG (mg/day) | 130 (202, 90) | 120 (198, 79) | 131 (203, 92) | 0.447 |
| Presence of carotid plaque (%) | 255/332 (76.8) | 72/133 (54.1) | 183/199 (92.0) | <0.001 |
| CIMT (mm) | 0.889 ± 0.185 | 0.817 ± 0.188 | 0.938 ± 0.167 | <0.001 |
| Plaque score (mm) | 4.2 (8.9, 1.3) | 1.3 (4, 0) | 6.6 (10.7, 3.8) | <0.001 |
| Maximum thickness of plaque (mm) | 2.1 (3, 1.1) | 1.2 (2, 0) | 2.6 (3,2) | <0.001 |
Nephropathy: persistent proteinuria (diabetic nephropathy stage 3). Retinopathy: simple or proliferative retinopathy. Neuropathy: peripheral or autonomic neuropathy. The values of triglyceride (TG), plaque score and maximum thickness of plaque were expressed as median (75th percentile, 25th percentile). CAD, coronary artery disease; CAD(+), presence of obstructive (50%) coronary artery disease; CIMT, common carotid artery intima‐media thickness; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; TC, total cholesterol.
MSCT coronary angiography showed that 200 patients (60%) had obstructive CAD, of which 106 patients (39.8%) had more than 75% stenosis. A total of 5,170 coronary segments were included in the analysis, whereas 55 segments were excluded because of quality insufficient to provide a diagnosis. Plaques were observed in 2,248 segments (43.9%), of those, non‐obstructive plaques were found in 1,566 segments (30.6%) and obstructive plaques in 682 segments (13.3%), of which 207 segments (4%) showed more than 75% stenosis.
The percentage of obstructive segments and Gensini score in the coronary artery both showed significant correlations with the standard risk factors, such as age, smoking, duration of diabetes, systolic blood pressure, low‐density lipoprotein cholesterol and HbA1c. We also found even closer correlative relationships among the CIMT (r = 0.336, P < 0.001), P‐max (r = 0.436, P < 0.001) and PS (r = 0.470, P < 0.001) in the carotid artery (Table S1). In a multiple, stepwise regression analysis for the extent (percentage of obstructive segments) and for the severity (Gensini score) in the coronary artery, the incorporation of CIMT to the RFs contributed significantly (regression coefficients [β] = 0.252, P < 0.001; R 2 = 0.166, P < 0.001 and β = 0.250, P < 0.001; R 2 = 0.201, P < 0.001), as did the incorporation of P‐max (β = 0.377, P < 0.001; R 2 = 0.237, P < 0.001 and β = 0.323, P < 0.001; R 2 = 0.237, P < 0.001, respectively). The incorporation of PS also greatly contributed (β = 0.458, P < 0.001; R 2 = 0.255, P < 0.001 and β = 0.394, P < 0.001; R 2 = 0.283, P < 0.001, respectively). The highest R 2 was obtained by incorporating PS or P‐max to the RFs (0.269 and 0.224, respectively; Table S2).
Multiple logistic regression analysis for the presence of obstructive (50%) CAD was carried out to investigate the added effects of CIMT and the markers of carotid atherosclerosis, such as PS and P‐max, to the RFs (Table 2). The NRI and IDI are shown in Table 3. In multiple logistic regression analysis, the RFs such as age, hypertension, hyperlipidemia and HbA1c significantly contributed, and the AUC was 0.720 (95% confidence interval [CI] 0.659–0.774). In the model with the addition of CIMT to the RFs, the CIMT was significantly associated with the presence of obstructive CAD (β = 2.319, P = 0.005) with the AUC increasing to 0.740 (95% CI 0.679–0.793; model B in Table 2). This model significantly improved the ability to classify the presence or absence of obstructive CAD (NRI index 0.230, 95% CI 0.010–0.450, P = 0.041; and IDI index 0.019, 95% CI 0.004–0.033, P = 0.013; A : B in Table 3). With the addition of P‐max to the RFs, P‐max was significantly associated with the presence of obstructive CAD (β = 0.875, P < 0.001), and the AUC increased greatly to 0.820 (95% CI 0.767–0.864; model C in Table 2). This model also significantly improved the ability to classify the absence or the presence of obstructive CAD (NRI index 0.775, 95% CI 0.572–0.979, P < 0.001; and IDI index 0.129, 95% CI 0.095–0.163, P < 0.001; A : C in Table 3). In the model with the addition of PS to the RFs, the PS was significantly associated with the presence of obstructive CAD (β = 0.245, P < 0.001), and the AUC increased greatly to 0.827 (95% CI 0.774–0.870; model D in Table 2). This model also showed significant improvement for the classification of the absence or presence of obstructive CAD (NRI index 0.652, 95% CI 0.454–0.849, P < 0.001; and IDI index 0.085, 95% CI 0.059–0.111, P < 0.001; A : D in Table 3).
Table 2.
Multiple logistic regression analysis of diabetic patients for the presence of obstructive disease in the coronary artery
| Obstructive (50%) CAD | |||
|---|---|---|---|
| β (95% CI) | P‐value | AUC(95% CI) | |
| A. Standard risk factors only | 0.720 (0.659–0.774) | ||
| B. Standard risk factors | |||
| +CIMT | 2.319 (0.708–3.99) | 0.005 | |
| Overall | 0.740 (0.679–0.793) | ||
| C. Standard risk factors | |||
| +P‐max | 875 (0.631–1.141) | <0.001 | |
| Overall | 0.820 (0.767–0.864) | ||
| D. Standard risk factors | |||
| +PS | 0.245 (0.171–0.328) | <0.001 | |
| Overall | 0.827 (0.774–0.870) | ||
| E. Standard risk factors | |||
| +CIMT | 0.740 (−1.049 to 2.562) | 0.419 | |
| +P‐max | 0.842 (0.589–1.116) | <0.001 | |
| +(CIMT‐0.888)*(Pmax‐2.1) | −1.399 (−2.677 to −0.111) | 0.031 | |
| Overall | 0.823 (0.770–0.866) | ||
| F. Standard risk factors | |||
| +CIMT | 0.025 (−1.855 to 1.912) | 0.979 | |
| +PS | 0.282 (0.197–0.376) | <0.001 | |
| +(CIMT −0.888)*(PS −5.878) | −0.535 (−0.806 to −0.252) | 0.001 | |
| Overall | 0.833 (0.780–0.874) | ||
Multiple logistic regression analysis for the presence of obstructive (50%) coronary artery disease (CAD) was first constructed to include only standard risk factors (A) – those factors being sex, age, body mass index, duration of diabetes, smoking, presence of hypertension or hyperlipidemia and glycated hemoglobin – and then standard risk factors plus common carotid artery (CIMT) (B) or standard risk factors plus maximum plaque thickness (P‐max) (C) or standard risk factors plus sum of the plaque thickness (PS) (D). In addition to standard risk factors, interaction variables between the markers of carotid atherosclerosis, such as PS, P‐max and CIMT (CIMT* P‐max and CIMT* PS) were also incorporated into this model. β (Log odds ratio), partial regression coefficient; AUC, area under curve; CI, confidence interval.
Table 3.
Statistical net reclassification improvement and integrated discrimination improvement analysis between the groups shown in Table 2
| NRI (95%CI) | P‐value | IDI (95%CI) | P‐value | |
|---|---|---|---|---|
| A : B | 0.230 (0.010–0.450) | 0.041 | 0.019 (0.004–0.033) | 0.013 |
| A : C | 0.775 (0.572–0.979) | <0.001 | 0.129 (0.095–0.163) | <0.001 |
| A : D | 0.652 (0.454–0.849) | <0.001 | 0.085 (0.059–0.111) | <0.001 |
| C : E | 0.269 (0.053–0.484) | 0.014 | 0.025 (0.010–0.040) | 0.001 |
| D : F | 0.411 (0.196–0.626) | <0.001 | 0.042 (0.024–0.060) | <0.001 |
Statistical significance between the groups (A, B, C, D, E and F, shown in Table 2) was examined using net reclassification improvement (NRI) and integrated discrimination improvement (IDI) analysis. A, standard risk factors only; B, standard risk factors + common carotid artery; C, standard risk factors + maximum of the plaque thickness; D, standard risk factors + sum of the plaque thickness; E, standard risk factors + common carotid artery * maximum of the plaque thickness; F, standard risk factors + common carotid artery * sum of the plaque thickness. CI, confidence interval.
Because of the significant effects of the interaction terms between P‐max* and CIMT, or between PS* and CIMT being observed (Table S3), the effects of the addition of interaction terms between P‐max* and CIMT or PS* and CIMT to the corresponding model were further investigated (model E and F in Table 2). With the addition of the interactive term between P‐max* and CIMT to the corresponding model, the AUC increased to 0.823 (95% CI 0.770–0.866; model E in Table 2), and the model improved significantly for the classification of the presence or absence of CAD (NRI index 0.269, P = 0.0145; and IDI index 0.025, 95% CI 0.01–0.04, P = 0.001; C : E in Table 3). Also, by adding the interaction terms between PS * and CIMT to the corresponding model, the AUC increased to 0.833 (95% CI 0.780–0.874; model F in Table 2), and the model was significantly improved for the classification of the presence or absence of obstructive CAD (NRI index 0.411, 95% CI 0.196–0.626, P < 0.001; and IDI index 0.042, 95% CI 0.024–0.060; D : F in Table 3).
To make use of these results in practice, partitioning was carried out, for the presence and absence of obstructive CAD, in PS and CIMT, together with RFs in 332 asymptomatic patients with diabetes (Figure 1a).
Figure 1.
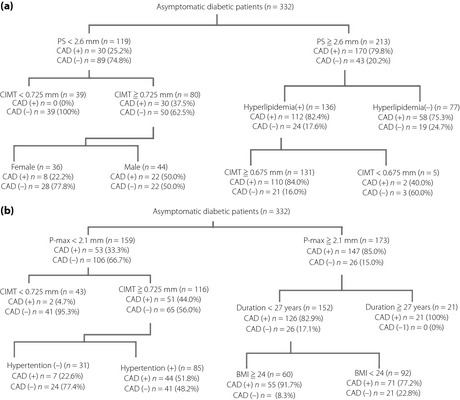
Partitioning of (a) common carotid artery (CIMT) and plaque score (PS), (b) CIMT and maximum thickness of the plaque (P‐max), together with the standard risk factors for the presence or absence of obstructive coronary artery disease (CAD). BMI, body mass index; CAD(+), presence of obstructive coronary artery disease; CAD(−), absence of obstructive coronary artery disease.
At the first stage, patients were partitioned at a value of PS of 2.6 mm, which was equivalent to the value of cut‐off in the ROC curve for the presence of obstructive CAD (Table S4, Figure S2). In patients with PS <2.6 mm (n = 119), 89 patients (74.8%) did not have obstructive CAD. For the patients in whom CIMT was <0.725 mm, all patients (100%) did not have obstructive CAD. In contrast, among the 213 patients in whom PS was more than 2.6 mm, 170 patients (79.8%) had obstructive CAD, and approximately 84% of patients with the presence of hyperlipidemia and CIMT 0.675 mm had obstructive CAD (Figure 1a).
Next, the partitioning of P‐max and CIMT, together with RFs was carried out (Figure 1b). At the first stage, patients with a P‐max value of 2.1 mm were selected, which was equivalent to the cut‐off value of P‐max in the ROC curve for obstructive CAD in Table S4 and Figure S2. Of the 159 patients with a P‐max value <2.1 mm, 106 patients (66.7%) did not have obstructive CAD, and almost all patients (95.3%) did not have obstructive CAD when the CIMT was at values of <0.725 mm. In contrast, among the 173 patients in whom the P‐max was more than 2.1 mm, 147 patients (85%) had obstructive CAD. Furthermore, patients with a duration of diabetes 27 years (100%), or patients with a duration of diabetes <27 years and a BMI 24 (91.7%), had obstructive CAD (Figure 1b).
Discussion
Our present study shows that although markers of carotid plaque, such as the P‐max and PS, are extremely useful in the detection of the presence of obstructive CAD, combining the markers of carotid plaque (P‐max and PS) with CIMT even further increases the delectability of obstructive CAD.
Therefore, we have determined this to be a very useful method as a first‐line screening test in identifying CAD in asymptomatic patients with diabetes.
Previous studies to examine the correlation between CIMT and the extents (or severity) of CAD in general patients with CAD have reported a significant, but weak, correlation (r = 0.29 for Gensini score)14, and between the IMT of the internal carotid artery and Gensini score was also weak (r = 0.26)15. Those results were similar to ours in asymptomatic patients with diabetes (r = 0.296, P < 0.001, in between CIMT and Gensini score, as shown in Table S1. In multiple regression analysis by Adams et al.14 in general patients, CIMT did not significantly contribute after adjustment with the conventional risk factors for the detection of severity of CAD (Gensini score). In our asymptomatic patients with diabetes, CIMT was still weak, but significantly contributed to the extent (percentage of number of obstructive segments) after adjustment with RFs. In addition, as new findings, we showed that P‐max and PS greatly contributed to the extents of obstructive CAD (percentage of numbers of obstructive segments) and also to the severity of CAD (Gensini score; Table S2). In the present results, we showed that PS and P‐max greatly contributed the extents and severity of CAD.
Second, we investigated the presence of obstructive (50%) CAD using a multiple logistic regression model (Tables 2 and 3). With the addition of CIMT to the RFs, the CIMT was significantly associated with the presence of obstructive CAD (β = 2.319, P = 0.005) and the AUC increased to 0.740 (95% CI 0.679–0.793; model B in Table 2). Our finding is consistent with the recent report that in asymptomatic patients with diabetes, after adjusting the RFs, CIMT independently contributes16, showing its usefulness in predicting CAD.
Furthermore, when the markers of carotid plaque, such as P‐max or PS, were included in the model, the AUC greatly increased by 0.1 (13.9%) or 0.107 (14.9%) in comparison with that of the RFs, whereas the AUC of the CIMT increased by just 0.02 (2.8%). Irie et al.6 reported similar findings in asymptomatic diabetic patients with the presence of carotid plaque (>1.1 mm) using max‐IMT; namely, the equivalent to P‐max in our study, and that the AUC significantly increased from 0.64 to 0.74, by 0.1 (15.6%), and was higher than that of CIMT. Both these studies show the clinical application of measuring markers of carotid plaque, such as P‐max along with the RFs, as being superior to CIMT in detecting obstructive CAD in patients with diabetes. Furthermore, the AUS in PS (0.833) was higher than that in P‐max (0.824). In this regard, the present study concluded that the PS, a simple and semiquantitative estimation of the carotid plaque, was the most effective marker in the detection of obstructive CAD.
In addition to this, we saw a significant interaction between PS* and CIMT, and also between P‐max* and CIMT (Table S3). In incorporating the interaction terms, such as PS* and CIMT, with the RFs, there was a further significant increase in the AUC from 0.827 to 0.833 by 0.006 (0.7%; model F in Table 2), and an improved NRI of 0.411 (approximately 41%; D : F in Table 3). Although an increase of 0.7% in the AUC might seem to be small, the interaction terms between the PS* and the CIMT caused a 41% improvement in the detection of obstructive CAD in comparison with that of just PS with the RFs. Similarly, with the addition of interaction terms between the P‐max* and the CIMT to the RFs, the AUC increased from 0.820 to 0.823 by 0.003 (0.3%) when compared with that of P‐max with the RFs (Table 2), and improved the NRI by 0.269, which was roughly interpreted as a 27% improvement in the detection of obstructive CAD (C : E in Table 3). Taking all metrics into consideration, we concluded that the evaluation of PS or P‐max in combination with CIMT proved to be the most effective method for detecting obstructive CAD.
Our findings in which the highest AUCs were obtained by combining the markers of carotid plaque and the CIMT also addressed the importance of the individual measurement of the IMT and plaque in the carotid artery. Although max‐IMT (maximum thickness of either IMT or plaque) has been used in many studies5, 6, 7, the importance of its relationship to the interaction terms between the plaques and the IMT had not been clarified, and the importance of their association with CAD had been underestimated. The thickening of IMT mainly represents hypertensive medical hypertrophy, which is biologically distinct from plaque. Whereas, the presence of plaque represents a distinctive phenotype of atherosclerosis, which creates a condition with higher possibilities of thrombosis and rupture, thus leading to cardiovascular events17. Disagreements or conflicts with negative or positive results in a population‐based study on the association between IMT and future myocardial infarction might depend on the evaluation of IMT including or not including plaque. Inaba et al.18 clarified this point in their meta‐analysis, reporting that IMT including plaque (i.e., probably might reflect plaque thickness) was superior to IMT not containing plaque. Editorials on that study commented that neither approach to IMT measurement should be called “atherosclerosis,” rather, they should be called IMT, and that IMT including plaque should be called plaque thickness19.
To test the clinical usefulness of the combination of the markers, a set of PS and CIMT with RFs (Figure 1a), and a set of P‐max and CIMT with RFs (Figure 1b) was carried out in 332 patients with diabetes using the partitioning method. In partition of PS and CIMT with RFs, the value of 2.6 mm for PS was selected at the first step, which corresponded to the cut‐off value in the ROC curve of PS for obstructive CAD (Table S4). Out of 119 patients with PS of <2.6 mm, 74.8% of patients did not have obstructive CAD. In the patients with PS of <2.6 mm and CIMT of <0.725 mm, none had obstructive CAD. In contrast, in 213 patients with PS 2.6 mm, 79.8% had obstructive CAD. Furthermore, in the presence of hyperlipidemia and CIMT of 0.675 mm, most patents (84%) had obstructive CAD.
Next, partitioning was carried out for P‐max and CIMT combined with RFs (Figure 1b). At the first stage, 2.1 mm of P‐max, which corresponded to the cut‐off value of P‐max in ROC for obstructive CAD (Table S4) was partitioned. The value (2.1 mm) can be compared with the research carried out by Kasami et al.5 and Irie et al.7, who determined cut‐off values of IMT‐max for the obstructive CAD at 1.9 mm and severe CAD at 2.45 mm, respectively, in the patients with the presence of carotid plaque (>1.1 mm). Out of 159 patients with a P‐max of <2.1 mm, 66.7% did not have obstructive CAD, and the vast majority of patients (95.4%) did not have obstructive CAD when the CIMT was <0.725 mm. In contrast, out of 173 patients with a P‐max over 2.1 mm, 85% had obstructive CAD in the patients who had diabetes for over 27 years, all (21 patients) had obstructive CAD; and in the 60 patients with a body mass index above 24 who had diabetes for <27 years, approximately 92% had obstructive CAD.
Using partitioning, we were able to confirm the practical benefits of combining PS or P‐max with CIMT, together with the standard risk factors. By using these markers, we can effectively predict the absence or presence of obstructive CAD in the most asymptomatic patients with diabetes.
The present study did have some limitations, however, in that it did not include subjects with non‐diabetes, so the usefulness of this method in the general population still remains unclear. Also, our participants might not represent general diabetic patients as a whole, as half of the patients in the present study had symptoms of chest discomfort. Carotid plaque area or volume8 were not included in the present study. This measurement is considered to be a more precise estimation of plaque.
In conclusion, although the markers of carotid plaque, such as the P‐max and PS, in the carotid artery are useful in the detection of CAD, we found the combination of the markers of carotid plaque with CIMT to be the most useful first‐line screening test for the presence or absence of CAD in asymptomatic patients with diabetes.
Disclosure
The authors declare no conflict of interest.
Supporting information
Table S1 ¦ Correlation between the extent (percentage of obstructive segments) and the severity (Gensini score) of coronary artery disease and each parameter in patients with diabetes.
Table S2 ¦ Multiple regression analysis for the extents (percentage of obstructive segments) and the severity (Gensini score) of coronary artery disease in patients with diabetes.
Table S3 ¦ Interaction between and markers of carotid atherosclerosis such as plaque number (PN), maximum plaque thickness (P‐max), plaque score (PS) and intima‐media thickness in common artery (CIMT) for the presence of obstructive coronary artery disease.
Table S4 ¦ Area under curve (AUC) of intima‐media thickness in common carotid artery (CIMT) and markers of carotid atherosclerosis for the presence of obstructive coronary artery disease in asymptomatic diabetic patients.
Figure S1 ¦ Diagrammatic representation of markers of carotid plaque.
Figure S2 ¦ Receiver operating characteristic curve for the presence of (50%) coronary artery disease in intima‐media thickness in common carotid artery (CIMT), number of the plaque (PN), maximum thickness of the plaque (P‐max) and plaque score (PS).
Acknowledgments
The authors thank Yuko Kosaka for her assistance in preparing the manuscript.
J Diabetes Investig 2016; 7: 396–403
References
- 1. Kahn CR, King GL, Jacobson AM, et al, editor. Joslin's Diabetes Mellitus, 14th edn Philadelphia, Baltimore, New Yolk, London, Buenos Aires, Hong Kong, Sydney, Tokyo: Lipplincott Williams & Wilkins, 2005. [Google Scholar]
- 2. Bots ML, Hoes AW, Koudstaal PJ, et al Common carotid intima‐media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation 1997; 96: 1432–1437. [DOI] [PubMed] [Google Scholar]
- 3. Polak JF, Pencina MJ, Pencina KM, et al Carotid‐wall intima‐media thickness and cardiovascular events. N Engl J Med 2011; 365: 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Den Ruijter HM, Peters SA, Anderson TJ, et al Common carotid intima‐media thickness measurements in cardiovascular risk prediction: a meta‐analysis. JAMA 2012; 308: 796–803. [DOI] [PubMed] [Google Scholar]
- 5. Kasami R, Kaneto H, Katakami N, et al Relationship between carotid intima‐media thickness and the presence and extent of coronary stenosis in type 2 diabetic patients with carotid atherosclerosis but without history of coronary artery disease. Diabetes Care 2011; 34: 468–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Irie Y, Katakami N, Kaneto H, et al Maximum carotid intima‐media thickness improves the prediction ability of coronary artery stenosis in type 2 diabetic patients without history of coronary artery disease. Atherosclerosis 2012; 221: 438–444. [DOI] [PubMed] [Google Scholar]
- 7. Irie Y, Katakami N, Kaneto H, et al The utility of carotid ultrasonography in identifying severe coronary artery disease in asymptomatic type 2 diabetic patients without history of coronary artery disease. Diabetes Care 2013; 36: 1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spence JD. Technology Insight: ultrasound measurement of carotid plaque‐patient management, genetic research, and therapy evaluation. Nat Clin Pract Neurol 2006; 2: 611–619. [DOI] [PubMed] [Google Scholar]
- 9. Handa N, Matsumoto M, Maeda H, et al Ultrasonic evaluation of early carotid atherosclerosis. Stroke 1990; 21: 1567–1572. [DOI] [PubMed] [Google Scholar]
- 10. Akazawa S, Tojikubo M, Nakano Y, et al Usefulness of sum of the thickness of plaque in the carotid artery for predicting the presence and the extent of the coronary artery disease in patients with type 2 diabetes mellitus without known coronary artery disease. Diabetes Res Clin Pract 2012; 96: 111–118. [DOI] [PubMed] [Google Scholar]
- 11. Austen WG, Edwards JE, Frye RL, et al A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation 1975; 51: 5–40. [DOI] [PubMed] [Google Scholar]
- 12. Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol 1983; 51: 606. [DOI] [PubMed] [Google Scholar]
- 13. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, et al Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008; 27: 157–172; discussion 207–112. [DOI] [PubMed] [Google Scholar]
- 14. Adams MR, Nakagomi A, Keech A, et al Carotid intima‐media thickness is only weakly correlated with the extent and severity of coronary artery disease. Circulation 1995; 92: 2127–2134. [DOI] [PubMed] [Google Scholar]
- 15. Kotsis VT, Pitiriga V, Stabouli SV, et al Carotid artery intima‐media thickness could predict the presence of coronary artery lesions. Am J Hypertens 2005; 18: 601–606. [DOI] [PubMed] [Google Scholar]
- 16. Djaberi R, Schuijf JD, de Koning EJ, et al Usefulness of carotid intima‐media thickness in patients with diabetes mellitus as a predictor of coronary artery disease. Am J Cardiol 2009; 104: 1041–1046. [DOI] [PubMed] [Google Scholar]
- 17. Finn AV, Kolodgie FD, Virmani R. Correlation between carotid intimal/medial thickness and atherosclerosis: a point of view from pathology. Arterioscler Thromb Vasc Biol 2010; 30: 177–181. [DOI] [PubMed] [Google Scholar]
- 18. Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima‐media thickness, more accurately predicts coronary artery disease events: a meta‐analysis. Atherosclerosis 2012; 220: 128–133. [DOI] [PubMed] [Google Scholar]
- 19. Editorial . Caroid plaque measurement is superior to IMT invited editorial comment on: carotid plaque, compared with carotid intoma‐media thickness, more accurately predicts coronary artery disease events: a meta‐analysis‐Yoichi Inaba,M.D.,Jennifer A. Chen M.D., Steve R. Bergmann M.D., Ph. D. Atherosclerosis 2012; 220: 34–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 ¦ Correlation between the extent (percentage of obstructive segments) and the severity (Gensini score) of coronary artery disease and each parameter in patients with diabetes.
Table S2 ¦ Multiple regression analysis for the extents (percentage of obstructive segments) and the severity (Gensini score) of coronary artery disease in patients with diabetes.
Table S3 ¦ Interaction between and markers of carotid atherosclerosis such as plaque number (PN), maximum plaque thickness (P‐max), plaque score (PS) and intima‐media thickness in common artery (CIMT) for the presence of obstructive coronary artery disease.
Table S4 ¦ Area under curve (AUC) of intima‐media thickness in common carotid artery (CIMT) and markers of carotid atherosclerosis for the presence of obstructive coronary artery disease in asymptomatic diabetic patients.
Figure S1 ¦ Diagrammatic representation of markers of carotid plaque.
Figure S2 ¦ Receiver operating characteristic curve for the presence of (50%) coronary artery disease in intima‐media thickness in common carotid artery (CIMT), number of the plaque (PN), maximum thickness of the plaque (P‐max) and plaque score (PS).


