Abstract
Aims/Introduction
To measure the elasticity of the tibial nerve using sonoelastography, and to associate it with diabetic neuropathy severity, the cross‐sectional area of the tibial nerve and neurophysiological findings in type 2 diabetic patients.
Materials and Methods
The elasticity of the tibial nerve was measured as the tibial nerve:acoustic coupler strain ratio using high‐resolution ultrasonography in 198 type 2 diabetic patients stratified into subgroups by neuropathy severity, and 29 control participants whose age and sex did not differ from the diabetic subgroups.
Results
The elasticity of the tibial nerve in patients without neuropathy (P < 0.001) was reduced compared with controls (0.76 ± 0.023), further decreasing (0.655 ± 0.014 to 0.414 ± 0.018) after developing neuropathy. The cut‐off value of elasticity of the tibial nerve that suggested the presence of neuropathy was 0.558. The area under the curve (0.829) was greater than that for the cross‐sectional area (0.612). The cross‐sectional area of the tibial nerve in diabetic patients without neuropathy (6.11 ± 0.13 mm2) was larger than that in controls (4.84 ± 0.16 mm2), and increased relative to neuropathy severity (P < 0.0001). The elasticity of the tibial nerve was negatively associated with neuropathy severity (P < 0.0001), cross‐sectional area (P = 0.002) and 2000 Hz current perception threshold (P = 0.011), and positively associated with nerve conduction velocities (P < 0.0001).
Conclusions
Determining the elasticity of the tibial nerve in type 2 diabetic patients could reveal early biomechanical changes that were likely caused by thickened fibrous sheaths of peripheral nerves, and might be a novel tool for characterizing diabetic neuropathy.
Keywords: Diabetic neuropathy, Elasticity of tibial nerve, Sonoelastography
Introduction
Diabetic neuropathy is primarily diagnosed using characteristic signs and symptoms, and is confirmed by neurophysiological tests, such as nerve conduction studies1, 2. Because the nerve biopsy required for the histological diagnosis of diabetic neuropathy in the peripheral nerves is invasive3, it is not routinely carried out. The development of high‐resolution ultrasonography and a high‐frequency linear probe has enabled the evaluation of the fine structure of the peripheral nerves. An enlarged cross‐sectional area (CSA), hypoechoic area and nerve fascicles of the peripheral nerves have been observed in type 2 diabetic patients with neuropathy4, 5, 6. The biomechanical changes in peripheral nerves have been assessed in few diabetic animal models. The sciatic nerves of diabetic rats were stiffer than those of control rats, and showed reduced blood perfusion of nerves with milder compression7. The elasticity of soft tissue is assessed by sonoelastography as the strain ratio for the soft tissue of interest to the standard material attached to an acoustic coupler (AC)8, 9. In a study using the AC:median nerve strain ratio, the median nerves of patients with carpal tunnel syndrome were stiffer than those of control subjects10. However, there have been no studies assessing changes in the elasticity of the peripheral nerves in diabetic patients with or without diabetic neuropathy.
The aim of the present study was to determine the elasticity of the tibial nerve using sonoelastography in patients with type 2 diabetes, and examine the independent relationship between the severity of diabetic neuropathy and the elasticity or CSA of the tibial nerve.
Materials and Methods
Participants
A total of 198 Japanese patients with type 2 diabetes and 29 non‐diabetic control individuals (glycated hemoglobin [HbA1c] <5.7% and fasting plasma glucose <5.5 mmol/L or casual postprandial plasma glucose <7.7 mmol/L) were enrolled from April 2013 to November 2014 at the Ishibashi Clinic, Hiroshima, Japan. The age and sex of the control subjects did not differ from those of the diabetic subgroups stratified by the severity of diabetic neuropathy. Participants were excluded if their alcohol consumption was >20 g/day, they had been diagnosed with neuropathy due to another cause, or their symptoms occurred as a result of carpal or tarsal tunnel syndrome. Written informed consent was obtained from all participants. The ethics committee of the Ishibashi Clinic approved the protocol of this study.
Assessment of diabetic neuropathy
Diabetic neuropathy was diagnosed in patients with type 2 diabetes, as recommended in the simplified diagnostic criteria proposed by the Diabetic Neuropathy Study Group in Japan11, based on the presence of two of the following three factors: (i) subjective symptoms in the bilateral lower limbs or feet; (ii) absent or reduced ankle jerk; and (iii) decreased vibration perception, assessed using a C128 tuning fork and measured bilaterally at the medial malleoli. Patients with diabetes were classified into one of five groups according to the stages of diabetic neuropathy defined in the Diabetic Neuropathy Study Group in Japan criteria11. Stage I represents the absence of neuropathy and includes patients who do not meet the diagnostic criteria for diabetic neuropathy. Stage II represents asymptomatic diabetic neuropathy, in which patients are asymptomatic but meet the diagnostic criteria for diabetic neuropathy. At stage III, subjective symptoms are positive, but either the ankle jerk reflex or vibration sensation is within the normal range. At stage IV, patients show clinical manifestations of autonomic neuropathy, such as orthostatic hypotension. Motor neuropathy appears at stage V. The latter two stages contained small numbers of patients; we therefore grouped stages IV and V together (IV+V).
Determination of the elasticity and CSA of the tibial nerve
Ultrasonographic examinations of the tibial nerve were carried out using high‐resolution ultrasonography equipped with an 18.0‐MHz linear array probe (HIVISION Ascendus; Hitachi Medical, Tokyo, Japan). The participants sat on a table with knees and ankles maintained at 90 degrees of flexion, and both feet placed on a low height‐adjustable stool to ensure that the tibial nerve was relaxed. Ultrasonographic transverse scans were obtained 3‐cm proximal to the cephalad border of the medial malleolus4. To determine the elasticity of the tibial nerve, an AC (EZU‐TECPL1; Hitachi‐Aloka, Tokyo, Japan) with a standardized elasticity was attached to the transducer as a reference medium. Sonoelastographic images were obtained by repeated manual compressions with the probe perpendicular to the tibial nerve. The strength and frequency of compression and decompression were adjusted to appropriate ranges according to the strain indicator (Figure 1a,b; lower left) on the monitor. The elastogram appeared within a rectangular region of interest as translucent color‐coded, real‐time images superimposed on the B‐mode image. The color‐code (Figure 1a,b; upper left) represented the relative stiffness levels of the various tissues within the region of interest. Red indicated soft, blue indicated hard, and green and yellow indicated medium elasticity. The strain indicator on the monitor specified whether the displacement was sufficient to obtain local strains within the region of interest. The elastograms were constructed automatically. The images were stored as cine loops in the memory of the sonographic machine. Representative sonoelastographic images, produced by appropriate compression, were chosen. The apparatus calculated the strain ratio between the tibial nerve and the AC. The measurements were repeated three times, and the averaged strain ratio was obtained.
Figure 1.
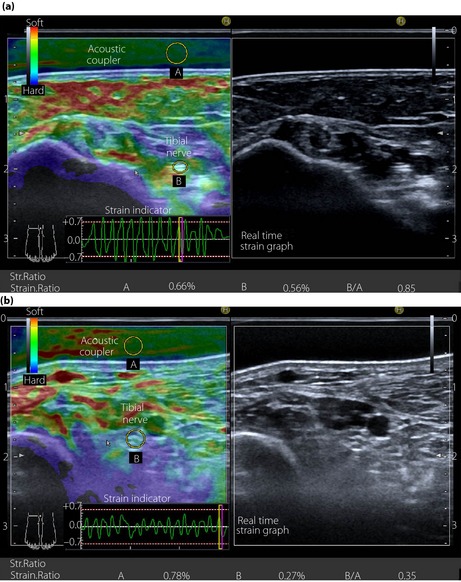
Transverse sectional image of the tibial nerve in (a) a 48‐year‐old male control participant and (b) a 52‐year‐old male patient with type 2 diabetes at stage III neuropathy, visualized by high‐resolution ultrasonography using an 18.0‐MHz linear array probe (HIVISION Ascendus; Hitachi Medical, Tokyo, Japan) attached with an acoustic coupler (EZU‐TECPL1; Hitachi‐Aloka Medical, Tokyo, Japan). A translucent color‐coded image represents the relative stiffness of tissues. Mild compression and decompression using a probe attached with an acoustic coupler were repeated on the tibial nerve. Representative sonoelastographic images were chosen from images stored as cine loops. The tibial nerve and region of the acoustic coupler located directly above the tibial nerve are shown with a circle. The elastograms were constructed automatically. The elasticity of the tibial nerve was assessed as the tibial nerve:acoustic coupler strain ratio. The measurements were repeated three times and averaged.
The CSA of the tibial nerve was evaluated by tracing the inner border of the thin hyperechoic epineurial rim. The pixel numbers were counted using Photoshop Elements 8.0 (Adobe Systems Inc., San Jose, CA, USA).
Assessment of nerve function
Motor (MCV) and sensory (SCV) nerve conduction velocities and action potential amplitudes in the forearm segment were determined by conventional procedures using an electromyography instrument (Neuropak S1; NIHON KOHDEN, Tokyo, Japan). A motor nerve study was carried out on the median nerve, and a sensory nerve study was carried out on the ulnar nerve. The current perception threshold (CPT) was measured using a neurometer (Neurotron, Baltimore, MD, USA) as reported previously12. The electrodes were aligned along the axis of the great toe on the medial side, and positioned at the plantar aspect of the great toe. The lowest stimulus perceivable by the participant was defined as the CPT for the relevant frequency (2,000, 250 and 5 Hz) in each individual. The vibration perception threshold was measured at the left medial malleolus using a biothesiometer (Biomedical Instruments, Newbury, OH, USA). Eight readings were obtained and averaged.
To assess the cardiovagal function of the autonomic nervous system, the coefficient of variation (CV) of R‐R intervals (CVR‐R) was calculated from the R‐R intervals for 200 samples on an electrocardiogram using the following formula: CVR‐R (%) = SD/mean R‐R intervals × 100.
Laboratory data
HbA1c levels were converted to National Glycohemoglobin Standardization Program units by adding 0.4% to the measured values13. Serum creatinine levels, lipid levels and the urinary albumin creatinine ratio were also determined.
Statistical analyses
All statistical analyses were carried out using the spss medical package (SPSS, Chicago, IL, USA) except for the receiver operating characteristic (ROC) curve analysis. Data are presented as the mean ± standard error of the mean. An analysis of variance (anova) was used to compare the control participants and diabetic subgroups stratified by the severity of diabetic neuropathy, graded with Diabetic Neuropathy Study Group in Japan staging11. The differences in age and sex ratio between diabetic subgroups and control participants were examined by anova and χ2‐tests with Bonferroni corrections, respectively. Multivariate regression analysis was used to determine the relationship between either of the elasticity or CSA of the tibial nerve, and stages of diabetic neuropathy, clinical factors and neurophysiological tests. The independence of the relationship between two factors out of the severity of neuropathy, the elasticity and CSA of the tibial nerve was assessed by the partial correlation analysis excluding the influence of a third confounding variable. The ROC curve analysis using Ekuserutohkei 2015 (add‐in software for Excel 2010; Social Survey Research Information Co. Ltd., Tokyo, Japan) established a cut‐off level for the elasticity and CSA of the tibial nerve to be suggestive of the presence of diabetic neuropathy. The sensitivity and specificity were equally weighted. The area under the curve (AUC) of the elasticity and CSA of the tibial nerve was compared by χ2‐test. A P‐value of < 0.05 was considered significant.
Results
Table 1 shows the clinical and demographic data of the control participants and subgroups of type 2 diabetic patients stratified by the severity of diabetic neuropathy. The sex ratio and age of control participants did not differ from those of the diabetic subgroups. The body mass index in patients at stage II neuropathy was higher than that in control participants. The systolic blood pressure in patients at stage IV+V neuropathy was higher than that in control participants and patients at stage I neuropathy. Angiotensin receptor blockers or angiotensin‐converting enzyme inhibitors were prescribed more frequently for diabetic patients at stage I–III neuropathy than for the control participants, whereas the prescription rates did not differ between diabetic subgroups. The HbA1c levels in the control participants were lower than those in the diabetic subgroups and higher in patients at stage IV+V neuropathy than those at stage I neuropathy. Statins were prescribed more frequently for patients at stage II neuropathy than control participants. The high‐density lipoprotein cholesterol level in the control participants was higher than that in patients at stage II neuropathy.
Table 1.
Clinical characteristics of control participants and subgroups of type 2 diabetic patients stratified by the stages of diabetic neuropathy
| Control participants | Stage of diabetic neuropathy | ||||
|---|---|---|---|---|---|
| Stage I | Stage II | Stage III | Stage IV+V | ||
| n (M/F) | 29 (20/9) | 50 (32/18) | 71 (46/25) | 43 (29/14) | 34 (23/11) |
| Age (years) | 50.6 ± 1.4 | 51.6 ± 1.3 | 55.0 ± 0.9 | 56.1 ± 1.3 | 55.0 ± 1.7 |
| BMI (kg/m2) | 23.0 ± 0.6 | 24.7 ± 0.5 | 25.6 ± 0.5* | 24.9 ± 0.7 | 25.7 ± 0.8 |
| SBP (mmHg) | 138.4 ± 2.7 | 141.8 ± 2.8 | 145.0 ± 1.9 | 144.1 ± 3.3 | 154.7 ± 4.7**, # |
| DBP (mmHg) | 82.1 ± 1.3 | 83.6 ± 1.4 | 85.8 ± 1.0 | 84.1 ± 1.5 | 88.2 ± 2.2 |
| No. treated with ARB/ACEI (%) | 2 (6.9) | 14 (28.0)* | 24 (33.8)** | 12 (27.9)* | 8 (23.5) |
| HbA1c (NGSP,%) | 5.6 ± 0.06 | 7.3 ± 0.21*** | 7.8 ± 0.20*** | 8.1 ± 0.24*** | 8.7 ± 0.38***, ### |
| LDL‐C (mmol/L) | 3.41 ± 0.14 | 3.46 ± 0.12 | 3.42 ± 0.11 | 3.64 ± 0.13 | 3.28 ± 0.17 |
| No. treated with statins (%) | 0 (0) | 6 (12.0) | 11 (15.5)* | 4 (9.3) | 5 (14.7) |
| HDL‐C (mmol/L) | 1.72 ± 0.085 | 1.50 ± 0.057 | 1.47 ± 0.046* | 1.50 ± 0.060 | 1.53 ± 0.09 |
| Triglycerides (mmol/L) | 1.87 ± 0.28 | 1.85 ± 0.18 | 1.92 ± 0.13 | 2.05 ± 0.19 | 1.96 ± 0.22 |
| ACR (mg/gCr) | 10.7 ± 2.6 | 25.8 ± 10.0 | 67.8 ± 32.5 | 56.5 ± 19.5 | 116.1 ± 39.7 |
| eGFR (ml/min) | 82.1 ± 1.5 | 83.1 ± 3.5 | 80.2 ± 2.0 | 85.3 ± 2.5 | 85.2 ± 3.9 |
| Duration of diabetes (years) | 9.7 ± 1.2 | 9.7 ± 0.8 | 9.7 ± 1.0 | 11.4 ± 1.4 | |
ACEI, angiotensin‐converting enzyme inhibitor; ACR, urinary albumin:creatinine ratio; ARB, angiotensin receptor blocker; BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate, HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure.
Data are the means ± standard error of the mean in control participants and subgroups of type 2 diabetes stratified by the stages of diabetic neuropathy according to the criteria of the Diabetic Neuropathy Study Group in Japan11.
*P < 0.05, **P < 0.01, ***P < 0.001 compared with control participants, # P < 0.05, ### P < 0.001 compared with patients at stage I neuropathy. The difference between control participants and diabetic subgroups stratified by the severity of neuropathy was tested with analysis of variance for continuous variables, and with χ2‐tests with Bonferroni corrections for categorical variables. To standardize the glycated hemoglobin (HbA1c) values to National Glycohemoglobin Standardization Program (NGSP) units, 0.4% was added to the measured HbA1c values13.
Figure 1 shows the representative sonoelastography of the tibial nerve in a control participant (Figure 1a) and a patient with type 2 diabetes at stage III neuropathy (Figure 1b). The ratios of strain (elasticity) produced by repeated compression and decompression of the tibial nerve (0.56%, 0.27%) to that of the AC (0.66%, 0.78%) were 0.85 and 0.35 in a control participant and a diabetic patient, respectively. The CSA of the tibial nerve in a diabetic patient (8.2 mm2) was larger than that in a control participant (5.6 mm2). The average CV for the elasticity of the tibial nerve, determined on three occasions in 20 control participants, was 12.7 ± 1.0%.
The elasticity of the tibial nerve in patients without neuropathy was reduced compared with control participants, and decreased further after developing the neuropathy. The CSA of the tibial nerve in patients without diabetic neuropathy was larger than that in control participants, and became larger in proportion to the severity of neuropathy (Table 2). When neurological functions between the controls and diabetic patients with stratified neuropathy were compared (Table 2), the results in diabetic patients without neuropathy did not significantly differ from those in the control participants. After developing neuropathy, the deterioration of neurological function was related to the severity of neuropathy. The cut‐off levels of the elasticity and CSA of the tibial nerve that suggested the presence of diabetic neuropathy were 0.558 and 6.48 mm2, with a sensitivity of 0.860 and 0.620, and a specificity of 0.696 and 0.568, respectively. The AUC for the elasticity (0.829) was significantly (P < 0.0001) greater than that for the CSA (0.612) of the tibial nerve, suggesting that the evaluation of the elasticity was a better tool than the CSA of the tibial nerve for characterizing the presence of diabetic neuropathy (Figure 2).
Table 2.
Comparison of elasticity and cross‐sectional area of the tibial nerve, and neurophysiological tests between control participants and subgroups of type 2 diabetic patients stratified by the stages of diabetic neuropathy
| Control participants | Stage of diabetic neuropathy | ||||
|---|---|---|---|---|---|
| Stage I | Stage II | Stage III | Stage IV+V | ||
| Tibial nerve | |||||
| Elasticity | 0.760 ± 0.0235 | 0.655 ± 0.0146*** | 0.542 ± 0.0144 ### | 0.475 ± 0.0193 & | 0.414 ± 0.0176 |
| Cross‐sectional area (mm2) | 4.84 ± 0.16 | 6.11 ± 0.13*** | 6.20 ± 0.13 | 6.63 ± 0.14 # | 7.49 ± 0.21 $ |
| Neurophysiological test | |||||
| MCV of median nerve (m/s) | 57.7 ± 0.65 | 57.4 ± 0.56 | 53.9 ± 0.46 ### | 51.5 ± 0.52 & | 46.8 ± 0.70 $$$ |
| Amplitude of median nerve (mV) | 6.4 ± 0.59 | 5.1 ± 0.49 | 3.9 ± 0.35*** | 3.7 ± 0.37 | 3.2 ± 0.38 # |
| SCV of ulnar nerve (m/s) | 63.5 ± 0.75 | 63.2 ± 0.59 | 60.2 ± 0.52 ## | 58.1 ± 0.60 | 54.1 ± 0.82 $$$ |
| Amplitude of ulnar nerve (μV) | 28.8 ± 2.9 | 22.6 ± 2.0 | 18.8 ± 1.5** | 14.1 ± 1.4 # | 12.6 ± 2.2 ## |
| Vibration perception threshold (μ/120 c/s) | 1.59 ± 0.15 | 2.25 ± 0.25 | 3.72 ± 0.44** | 3.63 ± 0.45 | 4.48 ± 0.60 ## |
| CPT 2000 Hz (mA) | 3.00 ± 0.14 | 3.13 ± 0.14 | 3.70 ± 0.13 | 4.52 ± 0.30*** | 5.54 ± 0.38 $ |
| CPT 250 Hz (mA) | 1.36 ± 0.07 | 1.43 ± 0.08 | 1.72 ± 0.07 | 2.14 ± 0.27 # | 2.77 ± 0.39 &&& |
| CPT 5 Hz (mA) | 0.76 ± 0.05 | 0.88 ± 0.05 | 0.97 ± 0.05 | 1.43 ± 0.21* | 1.69 ± 0.33 & |
| CVR‐R (%) | 3.77 ± 0.25 | 3.84 ± 0.20 | 3.75 ± 0.20 | 3.08 ± 0.23 | 2.66 ± 0.25 && |
CPT, current perception threshold; CV, coefficient of variation; CVR‐R, coefficient of variation of R‐R intervals MCV, motor nerve conduction velocity; SCV, sensory nerve conduction velocity.
The data represent the means ± standard error of the mean in control participants and subgroups of type 2 diabetic patients stratified by the stages of diabetic neuropathy.
*P < 0.05, **P < 0.01, ***P < 0.001 compared with control participants, # P < 0.05, ## P < 0.01, ### P < 0.001 compared with patients at stage I neuropathy, & P < 0.05, && P < 0.01, &&& P < 0.001 compared with patients at stage II neuropathy, $ P < 0.05, $$$ P < 0.001 compared with patients at stage III neuropathy. The difference between control participants and diabetic subgroups was tested with analysis of variance.
Figure 2.
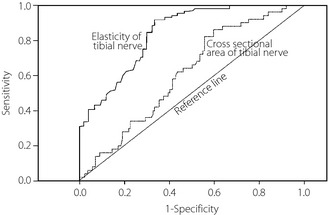
The receiver operating characteristic curve analysis establishing a cut‐off level of the elasticity and cross‐sectional area of the tibial nerve suggesting the presence of diabetic neuropathy (stages of the neuropathy; II–VI+V [n = 148] vs stage I [n = 50]). The elasticity and cross‐sectional area of the tibial nerve were determined by high‐resolution ultrasonography using an 18.0‐MHz linear array probe (HIVISION Ascendus; Hitachi Medical, Tokyo, Japan) attached with an acoustic coupler (EZU‐TECPL1; Hitachi‐Aloka Medical, Tokyo, Japan). The sensitivity and specificity were equally weighted. The area under curve of the elasticity and cross‐sectional area of the tibial nerve was compared by χ2‐test. The receiver operating characteristic analysis was carried out by Ekuserutohkei 2015 (add‐in software for Excel 2010; Social Survey Research Information Co. Ltd., Tokyo, Japan).
With respect to clinical factors, female sex was negatively associated with the CSA of the tibial nerve, and age was negatively associated with the elasticity of the tibial nerve. In addition, inverse associations were observed between the elasticity of the tibial nerve and the severity of neuropathy, CSA of the tibial nerve and 2000‐Hz CPT. The MCV of the median nerve and the SCV of the ulnar nerve were closely associated with the elasticity of the tibial nerve. In contrast, the CSA of the tibial nerve was positively associated with the severity of neuropathy and 2000‐Hz CPT, and showed an inverse association with the MCV of the median nerve and the SCV of the ulnar nerve (Table 3).
Table 3.
Relationship between either of the elasticity or cross‐sectional area of the tibial nerve and clinical factors, severity of diabetic neuropathy, cross‐sectional area of the tibial nerve or neurophysiological tests in type 2 diabetic patients
| Elasticity of tibial nerve | CSA of tibial nerve | |||
|---|---|---|---|---|
| Standardized β | P | Standardized β | P | |
| Sex | 0.044 | 0.555 | −0.188 | 0.018 |
| Age | −0.163 | 0.035 | 0.112 | 0.134 |
| Body mass index | −0.028 | 0.729 | 0.064 | 0.404 |
| Duration of diabetes mellitus | −0.044 | 0.552 | 0.061 | 0.393 |
| Diastolic blood pressure | −0.021 | 0.788 | 0.015 | 0.873 |
| HbA1c | −0.100 | 0.193 | 0.034 | 0.646 |
| Low‐density lipoprotein cholesterol | 0.010 | 0.894 | −0.097 | 0.192 |
| High‐density lipoprotein cholesterol | −0.041 | 0.618 | −0.009 | 0.905 |
| Triglycerides | −0.025 | 0.753 | 0.133 | 0.085 |
| Severity of diabetic neuropathy | −0.596 | <0.0001 | 0.306 | <0.0001 |
| Cross‐sectional area of tibial nerve | −0.234 | 0.002 | ||
| Motor nerve conduction velocity of median nerve | 0.296 | <0.0001 | −0.360 | <0.0001 |
| Amplitude of median nerve | 0.046 | 0.551 | −0.076 | 0.311 |
| Sensory nerve conduction velocity of ulnar nerve | 0.324 | <0.0001 | −0.200 | 0.006 |
| Amplitude of ulnar nerve | 0.123 | 0.110 | −0.102 | 0.168 |
| Vibration perception threshold | −0.067 | 0.391 | 0.058 | 0.437 |
| Current perception threshold 2000 Hz | −0.191 | 0.011 | 0.147 | 0.043 |
| Current perception threshold 250 Hz | −0.107 | 0.144 | 0.113 | 0.111 |
| Current perception threshold 5 Hz | −0.081 | 0.264 | 0.090 | 0.200 |
| Coefficient of variation in R‐R interval | 0.136 | 0.080 | −0.029 | 0.698 |
HbA1c, glycated hemoglobin.
Multivariate regression analysis was used to determine the independent relationship between either of the elasticity or cross‐sectional area (CSA) of the tibial nerve and clinical factors, stages of diabetic neuropathy, cross‐sectional area of the tibial nerve, or neurophysiological examinations.
The explanatory role of the severity of diabetic neuropathy for the elasticity and CSA of the tibial nerve was evaluated by partial correlation analysis examining the independent relationship between the two variables out of the elasticity and CSA of tibial nerve and the stages of diabetic neuropathy. The stages of diabetic neuropathy had an independent positive relationship with the CSA of the tibial nerve and a negative relationship with the elasticity of the tibial nerve. In contrast, there was no significant independent relationship between the CSA and elasticity of the tibial nerve (Table 4).
Table 4.
Independent relationship between the two variables out of the elasticity and cross‐sectional area of the tibial nerve and the stages of diabetic neuropathy in type 2 diabetic patients
| Control variable | |
|---|---|
| Elasticity of tibial nerve | Between CSA of tibial nerve and stages of diabetic neuropathy |
| Partial correlation coefficient 0.266 | |
| P < 0.0001 | |
| d.f. 195 | |
| CSA of tibial nerve | Between elasticity of tibial nerve and stages of diabetic neuropathy |
| Partial correlation coefficient −0.546 | |
| P < 0.0001 | |
| d.f. 195 | |
| Stages of diabetic neuropathy | Between CSA of tibial nerve and elasticity of tibial nerve |
| Partial correlation coefficient −0.051 | |
| P < 0.478 | |
| d.f. 195 |
d.f., Degrees of freedom.
Partial correlation analysis was used for studying the independent relationship between two variables out of three variables (the elasticity and cross‐sectional area [CSA] of the tibial nerve and the stages of diabetic neuropathy) after excluding the influence of the third confounding variable.
Discussion
Diabetic neuropathy is the most common complication of diabetes14, and is diagnosed based on characteristic symptoms and signs. Confirmation of the diagnosis by neurophysiological examinations has been recommended1, 2. However, these examinations do not provide information regarding the pathological or biomechanical changes of the affected nerves. Mechanical and physiological events are dynamically interdependent in the nervous system. For example, the mechanical stress to nerves evokes physiological responses, such as alteration of intraneural blood flow, impulse traffic or axonal transport15. Sustained increases in tension could adversely affect the electrophysiological properties of the nerve16.
Long‐standing hyperglycemia alters the structure of the peripheral nerves. In addition to axonal degeneration17, demyelination and subsequent remyelination18, the ischemia as a result of denatured endoneurial microvessels alters the sheaths of the peripheral nerves19. The thickening of the peri‐ and epineurial ensheathment and endoneurial fibrosis have been reported in the sural nerve of type 2 diabetic patients20. Nerve fibers are hermetically sealed between the basement membrane of endoneurial capillaries and perineurial sheaths. Therefore, changes that occur in the neural layers could influence the function of nerve fibers20. This fibrous thickening in the sheaths of the peripheral nerves might result in mechanical stress. When assessed by the quasistatic circular compression method, sciatic nerves in streptozocine‐diabetic rats were stiffer and showed decreased blood perfusion with less pressure than control rats7. The collagen diameters of end‐ and epineurial tissue of the sciatic nerve were larger in streptozocine and biobleeding diabetic rats than those in control rats21. The optic nerve heads of streptozocine‐diabetic rats, assessed by a biomaterials tester, were stiffer than those of control rats, likely as a result of non‐enzymatic collagen cross‐linking. Advanced glycation end‐products might mediate the latter process22. Boyd et al.23 reported altered biomechanics of the tibial nerve in patients with type 2 diabetes, evidenced by its reduced excursion at the ankle. They speculated that an altered elasticity of the tibial nerve could be responsible for the reduced excursion. However, to the best of our knowledge, no studies have assessed the elasticity of peripheral nerves in patients with diabetes.
High‐resolution ultrasonography of the peripheral nerves can show pathological changes with relative ease and excellent resolution. Increases in the CSA4, hypoechoic area5 and the maximum thickness of nerve fascicles6 of the peripheral nerves have been observed in patients with diabetic neuropathy. The in situ elasticity of soft tissue can be evaluated by sonoelastography using ultrasound. This is the basis of elastography, in which a region of tissue is compressed, and the degree to which it distorts (known as strain) is assessed. For achieving this aim, ultrasound has advantages over other imaging methods in terms of resolution in space and time, and safety. Therefore, it has emerged as the dominant technique in elastography24. With its semiquantitative values for strain ratios, sonoelastography has shown promising results in the diagnosis of mass lesions in various organs8, 9, 25, 26, 27. However, very few studies have been carried out to assess the elasticity of the peripheral nerves. Using the AC:median nerve strain ratio, Miyamoto et al.10 recently reported that the median nerve was stiffer in patients with carpal tunnel syndrome than that in healthy subjects. Furthermore, ROC analysis of the elasticity of the median nerve improved diagnostic accuracy for carpal tunnel syndrome compared with the CSA of the median nerve alone. However, as the authors did not carry out neurophysiological tests, the influence of increased nerve stiffness on neurological functions has not been clarified. Shear wave elastography showed that the stiffness of the median nerve in patients with carpal tunnel syndrome increases with the severity of the disease28.
The present study determined the elasticity (stiffness) of the tibial nerve by the tibial nerve:AC strain ratio in 198 patients with type 2 diabetes, subgrouped by the neuropathy severity, and control participants whose age and sex ratio did not differ from diabetic subgroups. The reproducibility of the tibial nerve: AC strain ratio in control participants was good (CV; 12.7 ± 1.0%). The tibial nerve was stiffer in patients without neuropathy than in control participants, and further deterioration of the elasticity was closely associated with the severity of neuropathy. The tentative cut‐off level of the elasticity of the tibial nerve suggesting the presence of the diabetic neuropathy, its sensitivity, and specificity assessed using an ROC curve were 0.558, 0.860 and 0.696, respectively. As the nerve functions in diabetic patients without neuropathy were similar to those in control participants, the stiffening of the peripheral nerves in type 2 diabetes occurred before the onset of neurophysiological anomalies. Therefore, the determination of the elasticity of peripheral nerves facilitates the characterization of diabetic neuropathy in the early stage, from a biomechanical perspective, with high sensitivity and modest specificity. The elasticity of the tibial nerve was negatively associated with the CSA of the tibial nerve, severity of neuropathy and 2000‐Hz CPT, and positively associated with the MCV and SCV in diabetic patients. In contrast, the CSA of the tibial nerve was positively associated with the severity of neuropathy and the 2000‐Hz CPT, and inversely associated with the MCV and SCV. These results suggest that biomechanical changes in the peripheral nerves might be relevant to dysfunction and the expansion of the CSA of the peripheral nerves in diabetic neuropathy. In contrast, there was no significant independent relationship between the CSA and elasticity of the tibial nerve. Therefore, the inverse association between the elasticity and CSA of the tibial nerve might be confounded by the influence of the neuropathy severity on them. Because the AUC for the elasticity (0.829) was significantly (P < 0.0001) greater than that for the CSA (0.612) of the tibial nerve, the evaluation of the elasticity might be a better tool than the CSA of the tibial nerve for biomechanical characterization of diabetic neuropathy. Furthermore, the elasticity of the tibial nerve had a better partial correlation coefficient (−0.546) than that of CSA (0.266) for the severity of diabetic neuropathy. Therefore, the elasticity of the tivial nerve might be more representative of the severity of neuropathy compared with the CSA of the tibial nerve.
We acknowledge a number of limitations to the present study, which could affect the interpretation of the results. First, although we measured the elasticity of the tibial nerve semiquantitatively as the tibial nerve:AC strain ratio, several quantitative methods (shear wave elastography, transient elastography and acoustic force elastography) are available29, 30, 31. However, these three techniques were not suitable for the present purpose, as they are either unsuitable for the depiction of anatomical images or only available for regional measurement with limited depth. Second, we measured the elasticity of the tibial nerve, but did not measure that of other peripheral nerves, such as the median nerve. The median nerve is subject to compression as a result of frequent dorsiflexion while using a computer mouse, and the flexor tendons prevent the smooth compression of the median nerve by a probe. Diabetic neuropathy is a length‐dependent disease, and we chose to examine the tibial nerve, which is located in the lower extremities. However, the increased stiffness in multiple peripheral nerves should be confirmed using sonoelastography to establish the reduced elasticity in peripheral nerves as a robust biomechanical marker for diabetic neuropathy. Third, because we associated the elasticity of nerves in the lower extremities (tibial nerve) with nerve conduction velocity in the upper extremities, the elasticity and the conduction velocity of the tibial nerve should be associated. However, the examination of the conduction velocity of the tibial nerve (lower extremity) might require technical experience to some extent compared with that of nerves in the upper extremities. Therefore, we measured the conduction velocity of nerves in the upper extremities. In light of this, the close relationship between the elasticity of the tibial nerve and nerve conduction velocity might be tentative. In contrast, the 2000‐Hz CPT, determined at an area innervated by a branch of the tibial nerve (medial plantar nerve), was negatively associated with the elasticity of the tibial nerve. Therefore, the stiffness of the tibial nerve, determined using sonoelastography, could be relevant to dysfunction of the tibial nerve. However, the present imaging study did not directly show the consequence of reduced elasticity of peripheral nerves. We could not rule out the possibility that the reduced elasticity of the tibial nerve was coincidental. A prospective study showing changes in elasticity and function of peripheral nerves before and after long‐term glycemic control would confirm the findings of the present study.
Finally, because nerve biopsies were not carried out, we could not identify the histological findings responsible for the increased stiffness of the tibial nerve.
In conclusion, the tibial nerve in patients with type 2 diabetes was stiffer than that in control participants before the onset of diabetic neuropathy, and deteriorated in proportion to the severity of neuropathy. The reduced elasticity of the peripheral nerves could be an early biomechanical change in diabetic neuropathy in patients with type 2 diabetes.
Disclosure
The authors declare no conflicts of interest.
Acknowledgments
The present study received no financial support.
J Diabetes Investig 2016; 7: 404–412
References
- 1. Dyck PJ, Albers JW, Andersen H, et al Diabetic polyneuropathies: update on research definition, diagnostic criteria and estimation of severity. Diabetes Metab Res Rev 2011; 27: 620–628. [DOI] [PubMed] [Google Scholar]
- 2. England JD, Gronseth GS, Franklin G, et al Distal symmetric polyneuropathy: a definition for clinical research: report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2005; 64: 199–207. [DOI] [PubMed] [Google Scholar]
- 3. Malik RA, Tesfaye S, Newrick PG, et al Sural nerve pathology in diabetic patients with minimal but progressive neuropathy. Diabetologia 2005; 48: 578–585. [DOI] [PubMed] [Google Scholar]
- 4. Riazi S, Bril V, Perkins BA, et al Can ultrasound of the tibial nerve detect diabetic peripheral neuropathy? A cross‐sectional study. Diabetes Care 2012; 35: 2575–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Watanabe T, Ito H, Sekine A, et al Sonographic evaluation of the peripheral nerve in diabetic patients. The relationship between nerve conduction studies, echo intensity, and cross‐sectional area. J Ultrasound Med 2010; 29: 697–708. [DOI] [PubMed] [Google Scholar]
- 6. Liu F, Zhu J, Wei M, et al Preliminary evaluation of the sural nerve using 22‐MHz ultrasound: a new approach for evaluation of diabetic cutaneous neuropathy. PLoS ONE 2012; 7: e32730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen RJ, Lin CCK, Ju MS. In situ biomechanical properties of normal and diabetic nerves: an efficient quasi‐linear viscoelastic approach. J Biomech 2010; 43: 1118–1124. [DOI] [PubMed] [Google Scholar]
- 8. Onur MR, Poyraz AK, Ucak EE, et al Semiquantitative strain elastography of liver masses. J Ultrasound Med 2012; 31: 1061–1067. [DOI] [PubMed] [Google Scholar]
- 9. Fischer T, Peisker U, Fiedor S, et al Significant differentiation of focal breast lesions: raw data‐based calculation of strain ratio. Ultraschall Med 2012; 33: 372–379. [DOI] [PubMed] [Google Scholar]
- 10. Miyamoto H, Halpern EJ, Kastlunger M, et al Carpal tunnel syndrome: diagnosis by means of median nerve elasticity‐improved diagnostic accuracy of US with sonoelastography. Radiology 2014; 270: 481–486. [DOI] [PubMed] [Google Scholar]
- 11. Yasuda H, Sanada M, Kitada K, et al Rationale and usefulness of newly devised abbreviated diagnostic criteria and staging for diabetic polyneuropathy. Diabetes Res Clin Pract 2007; 77(Suppl. 1): S178–S183. [DOI] [PubMed] [Google Scholar]
- 12. Ishibashi F, Okino M, Ishibashi M, et al Corneal nerve fiber pathology in Japanese type 1 diabetic patients and its correlation with antecedent glycemic control and blood pressure. J Diabetes Investig 2012; 3: 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kashiwagi A, Kasuga M, Araki E, et al International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig 2012; 3: 39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tesfaye S, Selvarajah D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes Metab Res Rev 2012; 28(Suppl. 1): 8–14. [DOI] [PubMed] [Google Scholar]
- 15. Shacklock M. Neurodynamics. Physiotherapy 1995; 81: 9–16. [Google Scholar]
- 16. Kwan MK, Wall EJ, Massie J, et al Strain, stress and stretch of peripheral nerve. Rabbit experiments in vitro and in vivo . Acta Orthop Scand 1992; 63: 267–272. [DOI] [PubMed] [Google Scholar]
- 17. Yagihashi S, Matsunaga M. Ultrastructural pathology of peripheral nerves in patients with diabetic neuropathy. Tohoku J Exp Med 1979; 129: 357–366. [DOI] [PubMed] [Google Scholar]
- 18. Ballin RH, Thomas PK. Hypertrophic changes in diabetic neuropathy. Acta Neuropathol 1968; 11: 93–102. [DOI] [PubMed] [Google Scholar]
- 19. Dyck PJ, Giannini C. Pathologic alterations in the diabetic neuropathies of humans: a review. J Neuropathol Exp Neurol 1996; 55: 1181–1193. [DOI] [PubMed] [Google Scholar]
- 20. Kundalić B, Ugrenović S, Jovanović I, et al Morphometric analysis of connective tissue sheaths of sural nerve in diabetic and nondiabetic patients. BioMed Res Int 2014; 2014; Article ID 870930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang H, Layton BE, Sastry AM. Nerve collagens from diabetic and nondiabetic Sprague‐Dawley and biobreeding rats: an atomic force microscopy study. Diabetes Metab Res Rev 2003; 19: 288–298. [DOI] [PubMed] [Google Scholar]
- 22. Terai N, Spoerl E, Haustein M, et al Diabetes mellitus affects biomechanical properties of the optic nerve head in the rat. Ophthalmic Res 2012; 47: 189–194. [DOI] [PubMed] [Google Scholar]
- 23. Boyd BS, Dilley A. Altered tibial nerve biomechanics in patients with diabetes mellitus. Muscle Nerve 2014; 50: 216–223. [DOI] [PubMed] [Google Scholar]
- 24. Cui XW, Friedrich‐Rust M, De Molo C, et al Liver elastography, comments on EFSUMB elastography guidelines 2013. World J Gastroenterol 2013; 19: 6329–6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee TH, Cho YD, Cha SW, et al Endoscopic ultrasound elastography for the pancreas in Korea: a preliminary single center study. Clin Endosc 2013; 46: 172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Salomon G, Drews N, Autier P, et al Incremental detection rate of prostate cancer by real‐time elastography targeted biopsies in combination with a conventional 10‐core biopsy in 1024 consecutive patients. BJU Int 2014; 113: 548–553. [DOI] [PubMed] [Google Scholar]
- 27. Kwak JY, Kim EK. Ultrasound elastography for thyroid nodules: recent advances. Ultrasonography 2014; 33: 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kantarci F, Ustabasioglu FE, Delil S, et al Median nerve stiffness measurement by shear wave elastography: a potential sonographic method in the diagnosis of carpal tunnel syndrome. Eur Radiol 2014; 24: 434–440. [DOI] [PubMed] [Google Scholar]
- 29. Parker KJ, Fu D, Graceswki SM, et al Vibration sonoelastography and the detectability of lesions. Ultrasound Med Biol 1998; 24: 1437–1447. [DOI] [PubMed] [Google Scholar]
- 30. Sandrin L, Catheline S, Tanter M, et al Time‐resolved pulsed elastography with ultrafast ultrasonic imaging. Ultrason Imaging 1999; 21: 259–272. [DOI] [PubMed] [Google Scholar]
- 31. Walker WF. Internal deformation of a uniform elastic solid by acoustic radiation force. J Acoust Soc Am 1999; 105: 2508–2518. [DOI] [PubMed] [Google Scholar]


