ABSTRACT
Menin is encoded by the MEN1 gene, which is mutated in an inherited human syndrome, multiple endocrine neoplasia type 1(MEN1). Menin is primarily nuclear protein, acting as a tumor suppressor in endocrine organs, but as an oncogenic factor in the mixed lineage leukemia, in a tissue-specific manner. Recently, the crystal structures of menin with different binding partners reveal menin as a key scaffold protein that functionally interacts with various partners to regulate gene transcription in the nucleus. However, outside the nucleus, menin also regulates multiple signaling pathways that traverse the cell surface membrane. The precise nature regarding to how menin associates with the membrane fraction is poorly understood. Here we show that a small fraction of menin associates with the cell membrane fraction likely via serine palmitoylation. Moreover, the majority of the membrane-associated menin may reside inside membrane vesicles, as menin is protected from trypsin-mediated proteolysis, but disruption of the membrane fraction using detergent abolishes the detection. Consistently, cellular staining for menin also reveals the distribution of menin in the cell membrane and the punctate-like cell organelles. Our findings suggest that part of intracellular menin associates with the cell membrane peripherally as well as resides within the membrane vesicles.
KEYWORDS: Cell membrane, menin, palmitoylation, secretory pathway
Introduction
Menin is encoded by the MEN1 gene, which is mutated in human inherited syndrome, multiple endocrine neoplasia type 1 (MEN1)1-3 Menin is primarily nuclear protein, acting as a tumor suppressor in endocrine organs, but as an oncogenic factor in the mixed lineage leukemia, in a tissue-specific manner.4-6 Menin possesses these dichotomous functions suggesting that it positively and negatively regulates gene expression in a cell or tissue dependent manner. It also interacts with a multitude of proteins with diverse functions. Recently, the crystal structures of menin with different binding partners reveal that menin is a key scaffold protein that functionally crosstalks with various partners to regulate gene transcription in the nucleus.7,8
Menin also regulates the interplay with multiple signaling pathways that traverse the cell surface membrane.9 While we and others have shown that menin is also detectable in cell membrane fraction,10 precise nature regarding to how menin associates with membrane is poorly understood. Understanding of how menin associates with the cell membrane compartment will likely provide new insights into how menin can regulate various cell membrane-traversing signaling pathways. Here we showed that a small fraction of menin associates with the cell membrane fraction likely via serine palmitoylation. Moreover, the majority of the membrane-associating menin may reside inside membrane vesicles, as menin is protected from trypsin-mediated proteolysis, but disruption of the membrane fraction using detergent abolished the resistance to protease digestion. Consistently, cellular staining for menin also reveals the distribution of menin in the cell membrane and the punctate-like cell organelles.
Results
Menin localization in the cell membrane fraction
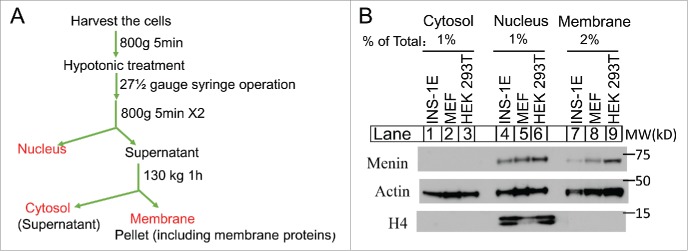
To determine whether and/or how much menin protein is localized in the cell membrane fraction, we cultured INS-1E, a derivative of insulin-secreting rat insulinoma cell line,11 a mouse embryonic fibroblast (MEF) cell line that behaves like endocrine cells in response to menin expression,12 and human embryonic kidney (HEK) 293 T cells. The cells were harvested and lysed using hypotonic buffer, and the membrane fraction, including the cell plasma membranes and membranes from other organelles, such as endoplasmic reticulum (ER), Golgi apparatus, and mitochondria, were collected by ultracentrifugation (Fig. 1A). Various cell fractions from the 3 cell lines, including the cytosol fraction post-nucleus/membrane (Fig. 1B, lane 1-3), the nuclear fractions (lanes 4-5), and the membrane fractions (lanes 7-9), were separated on SDS-PAGE, and then probed for the presence of menin in each of the fractions using an anti-menin antibody by Western blot analysis. We found that menin is barely detectable in the cytosol in the 3 cell lines (Fig. 1B, lanes 1-3, top). As expected, menin was easily detectable in the nuclear fractions in each of the 3 cell lines (Fig. 1B, lanes 4-5). Notably, menin was also detected in the membrane fraction of the 3 cell lines (Fig. 1B, lane 7-8), with menin in the membrane fraction of HEK293T cells accounting for approximately 25% of the nuclear menin (lane 9 vs 6). As a control, comparable amount of actin in each of the 3 fractions among the 3 cell lines was detected, and also nuclear protein Histone H4 was only detectable in the nuclear fraction.(lanes 4-6).
Figure 1.
Menin is localized to the cell membrane. (A). A Schematic diagram of the separation of cytosol, nucleus and membrane components. (B). Immunoblot analysis of the extraction of cytosol, nucleus and membrane fractions from the INS-1E, MEF and HEK 293T cell lines.
A fraction of menin is labeled with palmitic acid in cells
As a substantial amount of menin associates with the membrane fraction, we determined whether there was any lipid modification of the menin protein. To this end, we purified the TAP-tagged menin in β HC9 cells that expressed the tagged protein,13 and subjected the purified menin for mass spectrometry analysis. We found that there were 3 peptides from menin showing the potential palmitic modifications, based on fragment ions mass shifts in MS/MS of the peptides (Fig. 2A). There were modifications in serine/threonine, but not in more commonly observed cysteine palmitoylation, i.e., S593, S427/T429, S543/S555.(Fig. 2A)
Figure 2.
Menin is partly modificated by palmitoylation. (A). Mass spectrometry analysis of Menin protein transfected in HEK293T cells. (B). Schematic diagram of palmitoylation detection. (C). Immunoblot analysis of palmitoylated Menin by biotin-IP as shown in B, bands highlighted with a star were potential degraded proteins or crossactive.
To further determine whether part of menin can be modified by lipids such as palmitic acid in cells, we cultured 293T cells with azide-labeled palmitic acid.14 Then the labeled cells were processed and subjected to biotin alkyne in vitro, which can biorthogonal-chemically react with azide.15,16 The processed cell lysates, together with the control lysate without treatment with the azide palmitic acid, were used for binding to streptavidin beads, which bind specifically to biotinylated proteins (Fig. 2B). The precipitated proteins were separated on SDS-PAGE, and probed with the anti-menin antibody for Western blot analysis. We found that treatment of the cells with azide palmitic acid, but not the control, led to detection of a very small amount of menin from the treated cell lysate (Fig. 2C, lane 4). As a control, the cells not treated with azide-labeled palmitic acid failed to show any detection of menin in the biotinylated cell lysate (Fig. 2C, lane 3), indicating the specific pulldown of the biotinylated palmitic acid.
To determine the biochemical nature of menin association with the membrane fraction, we isolated the post-nuclear membrane fraction, and treated it with or without hydroxylamine, which acts as a reducing reagent for fatty acids such as palmitic acid.17 The control or hydroxylamine treated post-nuclear fractions were further centrifuged to isolate the membrane fraction and the post-membrane cytosol, and the membrane associating menin and the wash-off menin in the cytosol (Fig. 3A) were further analyzed with an anti-menin antibody in Western blot analysis. The result indicated that hydroxylamine treatment did not change overall amount of menin in the membrane fraction (Fig. 3B, lane 2, top), but washed off a very small amount of menin from the post-nuclear membrane fraction (lane 2, bottom 2 panels). These results suggest that only a very small amount of palmitoylated menin, either through cysteine or serine/threonine, exists in the membrane fractions.
Figure 3.
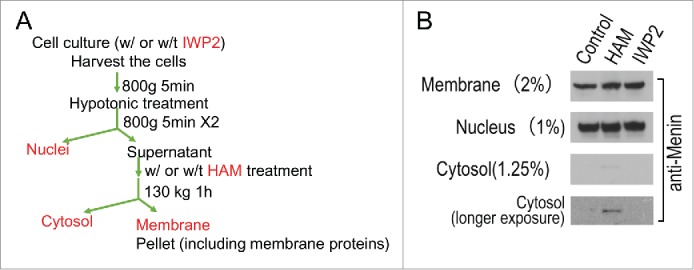
Menin was partially washed off from the membrane fraction by hydroxylamine (HAM), but a serine palmitoylation inhibitor (IWP2) failed to influence the distribution of Menin in the membrane fraction. (A). A diagram of experimental procedures of biochemical fractionation of cells, including treatment with or without IWP2, or with or without HAM. (B). Immunoblot analysis of Menin in the cytosol, nuclear and membrane fractions.
As our data suggest that menin may be partly modified by palmitic acid via serine or threonine residues, and it has been reported that the serine palmitoylation of Wnt could regulate its signaling and secretion,18,19 we treated 293T cells with IWP2, an inhibitor to the serine palmitoyl transferase of Wnt, followed by detection of the potential change of the membrane association of menin. The result indicated that treatment of the cells with IWP2 did not result in any change in the amount of menin in the membrane, nucleus, and cytosol (Fig. 3B, lane 3). These results indicate that IWP2-insensitive palmitoyl transferase might be involved in modifying menin, or majority of menin palmitoylation was not caused by serine-mediated palmitoylation.
Menin is likely localized in the lumen of the membrane vesicle
That neither hydroxylamine nor IWP2 could release the majority of the membrane-associating menin from the membrane prompted us to explore whether menin also associated with the membrane fraction via additional modes. To address this question, we examined whether treatment of the membrane fraction with high pH solution such as sodium bicarbonate, which can break the membrane vesicle integrity, but still leave the lipid bi-layers intact,20 can affect the detection of menin in the cell membrane faction. As such, we treated post-nuclear membrane pellets (Fig. 4A) with various conditions such sodium bicarbonate (pH11) and/or hydroxylamine, and then analyzed the membrane-associated or membrane-detached menin using Western blot analysis. We found that hydroxylamine washed off a very small amount of menin from the membrane fraction (lane 2 vs. lane 6), as previously described. Notably, sodium bicarbonate washed off a substantial amount of menin from the membrane fraction (lane 3 vs lane 6). The membrane fraction was free from nuclear contamination as no nuclear histone H4 was detected in the Western blot analysis (data not shown). Collectively, these results strongly suggest that vast majority of the membrane associated menin is localized inside the membrane vesicles.
Figure 4.
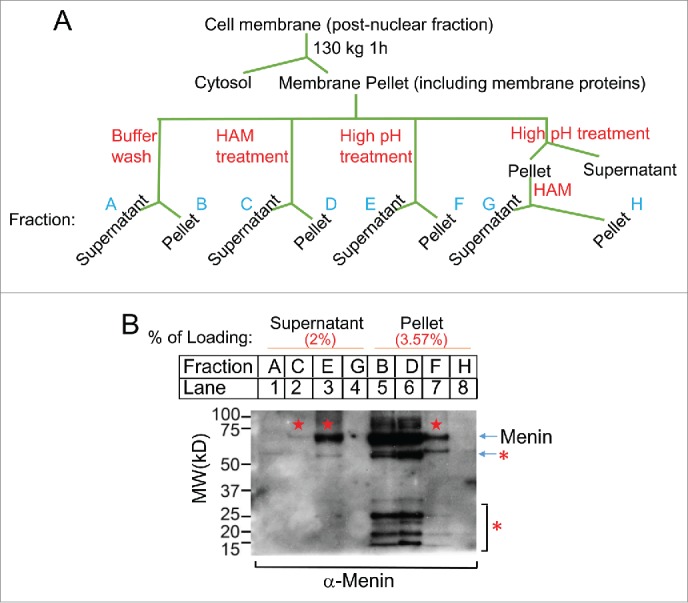
Biochemical detetion of the Menin localization. (A). A diagram for experimental procedures of biochemical cell fractionation. (B). Immunoblot analysis of Menin extracted from post-muclear fraction as shown in Figure. 4A. Pentagram denotes menin. The bands highlighted with a star were potential degraded menin or crossactive proteins.
Membrane-associated menin is protected from trypsin digestion, but the protection is abolished when the membrane integrity is compromised by Triton X-100
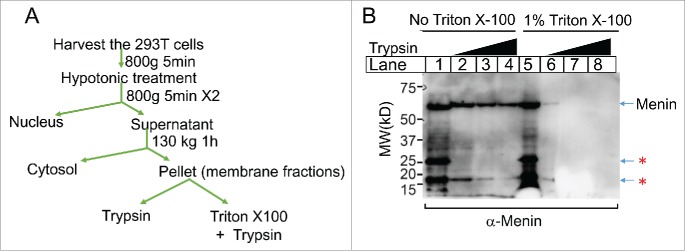
If part of menin is localized inside the membrane vesicle, then menin should be protected from proteolysis by outside protease. Indeed, when the membrane fraction was treated with an increasing concentrations of trypsin (Fig. 5A), the full-length menin remained detectable even in the presence of highest trypsin (Fig. 5B, lane 4). In contrast, when the membrane fraction was treated with Triton X-100, a detergent that breaks cell membranes by forming membrane/detergent micelles, even the lowest concentration of trypsin almost completely destroyed the menin protein (lane 6). Together, these results strongly suggest that the majority of membrane-associated menin resides inside the membrane vesicles.
Figure 5.
Menin in the membrane fraction is largely protected from digestion by trypsin. A. diagram for experimental procedures of the extraction of membrane-associated Menin and the digestion by trypsin with or without Triton X-100. B. Immunoblot analysis of Menin after the treatment of Trypsin with of without Triton X-100. Bands highlighted with a star were potential degraded proteins or crossactive.
Glycosylation signals on the menin surface fail to induce menin glycosylation in cellular organelles
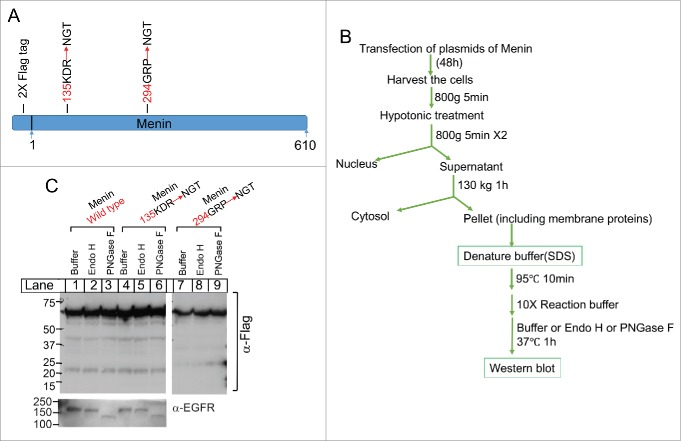
We showed that menin was protected from trypsin digestion in the membrane fraction. Generally, proteins in ER or Golgi apparatus, i.e., proteins in the membrane organelles and secretory pathway, are often glycosylated with various types of glycosylation, at the NGT or NGS site.21,22 To determine whether menin is located inside the ER or Golgi apparatus, and thus can be modified by glycosylation, we mutated triple amino acid resides on the surface of menin protein, based on the crystal structure of menin,8 i.e. 135KDR-NGT and 294GRP-NGT, in the menin cDNA sequence using site-directed mutagenesis (Fig. 6A). The resulting wild type or mutant menin cDNA in a mammalian expression vector were transduced to 293T cells, and the membrane fractions from the cells were isolated (Fig. 6B). The membrane fractions were digested with Endo H, which cleaves certain sugar molecules from the proteins in ER that have not yet trafficked to Golgi apparatus,22,23 leading to reduction of the molecule size on SDS-PAGE. On the other hand, the membrane fractions were also digested with PNGase F, which removes all the carbohydrate molecules on the proteins in either ER or Golgi complex,24 usually substantially reducing the protein size on gel. We found that the molecule size of wild type menin was not changed following digestion with either Endo H or PNGase F (Fig. 6C, lanes 2-3 vs 1). Similarly, incorporation of NGT glycosylation on the surface of menin, 135KDR-NGT (lanes 4-5), and 294GRP-NGT (lanes 7-9), did not change the size of menin, nor did Endo H and PNGase reduced the menin size on the gel. In contrast, as expected, PNGase substantially reduced the size of epidermal growth factor receptor (EGFR), a glycosylated type I transmembrane protein with the extracellular domain residing inside the lumen of ER and Golgi complex (Fig. 6C, lanes 3 and 6). These results indicate that either the ectopically introduced NGT in ER or Golgi complex failed to be glycosylated, or alternatively, menin localizes inside the membrane compartment without exposure to the glucose transferases. Further, it is also possible that membrane-associating menin is not localized inside the membrane fraction in a conventional way.
Figure 6.
Analysis of potential glycosylation of Menin. (A). A schematic diagram of the introduction of glycosylation sites on the surface of the menin protein. These sites were the loop regions on the surface base on the crystal structure of Menin. (B). A diagram for experimental procedures of cell fractionation and the treatment of the membrane fraction with various glycosidases. C. Immunoblot analysis of the potential size change of Menin after the treatment of glycosidases. EGFR was used as a positive control for glycosylase activity (bottom).
Immunofluorescence staining shows menin localization in the cell membrane compartment
To further determine whether we can observe menin localization inside membrane organelles, we developed a menin-specific antibody that specifically targets the C-terminal part of menin.25 We performed immunofluorescence staining of mouse insulinoma cell line, TGP61,26 and found that besides staining the nucleus with multiple foci, the menin antibody also stained the cellular membrane (Fig. 7A). Moreover, between the strongly stained nucleus and the cell membranes, there was also multiple fine dot-like staining, likely reflecting the membrane organelles such as ER and Golgi complex. As control, cells stained with control antibody (IgG) failed to show membrane staining. These results are consistent with the notion that part of menin associates with the membrane compartment.
Figure 7.
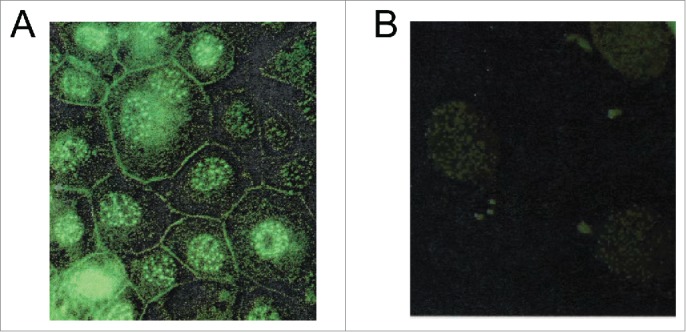
Immunofluorescence detection of Menin localization. (A and B). A Representative example of immunofluorescence staining for Menin in mouse insulinoma cell line TGP61 with the anti-menin antibody (Ab80) (A) or with IgG as a control (B).
Discussion
Although menin is primarily a nuclear protein,27 interacting with multiple nuclear proteins such as Smad protein, JunD, NFkB, FancD2, and MLL1/2,25,28-30 it is also detectable in cell membrane fraction.10 However, little is known as to how menin associates with the cell membrane fraction. Coupled with the fact that menin participates in various cell membrane-related functions such as regulating Ras signaling on the cell surface.31 and cell migration,13 it is important to investigate how menin associates with the cell membranes.
Our current studies show that, biochemically, menin can associate with the isolated cell membrane fraction through at least 2 ways. First, based on mass spectrometry analysis, we found that the palmitoylated signal from several menin peptides (Fig. 2A). Consistently, in vivo labeling of cellular proteins palmitic acid led to detection of the palmitoylated menin (Fig. 2C). However, only a very small fraction of the menin was modified by palmitic acid. Moreover, the hydroxylamine, which can reduce and remove palmitic group from the modified protein also released a fraction of the membrane-associated menin (Fig. 3). These results suggest that only a very small percentage of the membrane-associating menin is modified by palmitic acid, if any. We attempted to identify the potential palmitoylation site in menin based on the data of mass spectrometry analysis, and mutated the corresponding sites from serine to alanine, and transfected the wild type and the mutants into 293 cells. However, unexpectedly, these menin mutants show strong instability (data not shown), hindering further analysis of the potential palmitoylation sites. These results suggest that either the modification of these sites is crucial for menin stability, or the mutations led to instability of menin. Consistent with the latter case, it has been reported that mutation of one of the site, Ser555, led to menin instability and increased ubiquitin-mediated proteolysis.32,33
Second, we found that the majority of the membrane-associated menin was released from the isolated membrane by high PH sodium bicarbonate washing, suggesting that menin is localized within the membrane vesicles (Fig. 4). Supporting this notion, the full-length menin was largely protected from protease trypsin-mediated proteolysis, but disruption of the cell membrane by detergent Triton X-100 rendered completion digestion of menin by Trypsin (Fig. 5). Further, immunofluorescence staining for menin using affinity-purified menin also revealed that, besides localization in the nucleus, menin staining is also found on the cell surface membrane as well as certain punctate-like structure (Fig. 7A), likely reflecting certain membrane organelles such as ER or the Golgi apparatus. Consistent with our observation, Debelenko et al recently reported that menin is also detected within the secretory vesicles in β cells using electron microscopy.10 It is also possible that palmitoylated menin binds other molecules or undergoes other chemical modifications.
However, our further investigation by adding the potential glycosylation sites on the surface of menin failed to show that menin can be glycosylated in the ER or Golgi apparatus (Fig. 6). This may reflect the technical problem that the glycosylation sites were not placed correctly on the menin protein, or multiple sites are necessary to be placed on menin for the detectable glycosylation in the secretory pathway. Nevertheless, failure to observe glycosylation of the NGT-containing menin, coupled with the factor there is not signal peptide in the N-terminus of menin that is present in most of the secretory protein, raises the possibility that alternative mode of menin association with the cell membrane may also exist. Overall, our findings reveal that menin definitely associates with cell membrane via various biochemical analysis.
In addition, it is formally possible that menin goes through the secreted pathway (ER and Golgi), and becomes secreted, either in full-length protein or processed fragments or peptides, and then functions as a signal regulating extracellular functions. In this regard, we explored whether menin can be partly secreted in the cell culture medium or in bovine fetal serum. We analyzed the cell culture medium and serum by Western blot using the anti-menin antibody, however, we failed to detect the protein with size similar to menin using this approach.
It is natural to question the functional implications of menin membrane localization. In this regard, it is possible that menin association provides an advantage and efficiency in regulating various signaling pathways associating with the cell plasma membrane. For instance, menin represses Ras signaling, likely partly via regulating SOS,31 and it is conceivable that the membrane associating menin will be more effective to regulate membrane-associating SOS, a GTP exchange factor. The other example is menin binding to IQGAP1 and Rac1.13 Moreover, menin was also reported to suppress phosphorylation and activation of AKT, a membrane associated and myristoylated protein kinase,34 bringing menin within proximity of its target. Second, menin residing inside the secretory pathway, i.e., ER and the Golgi apparatus can either facilitate protein folding or secretion of other hormones such insulin. Consistently, menin is recently found to reside within the insulin-containing secretory vesicles.10 However, our inability at this stage to definitively identify the palmitoylation sites and to show its glycosylation in the secretory hampers our functional analysis of the 2 modes of menin association with the cell compartment.
It is clear that menin is primarily a nuclear protein and regulates expression of multiple targets in a tissue specific manner.2,9 Only recently has attention been drawn to its association with the membrane fraction. It is also important in the future to understand how the nuclear menin and menin in the membrane compartment are coordinated to regulate its diverse functions.
Materials and methods
The following antibodies and reagents were purchased from the indicated companies and used for the described studies: rabbit anti-Menin Antibody (A300-105A, Bethyl laboratories.inc); monoclonal anti-FLAG® M2 antibody produced in mice (F1804 SIGMA); Anti-Histone H4 antibody (ab177840, Abcam); monoclonal Anti-β-Actin antibody produced in mice (A5441 SIGMA); endo H (P0702S, NEB), PNGase F (P0704S, NEB), Click-IT® Palmitic Acid, Azide (15-Azidopentadecanoic Acid) (C10265, Life technologies); biotin Alkyne (PEG4 carboxamide-Propargyl Biotin) (B10185, Life technologies); Pierce™ Streptavidin Agarose (20353, Life technologies); Trypsin (T1426, Sigma) Soybean protease inhibitor (17075-029, Life technologies); QuikChange II XL Site-Directed Mutagenesis Kit (200521, Agilent Technology).
Cell culture and transfection
HEK 293T cells and Mouse embryonic fibroblasts (MEFs) were grown in 5% CO2 at 37°C in DMEM supplemented with 100 units/mL penicillin, 100 ug/mL streptomycin, and 10% (v/v) FBS. Rat INS-1E cells were grown in 5% CO2 at 37℃ in RPMI1640 supplemented with 100 units/mL penicillin, 100 ug/mL streptomycin, 10% (v/v) FBS, 10mM HEPES, 2mM L-Glutamine, 1mM pyruvate sodium, and 50uM b-Mercaptoethanol. HEK 293T cells were transfected with Calcium Phosphate precipitation method.(Molecular Clone 8th).
Cell fractionation of nucleus, cytosol, membrane
As shown in Fig. 1A, cells were harvested from the cell culture plates, followed by centrifugation at 800 g x 5 min in 4℃. The cell pellets were treated with hypotonic buffer (10 mM Hepes-KOH, pH 7.4, 10 mM KCl, 1.5 mM MgCl, 0.5mM EDTA, 0.5 mM EGTA) for 15 min on ice, then passed through a #27 ½ gauge syringe attached to a 5 ml syringe for 15 times. The resulting cell lysates were centrifuged at 800 g at 4℃ for 5 min. The pellets were mainly nucleus. The post-nuclear fraction at the supernatant was further centrifuged at 130,000 g at 4 ℃ for 60 min. The resulting supernatant contained cytosol, and the pellet was mainly cell membrane containing the plasma membranes, microsome membrane, Golgi apparatus, as well as mitochondrion membranes.
Mass spectrometry analysis of Menin modification
The purification of menin and further mass spectrometry analysis was carried out as previously described from our laboratory.13 Briefly, Men1-TAP cells were lysed in cell lysis buffer (250mM NaCl, 1mM MgCl2, 1mM CaCl2, 50mM Tris–HCl at pH 8.0, 0.5% NP-40, 5% glycerol, 2X ethylenediaminetetraacetic acid (EDTA)-complete protease inhibitor cocktail (Roche, Mannheim, Germany). The purification was performed as recommended by the manufacturer (InterPlay C-Terminal Mammalian TAP System Kit, Stratagene). The purified proteins were separated in the NuPAGE 4–12% Bis-Tris gel and visualized by silver stain (Silver Staining kit; Invitrogen). Menin (~70 kD) was excised and analyzed by mass spectrometry.
Biochemical analysis of Menin localization
As shown in Fig. 3A and 4A, HEK 293T cells were treated with or without 2.5uM of IWP2, which could inhibit the activity of porcupine, an O-palmitoyltransferase enzyme that catalyzes the palmitoylation of Wnt. Post-nuclear fraction was treated with 1M of hydroxylamine (HAM) for 1h at room temperature, which was a reducing reagent that could remove the palmitoyl group from protein. Alternatively, the post-nuclear fraction was treated under the high pH condition (Na2CO3 solution, pH 11), which could disrupt sealed membrane compartments without solubilizing or denaturing the lipid bilayer to reserve the membrane sheets but release the inner contents.
Trypsin proteolysis experiments
As shown in Fig. 5A, post-nuclear membrane fraction of HEK 293T cells were centrifuged at 130k g at 4°C for 60 min. The resulting pellet was re-suspended with hypotonic buffer and then divided into 8 aliquots. The aliquots were incubated with increasing concentrations (units/ml; 8.2, 16.4 and 32.8) of trypsin in the absence or presence of 1% (v/v) Triton X-100, followed by the incubation of 1 hour at 37°C. The proteolysis was stopped by addition of the soybean trypsin inhibitor (300 unit) to terminate the digestion, followed by separation with SDS-PAGE and immunoblotting.
Determining glycosidase sensitivity
As shown in Fig. 6A, post-nuclear membrane fraction of HEK 293T cells was centrifuged at 130k g at 4°C for 60 min. The resulting pellet was treated with carbohydrate-removing enzymes as recommended by the manufacturer (Endo H, PNGase F, NEB). Briefly, the pellet was resuspended by hypotonic buffer, followed by adding denaturing buffer and boiling at 95 °C for 10min. Then the 10X reaction buffer and Endo H or PNGase F wee added. The reaction mixtures were incubated at 37°C for 60 min, followed by detection of the menin protein by Western blot to detect Menin protein.
Site-directed mutagenesis
The loop regions on the surface of Menin protein were predicted based on the crystal structure. Oligonucleotide mutagenesis was carried out as instructed by the manufacturer (QuikChange II XL Site-Directed Mutagenesis Kit, 200521, Agilent Tech), and the primers were 135KDR-NGT_F: ggaacagcctcagccgctcctacttcAATGGTACTgcccacatccagtccctcttcag; 135KDR-NGT_R: ctgaagagggactggatgtgggcAGTACCATTgaagtaggagcggctgaggctgttcc; 294GRP-NSS_F: ctagaggagctggagcccacccctAACAGTAGCgacccactcaccctctaccacaagg; 294GRP-NSS_R: ccttgtggtagagggtgagtgggtcGCTACTGTTaggggtgggctccagctcctctag; the capital nucleic acids indicated the mutated ones.
Click-chemistry palmitoylation assay
HEK293T cells were grown in 6cm dishes for 36 hr and treated with 100µM palmitic acid azide for 6 hrs. The culture medium was removed and cells were washed 3 times with PBS. Cells were lysed in 200µl lysis buffer (50mM Tris pH 7.5, 1% SDS, and 1X protease cocktail inhibitor). Lysates were sonicated and centrifuged at 18,000 RPM for 10 min. Lysate (50µl) was reacted with biotin alkyne using the Click-IT assay in a 200 µl final reaction volume as instructed by the manufacturer. Biotinylated proteins were isolated using streptavidin agarose and washed 5 times in wash buffer (50mM Tris pH 7.5, 0.1%SDS), followed by SDS-PAGE and immunoblotting.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported in part by grants from the NIH (R01-DK085121) (1-R01-CA-178856 and R01 DK097555), Caring for Carcinoid Foundation-AACR Grant Care for Carcinoid Foundation (11-60-33), a pilot grant from ITMAT of the University of Pennsylvania. This work was also supported in part by the NIH/NIDDK Center for Molecular Studies in Digestive and Liver Diseases (P30DK050306) and its core facilities (Molecular Biology/Gene Expression Core). We also appreciate the immunofluorescent staining for menin expression in TGP61 cells by Dr. Hua Mao.
References
- 1.Yang Y, Hua X. In search of tumor suppressing functions of menin. Mol Cell Endocrinol 2007; 265:34-41; PMID:17222504; http://dx.doi.org/ 10.1016/j.mce.2006.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marx SJ. Molecular genetics of multiple endocrine neoplasia types 1 and 2. Nat Rev Cancer 2005; 5:367-75; PMID:15864278; http://dx.doi.org/ 10.1038/nrc1610 [DOI] [PubMed] [Google Scholar]
- 3.Brandi ML, Gagel RF, Angeli A, Bilezikian JP, Beck-Peccoz P, Bordi C, Conte-Devolx B, Falchetti A, Gheri RG, Libroia A, et al. Consensus: guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab 2001; 86:5658-71; PMID:11739416; http://dx.doi.org/ 10.1210/jcem.86.12.8070 [DOI] [PubMed] [Google Scholar]
- 4.Chen YX, Yan J, Keeshan K, Tubbs AT, Wang H, Silva A, Brown EJ, Hess JL, Pear WS, Hua X. The tumor suppressor menin regulates hematopoiesis and myeloid transformation by influencing Hox gene expression. Proc Natl Acad Sci USA 2006; 103:1018-23; PMID:16415155; http://dx.doi.org/ 10.1073/pnas.0510347103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yokoyama A, Somervaille TC, Smith KS, Rozenblatt-Rosen O, Meyerson M, Cleary ML. The menin tumor suppressor protein is an essential oncogenic cofactor for MLL-associated leukemogenesis. Cell 2005; 123:207-18; PMID:16239140; http://dx.doi.org/ 10.1016/j.cell.2005.09.025 [DOI] [PubMed] [Google Scholar]
- 6.Hughes CM, Rozenblatt-Rosen O, Milne TA, Copeland TD, Levine SS, Lee JC, Hayes DN, Shanmugam KS, Bhattacharjee A, Biondi CA, et al. Menin associates with a trithorax family histone methyltransferase complex and with the hoxc8 locus. Mol Cell 2004; 13:587-97; PMID:14992727; http://dx.doi.org/ 10.1016/S1097-2765(04)00081-4 [DOI] [PubMed] [Google Scholar]
- 7.Murai MJ, Chruszcz M, Reddy G, Grembecka J, Cierpicki T. Crystal structure of menin reveals binding site for mixed lineage leukemia (MLL) protein. J Biol Chem 2011; 286:31742-8; PMID:21757704; http://dx.doi.org/ 10.1074/jbc.M111.258186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang J, Gurung B, Wan B, Matkar S, Veniaminova NA, Wan K, Merchant JL, Hua X, Lei M. The same pocket in menin binds both MLL and JUND but has opposite effects on transcription. Nature 2012; 482:542-6; PMID:22327296; http://dx.doi.org/ 10.1038/nature10806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matkar S, Thiel A, Hua X. Menin: a scaffold protein that controls gene expression and cell signaling. Trends Biochem Sci 2013; 38:394-402; PMID:23850066; http://dx.doi.org/ 10.1016/j.tibs.2013.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Debelenko LV, Agarwal S, Du Q, Yan W, Erickson HS, Abu-Asab M, Raffeld MA, Libutti SK, Marx SJ, Emmert-Buck MR. Menin Immunoreactivity in Secretory Granules of Human Pancreatic Islet Cells. Appl Immunohistochem Mol Morphol 2014; 22(10):748-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asfari M, Janjic D, Meda P, Li G, Halban PA, Wollheim CB. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology 1992; 130:167-78; PMID:1370150; http://dx.doi.org/ 10.121210/endo.130.1.1370150 [DOI] [PubMed] [Google Scholar]
- 12.Schnepp RW, Chen YX, Wang H, Cash T, Silva A, Diehl JA, Brown E, Hua X. Mutation of tumor suppressor gene Men1 acutely enhances proliferation of pancreatic islet cells. Cancer Res 2006; 66:5707-15; PMID:16740708; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-4518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan J, Yang Y, Zhang H, King C, Kan HM, Cai Y, Yuan CX, Bloom GS, Hua X. Menin interacts with IQGAP1 to enhance intercellular adhesion of β-cells. Oncogene 2009; 28:973-82; PMID:19079338; http://dx.doi.org/ 10.1038/onc.2008.435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charron G, Wilson J, Hang HC. Chemical tools for understanding protein lipidation in eukaryotes. Curr Opin Chem Biol 2009; 13:382-91; PMID:19699139; http://dx.doi.org/ 10.1016/j.cbpa.2009.07.010 [DOI] [PubMed] [Google Scholar]
- 15.Sletten EM, Bertozzi CR. Bioorthogonal chemistry: fishing for selectivity in a sea of functionality. Angewandte Chemie Int Edition 2009; 48:6974-98; PMID:19714693; http://dx.doi.org/ 10.1002/anie.200900942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise huisgen cycloaddition process: copper (I)‐catalyzed regioselective “ligation” of azides and terminal alkynes. Angewandte Chemie 2002; 114:2708-11; http://dx.doi.org/ 10.1002/1521-3757(20020715)114:14<2708::AID-ANGE2708>3.0.CO;2-0 [DOI] [PubMed] [Google Scholar]
- 17.Bizzozero OA. Chemical analysis of acylation sites and species. Methods Enzymol 1995; 250:361-79; PMID:7651165; http://dx.doi.org/ 10.1016/0076-6879(95)50085-5 [DOI] [PubMed] [Google Scholar]
- 18.Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol 2009; 5:100-7; PMID:19125156; http://dx.doi.org/ 10.1038/nchembio.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takada R, Satomi Y, Kurata T, Ueno N, Norioka S, Kondoh H, Takao T, Takada S. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev Cell 2006; 11:791-801; PMID:17141155; http://dx.doi.org/ 10.1016/j.devcel.2006.10.003 [DOI] [PubMed] [Google Scholar]
- 20.Wu CC, MacCoss MJ, Howell KE, Yates JR 3rd. A method for the comprehensive proteomic analysis of membrane proteins. Nat Biotechnol 2003; 21:532-8; PMID:12692561; http://dx.doi.org/ 10.1038/nbt819 [DOI] [PubMed] [Google Scholar]
- 21.Moremen KW, Tiemeyer M, Nairn AV. Vertebrate protein glycosylation: diversity, synthesis and function. Nat Rev Mol Cell Biol 2012; 13:448-62; PMID:22722607; http://dx.doi.org/ 10.1038/nrm3383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annual Rev biochem 1985; 54:631-64; PMID:3896128; http://dx.doi.org/ 10.1146/annurev.bi.54.070185.003215 [DOI] [PubMed] [Google Scholar]
- 23.Robbins P, Trimble R, Wirth D, Hering C, Maley F, Maley GF, Das R, Gibson BW, Royal N, Biemann K. Primary structure of the Streptomyces enzyme endo-β-N-acetylglucosaminidase H. J Biol Chem 1984; 259:7577-83; PMID:6429133 [PubMed] [Google Scholar]
- 24.Tarentino AL, Gomez CM, Plummer TH Jr. Deglycosylation of asparagine-linked glycans by peptide: N-glycosidase F. Biochemistry 1985; 24:4665-71; PMID:4063349; http://dx.doi.org/ 10.1021/bi00338a028 [DOI] [PubMed] [Google Scholar]
- 25.Jin S, Mao H, Schnepp RW, Sykes SM, Silva AC, D'andrea AD, Hua X. Menin associates with FANCD2, a protein involved in repair of DNA damage. Cancer Res 2003; 63:4204-10; PMID:12874027 [PubMed] [Google Scholar]
- 26.Pettengill OS, Memoli VA, Brinck-Johnsen T, Longnecker DS. Cell lines derived from pancreatic tumors of Tg (Ela-1-SV40E) Bri18 transgenic mice express somatostatin and antigen. Carcinogenesis 1994; 15:61-5; PMID:7904904; http://dx.doi.org/ 10.1093/carcin/15.1.61 [DOI] [PubMed] [Google Scholar]
- 27.Guru SC, Goldsmith PK, Burns AL, Marx SJ, Spiegel AM, Collins FS, Chandrasekharappa SC. Menin, the product of the MEN1 gene, is a nuclear protein. Proc Natl Acad Sci 1998; 95:1630-4; PMID:9465067; http://dx.doi.org/ 10.1073/pnas.95.4.1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaji H, Canaff L, Lebrun J-J, Goltzman D, Hendy GN. Inactivation of menin, a Smad3-interacting protein, blocks transforming growth factor type β signaling. Proc Natl Acad Sci 2001; 98:3837-42; PMID:11274402; http://dx.doi.org/ 10.1073/pnas.061358098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agarwal SK, Guru SC, Heppner C, Erdos MR, Collins RM, Park SY, Saggar S, Chandrasekharappa SC, Collins FS, Spiegel AM, et al. Menin interacts with the AP1 transcription factor JunD and represses JunD-activated transcription. Cell 1999; 96:143-52; PMID:9989505; http://dx.doi.org/ 10.1016/S0092-8674(00)80967-8 [DOI] [PubMed] [Google Scholar]
- 30.Heppner C, Bilimoria KY, Agarwal SK, Kester M, Whitty LJ, Guru SC, Chandrasekharappa SC, Collins FS, Spiegel AM, Marx SJ, et al. The tumor suppressor protein menin interacts with NF-kappaB proteins and inhibits NF-kappaB-mediated transactivation. Oncogene 2001; 20:4917-25; PMID:11526476; http://dx.doi.org/ 10.1038/sj.onc.1204529 [DOI] [PubMed] [Google Scholar]
- 31.Wu Y, Feng ZJ, Gao SB, Matkar S, Xu B, Duan HB, Lin X, Li SH, Hua X, Jin GH. Interplay between Menin and K-Ras in Regulating Lung Adenocarcinoma. J Biol Chem 2012; 287:40003-11; PMID:23027861; http://dx.doi.org/ 10.1074/jbc.M112.382416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yaguchi H, Ohkura N, Takahashi M, Nagamura Y, Kitabayashi I, Tsukada T. Menin missense mutants associated with multiple endocrine neoplasia type 1 are rapidly degraded via the ubiquitin-proteasome pathway. Mol Cell Biol 2004; 24:6569-80; PMID:15254225; http://dx.doi.org/ 10.1128/MCB.24.15.6569-6580.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimazu S, Nagamura Y, Yaguchi H, Ohkura N, Tsukada T. Correlation of mutant menin stability with clinical expression of multiple endocrine neoplasia type 1 and its incomplete forms. Cancer Sci 2011; 102:2097-102; PMID:21819486; http://dx.doi.org/ 10.1111/j.1349-7006.2011.02055.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Ozawa A, Zaman S, Prasad NB, Chandrasekharappa SC, Agarwal SK, Marx SJ. The tumor suppressor protein menin inhibits AKT activation by regulating its cellular localization. Cancer Res 2011; 71:371-82; PMID:21127195; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-3221 [DOI] [PMC free article] [PubMed] [Google Scholar]