Abstract
Objective
Recent studies have reported an increased risk of fracture among patients with systemic lupus erythematosus (SLE) in comparison to the general population. The aim of this study was to examine associations between SLE status and bone geometry in Caucasian and African American women.
Methods
We compared hip bone mineral density (BMD) and bone geometry parameters among SLE women and controls using hip structure analysis (HSA). 153 DXA scans from the Study of Lupus Vascular and bone Long Term Endpoints (68.7% Caucasian and 31.3% African American) and 4920 scans from the third National Health and Nutrition Examination Survey (59.3% Caucasian and 40.7% African American) were analyzed. Linear regression was used to examine BMD and bone geometry differences by SLE status and by race/ethnicity after adjusting for age and body mass index.
Results
Significant differences were detected between SLE women and control. Among Caucasians, age-adjusted BMD (g/cm2), section modulus (cm3) and cross sectional areas (cm2) were lower among SLE subjects in comparison to control at the narrow neck (0.88 vs. 0.83, 1.31 vs. 1.11 and 2.56 vs. 2.40; p<0.001, <0.01 and <0.0001 respectively), while buckling ratio was increased (10.0 vs. 10.6, p<0.01). Likewise, BMD, section modulus and cross sectional areas were decreased among African American SLE women at all sub regions, while buckling ratios were increased.
Conclusions
There were significant bone geometry differences between SLE women and control at all hip sub-regions. Bone geometry profiles among SLE subjects were suggestive of increased fragility.
Keywords: Osteoporosis, Inflammation, Biomechanics, Modeling and Remodeling, Bone densitometry
Introduction
Osteoporotic fracture represents an enormous and growing public health concern 1, 2. In the United States, 1.5 million fractures are diagnosed annually, with an associated medical cost of 14 billion dollars per year 3, 4. Osteoporosis is a particular concern among certain populations, including patients with systemic lupus erythematosus (SLE), an autoimmune disease of unknown cause. SLE has a variety of manifestations affecting multiple organ systems and occurs primarily in women in their childbearing years. Treatment for SLE frequently includes corticosteroid to control symptoms, with anti-malarial, immunosuppressive or immunomodulatory medications often added. Supportive therapies to minimize side effects from disease manifestations or from medications include strategies to maintain a normal blood pressure and lipid profile, calcium, 25-hydroxyvitamin D, and other medications to maximize bone health5.
Data on the prevalence of osteoporotic fracture among patients with systemic lupus erythematosus (SLE) is limited, but recent studies demonstrate that SLE subjects are at a higher risk for fragility fracture than the general population6-8. Fractures among patients with SLE can occur at a younger age, including premenopausal females. Furthermore, African American race/ethnicity appears not to be protective against osteoporosis in SLE 9. There is recent evidence that autoimmunity and its associated inflammation and vitamin D deficiency play key roles in the pathogenesis of adverse skeletal effects in SLE 10. With recent advances in therapy, life expectancy has improved for patients with SLE 11 and osteoporosis will become an increasingly prevalent complication among patients with SLE.
The gold standard for osteoporosis diagnosis is by performing dual energy x-ray absorptiometry (DXA) scans of the hip and spine 12. Reduction of hip bone mineral density (BMD) by 1 standard deviation (SD) below age-adjusted mean predicts a 2.4-fold increase of fracture risk 13. Similarly, BMD improvements measured by DXA have been associated with fracture risk reduction in osteoporosis therapeutic trials 12. However, many studies have shown that BMD does not fully account for fracture risk 14, and BMD changes with osteoporosis treatment do not fully account for fracture risk reduction 15, suggesting that this clinical assessment does not adequately account for the complex mechanical characteristics of skeletal fragility.
Bones fracture when internal stresses exceed their load-bearing capacity 16,17. In osteoporosis, fracture occurs with minimal trauma because the load-bearing capacity of the skeleton is compromised. Reduced load-bearing capacity can result from deterioration in the material composition of the skeleton or from changes in the structural geometry of the bones. While there are no reliable non invasive ways to measure the material strength of the skeleton, we are not aware of any evidence suggesting that this parameter is altered in osteoporosis. Thus, changes in internal skeletal stresses are almost entirely a consequence of structural change. Hip structure analysis (HAS) allows for the determination of structural geometry, from which relevant strength information can be derived17. This essential information is not available from conventional DXA.
The value of HSA in predicting skeletal strength has been illustrated by some retrospective analyses of data from large fracture trials 16, 18. However the clinical applicability of HSA is currently limited by the high intolerance of the technique to skeletal positioning.
These observations are particularly pertinent for patients with SLE because the causes of increased bone fragility in patients with SLE are not fully elucidated. Indeed BMD measurements in patients with SLE who sustain fractures are often higher than those seen in postmenopausal women who sustain fractures19 In this study, we compared bone geometry parameters in patients with SLE from the Study of Lupus Vascular and bone Long Term Endpoints (SOLVABLE) study 20 to Caucasian and African American women from the third National Health and Nutrition Examination Survey21 using HSA. We hypothesized that the presence of SLE was associated with bone geometry profiles that predict increased skeletal fragility.
Methods
Study subjects
This study is a retrospective review of DXA and clinical data previously collected on SOLVABLE and NHANES III Caucasian and African American participants. The SOLVABLE cohort consists of women 18 years and older who met at least 4 of the 1982 (or updated 1997) American College of Rheumatology (ACR) classification criteria for SLE22,23. Designation of patient race/ethnicity was cultural (by self-declaration) rather than biological. There were three study visits in the protocol for SOLVABLE. The data from the first study visit for SOLVABLE which included interview for osteoporotic risk factors (including self-reported steroid use and 25 hydroxyvitamin D levels), physical examination for anthropometric measurements and SLE disease activity and severity, and imaging for BMD of the hip are included in this report. SOLVABLE patient enrolment occurred between December 2002 and August 2007.
The control population for this study consisted of non-pregnant Caucasian and African American women who participated in the NHANES III study. The NHANES III study was designed to assess the health and nutritional status of the civilian non-institutionalized Caucasian, African American and Mexican American members of the United States population, and was conducted between 1988 and 1994. Data collection was accomplished during household interviews and by direct physical examination at mobile examination centers. Relevant biochemical studies were done, including measurement of serum 25 hydroxyvitamin D levels. Men and non pregnant women 20 years and older in NHANES III who received physical examination and had not fractured both hips were eligible for a DXA scan of the left hip unless there was a history of previous fracture or surgery on the left side (1%), in which case the right side was scanned.
Our study was approved by the Institutional Review Boards of the Medical University of South Carolina and Northwestern University, Feinberg School of Medicine.
Bone Densitometry
Acquisition of DXA data at the hip on SOLVABLE subjects was conducted using a Hologic QDR-4500 (Hologic, Bradford, MA) as specified by the manufacturer with appropriate attention to quality control. Specifically, femur phantoms were measured to assess intra-instrument variation. The intra-instrument coefficient of variation was less than 0.46%. For NHANES, three mobile Hologic QDR1000 DXA scanners (Hologic, Waltham, MA) were used for BMD measurement, with a rigorous quality control program including the use of anthropometric phantoms and close scrutiny of individual scans at a central site. Scans were rejected if they did not meet quality criteria for conventional DXA processing (n=321) or if structural analysis was not possible. Specifically, scans were excluded from structural analysis if they had artifacts or were inappropriately positioned, resulting in excessive ante version or obscured (and cut-off) margins. A total of 2916 Caucasian and 2904 African American scans were available for HSA21.
Due to the time span between NHANESIII and SOLVABLE studies, our study compares DXA scans obtained using two different DXA generations. Hologic QDR1000 uses pencil beam technology while QDR4500 uses fan beam technology. The main consequence of these differences is a shorter scan time with QDR4500 than with QDR1000. While these technical differences are a potential limitation of the study, comparative studies have shown a high correlation between the two scanners (r2=0.99, 0.95 and 0.96 at the spine, femoral neck and total hip BMD respectively)24, implying that the practical consequences of the technical differences are probably minimal. Nevertheless, cross-calibration would have been desirable, but was not practical due to the retrospective nature of our study. For bone geometry measurement, the two scanners are reasonably consistent except at the proximal shaft. (Personal communication from HSA-developer).
Hip Structure Analysis (HSA)
The HSA program is based on a principle first described by Martin and Burr, namely that mineral profiles created during single photon absorptiometry (SPA) bone densitometry are a projection of the corresponding bone cross-section, and can be used to define its geometry at that location25. The program uses the distribution of mineral mass in a line of pixels across the bone axis to measure geometric properties of cross-sections in cut planes traversing the bone. Regions of interest include the narrow femoral neck (located at the narrowest point of the femoral neck), the intertrochanteric region (traversing the bisector of the neck and shaft axes), and the shaft (located 1.5 times the neck width distal to the intersection of the neck and shaft axes). These sites are clinically relevant since the majority of hip fractures involve the femoral neck or intertrochanter area.
Five parallel mass profiles, which are spaced about 1 mm apart along the bone axis (corresponding to a 5 mm section thickness) are generated and averaged using an algorithm developed by Thomas Beck26, to derive the following parameters: 1) bone mineral density (grams per square centimeter), 2) outer cortical diameters (centimeters), 3) bone cross sectional area (square centimeter), and 4) the cross-sectional moment of inertia. The section modulus (cm3) was calculated as cross-sectional moment of inertia divided by the maximum distance from the center of mass to the outer cortical margin. To estimate the average cortical thickness and buckling ratios, cortices of the narrow neck, inter-trochanter and shaft were modeled as circular annuli with 60%, 70%, and 100% of the measured mass in the cortex respectively. Buckling ratios were then calculated as the maximum distance from the center of mass to the outer diameter divided by the (estimated) mean cortical thickness. The program also measures neck shaft angle (the angle subtended by the neck and shaft axes) and neck length (the distance between the femoral head and the point of intersection between the neck and shaft axes).
The two studies relied on the same HSA operator, which should reduce systematic measurement error between the two studies. The HSA operator did not know that the SLE patients had SLE or that we would be using the NHANES data for comparison.
Statistical Analysis
Descriptive analyses were performed to examine bone density and geometry parameters among Caucasian and African American women from NHANES III (without SLE) and the SOLVABLE (SLE) studies. Linear regression was used to examine differences in bone density and geometry by SLE status and by race/ethnicity after adjusting for age and body mass index. In addition, the mean difference and 95 % confidence intervals between those with and without SLE were determined by race/ethnicity. Because the NHANES III population was compared to an external clinical population, NHANES sampling weights and design variables were not used to account for the survey design of the NHANES III population27 Data were analyzed using SAS system (version 9.1; SAS institute; Cary, NC).
Results
A total of 164 Caucasian and African American women from the Chicago Lupus Study were enrolled in the Study of Lupus Vascular and Bone Long-Term Endpoints (SOLVABLE), from whom 153 hip DXA scans (68.7% Caucasian and 31.3% African American) were adequate for HSA. Patients with analyzable scans (n=153) were of similar age and race/ethnicity distribution as those whose scans were not analyzable (n=11), with disease markers (C3, C4 and double-stranded DNA antibody levels) also being equally distributed. Smoking status and corticosteroids use were also similar between the two groups. From the NHANES cohort, a total of 4920 DXA scans (59.3% Caucasian and 40.7% African American) were available for analysis.
Demographic characteristics of the SLE patients and NHANES controls are presented in Table 1. Among Caucasian women, NHANES participants were older, had similar BMI and 25-hydroxyvitamin D levels, and were more likely to currently smoke, but less likely to be former smokers than participants with SLE. Among African American women, NHANES participants were a similar age and had similar BMI and 25-hydroxyvitamin D levels, but were more likely to currently smoke than women with SLE. Among women with SLE, disease duration was similar in African Americans and Caucasian women; however, the percent of African American women with American College of Rheumatology/Systemic Lupus International Collaborating Clinics-Damage Index (ACRI/SLICC-DI) scores greater than zero was higher in African American than Caucasian women (80.2% versus 55.7%) as was the percent with Systemic Lupus Activity Measure (SLAM) greater than 7 (49.0% versus 28.7%). However there were no significant differences in any of the HSA parameters measured in the hip between SLE patients with (SLAM>7) and without (SLAM<=) active disease, or with (SLICC>0) and without (SLICC=0) disease damage. Additionally, among women with SLE, African Americans were slightly (2 +/- SD years) younger with a higher mean BMI and a lower mean 25-hydroxyvitamin D level compared to Caucasians. African American women were also more likely to be current smokers, and less likely to be former smokers than Caucasian women.
Table 1.
Age-adjusted levels (95% CI) of population characteristics stratified by race and SLE status.
| White Women | Black Women | |||
|---|---|---|---|---|
| NHANES (n=2916) | SLE (n=105) | NHANES (n=2004) | SLE (n=48) | |
| Age* (years) | 53.8 (53.2, 54.5) | 44.8 (41.3, 48.3) | 43.4 (42.6, 44.2) | 42.3 (37.2, 47.4) |
| Menopausal*,a(%) | 55.1 (53.3, 56.9) | 40.0 (31.1, 49.6) | 34.6 (32.5, 36.7) | 39.6 (26.9, 53.9) |
| Age at menopause†,b (years) | 47.4 (47.1, 47.8) | 43.1 (41.0, 45.3) | 46.0 (45.5, 46.6) | 39.0 (35.8, 42.2) |
| Years since menopause†,c (years) | 21.9 (21.3, 22.5) | 11.4 (7.8, 14.9) | 16.8 (15.9, 17.6) | 11.5 (6.3, 16.7) |
| BMI (kg/m2) | 26.3 (26.1, 26.5) | 26.3 (25.1, 27.5) | 29.2 (28.9, 29.5) | 29.4 (27.6, 31.2) |
| 25-hydroxyvitamin D (ng/ml) | 29.9 (29.5, 30.3) | 31.1 (29.1, 33.1) | 17.7 (17.3, 18.2) | 16.4 (13.4, 19.4) |
| Obesed (%) | 22.8 (21.3, 24.4) | 20.5 (13.8, 29.4) | 38.9 (36.8, 41.1) | 43.9 (30.5, 58.3) |
| Severe vitamin D Deficiente (%) | 1.1 (0.8, 1.6) | 2.9 (0.9, 8.5) | 11.0 (9.6, 12.5) | 19.7 (10.5, 33.7) |
| Current Smoker (%) | 23.2 (21.6, 24.8) | 4.9 (2.2, 10.6) | 24.4 (22.5, 26.3) | 14.0 (7.0, 25.8) |
| Former Smoker (%) | 21.6 (20.1, 23.2) | 33.6 (25.1, 43.2) | 12.8 (11.4, 14.4) | 26.7 (15.9, 41.2) |
| SLE Duration (years) | ---- | 12.0 (10.4, 13.7) | ---- | 13.5 (11.0, 15.9) |
| SLICC > 0 (%) | ---- | 55.7 (45.6, 65.3) | ---- | 80.2 (66.6, 89.2) |
| SLAM > 7 (%) | ---- | 28.7 (20.8, 38.1) | ---- | 49.0 (35.1, 63.0) |
Unadjusted;
Limited to those who went through menopause;
Menopause is defined by the following algorithm. A women is considered menopausal if no menses in last 12 months (and not currently breastfeeding and no surgery done which could complicate interpretation of menopausal status); total hysterectomy and bilateral salpingo-oophorectomy; if no menses and had a hysterectomy, ovary status unknown or known to have at least one ovary, FSH >23 IU/L; or if menopausal status unknown and FSH unknown and age ≥ 50 years. Age 50 years is the median age of menopause in NHANES women who have not had a hysterectomy or oophorectomy;
Age of menopause is defined as age at last menses, or when a hysterectomy was preformed prior to menopause, age when FSH was measured > 23 IU/L or at 50 years of age;
Years since menopause, was defined as current age minus age at menopause plus 1 with the 1 added to differentiate between women who went through menopause the same year as their examination and women who are not menopausal;
Obesity is defined as BMI ≥ 30kg/m2;
Severely vitamin D deficient is defined as less than 10 ng/ml.
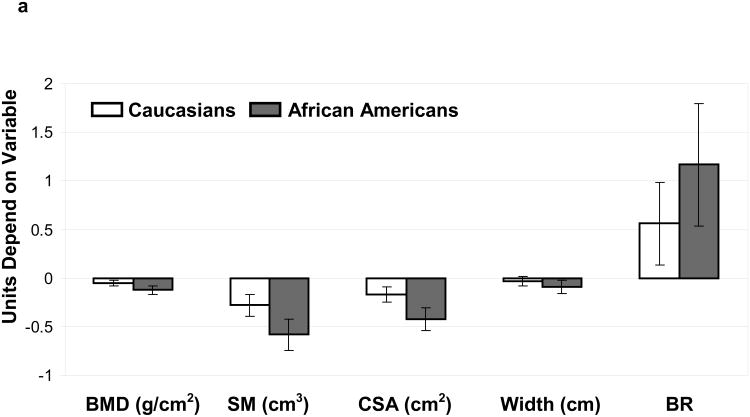
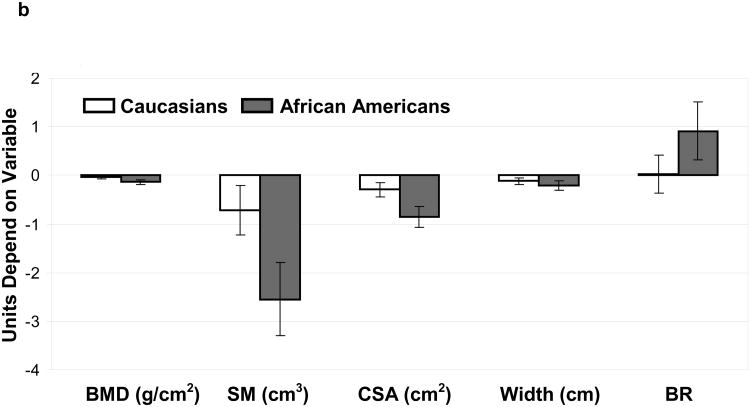
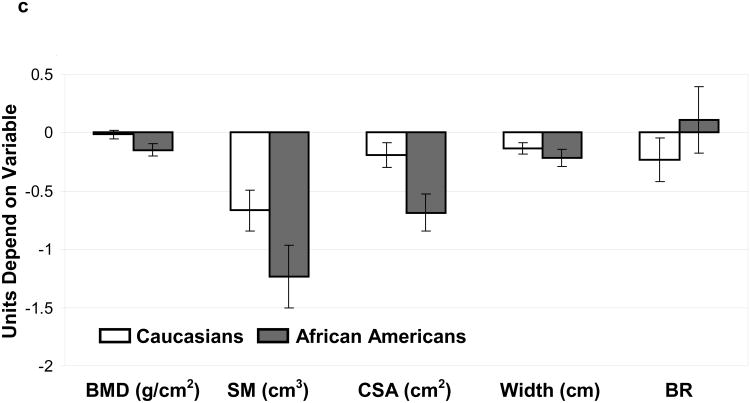
After stratifying by SLE status and race/ethnicity, age and BMI-adjusted means were determined for HSA parameters at the femoral neck, trochanter and proximal femoral shaft (Table 2). In general, BMD and bone geometry parameters were significantly different among both Caucasian and African American women with SLE compared to NHANES at all the areas of the skeleton, with some site-specific differences between the race/ethnic groups (figure 1 a, b & c). Specifically, BMD was reduced in both Caucasian and African American women with SLE compared to NHANES controls at all areas, although the difference between Caucasian women did not attain statistical significance at the femoral shaft region.
Table 2.
Age- and BMI-adjusted mean (95% CI) bone mineral density and bone structure levels stratified by race and SLE status.
| Whites | Blacks | |||
|---|---|---|---|---|
| NHANES (n=2916) | SLE (n=105) | NHANES (n=2004) | SLE (n=48) | |
| Narrow Neck Region | ||||
| BMD(g/cm2) | 0.88 (0.88, 0.89) | 0.83 (0.80, 0.86)† | 0.96 (0.95, 0.96) | 0.83 (0.79, 0.87)‡ |
| Section Modulus(cm3) | 1.31 (1.30, 1.32) | 1.11 (1.06, 1.17)* | 1.38 (1.37, 1.40) | 1.01 (0.93, 1.09)‡ |
| Cross Sectional Area (cm2) | 2.56 (2.55, 2.58) | 2.40 (2.32, 2.47)‡ | 2.74 (2.72, 2.76) | 2.32 (2.20, 2.44)‡ |
| Width (cm) | 3.07 (3.06, 3.08) | 3.04 (3.00, 3.09) | 3.03 (3.02, 3.04) | 2.94 (2.87, 3.01) |
| Buckling Ratio | 10.0 (9.94, 10.1) | 10.6 (10.2, 11.0)* | 9.13 (9.03, 9.23) | 10.3 (9.67, 10.9)† |
| Intertrochanter Region | ||||
| BMD (g/cm2) | 0.86 (0.86, 0.87) | 0.82 (0.79, 0.85)* | 0.94 (0.93, 0.94) | 0.80 (0.75, 0.84)‡ |
| Section Modulus (cm3) | 3.76 (3.74, 3.79) | 3.54 (3.40, 3.68)* | 3.81 (3.78, 3.85) | 3.03 (2.81, 3.24)‡ |
| Cross Sectional Area (cm2) | 4.35 (4.32, 4.37) | 4.05 (3.91, 4.20)‡ | 4.61 (4.58, 4.65) | 3.76 (3.55, 3.97)‡ |
| Width (cm) | 5.31 (5.30, 5.32) | 5.19 (5.13, 5.26)† | 5.20 (5.18, 5.22) | 4.98 (4.89, 5.08)‡ |
| Buckling Ratio | 9.25 (9.17, 9.32) | 9.27 (8.88, 9.66) | 8.58, 8.49, 8.67) | 9.49 (8.90, 10.1)* |
| Femur Shaft Region | ||||
| BMD (g/cm2) | 1.29 (1.29, 1.30) | 1.28 (1.24, 1.31) | 1.34 (1.33, 1.35) | 1.19 (1.14, 1.25)‡ |
| Section Modulus (cm3) | 2.26 (2.25, 2.28) | 1.93 (1.85, 2.00) | 2.32 (2.30, 2.34) | 1.67 (1.55, 1.79)‡ |
| Cross Sectional Area (cm2) | 3.78 (3.76, 3.80) | 3.59 (3.48, 3.69)‡ | 3.91 (3.89, 3.94) | 3.23 (3.06, 3.39)† |
| Width (cm) | 3.09 (3.08, 3.10) | 2.95 (2.90, 3.00)‡ | 3.08 (3.07, 3.09) | 2.86 (2.79, 2.94)‡ |
| Buckling Ratio | 3.62 (3.59, 3.70) | 3.39 (3.21, 3.57) | 3.46 (3.42, 3.51) | 3.57 (3.29, 3.86) |
P-values are for comparisons within racial group between NHANES participants and individuals with SLE:
p-value <0.01;
p-value <0.001;
p-value <0.0001.
Figure 1.
(A): Comparisons (SLE vs. NHANES) for bone mineral density (BMD) and bone geometry parameters (Section modulus-SM, cross-sectional area-CSA, Width and Buckling ratio-BR) were made for Caucasian(open bars) and African American(grey bars) subjects. Units depend on variable.
a. Narrow Neck: differences (95% Confidence Interval) between SLE and NHANES in Caucasians and African Americans adjusted for age and body mass index
(B): Comparisons (SLE vs. NHANES) for bone mineral density (BMD) and bone geometry parameters (Section modulus-SM, cross-sectional area-CSA, Width and Buckling ratio-BR) were made for Caucasian(open bars) and African American(grey bars) subjects. Units depend on variable.
b. Intertrochanter Region: differences (95% Confidence Interval) between SLE and NHANES in Caucasians and African Americans adjusted for age and body mass index
(C): Comparisons (SLE vs. NHANES) for bone mineral density (BMD) and bone geometry parameters (Section modulus-SM, cross-sectional area-CSA, Width and Buckling ratio-BR) were made for Caucasian(open bars) and African American(grey bars) subjects. Units depend on variable.
c. Femoral Shaft Region: differences (95% Confidence Interval) between SLE and NHANES in Caucasians and African Americans adjusted for age and body mass index
We examined our data for differences in skeletal outer diameter to determine if skeletal redistribution was associated with BMD reduction seen among women with SLE. In both Caucasians and African Americans the outer diameter was reduced at all areas (SLE vs. controls) although the difference was not significant at the narrow neck among Caucasians. Since a narrower bone with equivalent bone quantity would have a higher BMD these results suggest that reductions in the quantity of bone, rather than expanded diameter are responsible for the low BMD seen among SLE patients. In support of this theory, low BMD was associated with reductions in bone tissue cross sectional area among African American and Caucasian patients with SLE at all areas analyzed (figure 1, A-C).
We then examined associations between SLE status and skeletal resistance to bending forces, and to local buckling by looking at associations between disease status and section modulus and buckling ratio at common fracture sites (femoral neck and trochanter). Section modulus was reduced in both race/ethnic groups, suggesting reductions in bending resistance among women with SLE. This change was associated with reductions in outer diameter as noted above. At the same time buckling ratio was increased in both race/ethnic groups (non significant difference among Caucasians at the intertrochanter) suggesting an increased tendency for buckling. This finding suggests that cortical thinning proceeds faster among SLE patients than control given that SLE patients have a narrower outer skeletal diameter.
Discussion
Our study presents the first data available comparing bone geometry parameters between women with SLE and controls. Using HSA, we detected the presence of significant bone geometry differences between patients and controls, a finding that has major implications in the management of patients with SLE considering the impact of bone geometry on strength. Specifically, the presence of reduced section modulus and cross-sectional areas, along with increased buckling ratios at common fracture sites among SLE patients implies that SLE patients are more likely to suffer a hip fracture than the general population in the event of a fall.
This clinically important information, which is not available from conventional DXA is consistent with epidemiological data, and highlights the need for better ways for fracture prediction among patients with SLE. Our findings set the stage for studies to look for factors that underlie the differences in bone geometry and strength, a process which could pave the way for the development of therapeutic agents that specifically target these factors.
It is well documented that SLE is associated with reduced BMD at the hip and spine28, 29, but the mechanistic explanation for this difference has hitherto been lacking. BMD reduction can result from changes in skeletal mineralization, its distribution or quantity30. While we did not look at mineralization, the finding of reduced outer skeletal diameters at the proximal hip among women with SLE suggests that reduced bone quantity rather than skeletal redistribution by radial expansion is the mechanism responsible for reduced BMD at this skeletal site in SLE. This position is additionally supported by the presence of reduced proximal hip cross-sectional areas among the women with SLE.
The dimensional differences seen in our study suggest that the adaptive changes that normally preserve bone strength in the face of aging-associated bone loss may be altered in SLE. Specifically, as BMD declines with age, section modulus (a measure of bending resistance) is maintained by subperiosteal apposition, a process that results in the radial expansion of the skeleton while there is on-going endosteal bone loss, as was documented by Beck et al21. Section modulus scales exponentially with changes in bone diameter, making subperiosteal apposition a highly efficient mechanism to preserve bone strength since only a fraction of bone lost on the endocortical surface need be deposited on the subperiosteal surface for biomechanical equivalence. In our study BMD reduction was accompanied by significant reductions of section modulus among SLE patients, implying failure of subperiosteal apposition.
There are several reasons to account for alterations in bone geometry among patients with SLE, including effects of inflammation, corticosteroids, vitamin D deficiency, and altered skeletal loading from arthritis, myositis and/or osteonecrosis.
The finding of low BMD among steroid-naïve SLE patients has led to the hypothesis that inflammation by itself can lead to bone loss and fracture31, although the mechanisms by which this comes about are yet to be elucidated. In one study, markers of inflammation were correlated with markers of bone turnover, and bone loss was more severe among patients with higher levels of pro-inflammatory cytokines, highlighting the potential role of inflammation in the acceleration of bone turnover32. Recent studies using animal models of inflammatory arthritis have shown that inflammation can lead not only to low BMD, but also to unfavorable bone geometry parameters, raising the possibility that both skeletal remodeling and skeletal modeling can be impacted by inflammation33, 34, ultimately leading to fragility. While we did not directly examine inflammation in our study, the presence of low BMD and abnormal bone geometry supports the concept that skeletal modeling and remodeling can be simultaneously affected by SLE.
Corticosteroids represent a potent therapy for inflammation, and are commonly used in SLE. Corticosteroids have many adverse skeletal effects that can lead to bone loss and ultimately fracture35. It has been reported that up to 50% of patients on long term corticosteroids or patients with Cushing's syndrome develop fragility fracture36-39. However, a recent meta-analysis looking at fracture risk among corticosteroid-treated patients showed that steroid-associated BMD change did not fully account for the increased fracture risk among steroid treated patients40. Moreover, corticosteroid-treated patients tended to fracture at higher BMDs than control40, suggesting that other skeletal parameters are involved in fragility seen among steroid-treated patients. Kaji et al examined the effect of corticosteroids on bone geometry among middle-aged and elderly women41. Using Quantitative Computerized Tomography (QCT) scans of the distal radius, they demonstrated that a variety of bone geometry parameters including total skeletal area, periosteal circumference, cortical area and polar strength-strain index were significantly lower among steroid- treated pre-menopausal women compared to controls, findings that were similar to the effects of endogenous cortisol hyper secretion seen in Cushing's syndrome patients41. Moreover, corticosteroid-treated patients with vertebral fractures had lower total area, cortical area, periosteal circumference and polar strength-strain index compared to steroid-treated patients without vertebral fracture41. Collectively, these findings suggest that hypercortisolism can bring about changes of bone geometry that resemble the changes we detected among SLE patients.
Vitamin D is known to protect against fracture but its mechanisms of action have not been fully elucidated. A recent meta-analysis showed that supplementation with 700-800 IU of vitamin D significantly reduced the risk of hip and non vertebral fracture (Relative Risk reductions of 26% and 23% respectively)42. While these fracture benefits are partly a consequence of improved muscle function with reduced fall risk, it is widely believed that vitamin D also improves bone strength although mechanical studies in support of this assertion have not been available. In a 2 year randomized controlled trial that examined consequences of vitamin D (and calcium) supplementation versus placebo in middle-aged to elderly men using QCT, Daly et al showed that there was expansion of mid-femur medullary area among the placebo subjects, with significant reductions of cross-sectional area, while these parameters were preserved among subjects taking vitamin D and calcium43. Preservation of cross-sectional area has a direct consequence on compressional resistance, with potential implications on the buckling susceptibility as well. In addition, there was relative preservation of torsional resistance among the vitamin D and calcium recipients. Increased mid femur medullary area seen among placebo subjects probably resulted from increased endocortical resorption, highlighting the skeletal impact of vitamin D in high turnover situations. Several cross-sectional studies examining skeletal health in SLE have reported low levels of vitamin D44, raising the possibility that reduced bone strength due to abnormal bone geometry might be a factor in skeletal fragility.
Another potential explanation for the observed differences in section modulus lies in the possibility of differential skeletal loading between SLE patients and control. Mechanical loading of the skeleton is believed to be one of the most potent stimuli for skeletal adaptation during postnatal life45, with the greatest effect occurring during growth. According to Frost, muscle forces dominate a bone's postnatal structural adaptations to mechanical usage, modified somewhat by body weight and one's voluntary physical activities46. This assertion suggests that without exposure to optimal levels of muscle-generated bone strains one can fail to realize their full (genetically determined) mechanical potential. In SLE, there are a number of factors that can compromise skeletal loading, thereby preventing the full expression of the genetically-specified blueprint of modeling and remodeling, potentially resulting in reduced skeletal strength. These include inflammation, pain and fatigue and steroid-induced myopathy.
Overall, skeletal loading can be expected to have a more localized skeletal effect while the non mechanical factors can be expected to have a more global scope of action. As a result, it is very likely that the bone geometry differences seen at the proximal hip also exist at other skeletal sites, implying a global fracture risk in SLE. Furthermore, it has been suggested that the non mechanical factors act by either altering the mechanical regulation of bone cells (by altering the mechanical set-point of bone) or by a direct effect on bone cells themselves, independent of mechanical stimuli47. Accordingly, the non mechanical factors have the potential to affect both the structural and material properties of the skeleton, while skeletal loading would primarily alter the structural properties of bone47.
While bone geometry trends among SLE patients were in the direction consistent with increased fragility, proximal shaft buckling ratios trended in the opposite direction in our study. The reasons for these unexpected findings are not immediately clear to us although there was a slight difference in the location of this region of interest between the two studies. Additional studies are needed to clarify on the causes of, and consequences of the unexpected proximal shaft buckling ratios.
This study has some limitations that may have affected the results. First, we have used a 2-dimensional technique to determine cross-sectional diameters and assess bending properties in the image plane48. It is very likely that there are significant differences between patients and controls in other planes as well, which we are unable to capture. Second, cortical thickness component of this study is modeled on the assumption that the cortex occupies a fixed percentage of the wall, and that bone loss in SLE affects the cortical and trabecular components to the same degree as occurs with aging. Accordingly, our study would overestimate cortical thickness (and underestimate buckling ratios) if the percent involvement of the cortex is greater than it is the general population, and vice versa. Thirdly, BMD differences at the femur could also result from differences in fat distribution around the femur between patients and control49, however this is made less likely by the lack of a significant difference in BMI after controlling for race/ethnicity. Moreover, we were unable to perform scanner cross-calibration by scanning recommended hip phantoms at each of the DXA machine as has been recommended. Furthermore, slight incompatibilities exist between software versions used in NHANES and our study which could have affected geometry estimates.
Another area of potential concern pertains to the use of medications among the SOLVABLE population. Use of bisphosphonates in this young population was rare out of concerns for pregnancy. Steroids were used in less than half of the patients and the average dose is low. So though steroids can affect bone health, the low dose and reduced use in this population should minimize this concern.
In conclusion, our findings show that SLE is associated with changes in bone geometry that are suggestive of increased fracture risk. These changes are not captured by conventional DXA, and highlight the importance of including bone geometry on fracture-prediction models for patients with SLE. Our study focuses on the structural composition of the skeleton. Other aspects of bone quality including bone micro architecture, bone turnover and material composition which are known to influence bone strength were not studied. Considering that SLE patients often have multiple osteoporosis risk factors which could potentially alter these parameters, it will be worth examining these parameters in future studies to determine their impact on fracture risk in SLE.
Acknowledgments
We thank Dr. Steward Spies (Department of Radiology, Northwestern University for graciously providing access to the SOLVABLE DXA scans, Dr. Thomas Beck (Vice President and Chief Technology officer, Quantum Medical Metrics, LLC) for his generous assistance with hip structure analysis, and Dr. Craig Langman (Department of Pediatric Nephrology, Northwestern University and Children's Memorial Hospital) for laboratory assistance in completing the 25-hydroxyvitamin D assays.
We take full responsibility for the methods, data collection, data analysis and the writing of this article. All the authors had full access to the data in the study and take full responsibility for the integrity of the data and accuracy of data analysis.
Funding sources: MCRC pilot grant NIH NIAMS P60 AR049459 (JDA)
NIH K23 AR 052364 (DLK)
R01-MD004251 (KJH)
NIH K24 AR 002138, P60 2 AR30692 and UL1RR025741 (RRG)
Footnotes
Part of this work was presented at the 74th Annual Scientific Meeting of the American College of Rheumatology/ 45th Annual Scientific meeting of Association of Rheumatology Health Professionals, Atlanta, Georgia; November 6-11, 2010.
Disclosures: JDA has received Speaker's Bureau honoraria from Amgen and Novartis. All other authors state that they have no conflicts of interest.
Contributor Information
Jimmy D Alele, Email: alele@musc.edu.
Diane L Kamen, Email: kamend@musc.edu.
Kelly J Hunt, Email: huntke@musc.edu.
Rosalind Ramsey-Goldman, Email: rgramsey@northwestern.edu.
References
- 1.National Osteoporosis Foundation. America's Bone Health: The state of osteoporosis and low bone mass in our nation. Washington (DC): National Osteoporosis Foundation; 2002. [Google Scholar]
- 2.Bone health and osteoporosis: a report of the Surgeon General. Issued October 2004. Available on line at: www.surgeongeneral.gov/library/bonehealth. [PubMed]
- 3.Bouxsein ML. Biomechanics of age-related fractures. In: Marcus R, Feldman D, Kelsey J, editors. Osteoporosis. 2nd. San Diego: Academic Press; 2001. pp. 509–31. [Google Scholar]
- 4.Colon-Emeric CS, Saag KG. Osteoporotic fractures in older adults. Best Practice & Research in Clinical Rheumatology. 2006;20(4):695–706. doi: 10.1016/j.berh.2006.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gordon C, Ramsey-Goldman R. Systemic lupus erythematosus and lupus-like syndromes. In: Adebajo A, editor. ABC of Rheumatology. 4th. Blackwell Publishing; Oxford, UK: 2007. pp. 24–31. [Google Scholar]
- 6.Bultink IE, Lems WF, Kostense PJ, et al. Prevalence of and risk factors for low bone mineral density and vertebral fractures in patients with systemic lupus erythematosus. Arthritis Rheum. 2005;52(7):2044–50. doi: 10.1002/art.21110. [DOI] [PubMed] [Google Scholar]
- 7.Yee CS, Crabtree N, Skan J, et al. Prevalence and predictors of fragility fractures in systemic lupus erythematosus. Ann Rheum Dis. 2005;64(1):111–114. doi: 10.1136/ard.2003.018127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramsey-Goldman R, Dunn JE, Huang CF, et al. Frequency of fractures in women with systemic lupus erythematosus: comparison with United States population data. Arthritis Rheum. 1999;42(5):882–90. doi: 10.1002/1529-0131(199905)42:5<882::AID-ANR6>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 9.Lee C, Almagor O, Dunlop DD, et al. Association between African American race/ethnicity and low bone mineral density in women with systemic lupus erythematosus. Arthritis Rheum. 2007;57:585–592. doi: 10.1002/art.22668. [DOI] [PubMed] [Google Scholar]
- 10.Kamen DL, Alele JD. Skeletal manifestations of systemic autoimmune diseases. Curr Opin Endocrinol Diabetes Obes. 2010;17:540–545. doi: 10.1097/MED.0b013e328340533d. [DOI] [PubMed] [Google Scholar]
- 11.Bernatsky S, Boivin JF, Joseph L, et al. Mortality in systemic lupus erythematosus. Arthritis Rheum. 2006;54(8):2550–7. doi: 10.1002/art.21955. [DOI] [PubMed] [Google Scholar]
- 12.Blake GM, Fogelman I. Role of dual energy x-ray absorptiometry in the diagnosis and treatment of osteoporosis. J Clinical Densitom. 2007;10(1):102–110. doi: 10.1016/j.jocd.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Stone K, Seeley G, Lui L, et al. BMD at multiple sites and risk of fracture of multiple types: Long-term results from the study of osteoporotic fractures. J Bone Miner Res. 2003;18:1947–1954. doi: 10.1359/jbmr.2003.18.11.1947. [DOI] [PubMed] [Google Scholar]
- 14.Kleerekoper M, Nelson DA, Peterson EL, et al. Reference data for bone mass, calcitropic hormones, and biochemical markers for bone modeling in older (55-75) postmenopausal white and black women. J Bone Miner Res. 1994;9:1267–1276. doi: 10.1002/jbmr.5650090817. [DOI] [PubMed] [Google Scholar]
- 15.Laroche M. Treatment of osteoporosis: All the questions we still cannot answer. The American Journal of Medicine. 2008;121:744–747. doi: 10.1016/j.amjmed.2008.03.048. [DOI] [PubMed] [Google Scholar]
- 16.La Croix AZ, Beck TJ, Cauley JA, et al. Hip structural geometry and incidence of hip fracture in postmenopausal women: what does it add to conventional bone mineral density? Osteoporosis Int. 2010 Jun;21(6):919–29. doi: 10.1007/s00198-009-1056-1. Epub 2009 Sep 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beck TJ. Extending DXA beyond bone mineral density: understanding hip structure analysis. Current osteoporosis reports. 2007;5:49–55. doi: 10.1007/s11914-007-0002-4. [DOI] [PubMed] [Google Scholar]
- 18.Kaptoge S, Beck TJ, Reeve J, Stone KL, Hillier TA, Cauley JA, et al. Prediction of incident hip fracture risk by femur geometry variables measured by hip structural analysis in the study of osteoporotic fractures. J Bone Miner Res. 2008;23(12):1892–904. doi: 10.1359/JBMR.080802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee C, Almagor O, Dunlop DD, et al. Self-reported fractures and associated factors in women with systemic lupus erythematosus. J Rheumatol. 2007;34(10):2018–23. [PubMed] [Google Scholar]
- 20.Wu P, Rhew EY, Dyer AR, et al. 25-hydroxyvitamin D and cardiovascular risk factors in women with systemic lupus erythematosus. Arthritis Rheum. 2009;61:1387–1395. doi: 10.1002/art.24785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beck TJ, Looker AC, Ruff CB, et al. Structural trends in the aging femoral neck and proximal shaft: analysis of the third national health and nutrition examination survey dual-energy x-ray absorptiometry data. J Bone Miner Res. 2000;15:2297–2304. doi: 10.1359/jbmr.2000.15.12.2297. [DOI] [PubMed] [Google Scholar]
- 22.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25(11):1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 23.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 24.Kolta S, Ravaud P, Fetchenbaum J, et al. Follow-up of individual patients on two DXA scanners of the same manufacturer. Osteoporosis Int. 2000:11, 709–713. doi: 10.1007/s001980070070. [DOI] [PubMed] [Google Scholar]
- 25.Martin RB, Burr DB. Non-invasive measurement of long bone cross-sectional moment of inertia by photon absorptiometry. J Biomech. 1984;17:195–201. doi: 10.1016/0021-9290(84)90010-1. [DOI] [PubMed] [Google Scholar]
- 26.Beck TJ, Ruff CB, Warden KE, et al. Predicting femoral neck strength from bone mineral data. A structural approach. Invest Radiol. 1990;25:6–18. doi: 10.1097/00004424-199001000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Vargas CM, Ingram DD, Gillum RF. Incidence of hypertension and educational attainment: the NHANES I epidemiologic follow up study. First national health and nutrition examination survey. Am J Epidem. 2000;152(3):272–8. doi: 10.1093/aje/152.3.272. [DOI] [PubMed] [Google Scholar]
- 28.Kalla AA, Fataar AB, Jessop SJ, et al. Loss of trabecular bone mineral density in systemic lupus erythematosus. Arthritis Rheum. 1993;36(12):1726–34. doi: 10.1002/art.1780361212. [DOI] [PubMed] [Google Scholar]
- 29.Formiga F, Moga I, Nolla JM, et al. Loss of bone mineral density in premenopausal women who have systemic lupus erythematosus. Ann Rheum Dis. 1995;54(4):274–6. doi: 10.1136/ard.54.4.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uusi-Rasi K, Beck TJ, Semanick LM, et al. Structural effects of raloxifene on the proximal femur: results from the multiple outcomes of raloxifene evaluation trial. Osteoporosis Int. 2006;17:575–586. doi: 10.1007/s00198-005-0028-3. [DOI] [PubMed] [Google Scholar]
- 31.Teichman J, Lange U, Stracke H, et al. Bone metabolism and bone mineral density of systemic lupus erythematosus at the time of diagnosis. Rheumatol Int. 1999;18(4):137–40. doi: 10.1007/s002960050072. [DOI] [PubMed] [Google Scholar]
- 32.Korzocwa I, Olewicz-Gawilk A, Hrycaj P, et al. The effect of long term glucocorticoids on bone metabolism in systemic lupus erythematosus patients: the prevalence of its anti-inflammatory action upon bone resorption. Yale J Biol Med. 2003;76(2):45–54. [PMC free article] [PubMed] [Google Scholar]
- 33.Caetano-Lopes J, Nery AM, Henriques R, et al. Chronic arthritis directly induces quantitative and qualitative bone disturbances leading to compromised biomechanical properties. Clin Exp Rheum. 2009;27:475–482. [PubMed] [Google Scholar]
- 34.Pysklywec MW, Moran EL, Bogoch ER. Changes in the cross-sectional geometry of the distal femoral metaphysic associated with inflammatory arthritis are reduced by a biphosphonate (zoledronate) J Orthop Res. 2000;18(5):734–8. doi: 10.1002/jor.1100180509. [DOI] [PubMed] [Google Scholar]
- 35.Cunane G, Lane NE. Steroid-induced osteoporosis in systemic lupus erythematosus. Rheum Dis Clin North Am. 2000;26(2):1–14. doi: 10.1016/s0889-857x(05)70140-x. [DOI] [PubMed] [Google Scholar]
- 36.Van Staa TP, Leufkens HG, Abenhaim L, et al. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2004;15:993–1000. doi: 10.1359/jbmr.2000.15.6.993. [DOI] [PubMed] [Google Scholar]
- 37.Steinbuch M, Youket TE, Cohens S. Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporosis Int. 2004;15:323–328. doi: 10.1007/s00198-003-1548-3. [DOI] [PubMed] [Google Scholar]
- 38.Manelli F, Guistina A. Glucocorticoid-induced osteoporosis. Trends Endocrinol Metab. 2000;11:79–85. doi: 10.1016/s1043-2760(00)00234-4. [DOI] [PubMed] [Google Scholar]
- 39.Kaltas G, Manetti L, Grossman AB. Osteoporosis in Cushing's syndrome. Front Horm Res. 2002;30:60–72. doi: 10.1159/000061073. [DOI] [PubMed] [Google Scholar]
- 40.Kaji H, Yamauchi, Chihara K, et al. The threshold of bone mineral density for vertebral fracture in female patients with glucocorticoid-induced osteoporosis. Endocrine J. 2006;53:27–34. doi: 10.1507/endocrj.53.27. [DOI] [PubMed] [Google Scholar]
- 41.Kaji H, Yamauchi M, Chihara K, et al. Glucocorticoid excess affects cortical bone geometry in premenopausa, but not postmenopausal, women. Calcif Tissue Int. 2008;82:182–190. doi: 10.1007/s00223-008-9106-9. [DOI] [PubMed] [Google Scholar]
- 42.Bischoff-Ferrari HA, Willet WC, Wong JB, et al. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA. 2005;293:2257–2264. doi: 10.1001/jama.293.18.2257. [DOI] [PubMed] [Google Scholar]
- 43.Daly RM, Bass S, Nowson C. Long term effects of calcium-vitamin-D3-fortified milk on bone geometry and strength in older men. Bone. 2006;39:946–953. doi: 10.1016/j.bone.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 44.Kamen D, Aranow C. Vitamin D in systemic lupus erythematosus. Curr Opinion Rheum. 2008;20:532–537. doi: 10.1097/BOR.0b013e32830a991b. [DOI] [PubMed] [Google Scholar]
- 45.Burr DB. Muscle strength, bone mass and age-related bone loss. J Bone Miner Res. 1997;12:1547–1551. doi: 10.1359/jbmr.1997.12.10.1547. [DOI] [PubMed] [Google Scholar]
- 46.Frost HM. Perspective on our age-related bone loss: insights from a new paradigm. J Bone Miner Res. 1997;12:1539–1546. doi: 10.1359/jbmr.1997.12.10.1539. [DOI] [PubMed] [Google Scholar]
- 47.Van Der Meulen MCH, Carter DR, Beupre GS. Skeletal Development: Mechanical consequences of growth, aging and disease. In: Marcus R, Feldman D, Kelsey J, editors. Osteoporosis. 2nd. San Diego: Academic Press; 2001. pp. 471–487. [Google Scholar]
- 48.Nelson DA, Barondess DA, Hendrix SL, et al. (2000). Cross-sectional geometry, bone strength, and bone mass in the proximal femur in black and white postmenopausal women. J Bone Miner Res. 2000;15:1992–1997. doi: 10.1359/jbmr.2000.15.10.1992. [DOI] [PubMed] [Google Scholar]
- 49.Bolotin HH. 1998. A new perspective on the causal influence of soft tissue composition on DXA-measured in vivo bone mineral density. J Bone Miner Res. 1998;13:1739–1746. doi: 10.1359/jbmr.1998.13.11.1739. [DOI] [PubMed] [Google Scholar]