Abstract
Limited functional recovery can be achieved with rehabilitation after incomplete spinal cord injury. Eliminating the function of a repulsive Wnt receptor, Ryk, by either conditional knockout in the motor cortex or monoclonal antibody infusion, resulted in increased corticospinal axon collateral branches with pre-synaptic puncta in the spinal cord and enhanced recovery of forelimb reaching and grasping function following a cervical dorsal column lesion. Using optical stimulation, we observed that motor cortical output maps underwent massive changes after injury and the hindlimb cortical areas were recruited to control the forelimb over time. Furthermore, a greater cortical area was dedicated to control the forelimb in Ryk cKO. In the absence of weekly task-specific training, recruitment of ectopic cortical areas was greatly reduced without significant functional recovery even in Ryk cKO. Our study provides evidence that maximal circuit reorganization and functional recovery can be achieved by combining molecular manipulation and task-specific training.
A large proportion of spinal cord injury patients have incomplete lesions, where parts of the spinal cord tissues remain intact. Due to the strong inhibitory environment in the injured adult spinal cord, especially in the glial scar, and reduced growth potential of adult axons, the original connections are usually not restored. Nonetheless, the complex circuitry can undergo remodeling to achieve variable levels of functional recovery with rehabilitative training. The molecular and circuit mechanisms underlying such functional recovery are poorly understood. The study of both the circuit plasticity and its molecular control will provide important biological basis for treating paralysis.
Functional restoration of the corticospinal motor system after spinal cord injury is of principal importance since it is essential for recovery of voluntary motor control1. In rodents, however, the role of the corticospinal tract (CST) is more limited, with little effect on locomotion and hindlimb usage1. Nevertheless, the CST is crucial for skilled forelimb motor control1–3. Fine motor skills are lost after a dorsal column lesion of the main CST, with a varying extent of spontaneous recovery4,5. Therefore, we used a forelimb reaching and grasping task to study how skilled forelimb function is lost after lesion, how it recovers, and what limits its recovery.
Previous work in our lab has demonstrated that the Wnt signaling, which regulates axon guidance in development, has a profound effect on axon plasticity after injury in the adult spinal cord 6–9. To specifically test the function of Wnt-Ryk signaling in neurons, we generated a conditional allele of Ryk, encoding a repulsive Wnt receptor, performed motor cortex specific knockout and then lesioned the dorsal columns at cervical level 5 (C5). Following Ryk conditional knockout, mice recovered on a skilled forelimb reaching task to 81±7% of peak pre-injury levels at 12 weeks after dorsal column lesion, compared to only 60±5% in wild type control mice. This additional recovery depends on the segment of the main CST immediately rostral to the lesion (C3–C5), as a second dorsal column lesion at C3 reduces the functional recovery to control levels. Anatomical analyses showed significantly increased collateral sprouting of CST above and below the C5 injury and with pre-synaptic puncta in these axon sprouts.
Using an optogenetic approach, we monitored the output map of the motor cortex, We found that, immediately after C5 dorsal column lesion, forelimb elbow flexion can be activated by a much larger cortical area, whereas forelimb extension was lost. Over time, the area that activates forelimb flexion reduced back to the original size and a new area, which used to activate the hind limb, was recruited to activate forelimb extension. After the second lesion at C3, the control of forelimb flexion was lost but that of the new control of the forelimb extension was largely unaffected. In Ryk cKO, these changes are more gradual and persistent. Finally, mice that did not undergo weekly behavioral testing displayed only limited skilled forelimb recovery with performance similar to that of mice tested at one week after injury. In the absence of weekly testing, refinement of cortical motor maps was also impaired, irrespective of Ryk conditional deletion, highlighting the importance of targeted plasticity.
We demonstrate here that the cortical motor map undergoes dramatic changes to achieve recovery and this reorganization requires continued task-specific training. We also show genetic evidence demonstrating Wnt signaling as important regulator of axon plasticity in adult spinal cord using conditional knockout. Additionally, we demonstrate that a Ryk monoclonal antibody can be a therapeutic tool as blocking Ryk function after lesion leads to improved functional recovery. A large proportion of patients have incomplete spinal cord injuries, providing a substrate for recovery. Our work illustrates that promoting circuit plasticity is a promising approach to restore function.
RESULTS
Ryk cKO enhances recovery of fine motor control after SCI
Mice underwent two weeks of training for the reaching and grasping task, followed by a C5 dorsal column spinal cord lesion: a partial spinal cord injury model leaving the dorsal gray matter, lateral white matter and the entire ventral spinal cord intact (Fig. 1a, d). Immediately after dorsal column lesion, forelimb reaching and grasping function is lost (Fig. 1f). With continued training, the success rate of sugar pellet retrieval recovers due to reconfiguration of neural circuits5. It has been shown that the CST undergoes robust collateral sprouting after injury and some of the new sprouts are thought be responsible for new functional circuits10–13. However, axon sprouting is inhibited by molecular cues that limit axon plasticity14,15.
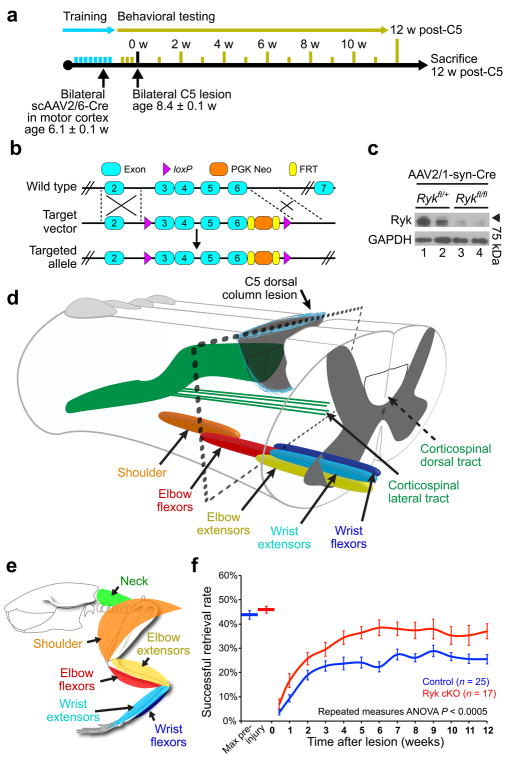
Figure 1. Ryk conditional deletion enhances motor function recovery from spinal cord injury.
(a) Timeline outlining experimental details of bilateral cervical level 5 (C5) dorsal column lesion. (b–c) Generation of Ryk conditional allele. (b) Exons 3–6 were flanked with loxP sites. (c) Western blot of postnatal day 7 motor cortex extract from mice infected at postnatal day 0 with AAV2/1 synapsin Cre. Full-length blot presented in Supplementary Fig. 8. (d–e) Schematic showing the level of the C5 lesion in relation to motor neuron pools for distinct forelimb muscle groups (adapted from McKenna, Prusky, and Whishaw, 200033). (f) Behavioral performance on forelimb reach skilled food-pellet retrieval task shows enhanced recovery after Ryk conditional deletion in bilateral motor cortex (n=25 mice (control), 17 mice (Ryk cKO), from 21 litters, repeated measures ANOVA P=0.0003, F(1,40)=16.0102). Data presented as mean±s.e.m.
Members of the Wnt glycoprotein family are phylogenetically conserved axon guidance molecules that direct the growth along the rostro-caudal axis of both ascending sensory axons and descending CST axons during development6,16. The repulsive Wnt receptor, Ryk, which mediates Wnt repulsion of the developing CST neurons is either not expressed in normal adult motor cortex and the CST neurons or expressed at extremely low levels to be detected by in situ hybridization or immunohistochemistry15. Spinal cord injury re-induces expression of Ryk mRNA and protein in the injured CST. By injecting function-blocking antibodies to Ryk and diffusible Wnt inhibitors, it was found that inhibiting Wnt-Ryk signaling enhanced the plasticity of both sensory and motor axons following injury7–9. However, Ryk antibodies or Wnt inhibitors may exert the effects by impacting on the environment, such as the glial cells around the lesion, rather than CST axons per se.
To specifically test the role of Ryk in neurons, we created a Ryk conditional allele (cKO) and crossed these mice with Ai14 B6.Cg mice containing a loxP-flanked stop cassette preventing tdTomato expression, in order to specifically label recombined corticospinal axons after viral transduction (Fig. 1b, c). Ryk cKO::tdTomato Ai14 mice were injected with an adeno-associated virus (AAV) that expresses Cre recombinase under the control of the cytomegalovirus (CMV) into the primary motor cortex and assess the enhancement of corticospinal circuit remodeling (Fig. 1a). AAV-Cre was injected to adult motor cortex an average of 2.3 weeks prior to C5 dorsal column lesion in order to ensure sufficient time for Cre expression, so that injury would not lead to Ryk expression. We found that Ryk deletion in the CST significantly enhances recovery of skilled forelimb function as assessed by forelimb reach over a period of 12 weeks (Repeated measures ANOVA P<0.005, Fig. 1f, Supplementary Fig. 1). The effects of Ryk deletion are observed early on, with a trend towards better performance at early testing sessions. This was consistently observed and may be due to reduced retraction of axons and collaterals as previously demonstrated at 5 weeks post-injury in animals infused with Ryk antibodies7. Following Ryk conditional deletion, mice recovered to 81±7% of peak pre-injury success rates, compared to only 60±5% in control mice.
Ryk cKO enhances CST collateral sprouting after SCI
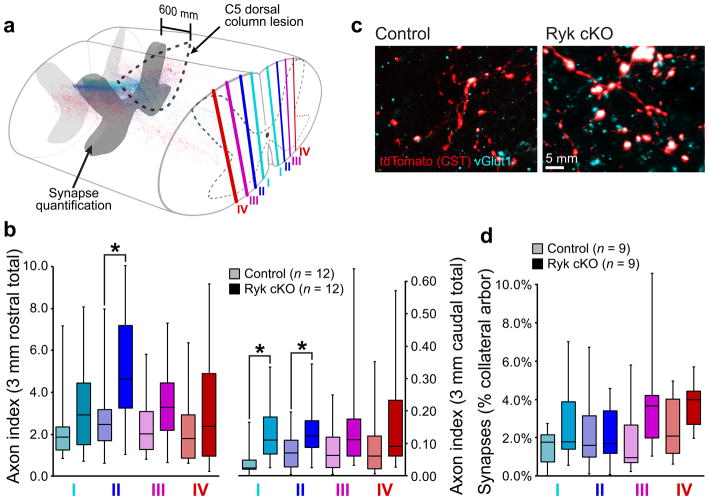
To begin to address the mechanisms underlying improved functional recovery in Ryk cKO mice, we analyzed CST collaterals and synapse density along collateral sprouts in the cervical spinal cord. We found that conditional Ryk deletion did not significantly reduce axonal die-back of the injured CST (one-tailed t-test P=0.12), but did lead to significantly increased numbers of CST collaterals within the spinal gray matter, both rostral and caudal to the site of C5 injury, at 12 weeks post-injury (one-tailed t-test P<0.05, Fig. 2e). In addition to a greater number of axon collaterals, mice with Ryk conditionally deleted from cortical pyramidal neurons exhibited pre-synaptic vesicular glutamate transporter 1 (vGlut1)-labeled puncta on identified corticospinal axon collaterals at 600μm rostral to the injury site, suggesting enhanced functional connectivity following Ryk conditional deletion (Fig. 3c, d).
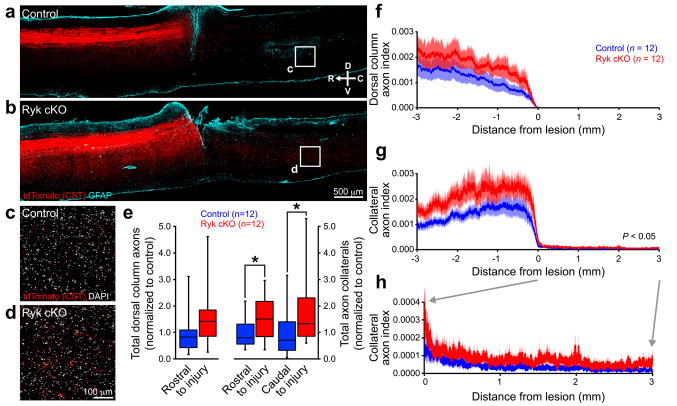
Figure 2. Ryk conditional deletion enhances corticospinal axon sprouting after spinal cord injury.
(a,b) Representative images of tdTomato-labeled CST axons from 8 serial sagittal cryosections spaced 140μm apart superimposed over GFAP astroglial staining at the center of injury (1 experiment, n=12 mice/group, from 11 litters; compass showing dorsal (D), ventral (V), rostral (R), and caudal (C)). Mice with Ryk conditional deletion had greater levels of collateralization both rostral and caudal to the lesion than control mice (one-tailed t-test *P<0.05). (c,d) Higher magnification of single confocal planes from boxed regions (1.5mm caudal to the lesion site) indicated in (a) and (b), respectively. (e) Sum of tdTomato labeled axons (normalized to pyramidal labeling) over 3mm rostral to lesion relative to control in the dorsal columns (n=12 mice/group, one-tailed t-test P=0.12, t(21)=1.198) and in the gray matter. Mice with Ryk conditional deletion had greater levels of collateralization both rostral and caudal to the lesion than control mice (n=12 mice/group, one-tailed t-test *P<0.05: rostral P=0.0499 t(19)=1.730, caudal P=0.0397 t(19)=1.855). (f–h) Distribution of corticospinal axons (axon index is thresholded pixels at every 0.411μm in 8 total saggital spinal cord cryosections divided by thresholded pixels in transverse pyramids) within the dorsal columns (f) or spinal gray matter (g,h). (h) Magnified view of rostral collaterals from (g). C5 injury site is at 0 μm, rostral is represented with negative numbers, caudal with positive. Data in (e) presented as median with inter-quartile range, data in (f–h) presented as mean±s.e.m.
Figure 3. Changes of corticospinal connectivity after C5 dorsal column lesion.
(a–b) Medio-lateral distribution of corticospinal axons shows highest increase in collaterals proximal to the main, dorsal corticospinal tract (regions I and II) after Ryk conditional deletion (n=12 mice/group, one-tailed t-test *P<0.05: II rostral P=0.0225 t(19)=2.146, II caudal P=0.0295 t(21)=1.996, I caudal P=0.0059 t(17)=2.819). (c) Both control and Ryk conditional deletion mice showed pre-synaptic densities (vGlut1 colocalization with tdTomato-labeled corticospinal axons) at 600μm rostral to the C5 injury site (1 experiment, n=9mice/group). (d) Medio-lateral distribution of corticospinal innervation at 600μm rostral to C5 injury site. All data presented as median and inter-quartile range.
The majority of CST axons reside within the dorsal columns and are lesioned by the C5 dorsal column injury. A sparse, minor, component of CST axons descend down the spinal cord within the lateral columns (lateral CST), which remained intact in our C5 lesion paradigm and may contribute to functional recovery5 (Fig. 1d). To test this, we first characterized the distribution of axon collaterals in the spinal cord both rostral and caudal to the C5 lesion. We observed an increase in axon collateral density in Ryk conditional deleted mice throughout the gray matter, with the highest density more medial, in close proximity to the principal dorsal column corticospinal tract (Fig. 3a, b), suggesting that increased branches may come from the dorsal column CST following Ryk deletion. No axons were present within the dorsal column CST caudal to C5 lesion in any of the mice studied.
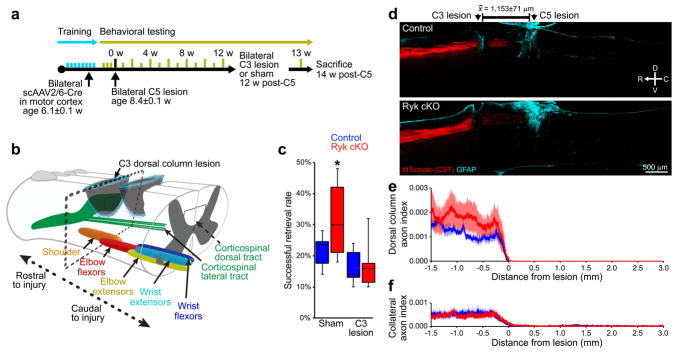
To further assess the contribution of the dorsal column CST to behavioral recovery, we performed a second dorsal column lesion in these animals 12 weeks after C5 injury at the C3 level, 1.15±0.07mm rostral to the original C5 injury (Fig. 4a, b). The lateral CST is again spared in this lesion. We waited one week before behavioral testing to allow the mice to recover from the immediate hyporeflexic stage of spinal shock17. The secondary injury at C3 ablated the enhanced functional recovery we observed in the Ryk conditional deletion mice, leaving only the modest levels of partial recovery achieved by control mice (Fig. 4c). This suggests that the axon sprouts from the dorsal column CST are indeed responsible for the enhanced functional recovery in the Ryk conditional knockout. This also suggests that the spared lateral CST axons may provide only a minor contribution to a basal level functional recovery independent of the dorsal column corticospinal tract. Quantification of axon distribution at 2 weeks after C3 lesion confirmed that the secondary C3 injury eliminated a majority of axon collaterals between the two injury sites, thereby disrupting the remodeled corticospinal circuit (Fig. 4d–f).
Figure 4. Secondary injury at cervical level 3 eliminates enhanced recovery.
(a) Timeline outlining experimental details of secondary C3 lesion experiments following recovery from bilateral C5 dorsal column lesion. (b) Schematic of secondary C3 injury, above the level of increased pre-synaptic density shown in (Fig. 3c–e). (c) Behavioral performance on forelimb reach skilled food-pellet retrieval task shows elimination of enhanced recovery after Ryk conditional deletion by second C3 dorsal column lesion (1 experiment, n=8 (control sham), 7 (control C3), 6 (Ryk cKO sham & C3) mice, mice from 14 litters, ANOVA P=0.0102 F(3)=4.7432, Bonferroni corrected t-test *P<0.05: 1.Ryk cKO sham v. control sham P=0.0106, 2.Ryk cKO sham v. Ryk cKO C3 P=0.0092). (d–f) Secondary C3 dorsal column lesion eliminated enhanced levels of collateralization in Ryk conditional deleted mice. (d) Representative images of tdTomato-labeled CST axons from 8 serial sagittal cryosections spaced 140μm apart superimposed over GFAP astroglial staining at the center of injury (1 experiment, 7 control, 6 Ryk cKO mice). (e,f) Distribution of corticospinal axons (axon index as described above) within the dorsal columns (e) or spinal gray matter (f). C3 injury site is at 0μm, rostral is represented with negative numbers, caudal with positive. Data in (c) presented as median and inter-quartile range, data in (e,f) presented as mean±s.e.m.
Monoclonal Ryk antibody promotes functional recovery
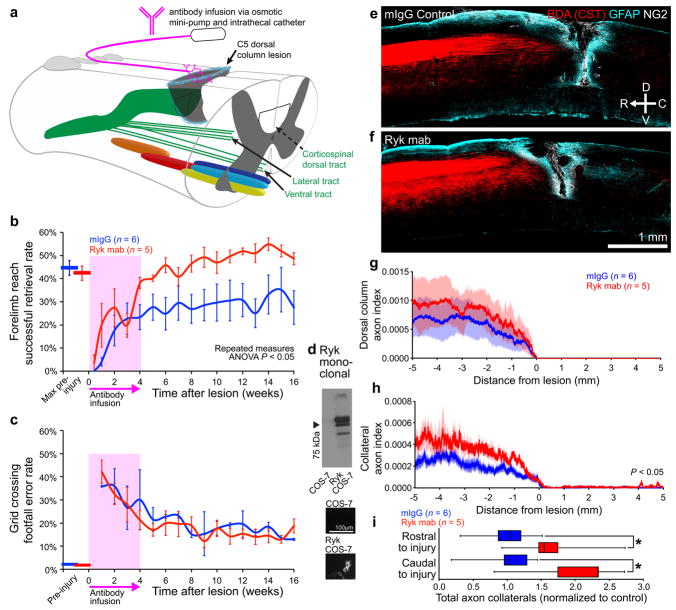
To test whether inhibition of the Wnt-Ryk signaling axis in the injured spinal cord after spinal cord injury is sufficient to increase CST remodeling and enhance behavioral recovery, we generated a new monoclonal Ryk antibody using half of the Wnt binding domain (amino acid range 90–183) as the antigen and infused into adult rats immediately following spinal cord injury (Fig. 5a, Supplementary Fig. 2). We have previously generated function-blocking polyclonal antibodies using the same region7. Following a C5 dorsal column wire-knife lesion, Ryk antibody infusion via osmotic minipump for 4 weeks promoted recovery of skilled forelimb function in the forelimb reach task with all rats recovering to peak pre-injury levels, as compared to only half of rats infused with IgG control (Fig. 5b). Ryk antibody did not enhance recovery in the grid crossing locomotor task since rats exhibited similar levels of forelimb stepping impairment irrespective of treatment group (Fig. 5c). Corticospinal axons were labeled with biotinylated dextran amine (BDA) injections into motor cortex. Consistent with the conditional Ryk deletion prior to injury, we found that the Ryk monoclonal antibody infusion at the time of injury resulted in an increase in corticospinal axon collaterals both rostral and caudal to the level of injury (one-tailed t-test P<0.05, Fig. 5e–i, Supplementary Fig. 3). The extent of increase of collateral sprouts after Ryk antibody infusion in rats was similar to that observed in CST axons lacking Ryk expression in mice (Fig. 2e, Fig. 5i). These results also suggest that Ryk signaling is a feasible therapeutic target, since functional recovery can be promoted by blocking its function after spinal cord injury.
Figure 5. Monoclonal Ryk antibody infusion promotes functional recovery from spinal cord injury.
(a) Schematic showing antibody infusion by intrathecal catheterization. (b) Behavioral performance on forelimb reach skilled food-pellet retrieval task shows enhanced recovery in rats infused with Ryk monoclonal antibody for 28 days starting at time of injury (n=6 rats (IgG control), 5 rats (Ryk monoclonal), repeated measures ANOVA P=0.0354, F(1,9)=6.113). (c) Behavioral performance on skilled locomotor grid crossing task was not affected by Ryk monoclonal antibody infusion. (d) Ryk monoclonal recognizes full-length Ryk protein expressed in transfected COS-7 cells by Western and immunocytochemistry. (e–h) BDA-labeled corticospinal axons in rats infused with Ryk monoclonal antibody had greater levels of collateralization than control mouse IgG infused rats. (e,f) Images of BDA-labeled CST axons are from 6 serial sagittal cryosections spaced 280μm apart superimposed over GFAP astroglial and NG2 staining at the center of injury. (g,h) Distribution of corticospinal axons (axon index is thresholded pixels at every 0.741μm in 6 total saggital spinal cord cryosections divided by thresholded pixels in transverse pyramids) within the dorsal columns (g) or spinal gray matter (h). C5 injury site is at 0μm, rostral is represented with negative numbers, caudal with positive. (i) Sum of normalized axon collaterals over 5mm relative to control. Rats infused with Ryk monoclonal antibody had greater levels of collateralization both rostral and caudal to the lesion than control mouse IgG infused rats (n=6 rats (IgG control), 5 rats (Ryk monoclonal), one-tailed t-test *P<0.05: rostral P=0.0446 t(6)=2.000, caudal P=0.0196 t(6)=2.594). Data in (b, c, g, h) presented as mean±s.e.m., data in (i) presented as median and inter-quartile range.
Cortical map re-organization during recovery
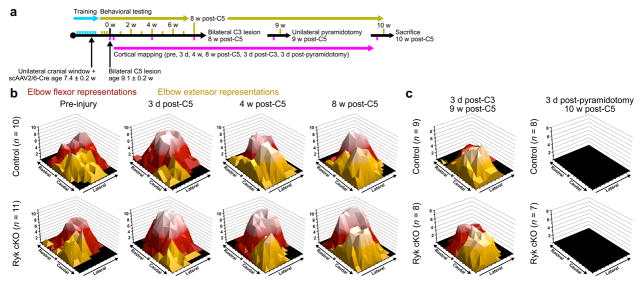
In order to address the circuit mechanisms with which the primary motor cortex regains control over the remodeled spinal cord, we used an optogenetics approach to monitor cortical output. Cortical motor maps have been studied using intracortical electrical stimulation in rodents and primates, as well as transcranial magnetic stimulation in humans18–21. Recent advances in optogenetic tools allow for the stimulation of specific neural populations in a minimally invasive manner22. Specifically, the expression of the light-activated, non-selective, cation channel channelrhodopsin-2 (ChR2) under control of the Thy1 promoter (Thy1-ChR2) allows for selective activation of layer V projection neurons within the motor cortex22. We performed unilateral craniotomies on Thy1-ChR2 mice contralateral to the dominant forelimb in order to investigate motor map changes through repeated optogenetic mapping of evoked motor output after injury23,24 (Supplementary Fig. 4). Following craniotomy, AAV-Cre was injected unilaterally into the motor cortex contralateral to the dominant forelimb as contralateral cortex exhibits motor plasticity in response to forelimb training25; and CST collateral plasticity, which supports functional recovery, was specifically induced in CST axons with Ryk deletion. We assessed motor map output by observing evoked, contralateral motor outputs in sedated mice.
We observed massive remapping of cortical motor output immediately after spinal cord injury in the mouse (Supplementary Fig. 5a–b). Acutely (3 days) after C5 injury, the total area of motor representations for limb muscles at or below the level of lesion was reduced or eliminated (Supplementary Fig. 5c). Conversely, motor maps expanded for muscle groups with motor neurons above the injury site, most notably elbow flexion mediated by biceps brachii and brachialis (Fig. 6b, Fig. 7e, Supplementary Fig. 5c). Over the next two months following spinal cord lesion, cortical maps underwent gradual changes with continued forelimb reaching and grasping training (Supplementary Fig. 5). Behavioral recovery after C5 spinal cord injury plateaued between 4 and 8 weeks post-injury (Fig. 1f, Supplementary Fig. 6), with a median time to reach 90% of peak, post-injury, performance of either 6 weeks in control mice or 5 weeks in Ryk conditional deletion. Therefore, we examined cortical motor maps before and after peak recovery at 4 weeks and 8 weeks, respectively, post-injury. At 4 weeks post-injury, we observed significant differences in the proportion of motor cortex allocated to forelimb extensor (biceps) or forelimb flexor (triceps) activation, with Ryk deleted mice exhibiting larger flexor motor maps at the expense of reduced extensor maps (P<0.05 one-tailed t-test, Fig 7e, f, Supplementary Fig. 5a–b). Expansion of elbow extensor areas at 4 weeks into regions originally occupied by the flexor was inversely correlated with behavioral recovery (n=21 mice: 10 (control), 11 (Ryk cKO), Spearman’s ρ = −0.5766, P=0.0062). By 8 weeks post-injury, Ryk deleted mice exhibited a similar pattern of extensor and flexor motor maps as controls, however the total area occupied by all elbow movements (flexor and extensor) was significantly larger in Ryk deleted mice (one-tailed t-test, P=0.0480 t(14)=1.79). Additionally, at 8 weeks post-injury, wrist flexor representations returned to (or exceeded) maximal pre-injury size in 64% of Ryk deleted mice compared to 10% of control mice (Wilcoxon rank sum P=0.0136 χ2=6.086, Supplementary Fig. 5c). Recovery of wrist flexor control correlated with improvement of forelimb reach performance at 8 weeks post-injury (Spearman’s ρ = 0.4555, P=0.0380). Over the course of the experiment, there was a strong correlation of wrist movement and skilled forelimb reach performance, regardless of injury or genotype (Pearson’s ρ = 0.665, P<0.0001, Fig. 8d).
Figure 6. Cortical map re-organization during recovery from spinal cord injury.
(a) Timeline outlining experimental details of optogenetic mapping with weekly behavioral testing following bilateral C5 dorsal column lesion. (b) Topographic representation of elbow flexor and extensor activation, relative to bregma (*) prior to and 3 days, 4 weeks, and 8 weeks after C5 dorsal column lesion. Data presented as total number of mice responding with evoked movements at each location, lighter color indicates a larger number of mice are responsive at a given location. Each tic mark represents 300μm. (c) Subsequent C3 dorsal column lesion disrupts remodeled circuitry, while subsequent pyradmidotomy eliminates unilateral evoked motor output. Both measured at 3 days after injury.
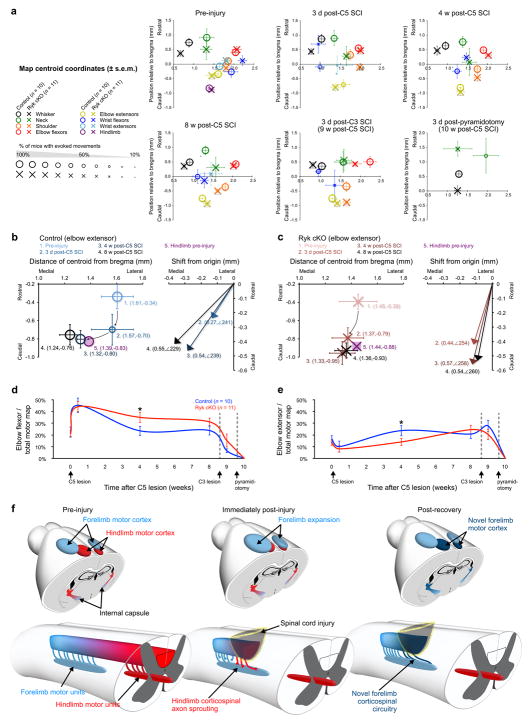
Figure 7. Forelimb motor map representations infiltrate quiescent former hindlimb cortical areas.
(a) Cortical map re-organization from spinal cord injury in mice that received weekly training demonstrates how map centroids shift after injury. Size of the marker is proportional to the percentage of mice with evoked motor movements of a given muscle group. (b,c) Elbow extensor motor maps shift caudal and medial towards cortex originally occupied by hindlimb representations. (d,e) Mice with Ryk conditional deletion have a greater proportion devoted to elbow flexor activation at 4 weeks post-C5 lesion (d) and conversely a smaller proportion of the motor cortex devoted to extensor activation (e) (n=10 (control) 11 (Ryk cKO) mice, one-tailed t-test *P<0.05: elbow flexion P=0.0347 t(19)=1.925, elbow extension P=0.0460 t(16)=-1.791, data presented as mean±s.e.m.). (f) Model for recruitment of ectopic cortical motor regions mediated by axon plasticity. After dorsal column injury, the immediate expansion of forelimb regions above the level of injury is likely mediated by lateral connectivity within the motor cortex. Increased axonal plasticity and connectivity after Ryk conditional deletion likely drives the formation of novel, ectopic areas of forelimb motor cortex.
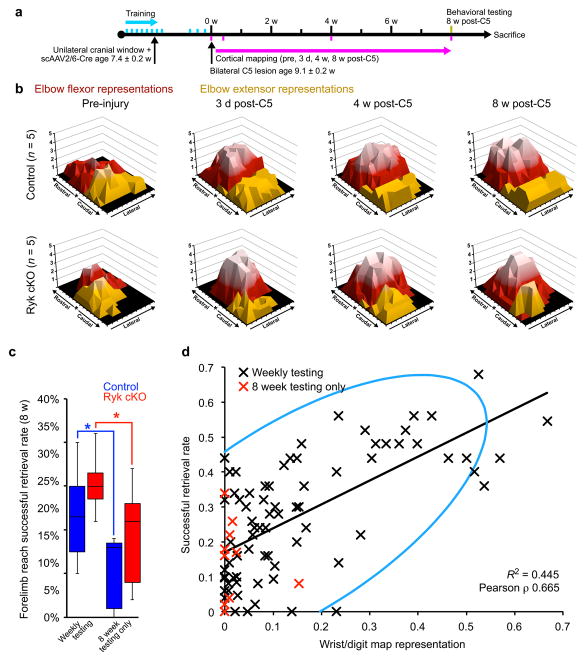
Figure 8. Cortical map re-organization and functional recovery from spinal cord injury are dependent upon rehabilitative training.
(a) Timeline outlining experimental details of optogenetic mapping with only terminal behavioral testing at 8 weeks post-injury. (b) Topographic representation of elbow flexor and extensor activation, relative to bregma (*) prior to and 3 days, 4 weeks, and 8 weeks after C5 dorsal column lesion in the absence of weekly behavioral testing. (c) At 8 weeks after C5 dorsal column lesion, mice with weekly behavioral testing, both Ryk cKO and controls, performed better than those only tested at 8 weeks (n=10 (control weekly testing), 11 (Ryk cKO weekly testing), 5 (control & Ryk cKO 8wk only testing) mice, ANOVA P = 0.0037 F(3) = 5.7157, Bonferroni corrected t-test *P<0.05: 1. Ryk cKO weekly v. 8 week only testing P = 0.0277, 2. control weekly v. 8 week only testing P = 0.0346, data presented as median and inter-quartile range). (d) In animals with weekly behavioral testing (black Xs), there was a strong correlation of wrist movement and skilled forelimb reach performance, regardless of injury or genotype (n=84 measurements (4 time points, 21 mice), bivariate Pearson correlation P, P<0.0001 ρ = 0.665). Light blue is density ellipse (α=0.95). Mice with no weekly behavioral testing are shown in red.
In order to further characterize the remapped cortical output, mice were subjected to a second dorsal column lesion at C3, rostral to the level of extensor motor units, at 8 weeks after C5 injury (Fig. 4b). We found that the C3 injury significantly reduced flexor motor maps in all mice but, surprisingly, had little effect on the recovered extensor motor maps, suggesting the flexor control is routed from connections rostral to C3 or from the lateral corticospinal tract (Fig. 6d, Supplementary Fig. 5a–b). Importantly, a subsequent unilateral pyramidotomy abolished unilateral forelimb responses to cortical stimulation and also the ability of mice to perform the forelimb reach task (Fig 6d, Supplementary Fig. 5a, b, Supplementary Fig. 6). Although plasticity of other supraspinal pathways, such as the rubrospinal tract or reticulospinal tract may also contribute to functional recovery, the effects of unilateral pyramidotomy suggest that a direct connection between the primary motor cortex with the cervical spinal cord is essential for the recovery of voluntary skilled forelimb movement (Supplementary Fig. 5a–b).
Cortical re-organization requires rehabilitative training
The repeated testing of skilled forelimb reach over the course of the experiment essentially constitutes a rehabilitative training paradigm that can promote motor recovery from spinal cord injury and cortical reorganization26,27. In order to determine if the induced axonal plasticity mediated by Ryk deletion alone was required to promote functional recovery, we tested the recovery of skilled forelimb reach at 8 weeks after C5 injury in another cohort of mice that did not undergo weekly behavioral testing after injury (Fig. 8a). Mice that did not undergo weekly behavioral testing displayed only limited skilled forelimb recovery with performance similar to that of mice tested at one week after injury (Fig. 8c, Fig. 1f). In the absence of weekly testing, refinement of cortical motor maps was also impaired, irrespective of Ryk conditional deletion (Fig. 8b, Supplementary Fig. 7).
DISCUSSION
In order to understand how neural circuits reorganize to regain function after injury, we performed functional, anatomical and behavioral analyses. We demonstrate here that the motor cortex remaps such that the cortical areas are no longer used for the hindlimb are recruited to control the forelimb to achieve functional recovery after a dorsal column lesion and this reorganization requires continued training. We also showed that removing Ryk, a receptor to axon guidance cues Wnts, results in greater CST axon plasticity and cortical circuit remodeling in conjunction with rehabilitative training, leading to maximal functional restoration. The more gradual and persistent changes in cortical control maps observed in Ryk conditional knockout injured mice are likely due to the enhanced changes in connectivity within the spinal cord and cortical circuits that occur in the absence of Wnt-Ryk signaling. We found previously that Wnt-Ryk signaling controls topographic map formation in the developing visual system28. Here we reveal a novel function of Wnt-Ryk signaling in controlling motor cortex remapping after spinal cord injury in adulthood.
Previous studies demonstrated that somatosensory cortical maps expand into affected neighboring regions after spinal cord injury10,11. We characterized the motor output map after spinal cord injury and show here that forelimb motor maps spread into adjacent regions affected by the injury. We observed an expansion of flexor control area caudally and medially towards cortical regions originally responsible for hindlimb movements (Fig. 7a, b, Supplementary Fig. 5a, b). These changes are likely stereotypical, because wrist flexor representations exhibited a medial shift as they recovered, similar to observed shifts of digit representations in primates29 (Fig. 7a, Supplementary Fig. 5a, b).
Alterations of motor output maps have been noted in spinal cord injury patients for many years but the neural circuit mechanisms remain unknown. It is known that naïve transected hindlimb-projecting corticospinal neurons sprout into cervical spinal cord as early as one week post spinal cord injury11. However it is unlikely that early expansion of motor maps above the level of the injury, which we observed only 3 days later, was due to sprouting and establishment of new connectivity patterns; rather, it is likely due to a loss of lateral inhibition within the cortex21. However, by 4 weeks after spinal cord injury cortical maps likely reflect the output to the remodeled corticospinal circuitry in the cervical spinal cord. We observed that the greater CST axon collateral numbers induced following Ryk deletion leads to slight increase of connections with motor units distal to the injury site but robust increase with those rostral to the injury (Fig. 2h, Fig. 3d). Therefore, the changes in connectivity above the level of injury could be the main source for the changes of cortical maps (Fig. 7f). For example, the initial expansion of the biceps at 4 weeks post-injury, and subsequent reduction at 8 weeks, may result from an initial sprouting of CST collaterals that normally project to forelimb motor units in the cervical spinal cord, followed by a subsequent pruning through Hebbian competition. The recruitment of the hindlimb cortical areas for triceps control may result from de novo connections from corticospinal neurons that originally controlled the hindlimb to the forelimb motor units of the cervical spinal cord. These sprouts may either directly contact motor units or form relays using propriospinal neurons. Conditional deletion of Ryk in corticospinal neurons enhances collateral sprouts and thus recruits more spinal cord circuitry, a process that likely underlies greater recovery of voluntary skilled forelimb control. The results of our antibody infusion experiments in the spinal cord suggest that circuit remodeling in the cervical spinal cord is sufficient to promote functional recovery. However, it is plausible that connectivity changes within the primary motor cortex also contribute to the remodeling of the entire circuit.
Other descending pathways are also involved in fine motor control and can partially compensate for the loss of CST input on a skilled forelimb reach task30. Additionally, animals with incomplete lesion of the pyramids have been shown to exhibit similar success rates of skilled forelimb reach to intact control animals through compensatory forelimb movements, indicating that a small proportion of spared CST is capable of restoring full, if altered, function on the skilled forelimb reach task31. In 59% of our Ryk cKO mice and 100% of Ryk monoclonal antibody-infused animals, we achieved recovery of skilled forelimb reach to levels at or above peak pre-injury levels. In our model, this full recovery clearly requires the novel CST connections rostral to the lesion, as a second C3 lesion abolishes the enhanced recovery. While the CST is not the sole component mediating control of skilled forelimb reach, it is required for the recovered function as pyramidotomy in our mouse model completely abolished the reaching and grasping behavior. These results suggest that restoring at least some CST function is a critical component in recovery of motor control after injury as compensatory plasticity of other tracts drives limited recovery in rodents, which are less dependent upon the CST for motor control than primates.
In this study, we also demonstrate that a Ryk monoclonal antibody can be a therapeutic tool as blocking Ryk function after lesion leads to improved functional recovery. We show here that maximal recovery of the forelimb can be achieved by combining targeted plasticity for the forelimb function (continued reaching and grasping training) and molecular manipulation. Therefore, we anticipate combining targeted plasticity of other functions with molecular manipulation may allow recovery of other motor or sensory functions. A large proportion of patients have incomplete spinal cord injuries, providing a substrate for recovery32. Our work illustrates that promoting circuit plasticity is a promising approach to restore maximal function following incomplete spinal cord injury.
Methods
All animal work in this research was approved by the University of California, San Diego (UCSD) Institutional Animal Care and Use Committee. Animals were housed on a 12hr light/dark cycle and behavioral analyses were done at consistent morning hours during the light cycle. Both mice and rats were group housed, except for mice with cranial windows which were singly housed after window implantation. Group sample sizes were chosen based upon previous studies and power analysis (25% effect size, α=0.05, α=0.2, power of 80%). One mouse was excluded from the study due to evidence of incomplete C5 lesion with labeled corticospinal axons present at and below the level of the injury and one mouse was excluded as it did not attempt to perform the behavioral task after injury. All procedures and methods (surgical procedures, behavioral assays, tissue processing, immunostaining, image analysis, COS-7 transfection, and cortical mapping) were performed by investigators blinded to genotype or treatment group.
Generation of transgenic mice
The target vector containing loxP-flanked exons 3–6 as well as the PKG-neo selection cassette was transfected into ES cells at the UCSD Transgenic Core facility. Cells were screened by Southern blot and PCR for integration of the targeting vector. Chimaeric mice were generated at the UCSD Transgenic Core facility. Ryk cKO mice were crossed with Ai14 B6.Cg mice containing a loxP-flanked stop cassette preventing tdTomato expression (The Jackson Laboratory, Bar Harbor, ME, RRID:IMSR_JAX:007914) and backcrossed into C57BL/6J for 6 generations. For cortical mapping experiments, Ryk cKO::tdTomato Ai14 mice were crossed with mice expressing channelrhodopsin (Chr2) behind the Thy1 promoter (B6.Cg-Tg(Thy1-COP4/EYFP)18Gfng/J) (The Jackson Laboratory).
Surgical procedures
Cortical AAV injection
Adult female C57BL/6J mice (6.1±0.1 weeks old) were deeply anaesthetized with isoflurane until unresponsive to toe and tail pinch and the area over the skull was shaved and cleaned with povidone-iodine before incision. The skull was thinned bilaterally over the motor cortex and self-complementary AAV2/6 Cre-HA (Salk Institute for Biological Studies Gene Transfer, Targeting and Therapeutics Core, La Jolla, CA) [1.49×1011 genome copies/ml] was injected into 10 sites per hemisphere (250nl/site) with a 36ga NanoFil needle (World Precision Instruments Inc., Sarasota, FL).
Cranial window
Adult female C57BL/6J mice (7.4±0.2 weeks old) were deeply anaesthetized with isoflurane. The skin over the skull was removed, the skull surrounding the motor cortex contralateral to the dominant forelimb was thinned, the skull over the motor cortex was removed, and self-complementary AAV2/6 Cre-HA was injected to 10 sites as above. The exposed cortex was covered with a 5mm #1 round glass coverslip (Warner Instruments, Hamden, CT) secured with VetBond (3M, St. Paul, MN). The exposed skull was covered with dental grip cement (Dentsply, York, PA).
C5 and C3 dorsal column lesion (mice)
Mice were deeply anaesthetized with isoflurane, spinal level C5 (or C3) was exposed by laminectomy and the dorsal columns were lesioned at a depth of 1mm with Vannas spring scissors (Fine Science Tools, Foster City, CA). The dorsal musculature was sutured with 4-0 silk sutures and the skin was closed with wound clips. Mice were randomly selected for C3 lesion or C3 sham (laminectomy only) groups.
Pyramidotomy
Mice were deeply anesthetized with ketamine [120mg/kg] and xylazine [12mg/kg], an incision was made and the ventral musculature was pushed aside to expose the pyramids. The dura was opened and the pyramid ipsilateral to the craniotomy was lesioned by 15° microscalpel (Electron Microscopy Sciences, Hatfield, PA) as previously described34.
C5 dorsal column lesion (rats)
Adult female Fischer 344 rats (120–135g) were deeply anaesthetized with 2ml/kg of ketamine cocktail (25mg per ml ketamine, 1.3mg per ml xylazine and 0.25mg per ml acepromazine). Spinal level C5 was exposed by laminectomy, the dura was punctured over the dorsal horn, and the dorsal columns were lesioned with 2 passes of a Scouten wire-knife (David Kopf Instruments, Tujunga, CA). The dorsal musculature was sutured with 4-0 silk sutures and the skin was closed with wound clips. Polyethylene intrathecal catheters (Durect Corp., Cupertino, CA) were pre-filled with either mouse IgG or Ryk monoclonal IgG (clone 25.5.5 generated against the ectodomain of Ryk, amino acid range 90–183, by Johns Hopkins Monoclonal Antibody Core, Baltimore, MD) [1mg/ml] in artificial cerebrospinal fluid, threaded through magna cisterna to the cervical spinal cord, secured with 4-0 silk sutures, and attached to model 2004 osmotic minipumps (Durect Corp.) filled with 200μl mouse IgG or Ryk IgG. Rats were randomly selected for mouse IgG or Ryk monoclonal IgG treatment. Osmotic minipumps were removed after 28 days. At 16 weeks after C5 spinal cord injury, rats were injected bilaterally with 10% wt/vol 10,000 MW biotinylated dextran amine (BDA) in sterile phosphate buffered saline at 20 sites per hemisphere (250nl/site); animals were sacrificed 2 weeks later.
Cortical mapping
Mice were lightly anaesthetized with ketamine [100mg/kg] xylazine [10mg/kg] mixture, still responsive to toe and tail pinch, and maintained during the course of stimulation with ketamine/xylazine mixture. Mice were fixed in a stereotaxic frame (David Kopf Instruments) and a fiber optic cable and cannulae (200μm diameter) affixed to the stereotax arm were used to stimulate motor cortex locations relative to bregma that were unobstructed by skull or dental cement (maximum of 165 sites). Stimulation was 3 pulses of 470nm light, 250ms duration at 1Hz from a single channel LED driver (Thorlabs, Newton, NJ). The intensity of stimulation was increased from 50mA up to 1000mA until movement was detected in 3 consecutive pulses. Sites with no evoked movements at 1000mA were scored as unresponsive. Only contralateral forelimb movements were scored; occasional, weaker, ipsilateral movements were observed as previously described in rats35.
Ryk knockout in postnatal day 0 pups
Pups were injected with 0.5μl AAV2/1-synapsin-Cre (Penn Vector Core, Philadelphia, PA) [1.99×1013 genome copies/ml] was injected into 2 sites in the motor cortex unilaterally with a 36ga NanoFil needle (World Precision Instruments Inc.). Mice were sacrificed 7 days later and the motor cortex was isolated and homogenized in lysis buffer (20 mM Tris HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 10 mM NaF, 10 mM β-glycerophosphate, 1 mM Na3VO4, 0.5% wt/vol sodium dodecyl sulfate, 1% vol/vol TritonX-100, and cOmplete protease inhibitor cocktail (Roche, Indianapolis, IN). Protein was analyzed by Western blot (40μg/well). Antibodies used for Western mouse anti-Ryk [20μg/ml] (Johns Hopkins Monoclonal Antibody Core), GAPDH [1:1000] (EMD Millipore, Billerica, MA, catalog #MAB374, RRID:AB_2107445).
Sacrifice and tissue processing
Animals were deeply anaesthetized with ketamine cocktail, transcardially perfused with ice-cold PBS followed by 4% wt/vol paraformaldehyde in PBS, and brains and spinal columns were post-fixed overnight at 4°C in 4% wt/vol paraformaldehyde. Tissue was cryoprotected in 30% wt/vol sucrose in PBS. Mouse spinal cords and brainstems, and rat brainstems were sectioned on a cryostat (Leica, Buffalo Grove, IL) at 20μm (saggital spinal cords, transverse brainstems) and mounted directly on Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA). Rat spinal cords were sectioned sagittally at 40μm thick and collected as free-floating sections. Sections were washed three times with PBS, blocked for one hour in PBS with 0.25% triton-X100 (PBST) and 5% donkey serum, then incubated overnight at 4°C with primary antibodies in PBST plus 5% donkey serum. The next day, sections were washed three times, incubated with Alexa Fluor conjugated secondary antibodies (Life Technologies, Grand Island, NY; Jackson ImmunoResearch, West Grove, PA) for 2.5 hours at room temperature, counterstained with DAPI [1μg/ml] (Sigma-Aldrich, St. Louis, MO) and washed three final times in PBS. Antibodies used for fluorescent immunohistochemistry were: rabbit anti-dsRed [1:1000] (Clontech Laboratories Inc., Mountain View, CA, catalog #632496, RRID:AB_10013483), monoclonal G-A-5 anti-GFAP [1:200] (Sigma-Aldrich, catalog #G3893, RRID:AB_2314539), guinea pig anti-vGlut1 [1:1000] (EMD Millipore, catalog #AB5905, RRID:AB_2301751), and rabbit anti-GFAP [1:750] (Dako, Carpinteria, CA, catalog #Z0334, RRID:AB_10013382).
COS-7 cell transfection
COS-7 cells were transfected with pcDNA4-Ryk using FuGene6 (Roche) to express full length Ryk. Cells were either fixed for 30min with ice-cold 4% wt/vol paraformaldehyde in PBS for immunocytochemistry, or lysed with lysis buffer for Western blot.
Image acquisition and analysis
Images were acquired on an inverted Zeiss LSM510 confocal microscope with LSM acquisition software (Carl Zeiss Microscopy, LLC, Thornwood, NY). Image density quantification was done on thresholded images using ImageJ (NIH, Bethesda, MD). An investigator blinded to the experimental group performed all analyses. Axon index is the total thresholded pixels at every 0.411μm in 8 total serial saggital spinal cord cryosections spaced 140μm apart for mice, or 0.741μm in 6 total serial saggital spinal cord cryosections spaced 280μm apart for rats, divided by thresholded pixels in transverse sections of the pyramid at the level of the obex. Lesion volume was calculated using the Cavalieri estimator tool in StereoInvestigator (MBF Bioscience, Williston, VT) on every seventh 40μm saggital section. For tdTomato and vGlut1 colocalization, all axons within the gray matter were quantified over a region 210μm wide at a distance of 600μm rostral to the C5 lesion in the 8 total serial saggital cryosections used for tdTomato quantification. The location of 600μm was chosen as we observed the highest density of axon collaterals in this region in both groups of animals (figure 1g) and it was located between the original C5 and secondary C3 lesions.
Behavioral testing
All animals were trained on skilled forelimb reach over a period of two weeks prior to bilateral spinal cord injury. Animals were food restricted during training and then for 24 hours prior to weekly training after injury. Animals reached through a vertical slot in the front of an acrylic chamber and over a small gap to retrieve a reward pellet. Mice performed 25 reaches per session for 20mg sucrose reward tablets (TestDiet, St. Louis, MO). Rats performed 50 reaches per session for 45mg sugar pellets (Bio-Serv, Flemington, NJ). Successful retrieval rate was calculated as the number of pellets that were retrieved and eaten divided by the number contacted by the forepaw. Forelimb reach was trained twice weekly by two independent investigators blind to genotype or experimental treatment; the two independent scores were averaged. Mice in the group without weekly training during recovery were only tested twice, at 8 weeks after injury. In addition to forelimb reach, rats were tested once weekly on a grid crossing task, where forelimb footfalls were calculated as a percentage of total forelimb steps in 3 passages over a 60 inch span of 1 inch equidistant wire grid.
Statistics
Statistical tests indicated in main text were performed using JMP 9 software (SAS Institute, Cary, NC). We have previously demonstrated that inhibition of repulsive Wnt signaling results in sprouting and plasticity of descending corticospinal and ascending dorsal column sensory axons after spinal cord injury7–9. In addressing our hypothesis that Ryk cKO or Ryk monoclonal antibody enhanced axon sprouting, we tested the increases by one-tailed t-test (figures 2e, 3b, 5i, and S3). In order to test longitudinal behavioral studies with multiple, equally spaced measurements, we utilized repeated measures ANOVA (figures 1f, 5b, S6). In testing multiple groups with continuous, parametric data, we utilized ANOVA with post-hoc Bonferroni correction on appropriate post-hoc comparisons (figures 4c and 8c). Bivariate correlation was performed to determine the relationship between forelimb function and cortical maps (figure 8d). Continuous data was tested with parametric tests and data was assumed to be normally distributed, but this was not formally tested.
Supplementary Material
Acknowledgments
We would like to thank Zhigang He, Binhai Zheng, Fan Wang, Kuanhong Wang and the Zou lab members for critical reading of the manuscript, as well as comments and suggestions. This work was supported by grants to Yimin Zou (RO1 NS047484, R21 NS081738, Wings for Life Foundation, and International Foundation for Research in Paraplegia).
Footnotes
Author contributions:
YZ and EH designed the experiments. EH, NI, TY, C-CL, AH, KT, AR, Anna Tury, MP, and EJ performed all the experiments under the supervision of YZ. YZ designed the antigen for Ryk monoclonal antibody. C-CL and AR prepared the antigen. S-HW generated the hybrodomas using the antigen under the supervision of AK. AT and EH screened for the hybridoma and tested the function of Ryk monoclonal antibody in vitro and in vivo.
References
- 1.Lemon RN, Griffiths J. Comparing the function of the corticospinal system in different species: Organizational differences for motor specialization? Muscle Nerve. 2005;32:261–279. doi: 10.1002/mus.20333. [DOI] [PubMed] [Google Scholar]
- 2.Whishaw IQ, Pellis SM, Gorny BP, Pellis VC. The impairments in reaching and the movements of compensation in rats with motor cortex lesions: an endpoint, videorecording, and movement notation analysis. Behav Brain Res. 1991;42:77–91. doi: 10.1016/S0166-4328(05)80042-7. [DOI] [PubMed] [Google Scholar]
- 3.Metz GAS, Dietz V, Schwab ME, van de Meent H. The effects of unilateral pyramidal tract section on hindlimb motor performance in the rat. Behavioural Brain Research. 1998;96:37–46. doi: 10.1016/S0166-4328(97)00195-2. [DOI] [PubMed] [Google Scholar]
- 4.Schmidlin E, Wannier T, Bloch J, Rouiller EM. Progressive plastic changes in the hand representation of the primary motor cortex parallel incomplete recovery from a unilateral section of the corticospinal tract at cervical level in monkeys. Brain Research. 2004;1017:172–183. doi: 10.1016/j.brainres.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 5.Weidner N, Ner A, Salimi N, Tuszynski MH. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proceedings of the National Academy of Sciences. 2001;98:3513–3518. doi: 10.1073/pnas.051626798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y, et al. Ryk-mediated Wnt repulsion regulates posterior-directed growth of corticospinal tract. Nat Neurosci. 2005;8:1151–1159. doi: 10.1038/nn1520. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, et al. Repulsive Wnt signaling inhibits axon regeneration after CNS injury. J Neurosci. 2008;28:8376–8382. doi: 10.1523/JNEUROSCI.1939-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollis ER, II, et al. Remodelling of spared proprioceptive circuit involving a small number of neurons supports functional recovery. Nat Commun. 2015;6 doi: 10.1038/ncomms7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hollis ER, II, Zou Y. Reinduced Wnt signaling limits regenerative potential of sensory axons in the spinal cord following conditioning lesion. Proceedings of the National Academy of Sciences. 2012;109:14663–14668. doi: 10.1073/pnas.1206218109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bareyre FM, et al. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- 11.Ghosh A, et al. Rewiring of hindlimb corticospinal neurons after spinal cord injury. Nat Neurosci. 2010;13:97–104. doi: 10.1038/nn.2448. [DOI] [PubMed] [Google Scholar]
- 12.Takeoka A, Vollenweider I, Courtine G, Arber S. Muscle Spindle Feedback Directs Locomotor Recovery and Circuit Reorganization after Spinal Cord Injury. Cell. 2014;159:1626–1639. doi: 10.1016/j.cell.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 13.van den Brand R, et al. Restoring Voluntary Control of Locomotion after Paralyzing Spinal Cord Injury. Science. 2012;336:1182–1185. doi: 10.1126/science.1217416. [DOI] [PubMed] [Google Scholar]
- 14.Giger RJ, Hollis ER, II, Tuszynski MH. Guidance Molecules in Axon Regeneration. Cold Spring Harb Perspect Biol. 2010 doi: 10.1101/cshperspect.a001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hollis ER., II Axon Guidance Molecules and Neural Circuit Remodeling After Spinal Cord Injury. Neurotherapeutics. 2015 doi: 10.1007/s13311-015-0416-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyuksyutova AI, et al. Anterior-Posterior Guidance of Commissural Axons by Wnt-Frizzled Signaling. Science. 2003;302:1984–1988. doi: 10.1126/science.1089610. [DOI] [PubMed] [Google Scholar]
- 17.Ditunno JF, Little JW, Tessler A, Burns AS. Spinal shock revisited: a four-phase model. Spinal Cord. 2004;42:383–395. doi: 10.1038/sj.sc.3101603. [DOI] [PubMed] [Google Scholar]
- 18.Leyton ASF, Sherrington CS. Observations on the excitable cortex of the chimpanzee, orang-utan, and gorilla. Experimental Physiology. 1917;11:135–222. doi: 10.1113/expphysiol.1917.sp000240. [DOI] [Google Scholar]
- 19.Streletz L, et al. Transcranial magnetic stimulation: Cortical motor maps in acute spinal cord injury. Brain Topogr. 1995;7:245–250. doi: 10.1007/BF01202383. [DOI] [PubMed] [Google Scholar]
- 20.Topka H, Cohen LG, Cole RA, Hallett M. Reorganization of corticospinal pathways following spinal cord injury. Neurology. 1991;41:1276–1283. doi: 10.1212/wnl.41.8.1276. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs KM, Donoghue JP. Reshaping the cortical motor map by unmasking latent intracortical connections. Science. 1991;251:944–947. doi: 10.1126/science.2000496. [DOI] [PubMed] [Google Scholar]
- 22.Arenkiel BR, et al. In Vivo Light-Induced Activation of Neural Circuitry in Transgenic Mice Expressing Channelrhodopsin-2. Neuron. 2007;54:205–218. doi: 10.1016/j.neuron.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayling OGS, Harrison TC, Boyd JD, Goroshkov A, Murphy TH. Automated light-based mapping of motor cortex by photoactivation of channelrhodopsin-2 transgenic mice. Nat Meth. 2009;6:219–224. doi: 10.1038/nmeth.1303. [DOI] [PubMed] [Google Scholar]
- 24.Hira R, et al. Transcranial optogenetic stimulation for functional mapping of the motor cortex. Journal of Neuroscience Methods. 2009;179:258–263. doi: 10.1016/j.jneumeth.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Kleim JA, Barbay S, Nudo RJ. Functional reorganization of the rat motor cortex following motor skill learning. J Neurophysiol. 1998;80:3321–3325. doi: 10.1152/jn.1998.80.6.3321. [DOI] [PubMed] [Google Scholar]
- 26.Girgis J, et al. Reaching training in rats with spinal cord injury promotes plasticity and task specific recovery. 2007;130 doi: 10.1093/brain/awm245. [DOI] [PubMed] [Google Scholar]
- 27.Fouad K, Tetzlaff W. Rehabilitative training and plasticity following spinal cord injury. Experimental Neurology. 2012;235:91–99. doi: 10.1016/j.expneurol.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Schmitt AM, et al. Wnt-Ryk signalling mediates medial-lateral retinotectal topographic mapping. Nature. 2006;439:31–37. doi: 10.1038/nature04334. [DOI] [PubMed] [Google Scholar]
- 29.Nishimura Y, et al. Time-dependent central compensatory mechanisms of finger dexterity after spinal cord injury. Science. 2007;318:1150–1155. doi: 10.1126/science.1147243. [DOI] [PubMed] [Google Scholar]
- 30.García-Alías G, Truong K, Shah PK, Roy RR, Edgerton VR. Plasticity of subcortical pathways promote recovery of skilled hand function in rats after corticospinal and rubrospinal tract injuries. Experimental Neurology. 2015;266:112–119. doi: 10.1016/j.expneurol.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piecharka DM, Kleim JA, Whishaw IQ. Limits on recovery in the corticospinal tract of the rat: Partial lesions impair skilled reaching and the topographic representation of the forelimb in motor cortex. Brain Research Bulletin. 2005;66:203–211. doi: 10.1016/j.brainresbull.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 32.Morawietz C, Moffat F. Effects of Locomotor Training After Incomplete Spinal Cord Injury: A Systematic Review. Archives of Physical Medicine and Rehabilitation. 2013;94:2297–2308. doi: 10.1016/j.apmr.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 33.McKenna JE, Prusky GT, Whishaw IQ. Cervical motoneuron topography reflects the proximodistal organization of muscles and movements of the rat forelimb: A retrograde carbocyanine dye analysis. The Journal of Comparative Neurology. 2000;419:286–296. doi: 10.1002/(sici)1096-9861(20000410)419:3<286::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 34.Lee JK, et al. Assessing Spinal Axon Regeneration and Sprouting in Nogo-, MAG-, and OMgp-Deficient Mice. Neuron. 2010;66:663–670. doi: 10.1016/j.neuron.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang F, Rouiller EM, Wiesendanger M. Modulation of sustained electromyographic activity by single intracortical microstimuli: comparison of two forelimb motor cortical areas of the rat. Somatosens Mot Res. 1993;10:51–61. doi: 10.3109/08990229309028823. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.