Abstract
For any given cardiac surgery, there are two invasive components: the surgical approach and the cardiopulmonary bypass circuit. The standard approach for cardiac surgery is the median sternotomy, which offers unrestricted access to the thoracic organs—the heart, lung, and major vessels. However, it carries a long list of potential complications such as wound infection, brachial plexus palsies, respiratory dysfunction, and an unpleasant-looking scar. The cardiopulmonary bypass component also carries potential complications such as end-organ dysfunction, coagulopathy, hemodilution, bleeding, and blood transfusion requirement. Furthermore, the aortic manipulation during cannulation and cross clamping increases the risk of dissection, arterial embolization, and stroke.
Minimally invasive cardiac surgery is an iconic event in the history of cardiothoracic medicine and has become a widely adapted approach as it minimizes many of the inconvenient side effects associated with the median sternotomy and bypass circuit placement. This type of surgery requires the use of novel perfusion strategies, especially in patients who hold the highest potential for postoperative morbidity. Cannulation techniques are a fundamental element in minimally invasive cardiac surgery, and there are numerous cannulation procedures for each type of minimally invasive operation. In this review, we will highlight the strategies and pitfalls associated with a minimally invasive cannulation.
Keywords: cardiopulmonary bypass, minimal invasive cardiac surgery, cannulation techniques and pitfall
Introduction
Minimally invasive cardiac surgery (MICS) has evolved rapidly over the last decade due to the development of specific strategies involving arterial venous cannulation,1–3 cardiopulmonary bypass (CBP), and myocardial preservation that have been tailored to the individual procedures.2,3
MICS procedures may be divided into two types: epicardial, such as a coronary artery bypass graft (CABG), and endocardial, such as valve procedures, cardiac mass resection, and atrial septal defect repair.3 Each of these procedures requires a specific approach for cannulation, cardiopulmonary bypass, and myocardial preservation.1–3 This review provides an overview of various MICS strategies and how they may be applied in individual procedures.
Arterial Cannulation
Options for arterial cannulation in MICS are the ascending aorta, femoral artery, or axillary artery.1–4 The choice is determined by the procedure being performed, the burden of atherosclerosis at the cannulation site, and patient factors such as body habitus.2,3 Central aortic and axillary cannulation has the advantage of antegrade flow, while femoral cannulation is convenient but carries a small risk of retrograde dissection, embolization, and ipsilateral limb ischemia.1–3,5–9
Preoperative computed tomography angiography (CTA) provides valuable information regarding arterial stenosis, tortuosity, and the presence of aneurysmal disease that can be useful in choosing a cannulation site.1–3,5–7,10 We advocate its use prior to all MICS procedures, especially when femoral arterial cannulation is planned. CT can also offer correlation with surface anatomy and help with more precise planning of incisions.2,3,10
Newer cannula designs combine smaller size with higher, laminar flows.1–3,5 The following describes current strategies when using each of the three sites for arterial cannulation.
Ascending Aorta
The cannula is usually inserted in the distal ascending aorta just inferior to the innominate artery (Figure 1).1–3,10 If central aortic cannulation is chosen as the arterial access site, intraoperative epiaortic ultrasound scanning is superior to palpation for locating the best site for cannulation and cross clamping. Ultrasound can identify soft, noncalcified plaques that are not easily felt and that present a high embolic risk.2,3,5,10 Central aortic cannulation mimics the approach used in sternotomy methods, and its use in MICS is limited by the degree of access to the ascending aorta. In addition, placing the cannula through a small incision can limit visibility.2,3,5,10
Figure 1.
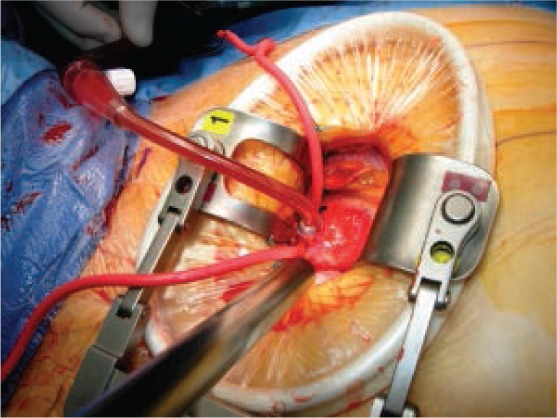
Central aortic cannula placed in the ascending aorta during minimally invasive coronary surgery.
The upper partial sternotomy approach for aortic valve replacement (AVR) offers good exposure of the entire ascending aorta and is most convenient for central cannulation.2,3,10 The right anterior thoracotomy approach for AVR offers good exposure of the proximal ascending aorta but less so of the distal segment, where cannulation is usually performed.2,3 Access to this segment can be improved with some simple maneuvers, but many surgeons simply choose an alternate site, usually femoral.2,3 The more lateral right chest incision that is required for MICS mitral valve procedures places the aorta slightly farther away, and most surgeons would opt for an alternate site when working on the aorta.2,3
One of the most feared complications of central aortic cannulation is dissection, which occurs in 0.01% to 0.09 % of patients.2,4,5 As a routine, the intraoperative transesophageal echocardiography (TEE) protocol should include examination for aortic dissection after cannulation and after instituting cardiopulmonary bypass (CPB).2,3,10 Difficult cannulation, a bluish hue to the aorta, or high arterial line pressure after instituting CPB should prompt suspicion.2,3 If TEE confirms the diagnosis of aortic dissection, CPB should be terminated immediately and alternate arterial cannulation performed, usually in the groin. CPB can then resume with systemic cooling to achieve deep hypothermic circulatory arrest. In these cases, the central cannula is left in place, and a median sternotomy is performed to replace the ascending aorta and proximal arch. The aortic valve is replaced while rewarming.2,3,5,10
The ideal cannula for central aortic cannulation in MICS should be easy to insert from a distance, reliably secured to prevent dislodgment, and safe to remove, with a built-in mechanism to seal the cannulation site.2,3,10
Femoral Artery
In the absence of significant aortoiliac disease, femoral arterial cannulation is the preferred choice of many surgeons for MICS procedures. It offers convenience and improved exposure through a limited chest incision.1–5,10 Exposure of the femoral artery and direct cannulation is probably the most common approach (Figure 2). The cannula is usually placed over a wire that is positioned in the true lumen of the aorta, as confirmed by TEE.1–3,5,11,12 In patients with small vessels, a 6- or 8-mm Dacron side graft may be used to provide inflow.2,3,5,11,12 Groin complications such as infection, hematoma, and lymphocele occur in a few cases and are more likely in obese patients.2,3,5,7,8
Figure 2.
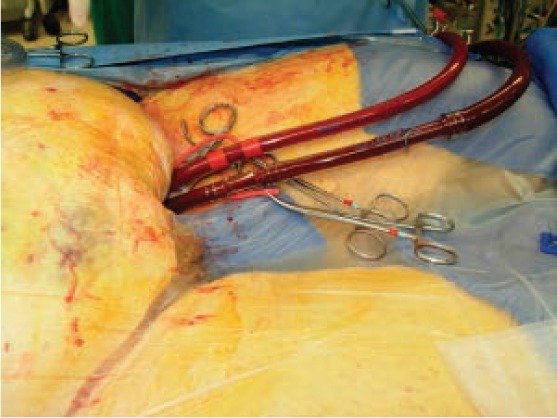
Percutaneous femoral artery and vein cannulation.
Percutaneous femoral cannulation may also be performed. This can be more difficult in obese patients who are most likely to benefit from this procedure. While infection and lymphocele are rare with this technique, hematomas may occur. Obtaining hemostasis after removing the cannula is facilitated by placement of sutures from percutaneous closure devices.1–3,5,7,11,12 Femoral artery cannulation may be performed prior to the chest incision. This is useful in redo chests where adhesions increase the risk of injury during dissection. It is often the quickest way to establish CPB in the event of an emergency.1–3,5
Femoral artery cannulation carries a small risk of retrograde aortic dissection, embolization, and ipsilateral limb ischemia. Antegrade perfusion of the limb using a small cannula reduces the risk of limb ischemia, as does the use of a side branch graft.2,3,6,7,13 We favor central cannulation in MICS AVR procedures and femoral or axillary cannulation in MICS mitral valve replacement (MVR) and MICS coronary artery bypass (CABG) cases.
Axillary Artery
Axillary artery cannulation offers the advantage of antegrade perfusion without crowding the operative field in MICS. While this site tends to be relatively disease free, its use is contraindicated in the presence of subclavian or innominate artery stenosis.8,9 Either side may be used, although the right is usually favored. This fragile vessel is accessed in the deltopectoral groove, and an 8-mm Dacron graft is sewn on as a side arm (Figure 3). This allows bidirectional flow in the axillary artery and eliminates the risk of ipsilateral limb ischemia that is associated with direct cannulation.1–3,9,14,15 The vessel may be cannulated prior to the chest incision, which is useful in redo MICS cases.1–3,9,14,15 Finally, in the event of a mishap that requires deep hypothermic circulatory arrest, the axillary artery may be used for antegrade cerebral perfusion during the period of circulatory arrest.2,3,9,14,15
Figure 3.
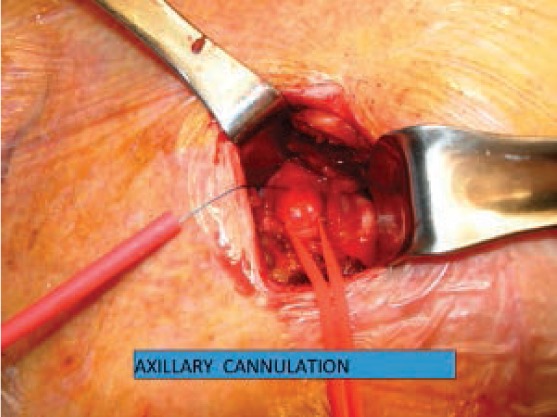
Direct cannulation of axillary artery.
Venous Cannulation
In a MICS procedure, venous drainage for CPB is performed via the femoral vein. Modern three-stage cannulas provide excellent drainage and decompression of the right heart with the aid of a kinetic drainage assist device.1–3,16–19 The latter is an important element of myocardial protection during cardioplegic arrest.2–3,16–19 The right femoral vein is favored because of the straighter alignment with the inferior vena cava.2,3,20,21 TEE is used to position the tip of the cannula in the superior vena cava, which is important for optimal drainage.2,3,12,19,20 This is effective for most MICS procedures including those involving the mitral valve.2,3,16 Similar to cannulation of the femoral artery, the cannula may be inserted open or percutaneously (Figure 4). The latter approach is preferred if the femoral artery is not being cannulated as this virtually eliminates groin complications.2,3,12,20,21
Figure 4.
Percutaneous cannulation of femoral vein with the tip of the cannula placed at the level of the right atrium and inferior vena cava.
Bicaval cannulation is necessary for MICS repair of atrial septal defect. In this procedure, the tip of the femoral cannula is positioned just below the junction of the inferior vena cava (IVC) and right atrium. The cannula for the superior vena cava (SVC) may be placed percutaneously from the neck or directly through the incision.1–3 The presence of an IVC filter or occlusion of the IVC is a contraindication for femoral venous cannulation and thus for MICS mitral valve cases or MICS CABG cases that require CPB. However, MICS AVR cases can still be performed by placing a low-profile cannula into the right atrium directly through the chest incision.2,3,17–19
Potential complications include perforation of the IVC during cannula insertion and entrapment of air with airlock during CPB. In addition, the length of the femoral vein cannula usually creates increased resistance to effective drainage. A vacuum-assisted device is often used in MICS cases to overcome this downfall and prevent any air entrapment during CBP.2,3,17–19
Myocardial Protection and Cardioplegia Administration
Cardioplegic arrest is required for intracardiac procedures. The ascending aorta may be cross-clamped through the primary incision or a separate smaller incision. The cross clamps used in MICS are longer, have a lower profile, and are readily available. However, surgeon preference usually determines their use.2,3,22–24
The IntraClude® balloon clamp (Edwards Lifesciences, Irvine, CA) allows end clamping of the aorta and is useful in redo cases. It is inserted from the femoral artery and requires TEE and frequently fluoroscopic guidance.2,3,22–24 Antegrade cardioplegia and venting of the aortic root is performed from the tip of the cannula. This clamp may not be used in the presence of aortoiliac disease. Some studies have shown an increased stroke rate with this technique, and balloon rupture during cardioplegic arrest can lead to complications.2,3,22–24
Conventional cardioplegia is used by most surgeons.2,3 Induction is usually achieved with an antegrade dose except when significant aortic regurgitation or coronary artery disease (especially left main artery) are present. This usually affects the distribution of antigrade cardioplegia and will not be sufficient to arrest the heart, thus retrograde may need to be added to achieve circulatory arrest.2,3,25,26 Cardioplegic arrest is maintained with repeat doses given at 15- to 20-minute intervals. These are usually given retrograde so as not to interrupt the flow of the operation.2,3,25–27 A special retrograde cannula is most conveniently placed percutaneously by the anesthesiologist. Although direct placement of a retrograde cannula by the surgeon is feasible, access is limited and would likely require TEE guidance.2,3 Alternatively, one may give intermittent administration of bolus antegrade cardioplegia directly into the coronary ostia in MICS AVR cases or the aortic root in MICS MVR cases.2,3,25,26 Studies have shown that a minimally invasive approach with antigrade perfusion does not result in increased neurologic consequences, while retrograde cardioplegia has shown to be associated with increased neurologic risk in older or high-risk patients.25,26
An alternative used by many is long-acting cardioplegia solutions. Del Nido cardioplegia offers 60 to 90 minutes of arrest before redosing is required, and custodial cardioplegia may offer even longer protection.27,28 Custodial solutions is routinely used for preservation of the harvested heart to be used for transplantation.29 Del Nido cardioplegia has a lower potassium content and is not a glucose-based product, thus it is considered more ideal for patients with renal failure and those with uncontrolled diabetes.26,27
Conclusion
There are many options for cannulation and myocardial protection in MICS. The procedure, patient variables, and surgical team experience will govern the correct choice. All of these require good communication and teamwork. Proper patient selection and preoperative planning of the specific minimally invasive approach are essential to overcome any obstacle that may present during the surgery.
Footnotes
Conflict of Interest Disclosure: The authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
References
- 1.Franco KL, Vinod HT. Cardiothoracic surgery review. Philadelphia: Lippincott Williams & Wilkins; c2012. p. 1770. p. [Google Scholar]
- 2.Gravlee GP, editor. Cardiopulmonary bypass: principles and practice. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; c2008. p. 783. p. [Google Scholar]
- 3.Mongero LB, Beck JR, editors. On bypass: advanced perfusion techniques. New York: Springer Science & Business Media; c2008. p. 576. p. [Google Scholar]
- 4.Grossi EA, Loulmet DF, Schwartz CF et al. Evolution of operative techniques and perfusion strategies for minimally invasive mitral valve repair. J Thorac Cardiovasc Surg. 2012 Apr;143(4 Suppl):S68–70. doi: 10.1016/j.jtcvs.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Murzi M, Glauber M. Central versus femoral cannulation during minimally invasive aortic valve replacement. Ann Cardiothorac Surg. 2015 Jan;4(1):59–61. doi: 10.3978/j.issn.2225-319X.2014.10.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gander JW, Fisher JC, Reichstein AR et al. Limb ischemia after common femoral artery cannulation for venoarterial extracorporeal membrane oxygenation: an unresolved problem. J Pediatr Surg. 2010 Nov;45(11):2136–40. doi: 10.1016/j.jpedsurg.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bisdas T, Beutel G, Warnecke G et al. Vascular complications in patients undergoing femoral cannulation for extracorporeal membrane oxygenation support. Ann Thorac Surg. 2011 Aug;92(2):626–31. doi: 10.1016/j.athoracsur.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 8.Preventza O, Bakaeen FG, Stephens EH, Trocciola SM, de la Cruz KI, Coselli JS. Innominate artery cannulation: an alternative to femoral or axillary cannulation for arterial inflow in proximal aortic surgery. J Thorac Cardiovasc Surg. 2013 Mar;145(3 Suppl):S191–6. doi: 10.1016/j.jtcvs.2012.11.061. [DOI] [PubMed] [Google Scholar]
- 9.Sinclair MC, Singer RL, Manley NJ, Montesano RM. Cannulation of the axillary artery for cardiopulmonary bypass: safeguards and pitfalls. Ann Thorac Surg. 2003 Mar;75(3):931–4. doi: 10.1016/s0003-4975(02)04497-1. [DOI] [PubMed] [Google Scholar]
- 10.Reser D, Holubec T, Scherman J, Yilmaz M, Guidotti A, Maisano F. Upper ministernotomy. Multimed Man Cardiothorac Surg. 2015 Nov 2;2015 doi: 10.1093/mmcts/mmv036. [DOI] [PubMed] [Google Scholar]
- 11.Pozzi M, Henaine R, Grinberg D et al. Total percutaneous femoral vessels cannulation for minimally invasive mitral valve surgery. Ann Cardiothorac Surg. 2013 Nov;2(6):739–43. doi: 10.3978/j.issn.2225-319X.2013.08.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazzitelli D, Guenzinger R, Schreiber C, Tassani-Prell P, Lange R. Percutaneous cannulation of the femoral vessels for cardiopulmonary bypass. Herz. 2008 Jul;33(5):374–6. doi: 10.1007/s00059-008-3043-2. [DOI] [PubMed] [Google Scholar]
- 13.Crooke GA, Schwartz CF, Ribakove GH et al. Retrograde arterial perfusion, not incision location, significantly increases the risk of stroke in reoperative mitral valve procedures. Ann Thorac Surg. 2010 Mar;89(3):723–9. doi: 10.1016/j.athoracsur.2009.11.061. discussion 729–30. [DOI] [PubMed] [Google Scholar]
- 14.Sabik JF, Nemeh H, Lytle BW et al. Cannulation of the axillary artery with a side graft reduces morbidity. Ann Thorac Surg. 2004 Apr;77(4):1315–20. doi: 10.1016/j.athoracsur.2003.08.056. [DOI] [PubMed] [Google Scholar]
- 15.Etz CD, Plestis KA, Kari FA et al. Axillary cannulation significantly improves survival and neurologic outcome after atherosclerotic aneurysm repair of the aortic root and ascending aorta. Ann Thorac Surg. 2008 Aug;86(2):441–6. doi: 10.1016/j.athoracsur.2008.02.083. [DOI] [PubMed] [Google Scholar]
- 16.Vistarini N, Aiello M, Mattiucci G et al. Port-access minimally invasive surgery for atrial septal defects: a 10-year single-center experience in 166 patients. J Thorac Cardiovasc Surg. 2010 Jan;139(1):139–45. doi: 10.1016/j.jtcvs.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 17.Colangelo N, Torracca L, Lapenna E, Moriggia S, Crescenzi G, Alfieri O. Vacuum-assisted venous drainage in extrathoracic cardiopulmonary bypass management during minimally invasive cardiac surgery. Perfusion. 2006 Nov;21(6):361–5. doi: 10.1177/0267659106071324. [DOI] [PubMed] [Google Scholar]
- 18.Vaughan P, Fenwick N, Kumar P. Assisted venous drainage on cardiopulmonary bypass for minimally invasive aortic valve replacement: is it necessary, useful or desirable? Interact Cardiovasc Thorac Surg. 2010 Jun;10(6):868–71. doi: 10.1510/icvts.2009.230888. [DOI] [PubMed] [Google Scholar]
- 19.De Somer F. Venous drainage--gravity or assisted? Perfusion. 2011 Sep;26(Suppl1):15–9. doi: 10.1177/0267659110394713. [DOI] [PubMed] [Google Scholar]
- 20.Jegger D, Chassot PG, Bernath MA et al. A novel technique using echocardiography to evaluate venous cannula performance perioperatively in CPB cardiac surgery. Eur J Cardiothorac Surg. 2006 Apr;29(4):525–9. doi: 10.1016/j.ejcts.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 21.Riley W, FitzGerald D, Cohn L. Single, percutaneous, femoral venous cannulation for cardiopulmonary bypass. Perfusion. 2007 May;22(3):211–5. doi: 10.1177/0267659107083021. [DOI] [PubMed] [Google Scholar]
- 22.Anderson MB, Shah SG, Arnold JL. Endoaortic occlusion with the intraclude device during the management of ascending aortic pseudoaneurysm. Innovations (Phila) 2015 Jan–Feb;10(1):71–2. doi: 10.1097/IMI.0000000000000117. [DOI] [PubMed] [Google Scholar]
- 23.Saito N, Matsumoto H, Yagi T et al. Evaluation of the safety and feasibility of resuscitative endovascular balloon occlusion of the aorta. J Trauma Acute Care Surg. 2015 May;78(5):897–903. doi: 10.1097/TA.0000000000000614. [DOI] [PubMed] [Google Scholar]
- 24.Bentala M, Heuts S, Vos R et al. Comparing the endo-aortic balloon and the external aortic clamp in minimally invasive mitral valve surgery. Interact Cardiovasc Thorac Surg. 2015 Sep;21(3):359–65. doi: 10.1093/icvts/ivv160. [DOI] [PubMed] [Google Scholar]
- 25.Murzi M, Cerillo AG, Miceli A et al. Antegrade and retrograde arterial perfusion strategy in minimally invasive mitral-valve surgery: a propensity score analysis on 1280 patients. Eur J Cardiothorac Surg. 2013 Jun;43(6):e167–72. doi: 10.1093/ejcts/ezt043. [DOI] [PubMed] [Google Scholar]
- 26.Grossi EA, Loulmet DF, Schwartz CF et al. Minimally invasive valve surgery with antegrade perfusion strategy is not associated with increased neurologic complications. Ann Thorac Surg. 2011 Oct;92(4):1346–9. doi: 10.1016/j.athoracsur.2011.04.055. discussion 1349–50. [DOI] [PubMed] [Google Scholar]
- 27.Matte GS, del Nido PJ. History and use of del Nido cardioplegia solution at Boston Children's Hospital. J Extra Corpor Technol. 2012 Sep;44(3):98–103. Erratum in: J Extra Corpor Technol. 2013 Dec;45(4):262. [PMC free article] [PubMed] [Google Scholar]
- 28.Kim K, Ball C, Grady P, Mick S. Use of del Nido Cardioplegia for Adult Cardiac Surgery at the Cleveland Clinic: Perfusion Implications. J Extra Corpor Technol. 2014 Dec;46(4):317–23. [PMC free article] [PubMed] [Google Scholar]
- 29.Zhidkov IL, Belianko IE, Sitnichenko NV, Paliulina MV, LaptiiIJ AV. [Effect of perfusion volume of the Custodiol solution on the efficiency of cardioplegia in experiment] Anesteziol Reanimatol. 2008 Sep–Oct;(5):42–7. [PubMed] [Google Scholar]


