Abstract
Unilateral agenesis of the pulmonary artery (UAPA) is a rare congenital anomaly. This report describes a 52-year-old female who gave a long history of chronic, recurrent, left-sided pulmonary infections related to UAPA. For many years, she was managed medically but the infection continued to recur. She eventually underwent left pneumonectomy and made a good recovery.
Keywords: pulmonary artery, pulmonary agenesis, congenital abnormalities, pneumonectomy
Introduction
Unilateral agenesis of the pulmonary artery (UAPA) occurs during embryogenesis due to an ipsilateral defect in the development of the sixth aortic arch.1 The condition is usually associated with congenital cardiac malformations, such as tetralogy of Fallot, ventricular septal defect, transposition of the great vessels, and aortic arch anomalies. This makes it easy to diagnose and treat early in life.1–3 We describe a case of isolated UAPA causing chronic recurrent chest infection, which failed to respond to medical management and was successfully treated via left pneumonectomy.
Case Presentation
The patient, a 52-year-old female, is a former chronic smoker with a long history of chronic cough, bronchitis, and recurrent pneumonia requiring multiple hospitalizations. She was eventually diagnosed with isolated left UAPA when a pulmonary perfusion test revealed excellent (95%) perfusion to the right lung but only 2% perfusion to the left lung. Throughout her life, her main symptom had been easy fatigability rather than shortness of breath. However, over the past 2 years she developed a worsening cough with occasional hemoptysis and paroxysmal nocturnal dyspnea (PND). She had no dyspnea at rest and did not require oxygen.
Recent computed tomographic angiography (CTA) (Figures 1, 2) showed a decrease in the left lung volume, hyperinflation of the right lung, mediastinal displacement to the left, left hemi diaphragm elevation, enlargement of the right pulmonary artery trunk, and absence of the left pulmonary artery. There was a right-sided aortic arch. These findings were confirmed via magnetic resonance image (MRI) (Figures 3, 4).
Figures 1, 2.
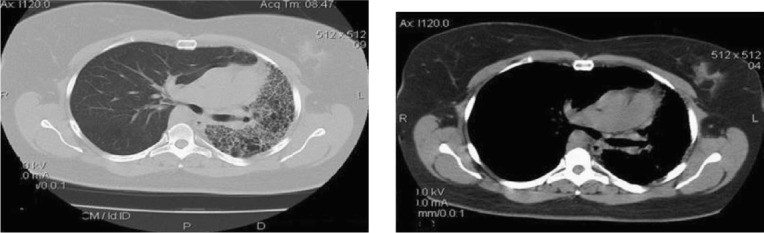
Computed tomographic angiography (CTA) showing hyperinflation of the right lung, decrease left lung volume, cardiac and mediastinal displacement to the left, enlargement of the pulmonary artery trunk, and absence of the left pulmonary artery.
Figures 3, 4.
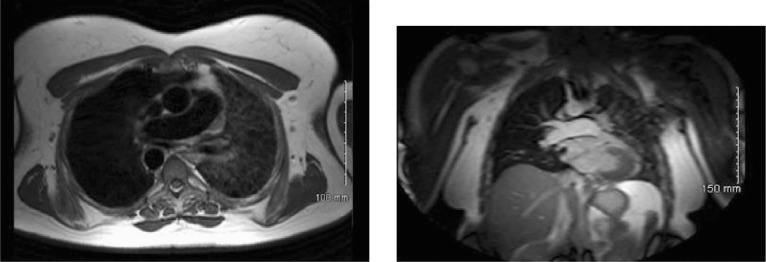
Right-side aortic arch and hypoplastic left pulmonary artery confirmed via magnetic resonance imaging.
On presentation, the patient's vitals were normal and she was afebrile. Examination revealed a hyperresonant right lung, diminished breath sounds, course crackles on the left side, and a slight left deviation of the trachea. All hematologic and biochemical profiles were normal. Pulmonary function testing showed a ratio of forced expiratory volume to forced vital capacity (FEV1/FVC) of 81%. FEV1, FVC, total lung capacity (TLC), and carbon monoxide diffusion capacity were 1.60 L, 1.98 L, 3.13, and 17.29, respectively. Elective pneumonectomy was recommended after carefully discussing the risks, benefits, possible complications, and alternative options with the patient. Shortly afterward, pneumonectomy via left anterior thoracotomy was performed.
During surgery the left lung was found to be scarred, fibrotic, and adherent to the chest wall. There were several enlarged bronchial collaterals and no pulmonary arterial branches exiting the mediastinum to feed the left lung. Both upper and lower pulmonary veins as well as collaterals were dissected and ligated. The diseased lung was removed, and the mediastinal pleura was then closed over the bronchial stump. Routine closure of the chest was done. The patient recovered well and was discharged home on postoperative day 10 with no complications. Six months later, the patient is doing well. No further hospitalization was needed, and she had no further chest infections.
Discussion
The proximal portion of the sixth aortic arch establishes the right and left pulmonary artery, and distorted development of any of those vessels will lead to ipsilateral absence of a pulmonary artery.1 The peripheral pulmonary vessels and vascular bed develop independently and receive blood supplied from a plexus of collateral vessels that usually originate from the aorta.1 These collateral vessels normally disappear early in life; however, in patients with UAPA, they usually remain open and supply blood to the intrapulmonary vessels.1 These include bronchial, intercostal, subclavian, intercostal, inferior phrenic artery, and subdiaphragmatic arteries.1,2 Approximately 4% of collaterals originate from the coronary arteries.3 The disease is estimated to be in about 1 in 200,000 individuals, and 98% of the cases will have the absent artery on the opposite side of the aortic arch.3,4
Right UAPA is more common than the left (67% vs 37%) and has a more benign course because it is rarely associated with other malformations.1 Since left UAPA is accompanied by congenital cardiovascular abnormalities, more than 75% of patients are diagnosed early in childhood; less than 25% of left UAPA cases exist in isolated fashion and are usually an incidental finding in adulthood.4 In isolated disease, patients can remain asymptomatic about 15% to 24% of the time, or they present with nonspecific symptoms.1,5 Symptoms in 40% of all UAPA cases include shortness of breath and exercise limitation, followed by frequent respiratory infections in 37% and recurrent hemoptysis in 20%.1 These symptoms are aggravated by factors such as smoking, pregnancy, or high altitude.1,5 Infections occur and are typically due to impairment of a mucociliary clearance mechanism and poor delivery of inflammatory cells to the site of inflammation.6 Hemoptysis is caused by excessive collateral circulation and fragility of the vessels.1,2,6 The inability of the vascular bed in the normal lung to cope with the increased blood perfusion leads to pulmonary hypertension in 44% of patients.1,6,7
Chest radiography can offer an important clue by showing asymmetry in the lung fields, a decrease in the size of the affected hemithorax, hyperinflation of the contralateral hemithorax, mediastinal deviation towards the affected side, and ipsilateral hemidiaphragm elevation.2,6 In addition, a contralateral pulmonary artery shadow and an ipsilateral reduction of the pulmonary vascular marking are usually noticed.2,6 Pulmonary function tests usually reveal a mild restrictive pattern with normal diffusion capacity, and ventilation-perfusion scintigraphy shows absence of perfusion on the affected side.3,8 Signs of chronic bronchitis are usually seen during bronchoscopy in those with recurrent lung infection, while blood vessel dilatation (mesh-like vascularization) is often seen in those with frequent episodes of hemoptysis.3,8 Although angiography was once the gold standard for diagnosing UAPA, it is rarely used given the improvement in CT and MRI quality.6–8 Hemoptysis is likely to resolve naturally; however, selective embolization is a valid alternative for patients with recurrent hemoptysis who are not eligible for surgery.1,3,8
Pneumonectomy is considered in cases of recurrent hemoptysis, recurrent pulmonary infections, and severe pulmonary hypertension (PH), and it is reported that 8% and 7% of patients undergo pneumonectomy and revascularization, respectively, for these three conditions.1,3,4,5,8 It is necessary that these patients be followed up closely, especially for observation of their pulmonary hemodynamics.4 Pharmacological treatment for PH is strongly recommended for patients unable to undergo surgical revascularization or in cases that are not improved after surgery. The overall mortality rate of UAPA was reported to be 7%, with common reasons of death being heart failure, respiratory failure, massive pulmonary hemorrhage, and pulmonary edema.3
Conclusion
UAPA is a rare congenital anomaly. The majority of cases are diagnosed and treated early in life because of the high association with congenital heart disease. In cases where UAPA occurs alone, patients can remain asymptomatic or may present with nonspecific symptoms. Patients are usually treated conservatively, however, surgery should be considered when patients do not respond well to conservative treatment.
Footnotes
Conflict of Interest Disclosure: The authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
References
- 1.Takahashi T, Endo H, Ito T, Takei T, Yagi K. Isolated unilateral absence of the left pulmonary artery: a case report. Ann Vasc Dis. 2014;7(2):178–82. doi: 10.3400/avd.cr.14-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Mello Junior WT, Coutinho N, ogueira JR, Santos M, Pelissari França WJ. Isolated absence of the right pulmonary artery as a cause of massive hemoptysis. Interact Cardiovasc Thorac Surg. 2008 Dec;7(6):1183–5. doi: 10.1510/icvts.2008.180430. [DOI] [PubMed] [Google Scholar]
- 3.Steiropoulos P, Archontogeorgis K, Tzouvelekis A, Ntolios P, Chatzistefanou A, Bouros D. Unilateral pulmonary artery agenesis: a case series. Hippokratia. 2013 Jan–Mar;17(1):73–6. [PMC free article] [PubMed] [Google Scholar]
- 4.Aypak C, Yıkılkan H, Uysal Z, Görpelioğlu S. Unilateral absence of the pulmonary artery incidentally found in adulthood. Case Rep Med. 2012;2012:942074. doi: 10.1155/2012/942074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Komatsu Y, Hanaoka M, Ito M et al. Unilateral absence of the pulmonary artery incidentally found after an episode of hemoptysis. Intern Med. 2007;46(21):1805–8. doi: 10.2169/internalmedicine.46.0257. [DOI] [PubMed] [Google Scholar]
- 6.Reading DW, Oza U. Unilateral absence of a pulmonary artery: a rare disorder with variable presentation. Proc (Bayl Univ Med Cent) 2012 Apr;25(2):115–8. doi: 10.1080/08998280.2012.11928802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koga H, Hidaka T, Miyako K, Suga N, Takahashi N. Age-related clinical characteristics of isolated congenital unilateral absence of a pulmonary artery. Pediatr Cardiol. 2010 Nov;31(8):1186–90. doi: 10.1007/s00246-010-9787-5. [DOI] [PubMed] [Google Scholar]
- 8.Bouros D, Pare P, Panagou P, Tsintiris K, Siafakas N. The varied manifestation of pulmonary artery agenesis in adulthood. Chest. 1995 Sep;108(3):670–6. doi: 10.1378/chest.108.3.670. [DOI] [PubMed] [Google Scholar]


