ABSTRACT
Protein phosphatase 2A (PP2A) is a heterotrimeric protein phosphatase consisting of a 36-kD catalytic C subunit (PP2Ac). This study aimed to explore the prognostic and biological significance of PP2Ac in human hepatocellular carcinoma (HCC). High PP2Ac expression was significantly (P < 0.01) associated with serum hepatitis B surface antigen positivity, serum hepatitis B e antigen positivity, liver cirrhosis, moderate to poor differentiation grade, advanced disease stage, intrahepatic metastasis, and early recurrence in HCC. Multivariate analysis revealed PP2Ac as an independent prognostic factor for overall survival. Enforced expression of hepatitis B virus X protein (HBx) and its carboxyl-terminal truncated isoform induced PP2Ac expression in HCC cells. Co-immunoprecipitation assay revealed a direct interaction between PP2Ac and HBx. Small interfering RNA-mediated knockdown of PP2Ac significantly inhibited in vitro cell proliferation, colony formation, migration, and invasion and reduced tumor growth in an xenograft mouse model. In contrast, overexpression of PP2Ac promoted HCC cell proliferation, colony formation, and tumorigenesis. Additionally, silencing of PP2Ac impaired the growth-promoting effects on HepG2 HCC cells elicited by overexpression of carboxyl-terminal truncated HBx. Gene expression profiling analysis showed that PP2Ac downregulation modulated the expression of numerous genes involved in cell cycle and apoptosis regulation. Collectively, PP2Ac upregulation has a poor prognostic impact on the overall survival of HCC patients and contributes to the aggressiveness of HCC. PP2Ac may represent a potential therapeutic target for HCC.
KEYWORDS: Growth, hepatitis B virus X protein, hepatocellular carcinoma, invasiveness, prognosis, protein phosphatase 2A
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third leading cause of cancer mortality worldwide.1 Chronic hepatitis B virus (HBV) infection is a major risk factor for HCC, especially in Asian countries.2 Hepatitis B virus X protein (HBx) encoded by the X gene of HBV plays a pivotal role in the onset and progression of HBV-associated HCC.3 HBx can regulate multiple biological events, including cell proliferation, apoptosis, migration and invasion.4 HBx gene is frequently integrated into the host genome in a 3′-end-deleted form, yielding a carboxyl-terminal truncated isoform (ct-HBx).5 Compared to full-length HBx (fl-HBx), the truncated HBx at 40 amino acid (ct-HBx3’-40) showed higher activities in promoting HCC cell growth and metastasis.6,7 HBx isoforms show the ability to regulate the expression of many genes involved in tumor development, such as p53, p21(WAF1), p14(ARF), MDM2, and Wnt5a.6,7
Protein phosphatase 2A (PP2A) is a heterotrimeric protein phosphatase consisting of a 36-kD catalytic C subunit (PP2Ac), a 65-kD structural A subunit, and a variable regulatory B subunit. PP2A is ubiquitously expressed and implicated in cell proliferation, development, survival, and tumorigenesis.8 In different types of human cancers, PP2A seems to play conflicting roles. While PP2A acts as a tumor suppressor in prostate cancer and breast cancer,9,10 pharmacological inhibition of PP2A leads to enhanced anticancer activity in head and neck squamous cell carcinoma and nasopharyngeal carcinoma. 11,12 A recent study has shown that PP2Ac is induced by transgenic expression of hepatitis C virus (HCV) proteins in the mouse liver, which inhibits DNA damage repair and contributes to diethylnitrosamine-induced hepatocellular carcinogenesis.13 However, the function of PP2Ac in human HCC, especially in HBV-related HCC remains elusive.
In this study, we explored the prognostic and biological significance of PP2Ac in human HCC. The regulatory effects of HBx isoforms on the expression of PP2Ac in HCC cells were investigated. We also checked the role of PP2Ac in mediating HBx-induced tumorigenesis of HCC cells.
Results
PP2Ac is upregulated in HCC and serves as an independent poor prognostic factor
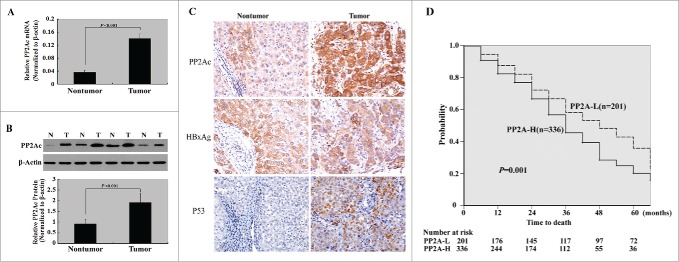
We first investigated the PP2Ac expression in 56 pairs of freshly resected HCC and adjacent nontumorous liver tissues. As illustrated in Figs. 1A and B, the PP2Ac mRNA and protein levels were consistently higher in HCC tissues than in adjacent nontumorous tissues.
Figure 1.
PP2Ac is upregulated in HCC and serves as an independent poor prognostic factor. (A) qPCR and (B) Western blot analysis of PP2Ac mRNA and protein levels in HCC and adjacent nontumorous liver tissues (n = 56), respectively. Representative Western blots show the PP2Ac protein expression in 4 pairs of HCC and nontumorous liver tissues. (C) Immunohistochemical staining for PP2Ac. A representative HCC case shows high cytoplasmic/membrane immunoreactivity for PP2Ac, but low PP2Ac staining in the adjacent nontumorous tissue. (D) Kaplan-Meier survival analysis. Overall survival curves were plotted for patients with high vs. low tumoral expression of PP2Ac (P = 0.001 by the log-rank test).
To validate the expression and clinical relevance of PP2Ac in HCC, we assessed its protein expression in an independent set of paraffin-embedded archival specimens (n = 537) using immunohistochemistry. Overall, 336 HCC specimens (62.6%) had high cytoplasmic/membrane immunoreactivity for PP2Ac, whereas only 23.6% of adjacent nontumorous tissues had high PP2Ac staining (Fig. 1C). Correlative analysis of PP2Ac expression and patients' clinical data revealed that high tumoral expression of PP2Ac was significantly associated with serum hepatitis B surface antigen (HBsAg; P = 0.006), serum hepatitis B e antigen (HBeAg; P = 0.001), liver cirrhosis (P = 0.003), histological grade (P < 0.001), TNM stage (P = 0.001), intrahepatic metastasis (P < 0.001), and early recurrence (P = 0.007) (Table 1).
Table 1.
Clinicopathologic factors and PP2Ac expression in hepatocellular carcinomas (n=537) based on immunohistochemistry.
| Variables | n | PP2Ac-L (n = 201) | PP2Ac-H (n = 336) | P |
|---|---|---|---|---|
| Sex | ||||
| Male | 380 | 136 | 244 | 0.222 |
| Female | 157 | 65 | 92 | |
| Age (y) | ||||
| <50 | 247 | 93 | 154 | 0.922 |
| ≥50 | 290 | 108 | 182 | |
| Serum AFP level (μg/l) | ||||
| <20 | 180 | 71 | 109 | 0.493 |
| ≥20 | 357 | 130 | 227 | |
| Serum HBsAg | ||||
| Positive | 402 | 137 | 265 | 0.006 |
| Negative | 135 | 64 | 71 | |
| Serum HBeAg | ||||
| Positive | 329 | 105 | 224 | 0.001 |
| Negative | 208 | 96 | 111 | |
| Tumor size | ||||
| ≤2 cm | 76 | 37 | 39 | 0.029 |
| >2 cm | 461 | 164 | 297 | |
| Histological grade | ||||
| Well differentiated | 127 | 68 | 59 | <0.001 |
| Moderately differentiated | 315 | 109 | 206 | |
| Poorly differentiated | 95 | 24 | 71 | |
| Liver cirrhosis | ||||
| Absent | 170 | 79 | 91 | 0.003 |
| Present | 367 | 122 | 245 | |
| Tumor capsule | ||||
| Intact | 116 | 48 | 68 | 0.193 |
| Absent or not intact | 421 | 143 | 268 | |
| Vascular invasion | ||||
| Absent | 116 | 50 | 66 | 0.154 |
| Present | 421 | 151 | 270 | |
| Intrahepatic metastasis | ||||
| Absent | 170 | 91 | 79 | <0.001 |
| Present | 367 | 110 | 257 | |
| TNM stage | ||||
| I + II | 115 | 58 | 57 | 0.001 |
| III + IV | 422 | 143 | 279 | |
| P53 | ||||
| − | 250 | 121 | 129 | <0.001 |
| + | 287 | 80 | 207 | |
| HBxAg | ||||
| − | 153 | 70 | 83 | 0.012 |
| + | 384 | 131 | 253 | |
| Early recurrence | ||||
| No | 230 | 101 | 129 | 0.007 |
| Yes | 307 | 100 | 207 |
NOTE: Significant P values are marked in bold.
Kaplan-Meier survival curves showed that high PP2Ac immunoreactivity was significantly associated with decreased overall survival (log-rank test, P = 0.001; Fig. 1D). We next performed multivariate survival analysis, using serum HBsAg level, serum HBeAg level, liver cirrhosis, intrahepatic metastasis, TNM stage, and PP2Ac expression as parameters. These parameters were found on univariate analysis (Table 2) to be predictive of overall survival. The results demonstrated that increased tumoral expression of PP2Ac was an independent prognostic parameter for decreased overall survival, with a relative risk of 2.67 ( 95% confidence interval, 1.49-6.38, P = 0.011; Table 3). Other independent prognostic factors were serum HBsAg (P = 0.047), serum HBeAg (P = 0.027), liver cirrhosis (P = 0.045), intrahepatic metastasis (P = 0.039), and TNM stage (P = 0.024).
Table 2.
Univariate analysis of overall survival in patients with hepatocellular carcinoma (n = 537).
| Overall survival |
||
|---|---|---|
| HR (95% CI) | P | |
| Sex | ||
| Male | 1.00 | |
| Female | 1.19 (0.57–2.73) | 0.567 |
| Age | ||
| <50 | 1.00 | |
| ≥50 | 1.39 (0.377–4.10) | 0.659 |
| Serum AFP level (μg /l) | ||
| <20 | 1.00 | |
| ≥20 | 1.09 (0.38-3.07) | 0.961 |
| Serum HBsAg | ||
| Negative | 1.00 | |
| Positive | 1.66 (1.17–2.82) | 0.031 |
| Serum HBeAg | ||
| Negative | 1.00 | |
| Positive | 2.16(1.34-5.14) | 0.027 |
| Tumor size | ||
| ≤2 cm | 1.00 | |
| >2 cm | 0.81 (0.44-3.56) | 0.424 |
| Histological grade | ||
| Well | 1.00 | |
| Moderate | 1.42 (0.46-8.84) | 0.683 |
| Poor | 1.39 ( 0.83-8.16) | 0.514 |
| Liver cirrhosis | ||
| Absent | 1.00 | |
| Present | 1.67 (0.78-3.44) | 0.047 |
| Tumor capsule | ||
| Intact | 1.00 | |
| Absent or not intact | 1.37 (0.63–3.56) | 0.217 |
| Intrahepatic metastasis | ||
| Not observed | 1.00 | |
| Observed | 2.39 (147-6.13) | 0.039 |
| TNM stage | ||
| I + II | 1.00 | |
| III + IV | 2.58 (1.31-5.78) | 0.021 |
| PP2Ac expression | ||
| Low (≤10%) | 1.00 | |
| High (>10%) | 2.86 (1.25–6.44) | 0.015 |
Abbreviations: HR, hazard ratio; 95% CI, 95% confidence interval.
Significant P values are marked in bold.
Table 3.
Multivariate analysis of overall survival in patients with hepatocellular carcinoma (n = 537).
| Overall survival |
||
|---|---|---|
| HR (95% CI) | P | |
| Serum HbsAg | ||
| Negative | 1.00 | |
| Positive | 1.81(1.33–7.62) | 0.047 |
| Serum HbeAg | ||
| Negative | 1.00 | |
| Positive | 2.34(1.49-6.01) | 0.027 |
| Liver cirrhosis | ||
| Absent | 1.00 | |
| Present | 1.84(1.21-4.37) | 0.045 |
| Intrahepatic metastasis | ||
| Not observed | 1.00 | |
| Observed | 239 (1.36–5.18) | 0.039 |
| TNM stage | ||
| I + II | 1.00 | |
| III + IV | 2.44(1.61-6.89) | 0.024 |
| PP2Ac expression | ||
| Low (≤10%) | 1.00 | |
| High (>10%) | 2.67(1.49-6.38) | 0.011 |
Abbreviations: HR, hazard ratio; 95% CI, 95% confidence interval.
Significant P values are marked in bold.
HBx isoforms promote the expression and phosphatase activity of PP2Ac in HCC cells
To check the effect of HBx isoforms on PP2Ac expression, HCC cells were transfected with fl-HBx- or ct-HBx3′-40-expressing plasmid and examined for the mRNA and protein levels of PP2Ac. As shown in Figs. 2A and B, enforced expression of fl-HBx significantly induced the expression of PP2Ac in both Huh7 and HepG2 cells. Overexpression of ct-HBx3′-40 also led to upregulation of PP2Ac in both the HCC cell lines. Additionally, PP2A phosphatase activity was significantly increased by the expression of exogenous fl-HBx and ct-HBx3′-40, compared with empty vector-transfected cells (Fig. 2C).
Figure 2.
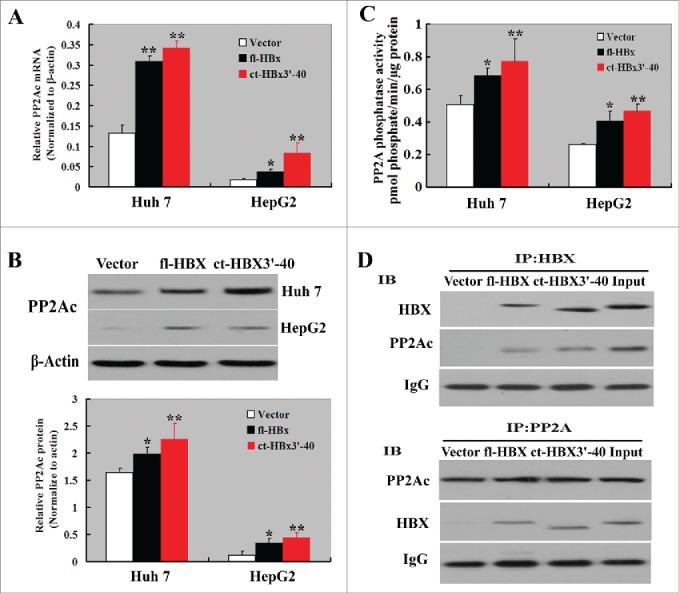
HBx isoforms promote the expression and phosphatase activity of PP2Ac in HCC cells. (A) qPCR and (B) Western blot analysis of the mRNA and protein levels of PP2Ac in HCC cells transfected with vector or fl-HBx- or ct-HBx-expressing plasmid, respectively. (C) PP2A phosphatase activity assay was performed to determine the effect of HBx isoforms on PP2Ac activity. Bar graphs indicate means ± standard deviation from 3 independent experiments. *P < 0.05, **P < 0.01 vs. empty vector-tansfected cells. (D) Interactions between PP2Ac and HBx isoforms detected by co-immunoprecipitation assay. Upper panels: Cell lysates were immunoprecipitated with anti-HBx antibody and Western blotted with anti-PP2Ac and anti-HBx antibody. Lower panels: Cell lysates were immunoprecipitated with anti-PP2Ac antibody and Western blots detected with anti-PP2Ac and anti-HBx antibody. The representative Western blots are shown from 3 independent experiments.
To test the possibility that HBx may physically interact with PP2A, Huh7 cells were transfected with HBx or ct-HBx3′-40 and then subjected to the co-immunoprecipitation experiment. As shown in Fig. 2D, PP2Ac was co-immunoprecipitated with HBx or ct-HBx3′-40. This co-immunoprecipitation was evident when cell lysates were immunoprecipitated with anti-HBx antibody and Western blotted with anti-PP2Ac antibody and also in reverse where lysates were immunoprecipitated with anti-PP2Ac antibody and Western blots detected with anti-HBx antibody. There was no evidence of co-immunoprecipitation of PP2Ac with HBx in empty vector-transfected Huh7 cells.
Downregulation of PP2Ac suppresses the aggressiveness of HCC cells
To explore the biological significance of PP2Ac in HCC cells, we specifically knocked down its expression in Huh7 cells. The delivery of different PP2Ac-specific shRNAs (shPP2Ac-A1 and shPP2Ac-A2) markedly reduced the expression of PP2Ac at both mRNA and protein levels, compared to control shRNA-transfected cells (Figs. S1A and S1B). PP2A phosphatase activity was also significantly (P < 0.01) decreased in PP2Ac-silenced Huh7 cells (Fig. S1C). The CCK-8 assay showed that downregulation of PP2Ac significantly reduced the proliferation of Huh7 cells within a 7-day period (P < 0.01; Fig. 3A). Colony formation assay demonstrated that depletion of PP2Ac significantly suppressed anchorage-independent colony formation of Huh7 cells (P < 0.01; Fig. 3B). Cell cycle analysis further revealed that PP2Ac silencing impaired the cell cycle progression, leading to a selective accumulation of cells in the G1 phase (Fig. 3C). BrdU assay showed that PP2Ac silencing resulted in a significant reduction in the number of S-phase cells, compared to control shRNA-transfected cells (P < 0.01; Fig. 3D). Quantification of apoptosis by annexin V/PI double staining indicated an about 2-fold higher apoptotic index for PP2Ac-silenced cells relative to control cells (Fig. 3E). The wound healing assay demonstrated that the mobility of PP2Ac-silenced Huh7 cells was significantly decreased compared to control cells (Fig. 3F). Consistently, the invasiveness of Huh7 cells was significantly (P < 0.01) reduced by PP2Ac downregulation (Fig. 3G).
Figure 3.
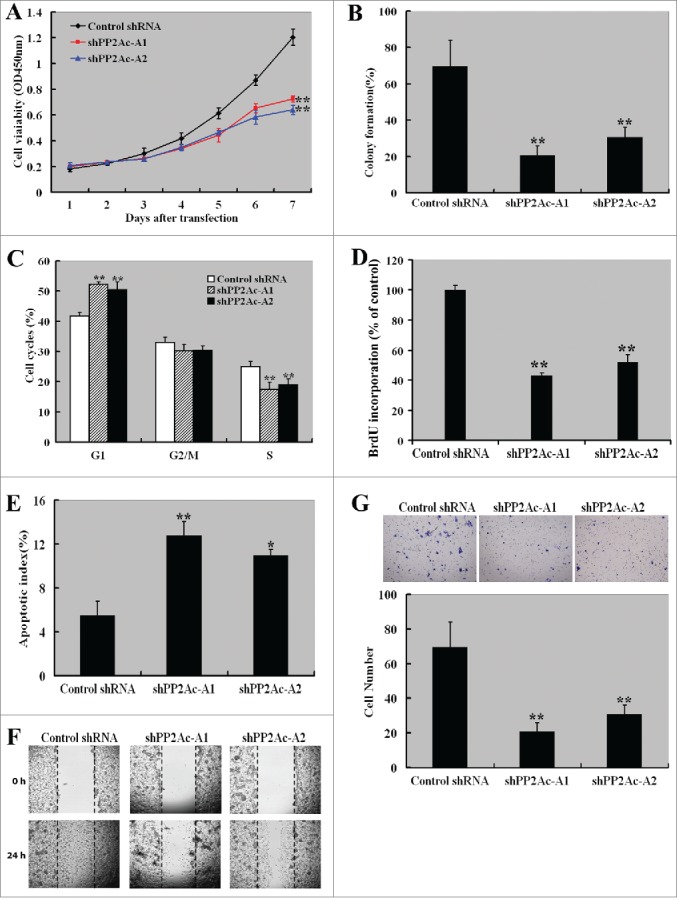
Downregulation of PP2Ac suppresses the aggressiveness of HCC cells. (A) CCK-8 assay showed that downregulation of PP2Ac significantly reduced the proliferation of Huh7 cells within a 7-day period. (B) Colony formation assay was performed to assess the effect of PP2Ac downregulation on the colony formation of Huh7 cells after 2-3 weeks of culture. (C) PI staining analysis of cell cycle distribution in Huh7 cells transfected with indicated shRNAs. (D) Assessment of the effect of PP2Ac knockdown on the number of S-phase cells by BrdU incorporation assay. The relative BrdU incorporation level was determined and reported as percentage of control. (E) Apoptosis detected by annexin-V/PtdIns staining. (F) Wound-healing assay was done to investigate the effect of PP2Ac downregulation on the migration of Huh7 cells. Representative images are shown from 3 independent experiments. (G) Transwell invasion assay was done to examine the effect of PP2Ac downregulation on the invasiveness of Huh7 cells. Bar graphs indicate means ± standard deviation from 3 independent experiments. *P < 0.05 , **P < 0.01 vs. control shRNA-tansfected cells. shPP2Ac-A1 and shPP2Ac-A2 represent 2 different PP2Ac-targeting shRNAs.
Depletion of PP2Ac inhibits tumor growth in an xenograft mouse model
Next, we checked whether manipulating PP2Ac affected tumor growth in a subcutaneous Huh7 xenograft model. Xenograft tumors were measured every 5 days. Statistically significant differences in tumor volume were noted from 5 days after cell injection (Fig. 4A). By the end of the 30-day period, there was about 40% decline in tumor volume in mice injected with PP2Ac-silenced Huh7 cells, compared to those injected with control cells (3950 ± 604 vs. 2315 ± 438 mm3, P < 0.01). At the end of the animal experiment, mice were killed and tumors were excised and weighed. Mice injected with shPP2Ac-A1-transfected cells had significantly lower mean tumor weight than those injected with control cells (0.92 ± 0.36 vs. 2.08 ± 0.30 g, P < 0.01; Fig. 4B). H&E staining analysis showed that shPP2Ac-A1 tumors had a marked reduction in stained tumor cells relative to control tumors (Fig. 4C). Immunohistochemical analysis revealed a clear decrease in Ki-67-immunoreactive proliferating cells in shPP2Ac-A1 tumors (64.6 ± 10.7% vs. 92.3 ± 11.8%, , P < 0.01; Fig. 4C).
Figure 4.
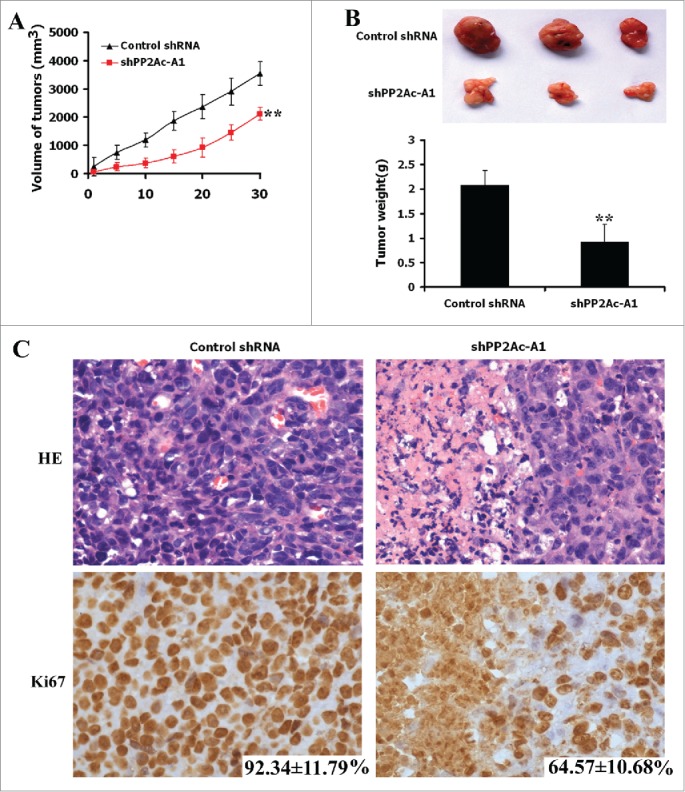
Downregulation of PP2Ac inhibits tumor growth in an Huh7 xenograft model. Huh7 cells stably transfected with shPP2Ac-A1 and control shRNA were injected into subcutaneous of BALB/c nude mice (n = 3) and tumor volume was measured every 5 days. The mice were observed for 30 days for tumor formation. (A and B) Downregulation of PP2Ac inhibits tumor growth as measured by tumor volume and tumor weight. *P < 0.05, **P < 0.01. (C) Representative images of H&E and Ki-67 staining of control shRNA- and PP2Ac-targeting shRNA-expressing Huh7 xenograft tumors.
Modulation of gene expression profiling by PP2Ac
To get more insight into the mechanism of PP2Ac action, we employed a real-time PCR array to profile the expression of genes involved in the signal transduction regulation. The gene expression profiling was analyzed in the shPP2Ac-A1 and control transfectants. Of the 84 genes examined, 17 showed a >5-fold change in expression level, 9 upregulated and 8 downregulated (Table 4). Interestingly, 9 upregulated genes >5-fold change, i.e. CDKN1A, WNT5A, CDKN1B, BBC3, NQO1, SLC2A1, RB1, FAS, and WNT2B. Among the down regulated genes <5-fold change were WNT3A, NOTCH1, FOSL1, CCND1, EGFR, MYC, ICAM1, and VEGF. To confirm the findings using the real-time PCR array, Western blot analysis was performed to check the expression changes at the protein level. p21, WNT5A, p27, BBC3, NQO1, SLC2A1, RB1, FAS, and WNT2B protein levels were significantly increased in PP2Ac-depleted cells. Conversely, the protein levels of WNT3A, NOTCH1, FOSL1, cyclin D1, EGFR, MYC, ICAM1, and VEGF were decreased by PP2Ac downregulation (Fig. 5).
Table 4.
Genes differentially expressed after knocked down of PP2Ac treatment in hepatoma 3B Cells (n = 3 )
| Refseq | Symbol | Description | Fold change of shPP2A-A1/Control shRNA |
|---|---|---|---|
| Up-regulated | |||
| NM_000389 | CDKN1A | Cyclin-dependent kinase inhibitor 1A (p21, Cip1) | 30.06 ± 2.354 |
| NM_003392 | WNT5A | Wingless-type MMTV integration site family, member 5A | 16.99 ± 1.427 |
| NM_004064 | CDKN1B | Cyclin-dependent kinase inhibitor 1B (p27, Kip1) | 15.73 ± 1.257 |
| NM_014417 | BBC3 | BCL2 binding component 3 | 10.23 ± 0.942 |
| NM_000903 | NQO1 | NAD(P)H dehydrogenase, quinone 1 | 6.59 ± 0.576 |
| NM_006516 | SLC2A1 | Solute carrier family 2 (facilitated glucose transporter), member 1 | 6.01 ± 0.533 |
| NM_000321 | RB1 | Retinoblastoma 1 | 5.89 ± 0.474 |
| NM_000043 | FAS | Fas (TNF receptor superfamily, member 6) | 5.65 ± 0.529 |
| NM_004185 | WNT2B | Wingless-type MMTV integration site family, member 2B | 5.46 ± 0.438 |
| Down-regulated | |||
| NM_033131 | WNT3A | Wingless-type MMTV integration site family, member 3A | 0.077 ± 0.019 |
| NM_017617 | NOTCH1 | Notch 1 | 0.135 ± 0.031 |
| NM_005438 | FOSL1 | FOS-like antigen 1 | 0.141 ± 0.027 |
| NM_053056 | CCND1 | Cyclin D1 | 0.146 ± 0.029 |
| NM_005228 | EGFR | Epidermal growth factor receptor | 0.189 ± 0.034 |
| NM_002467 | MYC | V-myc myelocytomatosis viral oncogene homolog (avian) | 0.196 ± 0.039 |
| NM_000201 | ICAM1 | Intercellular adhesion molecule 1 | 0.196 ± 0.048 |
| NM_003376 | VEGFA | Vascular endothelial growth factor A | 0.197 ± 0.057 |
Figure 5.
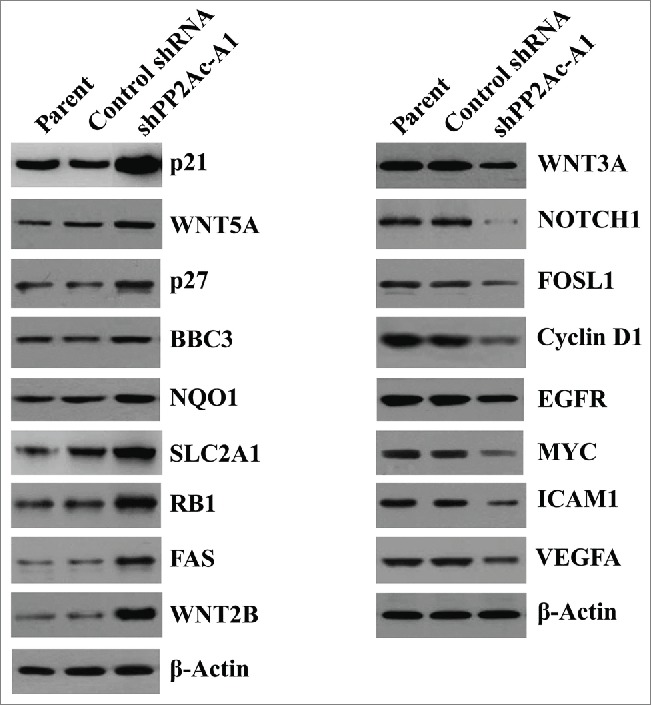
Modulation of gene expression by PP2Ac downregulation. Western blot analysis of indicated proteins in Huh7 cells transfected with control or PP2Ac-targeting shRNA and non-transfected parental cells. Representative blots are shown from 3 independent experiments.
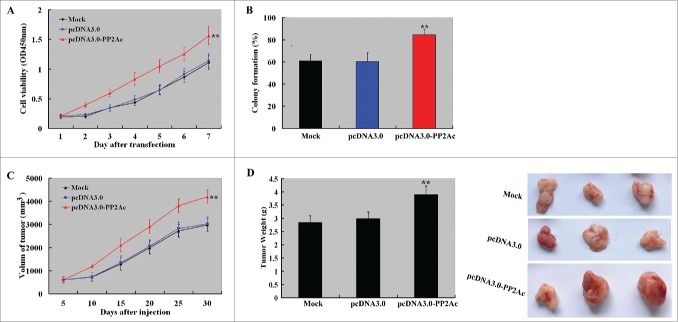
Overexpression of PP2Ac enhances the tumorigenecity of HCC cells
To examine the effect of PP2Ac overexpression on the tumorigenecity of HCC cells, we overexpressed PP2Ac in HepG2 cells with a low level of endogenous PP2Ac and tested cell proliferation, colony formation, and in vivo tumor formation. As shown in Figure 6A, PP2Ac-overexpressing cells displayed a significant increase in proliferation from 2 days after seeding, compared to empty vector-transfected cells (P < 0.01). PP2Ac-overexpressing cells also showed significantly higher colony formation efficiency than empty vector-transfected cells (P < 0.01; Fig. 6B). Consistent with the in vitro results, the volume of tumors arising from PP2Ac-overexpressing cells was significantly greater than those arising from empty vector-transfected cells at different time points examined (Fig. 6C). The tumor weight at the end of the experiment (30 days after cell injection) was also significantly raised in the PP2Ac-overexpressing group, compared to the vector control group (Fig. 6D).
Figure 6.
Overexpression of PP2Ac promotes the growth of HCC cells in vitro and in vivo. (A) CCK-8 assay showed that overexpression of PP2Ac significantly enhanced the proliferation of HepG2 cells within a 7-day period, compared to empty vector-transfected cells. (B) Colony formation assay revealed that PP2Ac overexpression significantly facilitated the formation of colonies after 2-3 weeks of culture. (C and D) Effects of PP2Ac overexpression on the growth of HepG2 xenograft tumors in nude mice (n = 3 mice for each group). Tumor volume was measured every 5 days and tumor volume-time curves were drawn (C). At the end of the experiment (30 days after cell injection, tumor xenografts were removed, weighted, and photographed (D). **P < 0.01 vs. empty vector group.
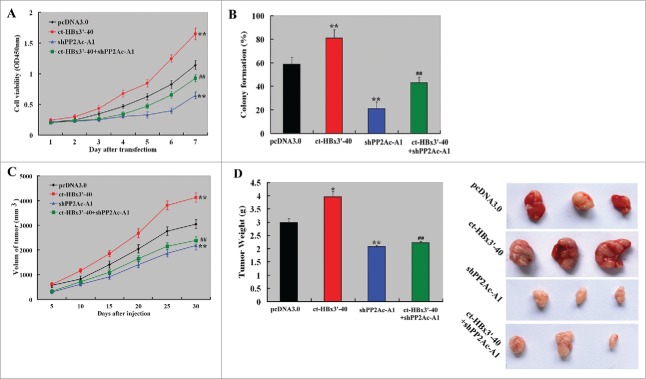
PP2Ac mediates the tumor-promoting role of HBx in HCC
Finally, we sought to check whether HBx-induced tumorigenesis in HCC was mediated through upregulation of PP2Ac. To this end, we co-transfected ct-HBx3′-40 and PP2Ac shRNA into HepG2 cells and evaluated the effect of PP2Ac silencing on HBx-induced tumor growth. We found that the delivery of ct-HBx3′-40 significantly enhanced in vitro cell proliferation (Fig. 7A) and colony formation (Fig. 7B), as well as in vivo tumor growth (Fig. 7C). Notably, the growth-promoting effects of overexpression of ct-HBx3′-40 were impaired by co-transfection of PP2Ac shRNA.
Figure 7.
PP2Ac knockdown abrogates HBx-induced tumorigenesis of HCC cells. (A) CCK-8 assay was done to measure the proliferation of HepG2 cells transfected with indicated constructs. (B) Colony formation assay demonstrated that co-transfection with PP2Ac shRNA significantly impaired the formation of colonies from ct-HBx3’-40-transfected HepG2 cells, compared to transfection of ct-HBx3’-40 alone. (C and D) Nude mice (n = 3 for each condition) were subcutaneously injected with HepG2 cells transfected with indicated constructs. Measurement of xenograft tumor volume was performed every 5 days and tumor volume-time curves were plotted (C). At the end of the experiment (30 days after cell injection), tumor xenografts were removed, weighted, and photographed (D). *P < 0.05, **P < 0.01 vs. empty vector group; ##P < 0.01 vs. transfection of ct-HBx3’-40 alone.
Discussion
A previous study reported that the PP2Ac mRNA abundance is significantly higher in human HCC tissue compared to non-tumorous surrounding tissues, as determined by quantitative PCR analysis.13 Our data confirmed the upregulation of PP2Ac in HCC tissue at the protein level by Western blot analysis and immunohistochemistry. HCV infection has been documented to upregulate the expression of PP2Ac in HCC cells.14,15 We found that high tumoral expression of PP2Ac was significantly correlated with serum HBsAg and HBeAg in HCC patients, suggesting a possible link between PP2Ac upregulation and HBV infection. This hypothesis was supported by in vitro data showing that overexpression of exogenous fl-HBx or ct-HBx led to a significant increase in the expression and activity of PP2Ac in HCC cell lines. Co-immunoprecipitation assays further validated the direct interaction of PP2Ac with HBx isoforms in HCC cells. HBx is an important oncoprotein involved in the development of HBV-related HCC.16 We found that HBx-induced HCC cell proliferation, colony formation, and tumor growth were significantly compromised by PP2Ac knockdown, suggesting a mediating role for PP2Ac in the pathogenesis of HBV-related HCC. Clinical data revealed that high PP2Ac expression was significantly associated with histological grade, TNM stage, intrahepatic metastasis, and early recurrence. Moreover, increased PP2Ac expression had a negative prognostic impact on HCC after complete resection. These findings suggest that PP2Ac upregulation contributes to the development and progression of HCC.
It has been documented that restoration of the α isoform of PP2Ac protein suppresses prostate tumor growth and metastasis in an orthotopic mouse model.17 Reactivation of PP2A has been found to block proliferation and induce caspase-dependent apoptosis in acute myeloid leukemia cells.18 Pharmacological activation of PP2A has been suggested as a promising strategy to treat c-KIT+ cancers.19 Despite these anticancer effects, PP2A may play a tumor-promoting role in some settings. For instance, targeting PP2A via antisense technology results in decreased proliferation of human multiple myeloma cells.20 In HCC, the function of PP2A also remains controversial. PP2A has been documented to mediate the proapoptotic effect of conjugated linoleic acid on SK-HEP-1 human HCC cells.21 In contrast, inhibition of PP2A enhances the cytotoxicity of chemotherapeutic drugs to HCC cells.22 To assess the direct role of PP2Ac in HCC cells, we specially knocked down its expression and examined its effects on aggressive phenotypes of HCC cells. Notably, we found that downregulation of PP2Ac inhibited the proliferation and colony formation, prevented S phase entry, and induced apoptosis in Huh7 cells. Moreover, PP2Ac silencing abrogated the migration and invasion of Huh7 cells. In vivo studies confirmed that PP2Ac downregulation delayed the growth of HCC xenograft tumors. Ectopic expression of PP2Ac in HepG2 cells with low endogenous PP2Ac led to increased cell proliferation, colony formation, and in vivo tumor growth. Taken together, our data uncover the critical role of PP2Ac in the aggressiveness of HCC. These findings also provide a biological explanation for the negative prognostic impact of PP2Ac on HCC.
PP2A has been shown to regulate the expression of many genes in various biological processes.8,23 We found that PP2Ac downregulation significantly increased the expression of p21, WNT5A, p27, BBC3, NQO1, SLC2A1, RB1, FAS, and WNT2B proteins. Both p21 and p27 act as cyclin-dependent kinase inhibitors and can induce cell cycle arrest at the G1 phase.24,25 The RB1 protein is also involved in the cell cycle G1 to S phase transition.26 WNT5A has been found to control the proliferation and migration of HCC cells.27 FAS is an apoptosis-inducing surface receptor involved in the pathogenesis of HBV-related HCC.28 BBC3 is a proapoptotic BH3-only protein that can sensitize HCC cells to sorafenib-induced apoptosis.29 Some tumor-promoting genes such as MYC,30 EGFR,31 VEGFA,32 and NOTCH133 are downregulated by PP2Ac silencing. These results offer a molecular insight into the regulation of the aggressiveness of HCC by PP2Ac. However, the direct mediators of the effects of PP2Ac downregulation are needed to further clarified.
In conclusion, our data demonstrate that PP2Ac upregulation is an independent poor prognostic factor for overall survival of HCC patients and PP2Ac contributes to the aggressive behaviors of HCC cells via alteration of gene expression profiling. We provide first evidence for the regulation of PP2Ac expression and activity by HBx. PP2Ac plays a mediating role in HBx-induced tumorigenesis of HCC cells. Further studies are warranted to explore the roles of the PP2Ac-HBx interactions in the pathogenesis of HBV-related HCC.
Materials and methods
Cell lines
Human HCC cell lines (HepG2 and Huh7) were purchased from the Institute of Cellular Research, Chinese Academy of Science, Shanghai, China. Cells were routinely cultured in RPMI1640 medium or Dulbecco's modified Eagle's medium with 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA), 50 U/mL penicillin, and 50 mg/mL streptomycin in a 5% CO2 incubator at 37°C.
Patient tissue samples
The study protocol was approved by the Ethics Committee of Second Military Medical University (Shanghai, China). Tumor samples from resection specimens were collected from 2 consecutive cohorts of patients with HCC, who underwent surgical resection for the disease at Changhai Hospital and Institute of Liver Diseases (Shanghai, China) between August 2007 and June 2009 for cohort A and between January 2001 and December 2005 for cohort B. The patients of both cohorts were selected on the basis of (a) distinctive pathologic diagnosis of HCC, (b) receiving curative resection, defined as macroscopically complete removal of the neoplasm, and (c) availability of detailed clinicopathologic data. Patients with preoperative anticancer treatment or with evidence of other malignancies were excluded from the study. Cohort A consisted of 56 patients, from whom fresh tumor samples coupled with adjacent nontumor liver tissues were obtained for gene expression analysis. After removal at surgery, fresh tissues were cut into small pieces, snap frozen in liquid nitrogen immediately, and stored at −80°C until RNA or protein extraction. Cohort B comprised 537 patients, whose paraffin-embedded tumor specimens were available at the surgical pathology archive of Changhai Hospital, allowing for immunohistochemical analysis. The clinicopathologic characteristics of both the study cohorts are summarized in Table S1. Of the 537 patients, 91 (17%) were lost to follow-up. The median follow-up time was 30 months (range, 6–72 months). By the end of follow-up, 344 patients (64%) died. Tumor recurrence was diagnosed on the basis of serum α-fetoprotein (AFP) elevation, imaging techniques including computed tomography, ultrasound, and magnetic resonance imaging, as well as histology.
Immunohistochemistry
Immunohistochemistry was performed by DAKO Envision+ Reagent (DakoCytomation, Carpinteria, CA, USA) as previously described. Briefly, paraffin sections (4-μm thick) were deparaffinized with xylene, rehydrated, and heated for 10 min in a steamer containing 10 mM of sodium citrate (pH 6.0) to retrieve antigen. Sections were incubated with anti-PP2Ac antibody (ab32141, Abcam, Cambridge, UK; dilution in 1:500) for 1 h, followed by the secondary reaction with DAKO Envision+ Reagent (DakoCytomation, Carpinteria, CA, USA). Negative controls were included by omitting the primary antibody, and a known positive control was included with each batch. Stained sections were independently assessed by 2 pathologists without prior knowledge of the clinical data. PP2Ac-high expression (PP2Ac-H) was defined as cytoplasmic staining of ≥10% of tumor cells and PP2Ac-low (PP2Ac-L) as no staining or staining of <10% of cells.
RNA isolation and quantitative PCR (qPCR) analysis
qPCR analysis was performed as previously described.34 Briefly, total RNA was extracted from cells or tissues with an RNeasy mini kit (Qiagen, Germany) according to the manufacturer's protocol. cDNA (cDNA) was synthesized from total RNA with the First-Strand cDNA Synthesis Kit for RT-PCR (Roche, Mannheim, Germany). The qPCR was performed using SYBR-green detection of PCR products in real time with the Light Cycler (Roche Diagnostics, Meylan, France). The cycling conditions were as follows: initial denaturation at 95°C for 10 min, and then 45 cycles of denaturation at 95°C for 5 s, annealing at 62°C for 20 s, and elongation at 72°C for 15 s. β-Actin was used as an internal control. The ratios of PP2Ac to β-actin represented normalized relative levels of PP2Ac expression. A no-template negative control was also included in each experiment. Analyses of all samples were carried out in triplicate, and the mean values were calculated. The primers were list as follows: PP2Ac, forward primer 5′- CCACAGCAAGTCACACATTGG -3′, reverse primer 5′- CAGAGCACTTGATCGCCTACAA -3′;13 β-actin, forward primer 5′-GAGCGGGAAATCGTGCGTGACATT-3′, reverse primer 5′-GATGGAGTTGAAGGTAGTTTCGTG-3′.
Protein extraction and Western blotting analysis
The following antibodies were used in this study: HBx (ab39716, 1:500), PP2Ac antibody (ab32141, 1:500), p21 ab80633, 1:500), WNT5A (ab110073, 1:1000), p27 (ab193379, 1:1000), BBC3 (ab33906, 1:500), NQO1 (ab28947, 1:500), SLC2A1 (ab652, 1:500), RB1 (ab24, 1:500), FAS (ab15285, 1:500), WNT2B (ab50575, 1:500), WNT3A (ab81614, 1:1000), NOTCH1 (ab128076, 1:500), cyclin D1 (ab6152, 1:500), MYC (ab56, 1:500), ICAM1 (ab2213, 1:500), VEGF (ab46154, 1:500), β-actin (ab8227, 1:2000; Abcam), EGFR (#4267,1:500), and FOSL1 (#5841,1:500; Cell Signaling Technology, Danvers, MA, USA).
Whole cell lysates were prepared from tissue samples and cultured cells with radioimmunoprecipitation assay buffer with freshly added 0.01% protease inhibitor cocktail (Sigma, St. Louis, MO, USA). Protein concentration was measured by BCA protein assay kit (Thermo Fisher Scientific). Proteins were resolved on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a nitrogen cellular membrane (Amersham life Science). After incubation with blocking solution (5% fat-free milk and 0.1% Tween 20) for 2 h at room temperature, the membrane was incubated with primary antibodies, followed by HRP-conjugated secondary antibody (Rockland, Gilbertsville, PA, USA). The signals were detected using the LAS4000 (GE healthcare).
Plasmid construction, stable transfection and detection
Construction of plasmids expressing fl-HBx or ct-HBx3′-40 was performed as previously described.6,7 Full-length human PP2Ac cDNA (GenBank accession number NM_002715) was amplified by PCR with the following primers: 5′-TAGAATTCCAATGGACGAGAAGGTGTTCAC-3′ and 5′-TAGGTACCTTACAGGAAGTAGTCTGGGGT-3′.35 The PCR products were purified and cloned into the pcDNA3.0 vector (Invitrogen, Carlsbad, CA, USA). Huh7 cells were transfected with HBX, HBX-40′, or empty vector using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. After transfection for 72 h, G418 (Invitrogen) was added to the medium with a final concentration of 0.6 mg/mL for positive cells selection, and positive cells were verified by Western blot analysis.
Two synthesized DNA nucleotide fragments (GenePharma Co. Ltd.) encoding short hairpin RNA (shRNA) for knockdown of endogenous PP2Ac (shPP2Ac-A1, CCTCTCGGTTTGGGAATAAGG; shPP2Ac-A2, TCCTGGAGTGTATCCTGTTTATT) were inserted into psiSTRIKE (shPP2Ac-A1, and shPP2Ac-A2). The same vectors with irrelevant nucleotides not targeting any annotated human genes (control shRNA) were used as negative controls. The constructs were transfected into HCC cells using Lipofectamine 2000. For establishing stable PP2Ac knockdown cells, shPP2Ac-A1, shPP2Ac-A2 and control shRNA plasmids were transfected into HCC cells. Transfected cells were selected for 2 weeks in the presence of 0.5 mg/mL of G418. The expression level of PP2Ac was determined by qPCR and Western blot analysis. G418-resistant cells were pooled and used in the subsequent experiments.
Cell proliferation assay
Transfected cells were seeded on 96-well plates at a density of 1×103 cells per well and after incubation for indicated times, cells were collected. Cell viability was measured using the Cell Counting Kit-8 (Dojindo Laboratories) according to the instructions of the manufacturer. Briefly, 10 μL of CCK-8 solution was added to each well of the plate, and the plate was incubated at 37°C for 1 h. The absorbance was measured at 450 nm to represent the cell viability. All experiments were independently repeated at least 3 times.
Colony formation assay
For colony formation, 5×104 cells were seeded onto a 10-cm plate and cultured with fresh media for 24 h. Cells were then cultured with media containing 1 mg/mL G418 in a humidified 5% CO2 atmosphere at 37°C for 2-3 weeks to allow colony formation. The medium was changed every 3 days. Colonies were fixed with methanol and stained with 1% crystal violet. Colonies having >50 cells were counted.
Cell cycle and apoptosis analysis
Cells were seeded on 6-well plates at 3×105 cells per well and after incubation for 72 h, cells were collected for cell cycle or apoptosis analysis. Cellular DNA content was analyzed by flow cytometry as described previously.34,36 Briefly, cells were fixed in ethanol, and incubated with 0.5 mg/ml of propidium iodide (PtdIns) along with 0.1 mg/ml of RNase A (200 KU; Calbiochem, San Diego, CA, USA). Apoptosis was measured with an Annexin-V/FITC kit (Trevigen, Gaithersburg, MD, USA) according to the manufacturer's protocol. Cells were analyzed on a FACScan flow cytometer with the CellQuest software (BD Biosciences).
BrdU incorporation assay
Cells transfected with control shRNA or PP2Ac specific shRNAs were seeded onto 96-well plates and and allowed to grow for 24 h. Cells were then incubated with 5-bromo-2'-deoxyuridine (BrdU) for 4 h and subjected to BrdU incorporation assays using the BrdU Cell Proliferation Assay kit according to the manufacturer's protocol (Cell Signaling Technology, Danvers, MA, USA).
Monolayer wound healing assay
Migration ability was measured using a wound-healing assay. Huh 7 cells transfected with shPP2Ac or control shRNA were allowed to grow to confluence. Confluent cell monolayers were scraped using a 200-μL pipette tip to form a scratch wound, and washed twice with medium, then cultured with new culture medium. After 24 h, the wound recovery was observed under a phase-contrast microscope (Leica, Solms, Germany).
Transwell invasion assay
Huh 7 cells with control shRNA and shPP2Ac were seeded on a 24-well plate with 5×104 cells per well. Cell invasion was assessed using 24-well inserts (Becton Dickinson, Franklin Lakes, NJ, USA) with 8-μm pores membranes, the membranes were coated with Matrigel (BD Biosciences, Bedford, MA, USA) that were prehydrated in serum-free medium according to the manufacturer's protocol. After 24 h of incubation, the cells in the upper chamber were removed, and the cells were fixed in 4% paraformaldehyde and stained with the Wright–Giemsa solution (Polysciences, Warrington, PA, USA). Digital images were obtained under a microscope. The invasion cells were quantified in 3 randomly selected fields at ×200 magnification in each membrane, and the average value was defined as the invasion index on 3 independent membranes.
Co-immunoprecipitation assay
All immunoprecipitation procedures were carried out at 4oC. Huh7 cells transfected with vector or fl-HBX or ct-HBX3’-40 were harvested, washed twice, and resuspended in lysis buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 1.0% Triton X-100, 1 mM EDTA and protease inhibitor cocktail). The lysates were incubated with anti-HBX (1:500) or anti-PP2Ac (1:100) antibody for 1 h, followed by incubation with protein A-Sepharose beads (Sigma-Aldrich Co. LLC) at 4°C overnight. The protein–antibody complexes that were recovered on beads were subjected to Western blot analysis after separation by SDS–PAGE.
PP2A phosphatase activity assay
Huh7 cells transfected with vector or fl-HBX or ct-HBX3’-40 were lysed in 25 mM Tris-HCl, 10 mM mercaptoethanol, 2 mM ethylenediaminetetra-acetic acid, 1mM benzamidine, 0.1 mM phenylmethanesulphonylfluoride. PP2A activity in whole cell extracts was measured using the Serine/Threonine Phosphatase Assay System (Promega, Wallisellen, Switzerland) according to the manufacturer's instructions.
Tumorigenicity in nude mice
Male BALB/c nude mice (5-6 week old) were purchased from Shanghai Experimental Animal Center of the Chinese Academy of Sciences (Shanghai, China). HCC cells with stable transfection of indicated constructs were injected into the subcutaneous tissue of mice (3 mice for each group). Kinetics of tumor formation was estimated by measuring tumor volume at every 5-d interval. Tumor volume was determined using the following formula: volume = 0.5 × width2 × length. The mice were observed for 30 days for tumor formation and then sacrificed. The number and diameter of tumors were measured. All experimental manipulations were undertaken in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, with the approval of the Scientific Investigation Board of the Shanghai University of Traditional Chinese Medicine, Shanghai, China.
Human Signal Transduction PathwayFinder RT2 Profiler™ PCR Array
To profile the gene expression associated with signal transduction pathway regulation, we employed the Human Signal Transduction PathwayFinger RT2 Profiler™ PCR Array (SuperArray, Frederick, MD, USA). RNA isolation, DNase treatment, and RNA clean-up were performed according to the manufacturer's protocol (Qiagen, Hilden, Germany) and as described previously.34 The isolated RNA was reverse transcribed into cDNA using the RT2 First Strand Kit (Invitrogen). PCR was performed using the RT2 SYBR Green qPCR Master Mix (Invitrogen) on an ABI PRISM7900 instrument (Applied Biosystems, Foster City, CA, USA). Data normalization was based on correcting all Ct values for the average Ct values of several constantly expressed housekeeping genes present on the array.37 The differential expressed genes were defined as fold change over 2 folds. The analysis was completed by Shanghai KangChen Bio-tech Company, Shanghai, China. Each assay was conducted in triplicate.
Statistical analysis
Data are presented as means ± standard deviation (SD). All statistical calculations were carried out using SPSS.11 software (SPSS, Chicago, IL, USA). The relationship between PP2Ac expression, HBsAg and HBx COOH-terminal deletion of HCCs was statistically analyzed by using the Pearson Chi-square test. Spearman's bivariate correlation was used to determine whether there is a positive or negative correlation between the HBx mutation and PP2Ac expression levels. Overall survival and recurrence-free survival were calculated using the Kaplan-Meier method and log-rank tests. The prognostic significance of clinicopathologic factors was determined using univariate Cox regression analysis. The variables that were found to be of prognostic significance in univariate analysis were put into a Cox proportional hazards model for multivariate analysis. A difference was defined as significant at P < 0.05 in Student's t- test, one-way ANOVA, or Chi-square test. A correlation coefficient >0.5 or <−0.5 was considered significant for Spearman's correlation.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported in part by grants from National Nature Science Foundation of China (No. 81072020 and 81172311 to S.H. Zhang, and No. 30973458 to A.M. Xu) and from ‘085’ first-class discipline construction of science and technology innovation in Shanghai University of Traditional Chinese Medicine (No. 085ZY1220 to S.H. Zhang).
References
- 1. Venook AP, Papandreou C, Furuse J, de Guevara LL: The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist 2010; 15 Suppl 4:5-13; PMID:21115576; http://dx.doi.org/ 10.1634/theoncologist.2010-S4-05 [DOI] [PubMed] [Google Scholar]
- 2. Nagaoki Y, Hyogo H, Aikata H, Tanaka M, Naeshiro N, Nakahara T, Honda Y, Miyaki D, Kawaoka T, Takaki S, et al. . Recent trend of clinical features in patients with hepatocellular carcinoma. Hepatol Res 2012; 42:368-75; PMID:22151896; http://dx.doi.org/ 10.1111/j.1872-034X.2011.00929.x [DOI] [PubMed] [Google Scholar]
- 3. Lau CC, Sun T, Ching AK, He M, Li JW, Wong AM, Co NN, Chan AW, Li PS, Lung RW, et al. . Viral-human chimeric transcript predisposes risk to liver cancer development and progression. Cancer Cell 2014; 25:335-49; PMID:24582836; http://dx.doi.org/ 10.1016/j.ccr.2014.01.030 [DOI] [PubMed] [Google Scholar]
- 4. Ng SA, Lee C: Hepatitis B virus X gene and hepatocarcinogenesis. J Gastroenterol 2011; 46:974-90; PMID:21647825; http://dx.doi.org/ 10.1007/s00535-011-0415-9 [DOI] [PubMed] [Google Scholar]
- 5. Quetier I, Brezillon N, Revaud J, Ahodantin J, DaSilva L, Soussan P, Kremsdorf D: C-terminal-truncated hepatitis B virus X protein enhances the development of diethylnitrosamine-induced hepatocellular carcinogenesis. J Gen Virol 2015; 96:614-25; PMID:25519169; http://dx.doi.org/ 10.1099/vir.0.070680-0 [DOI] [PubMed] [Google Scholar]
- 6. Liu X, Zhang S, Lin J, Zhang S, Feitelson MA, Gao H, Zhu M: Hepatitis B virus X protein mutants exhibit distinct biological activities in hepatoma Huh7 cells. Biochem Biophys Res Commun 2008; 373:643-7; PMID:18602370; http://dx.doi.org/ 10.1016/j.bbrc.2008.06.087 [DOI] [PubMed] [Google Scholar]
- 7. Liu X, Wang L, Zhang S, Lin J, Zhang S, Feitelson MA, Gao H, Zhu M: Mutations in the C-terminus of the X protein of hepatitis B virus regulate Wnt-5a expression in hepatoma Huh7 cells: cDNA microarray and proteomic analyses. Carcinogenesis 2008; 29:1207-14; PMID:18477650; http://dx.doi.org/ 10.1093/carcin/bgn111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seshacharyulu P, Pandey P, Datta K, Batra SK: Phosphatase: PP2A structural importance, regulation and its aberrant expression in cancer. Cancer Lett 2013; 335:9-18; PMID:23454242; http://dx.doi.org/ 10.1016/j.canlet.2013.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chien W, Sun QY, Lee KL, Ding LW, Wuensche P, Torres-Fernandez LA, Tan SZ, Tokatly I, Zaiden N, Poellinger L, et al. . Activation of protein phosphatase 2A tumor suppressor as potential treatment of pancreatic cancer. Mol Oncol 2015; 9:889-905; PMID:25637283; http://dx.doi.org/ 10.1016/j.molonc.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rincon R, Cristobal I, Zazo S, Arpi O, Menendez S, Manso R, Lluch A, Eroles P, Rovira A, Albanell J, et al. . PP2A inhibition determines poor outcome and doxorubicin resistance in early breast cancer and its activation shows promising therapeutic effects. Oncotarget 2015; 6:4299-314; PMID:25726524; http://dx.doi.org/ 10.18632/oncotarget.3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhu DW, Yuan YX, Qiao JK, Yu C, Yang X, Wang LZ, Zhang ZY, Zhong LP: Enhanced anticancer activity of a protein phosphatase 2A inhibitor on chemotherapy and radiation in head and neck squamous cell carcinoma. Cancer Lett 2015; 356:773-80; PMID:25449438; http://dx.doi.org/ 10.1016/j.canlet.2014.10.024 [DOI] [PubMed] [Google Scholar]
- 12. Lv P, Wang Y, Ma J, Wang Z, Li JL, Hong CS, Zhuang Z, Zeng YX: Inhibition of protein phosphatase 2A with a small molecule LB100 radiosensitizes nasopharyngeal carcinoma xenografts by inducing mitotic catastrophe and blocking DNA damage repair. Oncotarget 2014; 5:7512-24; PMID:25245035; http://dx.doi.org/ 10.18632/oncotarget.2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Duong FH, Dill MT, Matter MS, Makowska Z, Calabrese D, Dietsche T, Ketterer S, Terracciano L, Heim MH: Protein phosphatase 2A promotes hepatocellular carcinogenesis in the diethylnitrosamine mouse model through inhibition of p53. Carcinogenesis 2014; 35:114-22; PMID:23901063; http://dx.doi.org/ 10.1093/carcin/bgt258 [DOI] [PubMed] [Google Scholar]
- 14. Duong FH, Filipowicz M, Tripodi M, La Monica N, Heim MH: Hepatitis C virus inhibits interferon signaling through up-regulation of protein phosphatase 2A. Gastroenterology 2004; 126:263-77; PMID:14699505; http://dx.doi.org/ 10.1053/j.gastro.2003.10.076 [DOI] [PubMed] [Google Scholar]
- 15. Duong FH, Christen V, Lin S, Heim MH: Hepatitis C virus-induced up-regulation of protein phosphatase 2A inhibits histone modification and DNA damage repair. Hepatology 2010; 51:741-51; PMID:20043320; http://dx.doi.org/ 10.1002/hep.23388 [DOI] [PubMed] [Google Scholar]
- 16. Guerrieri F, Belloni L, Pediconi N, Levrero M: Molecular mechanisms of HBV-associated hepatocarcinogenesis. Semin Liver Dis 2013; 33:147-56; PMID:23749671; http://dx.doi.org/ 10.1055/s-0033-1345721 [DOI] [PubMed] [Google Scholar]
- 17. Bhardwaj A, Singh S, Srivastava SK, Arora S, Hyde SJ, Andrews J, Grizzle WE, Singh AP: Restoration of PPP2CA expression reverses epithelial-to-mesenchymal transition and suppresses prostate tumour growth and metastasis in an orthotopic mouse model. Br J Cancer 2014; 110:2000-10; PMID:24642616; http://dx.doi.org/ 10.1038/bjc.2014.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cristobal I, Garcia-Orti L, Cirauqui C, Alonso MM, Calasanz MJ, Odero MD: PP2A impaired activity is a common event in acute myeloid leukemia and its activation by forskolin has a potent anti-leukemic effect. Leukemia 2011; 25:606-14; PMID:21233840; http://dx.doi.org/ 10.1038/leu.2010.294 [DOI] [PubMed] [Google Scholar]
- 19. Roberts KG, Smith AM, McDougall F, Carpenter H, Horan M, Neviani P, Powell JA, Thomas D, Guthridge MA, Perrotti D, et al. . Essential requirement for PP2A inhibition by the oncogenic receptor c-KIT suggests PP2A reactivation as a strategy to treat c-KIT+ cancers. Cancer Res 2010; 70:5438-47; PMID:20551067; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kang HS, Choi I: Protein phosphatase 2A modulates the proliferation of human multiple myeloma cells via regulation of the production of reactive oxygen intermediates and anti-apoptotic factors. Cell Immunol 2001; 213:34-44; PMID:11747354; http://dx.doi.org/ 10.1006/cimm.2001.1861 [DOI] [PubMed] [Google Scholar]
- 21. Muzio G, Maggiora M, Oraldi M, Trombetta A, Canuto RA: PPARalpha and PP2A are involved in the proapoptotic effect of conjugated linoleic acid on human hepatoma cell line SK-HEP-1. Int J Cancer 2007; 121:2395-401; PMID:17691108; http://dx.doi.org/ 10.1002/ijc.23004 [DOI] [PubMed] [Google Scholar]
- 22. Bai X, Zhi X, Zhang Q, Liang F, Chen W, Liang C, Hu Q, Sun X, Zhuang Z, Liang T: Inhibition of protein phosphatase 2A sensitizes pancreatic cancer to chemotherapy by increasing drug perfusion via HIF-1alpha-VEGF mediated angiogenesis. Cancer Lett 2014; 355:281-7; PMID:25304380; http://dx.doi.org/ 10.1016/j.canlet.2014.09.048 [DOI] [PubMed] [Google Scholar]
- 23. Janssens V, Rebollo A: The role and therapeutic potential of Ser/Thr phosphatase PP2A in apoptotic signalling networks in human cancer cells. Curr Mol Med 2012; 12:268-87; PMID:22300139; http://dx.doi.org/ 10.2174/156652412799218930 [DOI] [PubMed] [Google Scholar]
- 24. Liu Y, Zhao J, Zhang W, Gan J, Hu C, Huang G, Zhang Y: lncRNA GAS5 enhances G1 cell cycle arrest via binding to YBX1 to regulate p21 expression in stomach cancer. Sci Rep 2015; 5:10159; PMID:25959498; http://dx.doi.org/ 10.1038/srep10159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Siriwardana G, Seligman PA: Iron depletion results in Src kinase inhibition with associated cell cycle arrest in neuroblastoma cells. Physiol Rep 2015; 3:1-10; PMID:25825542; http://dx.doi.org/ 10.14814/phy2.12341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schweyer S, Bachem A, Bremmer F, Steinfelder HJ, Soruri A, Wagner W, Pottek T, Thelen P, Hopker WW, Radzun HJ, et al. . Expression and function of protein phosphatase PP2A in malignant testicular germ cell tumours. J Pathol 2007; 213:72-81; PMID:17590861; http://dx.doi.org/ 10.1002/path.2203 [DOI] [PubMed] [Google Scholar]
- 27. Bi L, Liu X, Wang C, Cao Y, Mao R, Li P, Geng M: Wnt5a involved in regulation of the biological behavior of hepatocellular carcinoma. Int J Clin Exp Pathol 2014; 7:987-95; PMID:24696716 [PMC free article] [PubMed] [Google Scholar]
- 28. Zou C, Chen J, Chen K, Wang S, Cao Y, Zhang J, Sheng Y, Huang A, Tang H: Functional analysis of miR-181a and Fas involved in hepatitis B virus-related hepatocellular carcinoma pathogenesis. Exp Cell Res 2015; 331:352-61; PMID:25449696; http://dx.doi.org/ 10.1016/j.yexcr.2014.11.007 [DOI] [PubMed] [Google Scholar]
- 29. Fernando J, Sancho P, Fernandez-Rodriguez CM, Lledo JL, Caja L, Campbell JS, Fausto N, Fabregat I: Sorafenib sensitizes hepatocellular carcinoma cells to physiological apoptotic stimuli. J Cell Physiol 2012; 227:1319-25; PMID:21604268; http://dx.doi.org/ 10.1002/jcp.22843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cheng Q, Yuan F, Lu F, Zhang B, Chen T, Chen X, Cheng Y, Li N, Ma L, Tong T: CSIG promotes hepatocellular carcinoma proliferation by activating c-MYC expression. Oncotarget 2015; 6:4733-44; PMID:25749381; http://dx.doi.org/ 10.18632/oncotarget.2900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fang Z, Zhou L, Jiang S, Cao L, Yu L: UNC50 prompts G1/S transition and proliferation in HCC by regulation of epidermal growth factor receptor trafficking. PLoS One 2015; 10:e0119338; PMID:25738771; http://dx.doi.org/ 10.1371/journal.pone.0119338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang L, Wang JN, Tang JM, Kong X, Yang JY, Zheng F, Guo LY, Huang YZ, Zhang L, Tian L, et al. . VEGF is essential for the growth and migration of human hepatocellular carcinoma cells. Mol Biol Rep 2012; 39:5085-93; PMID:22161247; http://dx.doi.org/ 10.1007/s11033-011-1304-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yu LX, Zhou L, Li M, Li ZW, Wang DS, Zhang SG: The Notch1/cyclooxygenase-2/Snail/E-cadherin pathway is associated with hypoxia-induced hepatocellular carcinoma cell invasion and migration. Oncol Rep 2013; 29:362-70; PMID:23124652; http://dx.doi.org/ 10.3892/or.2012.2103 [DOI] [PubMed] [Google Scholar]
- 34. Liu AW, Cai J, Zhao XL, Jiang TH, He TF, Fu HQ, Zhu MH, Zhang SH: ShRNA-targeted MAP4K4 inhibits hepatocellular carcinoma growth. Clin Cancer Res 2011; 17:710-20; PMID:21196414; http://dx.doi.org/ 10.1158/1078-0432.CCR-10-0331 [DOI] [PubMed] [Google Scholar]
- 35. Barr RK, Lynn HE, Moretti PA, Khew-Goodall Y, Pitson SM: Deactivation of sphingosine kinase 1 by protein phosphatase 2A. J Biol Chem 2008; 283:34994-5002; PMID:18852266; http://dx.doi.org/ 10.1074/jbc.M804658200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhu Z, Luo Z, Li Y, Ni C, Li H, Zhu M: Human inhibitor of growth 1 inhibits hepatoma cell growth and influences p53 stability in a variant-dependent manner. Hepatology 2009; 49:504-12; PMID:19085961; http://dx.doi.org/ 10.1002/hep.22675 [DOI] [PubMed] [Google Scholar]
- 37. Livak KJ, Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25:402-8; PMID:11846609; http://dx.doi.org/ 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.