abstract
NANOG is a transcription factor that is involved in the self-renewal of embryonic stem cells (ES) and is a critical factor for the maintenance of the undifferentiated state of pluripotent cells. Extensive data in the literature show that the NANOG gene is aberrantly expressed during the development of malignancy in cancer cells. ES and cancer stem cells (CSCs), a subpopulation of cancer cells within the tumor, are thought to share common phenotypic properties.
This review describes the role of NANOG in cancer cell proliferation, epithelial-mesenchymal transition (EMT), apoptosis and metastasis. In addition, this paper illustrates a correlation between NANOG and signal transducer and activator of transcription 3 (STAT3) in the maintenance of cancer stem cell properties and multidrug resistance.
Together, the available data demonstrate that NANOG is strictly involved in the process of carcinogenesis and is a potential prognostic marker of malignant tumors.
KEYWORDS: Angiogenesis, apoptosis, cancer, cancer stem cells, metastasis, NANOG, STAT3
Abbreviations
- 4’-OHT
4’-hydroxytamoxifen
- AICAR
5‑Aminoimidazole-4-carboxyamide ribonucleoside
- ALDH1+
aldehyde dehydrogenase 1 positive
- AML
human acute myeloid leukemia
- AMPK
AMP‑activated protein kinase
- BMI1
B lymphoma Mo-MLV insertion region 1 homolog
- BMP
bone morphological proteins
- CSCs
cancer stem cells
- CTL
cytotoxic T, lymphocytes
- EGF
epidermal growth factor
- EMT
epithelial-mesenchymal transition
- ES
embryonic stem cells
- FAK
focal adhesion kinase
- FGF2
fibroblast growth factor 2
- FLK1
Fetal liver kinase-1
- GAC
gastric adenocarcinoma
- HA
hyaluronan
- HCC
hepatocellular carcinoma
- HDACI
histone deacetylase inhibitors
- Hh
hedgehog
- HIF1
hypoxia-inducible factor 1
- HNC
head and neck cancer
- HNSCC
head and neck squamous cell carcinoma
- HPC
hypopharyngeal cancer
- IGF1R
insulin-like growth factor 1 receptor
- IL-6
interleukin 6
- KLF4
krupper-like factor 4
- LIF
leukemia inhibitory factors
- MDR1
multidrug resistance 1 receptor
- miRNA
micro RNA
- mRNA
mRNA
- NEP1-40
Nogo-A inhibitory peptide 1-40
- NgR,
Nogo-66 receptor
- NPC
nasopharyngeal carcinoma
- NSCLS
non-small cells lung cancer
- OCT4
octamer-binding transcription factor
- OSCC
oral squamous cell carcinoma
- p53
protein 53
- PDCD4
programed cell death 4
- PDGF
platelet-derived growth factor
- PI-PLC
phosphatidylinositol-specific phospholipase C
- PTCH
parathyroid hormone receptor
- RFS
recurrence-free survival
- RNAi
RNA interference
- Shh
Binding of sonic Hh
- SHP-1
src-homology protein tyrosine phosphatase 1
- SMO
smoothened receptor
- SOX2
sex determing region Y HMG-box 2
- STAT3
signal transducer and activator of transcription 3
- TF
transcription factor
- TIC
tumor initiating cells
- Tregs
regulatory T cells
- VEGF
vascular endothelial growth factor
- VEGFR2
vascular endothelial growth factor receptor 2.
Introduction
NANOG is a transcription factor that is involved in the self-renewal of embryonic stem cells (ES). It was first discovered by Chambers et al.1 and Mitsui et al.2 in mouse ES cells and described as an important transcription regulator that both activates the repressors and suppresses the activators of differentiation. The name NANOG derives from Tìr nan Òg, the mythical Celtic land of youth. The NANOG protein possesses a homeobox sequence, containing a 60-63 amino acid motif, within the coded protein. The superfamily of homeobox genes is highly diverse and includes several classes which are subdivided into gene families. The best-known gene families among the ANTP class are the Hox, En, Dlx, Evx, NK-2 and Msx families. Conversely, the PRD class includes the Pax, Gsc and Otx gene families.3-5
The NANOG gene is located on chromosome 12 at 12p13.31. The chromosomal region containing the NANOG gene can undergo tandem duplication, which generates 2 copies of NANOG on chromosome 12. The two copies are identical in 97% of these events, although their transcripts are often differentially spliced. The second copy is a pseudogene, which is known as NANOGP1 or NANOG2. NANOGP1 possesses regions with high homology to NANOG introns and exons.
There are 10 additional known NANOG pseudogenes that develop as a result of mRNA (mRNA) retrotransposition, and they are characterized by the absence of introns and the 5′ promoter sequences. These pseudogenes often possess only a residual polyadenylation tract and flanking repeats. The NANOG pseudogenes are numbered from NANOGP2 to NANOGP11. Two of these NANOG pseudogenes are located on the X chromosome, while 2 are located on chromosome 6, and the rest are located on chromosomes 2, 7, 9, 10, 14, and 15. NANOGP1, P2, P4, P7, P8, P9 and P10 show 90% homology to NANOG, and NANOGP5 shows 85% homology to this parental gene.
The human NANOG protein consists of 305 amino acids and possesses 3 functional domains: the N-terminal domain, which contains 94 amino acids; the homeodomain, which contains 60 amino acids; and the C-terminal domain, which contains 151 amino acids. Along with NANOG, NANOGP1 is expressed in human ES cells and has a length of 232 amino acids. NANOGP2, P4, P5, P9 and P10 harbor premature stop codons, and as a result, the truncated proteins are translated in the same reading frame. NANOGP7 and P8 do not contain stop codons and are able to encode full-length proteins. NANOGP8 encodes a full-length protein with a length of 305 amino acids that differs from the NANOG gene by only 3 amino acids.6-10
NANOG, together with octamer-binding transcription factor 4 (OCT4) and sex-determining region Y HMG-box 2 (SOX2), is responsible for maintaining ES cells in an undifferentiated state. NANOG mRNA can only be detected in the epiblast. A small amount of NANOG mRNA is also present in germ cells, but NANOG mRNA is not observed in the native cells of adult organisms.5,6,11,12 One exception is human fibroblasts, which possess low levels of OCT4, SOX2 and NANOG mRNA. However, the NANOG protein can only be detected in human fibroblast CRL-2352 cells in the presence of fibroblast growth factor 2 (FGF2).13 It is thought that ES and cancer stem cells (CSCs), a subpopulation of cancer cells within the tumor, share some common phenotypic properties. For example, both cell types (ES cells and CSCs) are characterized by intensive growth and high expression of telomerase, which is responsible for the acquisition of immortality. Trophoblastic cells and cancer cells are also able to infiltrate local tissues. It has been demonstrated that expression of the NANOG gene occurs not only in embryonic-derived malignancies but also in breast cancer, ovarian cancer, cervical cancer and kidney cancer.14 The aim of the present review is to summarize the current body of literature on the role of NANOG in tumorigenesis, including cancer cell proliferation, epithelial-mesenchymal transition (EMT), apoptosis and metastasis.
Cancer stem cells
It is well established that both normal tissues and the tumors that develop within them contain heterogeneous cell types, such as immune, mesenchyme and endothelial cells. However, only a small percentage of tumor cells actually possess tumorigenic potential. The characteristic properties that distinguish normal tissues from malignant ones are attributed to cancer stem cells, which are cells within the tumor that have the capacity to self-renew and give rise to the heterogeneous lineage of cells that constitute the tumor. CSCs are alternatively referred to as “tumor-initiating cells” (TIC) and “tumorigenic cells” in the literature.15-17
CSCs were first discovered in human acute myeloid leukemia (AML). Lapidot et al. designed an in vivo experimental model in which AML-initiating cells were transplanted into immune-deficient mice. These experiments showed that AML consists of 2 fractions of AML cells: colony-forming cells and less mature leukemia- initiating cells.18 Since 2012, it has only been possible to experimentally define CSCs by their ability to generate a tumor based on transplantation assays. However, the existence of CSCs in undisturbed tumors has not been proven. Driessens et al. traced the growth of squamous skin tumors in vivo using genetic lineage tracing. To this end, they marked the different stages of tumor progression and found that the majority of the cells within the population show only a limited proliferative potential and are the non-tumorigenic progeny of CSCs. The minority fraction consists of 2 groups of cells: those with stem-cell-like properties that cycle twice a day, and those that produce differentiated cells and cycle more slowly.15,17,19
Years of investigations revealed that the NANOG gene and its isoforms, together with OCT4 and SOX2, play an important role in the development of a malignant phenotype in cells. NANOG belongs to the group of transcription factors (TF), which together with leukemia inhibitory factors (LIF) and bone morphogenetic proteins (BMPs), are responsible for the self-renewal and pluripotency of ES cells. However, NANOG can sustain pluripotency in ES cells even in the absence of LIF. In addition, pluripotency can be regulated independent of LIF by E-cadherin, which is able to regulate NANOG transcription via Signal transducer and activator of transcription 3 (STAT3) phosphorylation in ES cells.20,21,22
It is thought that ES cells and CSCs share some common phenotypic properties, such as intensive growth and high expression of telomerase, which is responsible for cell immortality. Trophoblastic cells and cancer cells are also able to infiltrate local tissues. Although ES cells and CSCs share common properties, there are some differences between them. Both cell types are able to self-renew, but ES cells promote differentiation, while CSCs promote proliferation. Overexpression of NANOG in ES cells maintains specific differentiation, whereas overexpression of this gene in CSCs results in inhibition of apoptosis.
NANOG mRNA can be detected only in the epiblast and in germ cells and is not observed in the healthy cells of adult organisms (apart from fibroblasts).15,23 Therefore, Shan et al. conducted an investigation of whether NANOG may serve as a biomarker for CSCs. Both western blotting and immunohistochemistry analyses showed that the expression of NANOG was absent in healthy liver tissues. Conversely, the expression of NANOG was high in hepatocellular carcinoma (HCC) tissues and moderately high in the surrounding (non-HCC) tissues. Cancer cells that express NANOG exhibit a high capacity for self-renewal and differentiation. Furthermore, NANOG maintains the self-renewal of CSCs through the insulin-like growth factor 1 receptor (IGF1R) signaling pathway. Knocking down the expression of NANOG in NANOG-positive cells decreases the expression of IGF1R. Furthermore, IGF1R inhibitors block the self-renewal of NANOG-positive CSCs.24
Nanog and the ability of cancer cells to metastasize
The formation of metastases is a process that requires the reduction of cell-cell interactions and the migration of cells through the extracellular matrix. This process requires changes in cell phenotypes, including reorganization of the cytoskeleton.24
Recent data show that CSCs with a high capacity for metastasis usually exhibit EMT markers. This phenomenon is defined by the loss of epithelial morphology and the acquisition of a mesenchymal phenotype. Loss of E-cadherin (which is necessary for maintaining the plasticity of epithelial cells) and increasing expression of N‑cadherin (which mediates calcium-dependent adhesion) are major hallmarks of EMT. This process is regulated by several TFs, including SNAIL1, SNAIL2 (SLUG) and TWIST. In addition, several micro RNAs (miRNAs) (predominantly miR-200) regulate EMT by forming double-negative feedback loops. miR-200 and miR-128 regulate the expression of the B lymphoma Mo-MLV insertion region 1 homolog (BMI1) protein, which has been reported to be overexpressed in several tumors and to be involved in cancer cell metastasis. BMI1 positively regulates the expression of the EMT marker SNAIL1.25,26 SNAIL1 can also be induced by certain TFs, such as TGFβ, or indirectly, by Notch. NANOG homodimers are able to regulate BMI1 directly through promoter occupancy.27 In cells with the EMT phenotype, such as non-small cell lung cancer (NSCLS), head and neck squamous cell carcinoma (HNSCC) or colon cancer cells, NANOG and SNAIL1 expression appears to be correlated.26,28,29 Liu et al. indicated that in A549 cells, SNAIL1 activates NANOG through the SMAD1/Akt/Gsk3β pathway.28 NANOG, together with OCT4, can therefore activate SNAIL2 and promote metastasis and the EMT phenotype in cancer cells. Double knock-down of the NANOG and OCT4 genes in A549 cells leads to a reduction of SNAIL-2 mRNA and elevation of E-cadherin protein levels.30 Signaling cascades involved in the EMT phenomenon and the invasive phenotype of cancer cells are shown in Fig. 1.
Figure 1.
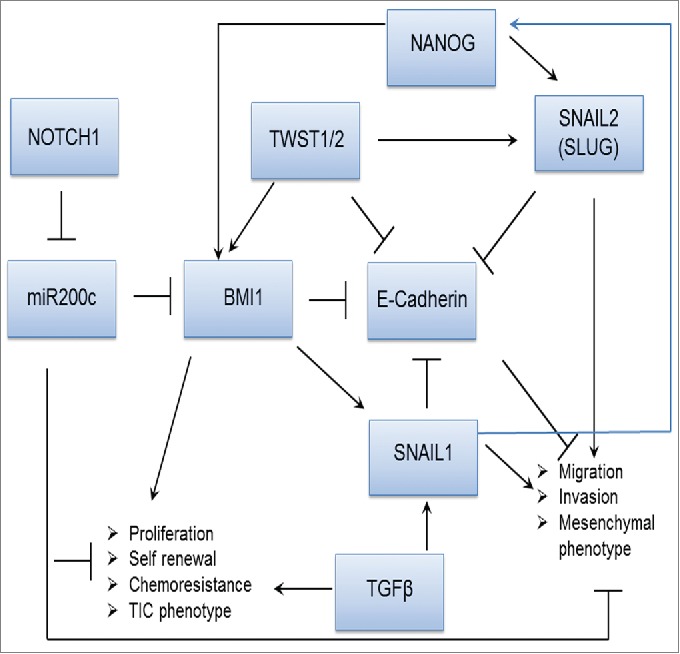
Signaling cascades known to play role in the EMT phenomenon: NOTCH1 and TGFβ. Upregulation of the promoters of EMT genes, such as TWIST1/2, BMI1, SNAIL1, OCT4 and NANOG, suppresses the expression of E-cadherin and results in loss of the epithelial phenotype.
According to Luo et al., in nasopharyngeal carcinoma (NPC), NANOG expression is inversely correlated with high expression of E-cadherin and positively correlated with high expression of N-cadherin. Expression of NANOG in NPC can promote tumor cell growth, anti-apoptosis properties and metastasis. In addition, EMT is associated with the development of stem cell-like properties in CSCs.31 Siu at al. showed that the excessive proliferation, migration and invasion of ovarian cancer cells with NANOG expression are related to the regulation of E-cadherin. Knock-down of NANOG results in a decreased metastatic potential of ovarian cancer cells and an increase in E-cadherin mRNA levels. Conversely, NANOG overexpression leads to enhanced cell proliferation and migration and decreased E‑cadherin mRNA levels.32 Taken together, these data provide strong evidence that NANOG may be the key factor in the development of the EMT phenotype via the TWIST-1/BMI1 pathway.
Shan et al. showed that HCC cancer cells expressing NANOG are highly metastatic and invasive. Additionally, these cells are resistant to chemotherapy with sorafenib and cisplatin.24 Yin et al. examined the role of OCT4 and NANOG within patients with HCC. The expression of NANOG and OCT4 was observed to be significantly correlated with larger tumor sizes and vascular invasion. Furthermore, the median recurrence-free survival (RFS) of patients with NANOG-positive tumors was 18 months, which was significantly shorter than that of patients with NANOG-negative tumors. These authors also examined the role of NANOG and OCT4 in HCC cells with different metastatic potentials. MHCC97-H and HCCLM3 cells with a high metastatic potential33 also exhibited the highest expression of the NANOG and OCT4 genes.
Lin et al. examined the role of the NANOG protein in the development of malignant gastric adenocarcinoma (GAC). Overexpression of NANOG was correlated with a poor overall 5-year survival rate in patients with GAC, and NANOG expression was positively correlated with tumor invasion in GAC patients. Furthermore, NANOG protein was found in 60% of lymph nodes containing metastases. Thus, the expression of NANOG can be correlated with TNM stage, including lymph node metastasis and tumor development, and this gene could play a prognostic role in GAC.34 Similar conclusions were reached by Xu et al., who examined patients with colorectal cancer. The found that NANOG expression was correlated with TNM stage and both lymph node and liver metastasis. Patients with NANOG expression exhibited a worse 5-year survival rate compared with patients not showing NANOG expression.35
Siu et al. performed wound healing assays that demonstrated that knocking down NANOG in a choriocarcinoma cell line (JEG-3) led to reduced migration and invasion of cancer cells. Furthermore, Real-TimeTM PCR experiments revealed a significant reduction in MMP-2 and MMP-9 expression in JEG-3 cells with reduced expression of NANOG.23
Borrull et al. observed that melanoma cells with a more aggressive phenotype express high levels of NANOG and OCT4. These researchers examined the correlations between NANOG overexpression and the migratory capacity of A375 melanoma cells. To this end, they used 3 experimental groups of A375 cells: the first group was transfected with an expression vector encoding NANOG (A375 NANOG); the second group was transfected with an expression vector encoding OCT4 (A375 OCT4); and the third group was transfected with an empty vector to serve as a control (A375 EV). In wound-healing assays, the A375 NANOG cells and A375 OCT4 cells completely filled the wound within 30 hours, in contrast to A375 EV cells. Similar results were obtained using an inhibitor of cell proliferation (mitomycin C). In transwell migration assays, it was observed that A375 NANOG and A375 OCT4 cells showed a 3.2-fold and 8-fold increases, respectively, in transmigration compared with A375 EV control cells. Moreover, the authors showed a connection between mesenchymal motility and the activity of MMPs. After application of the MMP inhibitor GM 6001, a significant reduction in the motility of cells in all 3 groups was observed in transwell assays. However, increases in NANOG/OCT4 expression did not correlate with increased expression of MT1-MMP in A375 cells.36
Imai et al. demonstrated a correlation between NANOG expression and a malignant phenotype. The authors indicated that hypopharyngeal cancer (HPC) cells, which express the transmembrane protein CD271 (CD271+), also express high levels of NANOG. These cells were characterized by self-renewal and the potential for tumor initiation and metastasis, as they expressed high levels of MMP-1, MMP-2 and MMP-10.37
Let 7a/mir-98 is a mammalian miRNA that is often downregulated in certain malignancies, including ovarian cancer, lung cancer, colon cancer and melanoma. Let 7a acts as a tumor suppressor, and reduction of this miRNA may lead to carcinogenesis. Yu et al. examined the correlation between LET-7A and CSCs in the development of malignant head and neck cancer (HNC). The expression of LET-7A was inversely correlated with CSC markers, including NANOG. Metastatic HNC tissues showed low expression of LET-7A and high expression of NANOG compared with normal tissues. Additionally, aldehyde dehydrogenase 1-positive (ALDH1+) HNC cells, which are chemoresistant to cisplatin, were transfected with a vector overexpressing let-7a. Overexpression of LET-7A in ALDH1+ HNC cells led to a decrease in NANOG protein levels and an increase in apoptosis. Overexpression of LET‑7A and silencing of NANOG expression using RNA interference (RNAi) increased the sensitivity of HNC cells to cisplatin.38
It has been shown that the expression of NANOG correlates with chemoresistance to cisplatin in oral squamous cell carcinoma (OSCC) cells. Cisplatin-resistant OC-2 cells exhibited enhanced invasive and migratory properties, which were examined using a Matrigel assay.39 Similarly, Watanabe et al. revealed a correlation between high expression of NANOG protein and a malignant phenotype of OSCC cells. High NANOG expression was correlated with a poor differentiation and metastatic potential of OSCC cells. Despite the use of adjuvant therapy, high NANOG protein levels persisted in metastatic foci of the examined cells.40
Up-regulation of NANOG expression is responsible for excessive proliferation, invasion and migration in human glioma tissues. Niu et al. found a connection between the overexpression of NANOG and low levels of a specific miRNA, miR-134, in the glioblastoma cell line U87. Matrigel transwell assays showed that U87 cells with upregulated miR-134 levels migrated more slowly compared with control cells. High expression of miR-134 in U87 cells also reduced the speed of wound closure compared with control cells.41 These findings show that NANOG is associated with excessive migration and metastasis of cancer cells, which might be connected to downregulation of certain MMPs. Furthermore, overexpression of NANOG is correlated with a poor prognosis of patients with various malignancies.
Nanog and its role in Apoptosis and Csc maintenance
NANOG plays a significant role in the cell cycle and in the process of apoptosis.
The hedgehog (Hh) pathway can be involved in tumorigenesis. Specifically, binding of sonic Hh (Shh) to the protein patched homolog receptor (PTCH) leads to disinhibition of the Smoothened receptor (SMO) and consequent activation of the GLI1 and GLI2 proteins. GLI1 binds to the NANOG promoter and activates NANOG gene transcription. It has been demonstrated that supersession of protein 53 (p53) expression determines the transcriptional activation of NANOG. Furthermore, NANOG (together with its pseudogene NANOGP8), p53 and GLI1 form a network that is involved in cell apoptosis and CSC maintenance. NANOG and p53 form a negative functional loop, and NANOG and GLI1 form a positive feedback loop.42,43 NANOG and p53 have also been shown to interact with Focal adhesion kinase (FAK), which plays role in cell survival. FAK is able to activate p53 degradation, and conversely, p53 negatively regulates FAK. Moreover, NANOG is able to bind to the FAK promoter and upregulate its activity, and FAK directly phosphorylates the NANOG protein.44 The roles of NANOG in the CSC phenotype and functions including apoptosis are shown in Fig. 2.
Figure 2.

The hedgehog (Hh) signaling pathway and FAK cooperation with NANOG and p53 in the CSC phenotype. In the absence of Hh, the PTCH1 and PTCH2 receptors inhibit the activity of the SMO transmembrane protein. When Hh binds to PTCH1/2, the inhibition of SMO is released. Activated GLI1 is transported to the nucleus, where it binds to the NANOG promoter. NANOG is also regulated positively by FAK (which forms a negative feedback loop) and negatively by p53 (which forms a positive feedback loop).
Chen et al. showed that knock-down of NANOG in mouse ES cells led to an increase in the percentage of cells in G0/G1 phase (from 30% to 32.8%). In contrast, the percentage of cells in S phase decreased (from 40% to 37.54%). It has therefore been shown that NANOG knock-down induces cell cycle arrest. Moreover, NANOG knock-down induces apoptosis. The number of apoptotic cells was observed to increase to 5.06% compared with the control level (2.9%). A significant increase in caspase-3 activation has also been detected in mouse ES cells subjected to NANOG knock-down.45
Overexpression of NANOG is correlated with low expression of miR-134. The percentage of apoptotic cells, determined via flow cytometry, was found to be significantly higher in glioblastoma U87 cells with increased expression of miR-134 compared with the control groups. Using DAPI staining and an inverted fluorescent microscope, nuclear condensation and chromatin migration were observed in U87 cells with miR-134 overexpression. It has been shown that miR-134 might play an important role in cell apoptosis.41
Chae et al. investigated the effect of 5‑aminoimidazole-4-carboxyamide ribonucleoside (AICAR), which is an activator of AMP‑activated protein kinase (AMPK). This kinase regulates cell growth, apoptosis and differentiation, mainly by impacting p53. It has been found that AMPK, activated by AICAR, can activate p53/p21, cause G1/S cycle arrest and suppress NANOG expression in both human and murine ES cells. As p53 phosphorylation is correlated with downregulation of NANOG expression, the role of AICAR has been investigated in the context of NANOG protein levels. Following the application of AICAR, NANOG expression was downregulated to approximately 56% of the control level. NANOG mRNA was reduced after 9 hours of AICAR treatment and had partially recovered 24 hours after AICAR treatment but remained at a low level. Interestingly, after knocking down p53 expression, AICAR treatment did not suppress the expression of NANOG, which indicates that AICAR impacts NANOG expression via p53, resulting in cell cycle arrest, without any effect on apoptosis. AICAR also does not impact miR-134 levels.46,47
You et al. assessed the ability of the histone deacetylase inhibitor (HDACI) apicidin to downregulate NANOG activity in specific human embryonic carcinoma cell lines. Apicidin suppressed NANOG expression and impacted apoptosis in NCCIT cells. Using flow cytometry, it was shown that apicidin increased the number of Annexin V-positive cells (35.4%) compared with the control (1.6%).48
Together, these data suggest that NANOG inhibits apoptosis and promotes cell cycle arrest mainly via p53 regulation. It has also been suggested that an inverse correlation between NANOG and miR-134 expression impacts tumor progression. Downregulation of miR-134 in certain tumors causes overexpression of NANOG and consequent suppression of apoptosis.
Nanog and its role in Hypoxia-Induced Tumor Growth and Angiogenesis
Angiogenesis is a process in which new capillaries are created from blood vessels. It is observed not only in normal physiological processes, such as embryogenesis or wound healing but also in cancer development. NANOG is able to regulate angiogenesis in ES cells through the activation of Vascular endothelial growth factor receptor 2 (VEGFR2), also known as Fetal liver kinase-1 (FLK1).49
It has been well documented that hypoxia, defined as oxygen deprivation, induces angiogenesis in various malignancies. Hypoxia plays a critical role in tumor development, mainly by increasing the expression of embryonic markers such as NANOG, OCT4 and SOX2. It has been suggested that NANOG is able to disturb the susceptibility of tumor cells to cytotoxic T lymphocyte (CTL)-mediated toxicity. Hasmim et al. showed that silencing NANOG expression using a specific siRNA restored the susceptibility of IGR-Heu lung carcinoma cells to lysis by CTL under hypoxic conditions.50 Further research by these authors demonstrated that NANOG can also regulate the recruitment of regulatory T cells (Tregs) within the B16-F10 melanoma tumor bed under hypoxic conditions. Silencing NANOG gene expression led to a reduction in intratumoral Tregs. Moreover, in B16-F10 cells under hypoxic stress, NANOG positively regulated the expression of TGFβ1, resulting in differentiation of naïve CD4+ T cells into Tregs. The supernatant from hypoxic B16-F10 cells with silenced NANOG expression did not induce the expansion of Tregs. However, supplementation of that supernatant with TGFβ1 re-established the expansion of Tregs. These data suggest that NANOG regulates the expansion of Tregs via TGFβ1 and promotes tumor growth through immunosuppression.51
NANOG expression is associated with gene activity directly connected with hypoxia and angiogenesis. Hypoxia-inducible factor 1 (HIF-1) activates Vascular endothelial growth factor (VEGF) as its major target gene, which stimulates vascular proliferation.52-54 HIF-1 and HIF-2 knock-down in hypoxic IGR-Heu lung carcinoma cells was shown to result in a decrease in NANOG protein levels. However, NANOG knock-down led to an increase of several proapoptotic as well as antiapoptotic genes. The positive correlation between NANOG and HIF-1 expression has also been observed in prostate cancer.55
Hypoxia in prostate and pancreatic cancer increases the expression of miR-21, miR-210, HIF-1 and VEGF. Bao et al. detected an increased amount of NANOG mRNA and miR-21 in AsPC-1/MiaPaCa-2 pancreatic cancer cells and PC-3/LNCaP prostate cells under hypoxic conditions.56,57 It is known that the HA-mediated NANOG-STAT3 complex binds to the miR-21 promoter.58 However, the role of NANOG in the activation of miR-21 in hypoxia-induced angiogenesis is unclear.
Correlation between the NANOG and STAT3 genes in the development of malignant phenotypes and multidrug resistance in cancer cells
STAT3 regulates cell growth, differentiation and survival. In physiological conditions, STAT3 is activated by numerous cytokines and growth factors, such as epidermal growth factor (EGF), platelet-derived growth factor (PDGF) and interleukin 6 (IL-6), as well as by oncogenic proteins such as SRC and Ras. STAT3 activation is regulated by the phosphorylation of a tyrosine residue at position 705 by receptor-associated and non-receptor-associated protein kinases. Phosphorylation of STAT3 in the cytoplasm leads to its dimerization and translocation to the cell nucleus. The dimerized STAT3 transcription factor then binds DNA, leading to the activation of genes responsible for proliferation, differentiation and apoptosis. Overexpression of the STAT3 gene may lead to carcinogenesis and is observed in many human malignancies, such as bladder cancer, ovarian cancer and breast cancer.59,60
NANOG and STAT3 can cooperate in the maintenance of ES cell properties, and it is thought that there is a functional link between these 2 genes. Approximately 55% of hypothetical STAT3 target genes also contain a binding site for NANOG. In turn, 41% of hypothetical NANOG target genes contain a binding site for STAT3. These data suggest that STAT3 and NANOG can cooperate in the regulation of gene expression, which may be important in maintaining an undifferentiated state. Among 24 STAT3 target genes analyzed by Bourillot et al., 21 were found to contain NANOG binding sites in their sequences. Moreover, NANOG regulates the transcriptional activity of these genes. After knock-down of NANOG expression in mouse embryonic stem cells (CGR8 cells), a significant reduction in the expression of 19 STAT3 target genes was identified; 14 of these 19 genes were also downregulated upon treatment with 4′-hydroxytamoxifen (4′-OHT), a NANOG inhibitor, in RCNHTKβ cells. This study showed that the vast majority of STAT3 target genes are also regulated transcriptionally by NANOG, and NANOG and STAT3 cooperate to inhibit mesoderm and endoderm differentiation.61 Stuart et al. showed that NANOG maintains native pluripotency by enhancing LIF/STAT3 signal transduction, resulting in the activation of Kruppel-like factor 4 (KLF4), which regulates cellular proliferation and differentiation.22
Yin et al. examined the impact of Src-homology protein tyrosine phosphatase 1 (SHP-1) on NANOG expression in the mouse F9 embryonal carcinoma cell line. Overexpression of SHP-1 reduced NANOG promoter activity through STAT3 regulation. To elucidate the relationship between these 3 genes, the authors knocked down or overexpressed SHP-1 in F9 cells expressing 2 different STAT3 proteins: dominant-negative STAT3 (Y705F), which cannot be activated by phosphorylation, or a phospho-mimetic STAT3 mutant (Y705D), which maintains constitutive activity. Overexpression of Y705D resulted in 1.5-1.6-fold higher activation of the NANOG promoter, regardless of whether SHP-1 was knocked down or overexpressed. This result likely occurred because when there is large amount of STAT3 present, the NANOG promoter is occupied by Y705D. Therefore, the NANOG gene was upregulated regardless of the inhibition or overexpression of SHP1. The overexpression of Y705F resulted in a 1.45-fold increase in the activation of the NANOG promoter when SHP-1 was knocked down. However, overexpression of Y705F or STAT3 knock-down led to the decrease of NANOG promoter activation to 50% of output level, when SHP-1 was overexpressed. As Y705F cannot be phosphorylated, NANOG is regulated directly only via endogenous STAT3. In cells in which SHP-1 was knocked down (but without STAT3 manipulation), NANOG promoter activation was decreased to only 42%. These results indicate that SHP-1 regulates NANOG via STAT3 signaling.62,63
Gao et al. determined that NANOG expression might be regulated via the STAT3 pathway together with the activity of the Nogo-66 receptor (NgR). Activated NgR phosphorylates STAT3 and increases NANOG mRNA and protein levels. An increased level of NANOG protein inhibits the differentiation of murine embryos. Application of the NgR inhibitors phosphatidylinositol-specific phospholipase C (PtdIns-PLC) and nogo-A inhibitory peptide 1-40 (NEP1-40) results in downregulation of NANOG. The same result was obtained upon application of the STAT3 phosphorylation inhibitors AG490 and rapamycin.64
Hyaluronan (HA) is a glycosaminoglycan that is a component of the extracellular matrix. In malignant tissues, the concentration of HA is generally higher than in normal tissues. HA interacts with the cell surface receptor CD44. This receptor is unregulated in several cancers and is a marker for the TIC phenotype. Binding of HA to CD44 results in the association of NANOG with CD44, translocation of the complex to nucleus and activation of target genes. It is thought that some NANOG proteins form a complex with STAT3 and induce its transcription, leading to tumor growth and multidrug resistance. Bourguignon et al. examined whether HA/CD44 signaling influences the correlation between STAT3 and NANOG expression in the development of multidrug resistance in breast and ovarian cancer. The expression of STAT3 was low in breast cancer cells (MCF-7) and ovarian cancer cells (SK-OV3) treated with anti-CD44 antibodies, regardless of concurrent treatment with HA. STAT3 expression was enhanced in both cell lines after treatment with HA. The expression of STAT3 was also reduced after transfection of MCF-7 and SK-OV3 cells with NANOG siRNA. HA/CD44-activated NANOG and STAT3 cooperate in tumor growth and cell survival. Treatment of tumor cells with ant-CD44 antibodies or STAT3/NANOG siRNAs blocked tumor growth. On the contrary, transfection with NANOG cDNA stimulated tumor growth. These data indicate that HA/CD44-activated NANOG regulates STAT3 activation in the MCF-7 and SK-OV3 cell lines, which results in tumor growth. Furthermore, MCF-7 and SK-OV3 cells transfected with STAT3 siRNA or NANOG siRNA showed inhibition of multidrug resistance 1 receptor (MDR1) expression induced by HA/CD44. The absence of HA increases the susceptibility of cells to chemotherapy with doxorubicin.65,66
Many miRNAs are thought to exhibit a connection with the development of some cancers, including HNSCC. MiR-21, an miRNA involved in the inhibition of the tumor suppressor protein Programed cell death 4 (PDCD4), is upregulated in HNSCC tissues. Inhibition of PDCD4 leads to tumor invasion, metastasis, chemoresistance and excessive tumor growth. Bourguignon et al. showed that HA/CD44-mediated NANOG/STAT-3 signaling regulates miR-21 production, PDCD4 expression, and the development of chemoresistance in HNSCC. Silencing of either the STAT3 or NANOG gene using specific siRNAs blocked HA-mediated NANOG/STAT-3 binding to the miR-21 promoter in the head and neck squamous cell carcinoma cell line HSC-3. These investigations were undertaken using chromatin immunoprecipitation assays and Real-TimeTM PCR. Moreover, an anti-miR-21 inhibitor was found to increase PDCD4 expression and block HA/CD44-mediated tumor development and chemoresistance.58 The HA/CD44 signaling pathway is shown in Fig. 3.
Figure 3.

The HA/CD44 signaling pathway leading to the malignant phenotype of cancer cells. HA binds to the CD44 receptor and promotes its association with NANOG and STAT3. NANOG binds to STAT3 and associates with the miR-21 promoter, resulting in miR-21 transcription, protein production and downregulation of PDCD4. Additionally, NANOG associated with CD44 translocates to the nucleus and, together with other transcriptional factors, activates genes related to self-renewal and the TIC phenotype. STAT3 associated with CD44, together with CyclinD1 and Survivin, activates genes associated with the survival of tumor cells.
Lee et al. identified NANOG as an important factor in CD24-mediated tumorigenicity. Moreover, regulation of NANOG by CD24 occurs via STAT3 phosphorylation. The initial hypothesis assumed that the transmembrane protein CD24 activates NANOG in the nucleus via the IL-6 pathway. The connection between STAT3 phosphorylation and CD24 was indicated by the observation that knocking down CD24 altered the expression of several genes downstream of STAT3. To test this hypothesis, Lee et al. examined STAT3 and its phosphorylated form (pSTAT3) in Huh-7 and PLC/PRF/5 HCC cell lines in which CD44 was knocked down. The level of pSTAT3 in CD24 knock-down cells was reduced compared with the parental form of the protein, and there was no change in the level of STAT3 mRNA in CD24 knock-down cells. Further experiments evaluated whether the application of a STAT3 inhibitor (S3I-201) in Huh-7 and PLC/PRF/5 cells would affect pSTAT3 and NANOG expression. As a result, downregulation of NANOG was observed, which was confirmed using a GFP-tagged NANOG promoter in PLC/PRF/5 cells. The GFP signal was found to be higher in CD24-positive PLC/PRF/5 cells and lower in CD24-negative cells, and the signal in CD44-positive cells was decreased upon application of S3I-201. Lee et al. also showed that CD24 leads to STAT3 phosphorylation via Src-associated kinase, and not via JAK2. These findings reveal a correlation between the expression of CD24, the activation of STAT3, and the expression of NANOG.67
Conlcusions
CSCs are widely known to be responsible for tumorigenicity and chemoresistance. CSCs share many properties with ES cells, and hence, they express “stemness genes," such as OCT4, SOX2 and NANOG. The transcription factor NANOG is expressed in many malignant human tumors, including breast,68 ovarian,69 and bladder70 cancers, gastric adenocarcinoma,34 melanoma36 and many others. NANOG expression is always correlated with a poor prognosis, as poorly differentiated tumors are more resistant to treatment. This transcription factor targets many genes connected with the malignant phenotype of cancer cells, including MMPs, STAT3, p53 and MDR1. Inhibition of NANOG expression in vitro leads to decreased cellular migration, invasiveness and proliferation.23,35,36,61 These data demonstrate that NANOG is specifically involved in the process of carcinogenesis and can be a potential biological and prognostic marker for malignant tumors.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by grants KNW-1-050/D/1/0, KNW-1-035/D/2/0, KNW-2-004/D/3/N, KNW-1-095/N/3/0, KNW-1-151/N/4/0 and KNW-1-028/N/5/0 funded by Medical University of Silesia, Katowice, Poland. The first author participates in the “DoktoRIS - scholarship program for innovative Silesia," co-financed by the European Union under the European Social Fund.
References
- 1. Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 2003; 113:643-55; PMID:12787505; http://dx.doi.org/ 10.1016/S0092-8674(03)00392-1 [DOI] [PubMed] [Google Scholar]
- 2. Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 2003; 113:631-42; PMID:12787504; http://dx.doi.org/ 10.1016/S0092-8674(03)00393-3 [DOI] [PubMed] [Google Scholar]
- 3. Theunissen TW, Silva JC. Switching on pluripotency: a perspective on the biological requirement of Nanog. Philos Trans R Soc Lond B Biol Sci 2011; 366:2222-29; PMID:21727127; http://dx.doi.org/ 10.1098/rstb.2011.0003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gauchat D, Mazet F, Berney C, Schummer M, Kreger S, Pawlowski J, Galliot B. Evolution of Antp-class genes and differential expression of Hydra Hox/paraHox genes in anterior patterning. Proc Natl Acad Sci U S A 2000; 97:4493-8; PMID:10781050; http://dx.doi.org/ 10.1073/pnas.97.9.4493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Booth HA, Holland PW. Eleven daughters of Nanog. Genomics 2004; 84:229-38; PMID:15233988; http://dx.doi.org/ 10.1016/j.ygeno.2004.02.014 [DOI] [PubMed] [Google Scholar]
- 6. Chang DF, Tsai SC, Xing CW, Ping X, Senadheera D, Lutzko C. Molecular characterization of the Human Nanog protein. Stem Cells 2009; 27:812-21; PMID:19350681; http://dx.doi.org/ 10.1634/stemcells.2008-0657 [DOI] [PubMed] [Google Scholar]
- 7. Ambady S, Malcuit C, Kashpur O, Kole D, Holmes WF, Hedblom E, Page RL, Dominko T. Expression of NANOG and NANOGP8 in a variety of undifferentiated and differentiated human cells. Int J Dev Biol 2010; 54:1743-54; PMID:21136380; http://dx.doi.org/ 10.1387/ijdb.103192sa [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eberle I, Pless B, Braun M, Dingermann T, Marschalek R. Transcriptional properties of human NANOG1 and NANOG2 in acute leukemic cells. Nucleic Acids Res 2010; 38:5384-95; PMID:20427424; http://dx.doi.org/ 10.1093/nar/gkq307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ishiguro T, Sato A, Ohata H, Sakai H, Nakagama H, Okamoto K. Differential expression of nanog1 and nanogp8 in colon cancer cells. Biochem Biophys Res Commun 2012; 418:199-204; PMID:22079639; http://dx.doi.org/ 10.1016/j.bbrc.2011.10.123 [DOI] [PubMed] [Google Scholar]
- 10. Scerbo P, Markov GV, Vivien C, Kodjabachian L, Demeneix B, Coen L, Girardot F. On the origin and evolutionary history of NANOG. PLoS One 2014; 9:e85104; PMID:24465486; http://dx.doi.org/ 10.1371/journal.pone.0085104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fairbanks DJ, Maughan PJ. Evolution of the NANOG pseudogene family in the human and chimpanzee genomes. BMC Evol Biol 2006; 6:12; PMID:16469101; http://dx.doi.org/ 10.1186/1471-2148-6-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee KB, Folger JK, Rajput SK, Smith GW. Temporal regulation of mRNAs for select bone morphogenetic proteins (BMP), BMP receptors and their associated SMAD proteins during bovine early embryonic development: effects of exogenous BMP2 on embryo developmental progression. Reprod Biol Endocrinol 2014; 12:67; PMID:25027287; http://dx.doi.org/ 10.1186/1477-7827-12-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Page RL, Ambady S, Holmes WF, Vilner L, Kole D, Kashpur O, Huntress V, Vojtic I, Whitton H, Dominko T. Induction of stem cell gene expression in adult human fibroblasts without transgenes. Cloning Stem Cells 2009; 11:417-26; PMID:19622035; http://dx.doi.org/ 10.1089/clo.2009.0015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Men CR, Badeaux M, Choy G, Chandra D, Patrawala L, Liu C, Calhoun-Davis T, Zaehres H, Daley GQ, Tang DG. Functional evidence that the self-renewal gene NANOG regulates human tumor development. Stem Cells 2009; 27:993-1005; PMID:19415763; http://dx.doi.org/ 10.1002/stem.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu A, Yu X, Liu S. Pluripotency transcription factors and cancer stem cells: small genes make a big difference. Chin J Cancer 2013; 32:483-7; PMID:23419197; http://dx.doi.org/ 10.5732/cjc.012.10282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells–perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res 2006; 66:9339-44; PMID:16990346; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-3126 [DOI] [PubMed] [Google Scholar]
- 17. Bernstein A. Cancer stem cells: the centrality of translational research to cancer control. CMAJ 2007; 176:29-30; PMID:17167103; http://dx.doi.org/ 10.1503/cmaj.061644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994; 367:645-8; PMID:7509044; http://dx.doi.org/ 10.1038/367645a0 [DOI] [PubMed] [Google Scholar]
- 19. Driessens G, Beck B, Caauwe A, Simons BD, Blanpain C. Defining the mode of tumour growth by clonal analysis. Nature 2012; 488:527-30; PMID:22854777; http://dx.doi.org/ 10.1038/nature11344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB, Wong E, Orlov YL, Zhang W, Jiang J, et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell 2008; 133:1106-17; PMID:18555785; http://dx.doi.org/ 10.1016/j.cell.2008.04.043 [DOI] [PubMed] [Google Scholar]
- 21. Hawkins K, Mohamet L, Ritson S, Merry CL, Ward CM. E-cadherin and, in its absence, N-cadherin promotes Nanog expression in mouse embryonic stem cells via STAT3 phosphorylation. Stem Cells 2012; 30:1842-51; PMID:22696497; http://dx.doi.org/ 10.1002/stem.1148 [DOI] [PubMed] [Google Scholar]
- 22. Stuart HT, van Oosten AL, Radzisheuskaya A, Martello G, Miller A, Dietmann S, Nichols J, Silva JC. NANOG amplifies STAT3 activation and they synergistically induce the naive pluripotent program. Curr Biol 2014; 24:340-6; PMID:24462001; http://dx.doi.org/ 10.1016/j.cub.2013.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Siu MK, Wong ES, Chan HY, Ngan HY, Chan KY, Cheung AN. Overexpression of NANOG in gestational trophoblastic diseases: effect on apoptosis, cell invasion, and clinical outcome. Am J Pathol 2008; 173:1165-72; PMID:18772339; http://dx.doi.org/ 10.2353/ajpath.2008.080288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shan J, Shen J, Liu L, Xia F, Xu C, Duan G, Xu Y, Ma Q, Yang Z, Zhang Q, et al. Nanog regulates self-renewal of cancer stem cells through the insulin-like growth factor pathway in human hepatocellular carcinoma. Hepatology 2012; 56:1004-14; PMID:22473773; http://dx.doi.org/ 10.1002/hep.25745 [DOI] [PubMed] [Google Scholar]
- 25. Fu J, Rodova M, Nanta R, Meeker D, Van Veldhuizen PJ, Srivastava RK, Shankar S. NPV-LDE-225 (Erismodegib) inhibits epithelial mesenchymal transition and self-renewal of glioblastoma initiating cells by regulating miR-21, miR-128, and miR-200. Neuro Oncol 2013; 15:691-706; PMID:23482671; http://dx.doi.org/ 10.1093/neuonc/not011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu CC, Lo WL, Chen YW, Huang PI, Hsu HS, Tseng LM, Hung SC, Kao SY, Chang CJ, Chiou SH. Bmi-1 Regulates Snail Expression and Promotes Metastasis Ability in Head and Neck Squamous Cancer-Derived ALDH1 Positive Cells. J Oncol 2011; 2011:pii: 609259; http://dx.doi.org/ 10.1155/2011/609259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xie X., Piao L, Cavey GS, Old M, Teknos TN, Mapp AK, Pan Q. Phosphorylation of Nanog is Essential to Regulate Bmi1 and Promote Tumorigenesis. Oncogene 2014; 33:2040-52; PMID:23708658; http://dx.doi.org/ 10.1038/onc.2013.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu CW, Li CH, Peng YJ, Cheng YW, Chen HW, Liao PL, Kang JJ, Yeng MH. Snail regulates Nanog status during the epithelial-mesenchymal transition via the Smad1/Akt/GSK3β signaling pathway in non-small-cell lung cancer. Oncotarget 2014; 5:3880-94. PMCID: PMC4116528; PMID:25003810; http://dx.doi.org/ 10.18632/oncotarget.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dang H, Ding W, Emerson D, Rountree CB. Snail1 induces epithelial-to-mesenchymal transition and tumor initiating stem cell characteristics. BMC Cancer 2011; 396; PMID:21929801; http://dx.doi.org/ 10.1186/1471-2407-11-396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chiou SH, Wang ML, Chou YT, Chen CJ, Hong CF, Hsieh WJ, Chang HT, Chen YS, Lin TW, Hsu HS, et al. Coexpression of Oct4 and Nanog enhances malignancy in lung adenocarcinoma by inducing cancer stem cell-like properties and epithelial-mesenchymal transdifferentiation. Cancer Res 2010; 70:10433-44; PMID:21159654; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-2638 [DOI] [PubMed] [Google Scholar]
- 31. Luo W, Li S, Peng B, Ye Y, Deng X, Yao K. Embryonic Stem Cells Markers SOX2, OCT4 and Nanog Expression and Their Correlations with Epithelial-Mesenchymal Transition in Nasopharyngeal Carcinoma. PLoS One 2013; 8:e56324; PMID:23424657; http://dx.doi.org/ 10.1371/journal.pone.0056324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Siu MK, Wong ES, Kong DS, Chan HY, Jiang L, Wong OG, Lam EW, Chan KK, Ngan HY, Le XF, et al. Stem cell transcription factor NANOG controls cell migration and invasion via dysregulation of E-cadherin and FoxJ1 and contributes to adverse clinical outcome in ovarian cancers. Oncogene 2013; 32:3500-9; PMID:22945654; http://dx.doi.org/ 10.1038/onc.2012.363 [DOI] [PubMed] [Google Scholar]
- 33. Yin X, Li YW, Zhang BH, Ren ZG, Qiu SJ, Yi Y, Fan J. Coexpression of stemness factors Oct4 and Nanog predict liver resection. Ann Surg Oncol 2012; 19:2877-87; PMID:22461131; http://dx.doi.org/ 10.1245/s10434-012-2314-6 [DOI] [PubMed] [Google Scholar]
- 34. Lin T, Ding YQ, Li JM. Overexpression of Nanog protein is associated with poor prognosis in gastric adenocarcinoma. Med Oncol 2012; 29:878-85; PMID:21336986; http://dx.doi.org/ 10.1007/s12032-011-9860-9 [DOI] [PubMed] [Google Scholar]
- 35. Xu F, Dai C, Zhang R, Zhao Y, Peng S, Jia C. Nanog: a potential biomarker for liver metastasis of colorectal cancer. Dig Dis Sci 2012; 57:2340-6; PMID:22562535; http://dx.doi.org/ 10.1007/s10620-012-2182-8 [DOI] [PubMed] [Google Scholar]
- 36. Borrull A, Ghislin S, Deshayes F, Lauriol J, Alcaide-Loridan C, Middendorp S. Nanog and Oct4 overexpression increases motility and transmigration of melanoma cells. J Cancer Res Clin Oncol 2012; 138:1145-54; PMID:22406932; http://dx.doi.org/ 10.1007/s00432-012-1186-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Imai T, Tamai K, Oizumi S, Oyama K, Yamaguchi K, Sato I, Satoh K, Matsuura K, Saijo S, Sugamura K, et al. CD271 defines a stem cell-like population in hypopharyngeal cancer. PLoS One 2013; 8:e62002; PMID:23626764; http://dx.doi.org/ 10.1371/journal.pone.0062002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yu CC, Chen YW, Chiou GY, Tsai LL, Huang PI, Chang CY, Tseng LM, Chiou SH, Yen SH, Chou MY, et al. MicroRNA let-7a represses chemoresistance and tumourigenicity in head and neck cancer via stem-like properties ablation. Oral Oncol 2011; 47:202-10; PMID:21292542; http://dx.doi.org/ 10.1016/j.oraloncology.2010.12.001 [DOI] [PubMed] [Google Scholar]
- 39. Tsai LL, Yu CC, Chang YC, Yu CH, Chou MY. Markedly increased Oct4 and Nanog expression correlates with cisplatin resistance in oral squamous cell carcinoma. J Oral Pathol Med 2011; 40:621-8; PMID:21342274; http://dx.doi.org/ 10.1111/j.1600-0714.2011.01015.x [DOI] [PubMed] [Google Scholar]
- 40. Watanabe M, Ohnishi Y, Inoue H, Wato M, Tanaka A, Kakudo K, Nozaki M. NANOG expression correlates with differentiation, metastasis and resistance to preoperative adjuvant therapy in oral squamous cell carcinoma. Oncol Lett 2014; 7:35-40; PMID:24348816; http://dx.doi.org/ 10.3892/ol.2013.1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Niu CS, Yang Y, Cheng CD. MiR-134 regulates the proliferation and invasion of glioblastoma cells by reducing Nanog expression. Int J Oncol 2013; 42:1533-40; PMID:23467648; http://dx.doi.org/ 10.3892/ijo.2013.1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zbinden M, Duquet A, Lorente-Trigos A, Ngwabyt SN, Borges I, Ruiz i Altaba A. NANOG regulates glioma stem cells and is essential in vivo acting in a cross-functional network with GLI1 and p53. EMBO J 2010; 29:2659-74; PMID:20581802; http://dx.doi.org/ 10.1038/emboj.2010.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Po A, Ferretti E, Miele E, De Smaele E, Paganelli A, Canettieri G, Coni S, Di Marcotullio L, Biffoni M, Massimi L, et al. Hedgehog controls neural stem cells through p53-independent regulation of Nanog. EMBO J 2010; 29:2646-58; PMID:20581804; http://dx.doi.org/ 10.1038/emboj.2010.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ho B, Olson G, Figel S, Gelman I, Cance WG, Golubovskaya VM. Nanog increases focal adhesion kinase (FAK) promoter activity and expression and directly binds to FAK protein to be phosphorylated. J Biol Chem 2012; 287:18656-73; PMID:22493428; http://dx.doi.org/ 10.1074/jbc.M111.322883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen T, Du J, Lu G. Cell growth arrest and apoptosis induced by Oct4 or Nanog knockdown in mouse embryonic stem cells: a possible role of Trp53. Mol Biol Rep 2012; 39:1855-61; PMID:21706347; http://dx.doi.org/ 10.1007/s11033-011-0928-6 [DOI] [PubMed] [Google Scholar]
- 46. Chae HD, Lee MR, Broxmeyer HE. 5-Aminoimidazole-4-carboxyamide ribonucleoside induces G(1)/S arrest and Nanog downregulation via p53 and enhances erythroid differentiation. Stem Cells 2012; 30:140-9; PMID:22076938; http://dx.doi.org/ 10.1002/stem.778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Han MK, Song EK, Guo Y, Ou X, Mantel C, Broxmeyer HE. SIRT1 regulates apoptosis and Nanog expression in mouse embryonic stem cells by controlling p53 subcellular localization. Cell Stem Cell 2008; 2:241-51; PMID:18371449; http://dx.doi.org/ 10.1016/j.stem.2008.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. You JS, Kang JK, Seo DW, Park JH, Park JW, Lee JC, Jeon YJ, Cho EJ, Han JW. Depletion of embryonic stem cell signature by histone deacetylase inhibitor in NCCIT cells: involvement of Nanog suppression. Cancer Res 2009; 69:5716-25; PMID:19567677; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-4953 [DOI] [PubMed] [Google Scholar]
- 49. Kohler EE, Cowan CE, Chatterjee I, Malik AB, Wary KK. NANOG induction of fetal liver kinase-1 (FLK1) transcription regulates endothelial cell proliferation and angiogenesis. Blood 2011; 117:1761-9; PMID:21119109; http://dx.doi.org/ 10.1182/blood-2010-07-295261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hasmim M, Noman MZ, Lauriol J, Benlalam H, Mallavialle A, Rosselli F, Mami-Chouaib F, Alcaide-Loridan C, Chouaib S. Hypoxia-dependent inhibition of tumor cell susceptibility to CTL-mediated lysis involves NANOG induction in target cells. J Immunol 2011; 187:4031-9; PMID:21911602; http://dx.doi.org/ 10.4049/jimmunol.1101011 [DOI] [PubMed] [Google Scholar]
- 51. Hasmim M, Noman MZ, Messai Y, Bordereaux D, Gros G, Baud V, Chouaib S. Cutting edge: Hypoxia-induced Nanog favors the intratumoral infiltration of regulatory T cells and macrophages via direct regulation of TGF-β1. J Immunol 2013; 191:5802-6; PMID:24227785; http://dx.doi.org/ 10.4049/jimmunol.1302140 [DOI] [PubMed] [Google Scholar]
- 52. Theodoropoulos GE, Lazaris AC, Theodoropoulos VE, Papatheodosiou K, Gazouli M, Bramis J, Patsouris E, Panoussopoulos D. Hypoxia, angiogenesis and apoptosis markers in locally advanced rectal cancer. Int J Colorectal Dis 2006; 21:248-57; PMID:16052307; http://dx.doi.org/ 10.1007/s00384-005-0788-4 [DOI] [PubMed] [Google Scholar]
- 53. Argaw AT, Zhang Y, Snyder BJ, Zhao ML, Kopp N, Lee SC, Raine CS, Brosnan CF, John GR. IL-1beta regulates blood-brain barrier permeability via reactivation of the hypoxia-angiogenesis program. Immunol 2006; 177:5574-84; http://dx.doi.org/ 10.4049/jimmunol.177.8.5574 [DOI] [PubMed] [Google Scholar]
- 54. Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med 2003; 9:677-84; PMID:12778166; http://dx.doi.org/ 10.1038/nm0603-677 [DOI] [PubMed] [Google Scholar]
- 55. Miyazawa K, Tanaka T, Nakai D, Morita N, Suzuki K. Immunohistochemical expression of four different stem cell markers in prostate cancer: High expression of NANOG in conjunction with hypoxia-inducible factor-1α expression is involved in prostate epithelial malignancy. Oncol Lett 2014; 8:985-92; PMID:25120646; http://dx.doi.org/ 10.3892/ol.2014.2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bao B, Ali S, Ahmad A, Azmi AS, Li Y, Banerjee S, Kong D, Sethi S, Aboukameel A, Padhye SB, Sarkar FH. Hypoxia-induced aggressiveness of pancreatic cancer cells is due to increased expression of VEGF, IL-6 and miR-21, which can be attenuated by CDF treatment. PLoS One 2012; 7:e50165; PMID:23272057; http://dx.doi.org/ 10.1371/journal.pone.0050165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bao B, Ahmad A, Kong D, Ali S, Azmi AS, Li Y, Banerjee S, Padhye S, Sarkar FH. Hypoxia induced aggressiveness of prostate cancer cells is linked with deregulated expression of VEGF, IL-6 and miRNAs that are attenuated by CDF. PLoS One 2012; 7:e43726; PMID:22952749; http://dx.doi.org/ 10.1371/journal.pone.0043726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bourguignon LY, Earle C, Wong G, Spevak CC, Krueger K. Stem cell marker (Nanog) and Stat-3 signaling promote MicroRNA-21 expression and chemoresistance in hyaluronan/CD44-activated head and neck squamous cell carcinoma cells. Oncogene 2012; 31:149-60; PMID:21685938; http://dx.doi.org/ 10.1038/onc.2011.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Aggarwal BB, Kunnumakkara AB, Harikumar KB, Gupta SR, Tharakan S, Koca C, Dey S, Sung B. Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann N Y Acad Sci 2009; 1171:59-76; PMID:19723038; http://dx.doi.org/ 10.1111/j.1749-6632.2009.04911.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Huang S. Regulation of metastases by signal transducer and activator of transcription 3 signaling pathway: clinical implications. Clin Cancer Res 2007; 13:1362-6; PMID:17332277; http://dx.doi.org/ 10.1158/1078-0432.CCR-06-2313 [DOI] [PubMed] [Google Scholar]
- 61. Bourillot PY, Aksoy I, Schreiber V, Wianny F, Schulz H, Hummel O, Hubner N, Savatier P. Novel STAT3 target genes exert distinct roles in the inhibition of mesoderm and endoderm differentiation in cooperation with Nanog. Stem Cells 2009; 27:1760-71; PMID:19544440; http://dx.doi.org/ 10.1002/stem.110 [DOI] [PubMed] [Google Scholar]
- 62. Yin S, Wu H, Lv J, Wu X, Zhang Y, Du J, Zhang Y. SHP-1 arrests mouse early embryo development through downregulation of Nanog by dephosphorylation of STAT3. PLoS One 2014; 9:e86330; PMID:24466030; http://dx.doi.org/ 10.1371/journal.pone.0086330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhou JJ, Chen RF, Deng XG, Zhou Y, Ye X, Yu M, Tang J, He XY, Cheng D, Zeng B, et al. Hepatitis C virus core protein regulates NANOG expression via the stat3 pathway. FEBS Lett 2014; 588:566-73; PMID:24462277; http://dx.doi.org/ 10.1016/j.febslet.2013.11.041 [DOI] [PubMed] [Google Scholar]
- 64. Gao Y, Wang B, Xiao Z, Chen B, Han J, Wang X, Zhang J, Gao S, Zhao Y, Dai J. Nogo-66 regulates nanog expression through stat3 pathway in murine embryonic stem cells. Stem Cells Dev 2010; 19:53-60; PMID:19400741; http://dx.doi.org/ 10.1089/scd.2008.0357 [DOI] [PubMed] [Google Scholar]
- 65. Bourguignon LY, Peyrollier K, Xia W, Gilad E. Hyaluronan-CD44 interaction activates stem cell marker Nanog, Stat-3-mediated MDR1 gene expression, and ankyrin-regulated multidrug efflux in breast and ovarian tumor cells. J Biol Chem 2008; 283:17635-51; PMID:18441325; http://dx.doi.org/ 10.1074/jbc.M800109200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wood NJ. Pancreatic cancer: pancreatic tumour formation and recurrence after radiotherapy are blocked by targeting CD44. Nat Rev Gastroenterol Hepatol 2014; 11:73; PMID:24445614; http://dx.doi.org/ 10.1038/nrgastro.2014.1 [DOI] [PubMed] [Google Scholar]
- 67. Lee TK, Castilho A, Cheung VC, Tang KH, Ma S, Ng IO. CD24(+) liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated NANOG regulation. Cell Stem Cell 2011; 9:50-63; PMID:21726833; http://dx.doi.org/ 10.1016/j.stem.2011.06.005 [DOI] [PubMed] [Google Scholar]
- 68. Ling GQ, Chen DB, Wang BQ, Zhang LS. Expression of the pluripotency markers Oct3/4, Nanog and Sox2 in human breast cancer cell lines. Oncol Lett 2012; 4:1264-8; PMID:23197999; http://dx.doi.org/ 10.3892/ol.2012.916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hoei-Hansen CE, Kraggerud SM, Abeler VM, Kaern J, Rajpert-De Meyts E, Lothe RA. Ovarian dysgerminomas are characterised by frequent KIT mutations and abundant expression of pluripotency markers. Mol Cancer 2007; 6:12; PMID:17274819; http://dx.doi.org/ 10.1186/1476-4598-6-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhang Y, Wang Z, Yu J, Shi JZ, Wang C, Fu WH, Chen ZW, Yang J. Cancer stem-like cells contribute to cisplatin resistance and progression in bladder cancer. Cancer Lett 2012; 322:70-7; PMID:22343321; http://dx.doi.org/ 10.1016/j.canlet.2012.02.010 [DOI] [PubMed] [Google Scholar]


