Abstract
Purpose
To determine the testability of Retinomax and IOLMaster ocular biometry in preschool children.
Design
Population-based study of inner city preschool children in Los Angeles County.
Participants
Two thousand five hundred forty-five Hispanic and 2178 African American children 6 to 72 months old.
Methods
Subjects were identified by door-to-door screening within previously identified contiguous census tracts. Pediatric ophthalmologists or optometrists performed comprehensive eye examinations on all subjects. Refractive error and keratometry measurements were attempted on all subjects with the Retinomax autorefractor after cycloplegia. Axial length measurements with the IOLMaster partial coherence interferometer were attempted on those subjects ages 30 to 72 months.
Main Outcome Measures
Ability to obtain high confidence autorefraction readings or axial length measurements on both eyes.
Results
Overall, 89% were testable in both eyes with the Retinomax device, and 91% of the children were testable with the IOLMaster. Testability rose sharply with age, so that by age 36 months 98% of children were testable with the Retinomax device and 90% were testable with IOLMaster. There were no consistent gender- or ethnicity-related differences in testability overall or when stratified by age for either device.
Conclusions
Young children can be reliably tested for ocular biometry with the Retinomax and IOLMaster devices. This may impact strategies for management of cataracts and refractive errors in preschool children.
There has been a recent emphasis on examining preschool children to detect refractive errors and risk factors for amblyopia, as well as for understanding normal development of the eye.1 Concomitantly, automated and portable noncontact technologies for purposes of ocular biometry have been developed.2,3 Such technologies may be intimidating and require patient cooperation. It is uncertain at what age they might be applied reliably to children, especially in circumstances of mass screening. Two such technologies that could be applied to preschool age children in assessing for amblyopia-related conditions are the Retinomax autorefractor (Nikon, Melville, NY) and the partial coherence interferometer for measurement of axial length (IOLMaster, Carl Zeiss Meditec, Dublin, CA). These devices can also be used in the management of amblyogenic cataracts for calculating intraocular lens (IOL) power and postoperative spectacle or contact lens correction. The reliability of these and other biometric technologies has been validated in adults but has not been thoroughly validated in preschool children. The purpose of this study is to determine the testability of these two instruments in a large population-based cohort of preschool Hispanic and African American children.
Materials and Methods
The Multi-ethnic Pediatric Eye Disease Study (MEPEDS) is a population-based study designed to (1) estimate and compare the prevalence of strabismus, amblyopia, and refractive error in a sample of African American, Asian American, Hispanic/Latino, and non-Hispanic Caucasian 6- to 72-month-olds and (2) evaluate the association of selected demographic, behavioral, biological, and ocular risk factors with these ocular disorders. To date, examination of the African American and Hispanic/Latino populations has been completed. Assessment of the other ethnic groups is being initiated. Thus, we are reporting the results for African American and Hispanic/Latino children only.
Subjects are identified by door-to-door screening of families within 35 previously identified contiguous census tracts in Los Angeles County. The details of the screening process have been reported elsewhere.4
Ophthalmologists or optometrists with subspecialty training in pediatrics perform comprehensive eye examinations on all MEPEDS subjects. This examination includes visual acuity, stereoacuity, assessment of ocular motility and alignment, and ophthalmoscopic examination. Refractive error and keratometry measurements are attempted on all subjects with the Retinomax autorefractor after cycloplegia with 2 sets of 1% cyclopentolate eyedrops. Subjects refusing cycloplegia were not included in the analysis. Axial length measurements with the IOLMaster partial coherence interferometer were attempted only on those subjects 30 to 72 months old. This was usually performed after cycloplegia unless the subjects refused dilating eyedrops. All children were examined in a clinic designed specifically for MEPEDS in Inglewood, California or in a customized van that was similarly equipped.
The Retinomax is a handheld device that measures refractive error along 2 axes while held against the subject’s forehead. The examiner attempts to focus the mire on the cornea of the eye while the child fixates on an internal lighted target. Multiple readings are made automatically. The device provides a confidence rating from 1 to 10 for all readings based on repeatability of measurements. A confidence rating of ≥8 was considered a successful test. Up to 3 tests were attempted on each eye until a successful test was achieved. The right eye was tested first. A subject was considered testable if a successful test was achieved on both eyes.
Age-eligible subjects were seated at the IOLMaster partial coherence interferometer and instructed to look at an internal fixation target with their heads positioned in the chin rest. The right eye was tested first. Five attempts were made to measure axial length of each eye. Testing was considered successful if ≥2 measurements within 0.1 mm of each other were obtained for both eyes in any subject.
Age-, gender-, and ethnicity-specific testability rates were calculated. Comparisons of testability among different groups were performed using chi-square analyses and the trend test (SAS software 9.1, SAS, Inc., Cary, NC). Testability was plotted against age by gender and ethnic groups with locally weighted regression lines using S-PLUS (Insightful Corp., Seattle, WA).
Results
Retinomax testing was attempted on 4723 children (2178 African American, 2545 Hispanic; 2324 female, 2399 male). Partial coherence tomography was attempted on 3126 children (1471 African American, 1655 Hispanic; 1552 female, 1574 male). A total of 125 subjects refused dilating eyedrops and were not included in the analysis. In children 30 months and older, 72 fewer subjects were tested with Retinomax than partial coherence tomography due to their refusal to have cycloplegic eyedrops instilled. Overall, 89% (88% African American, 91% Hispanic; 90% female, 89% male) were testable with the Retinomax device, and 91% of the children were testable (90% African American, 92% Hispanic; 92% female, 90% male) with the IOLMaster (Table 1). Overall, 91.6% of subjects were testable in either eye with the Retinomax device and 92.1% with the IOL Master.
Table 1.
Testability by Age and Ethnicity with Retinomax and IOLMaster Devices
| Retinomax | IOLMaster | |||
|---|---|---|---|---|
| Age (mos) | African American | Hispanic | African American | Hispanic |
| 6–11 | 108/188 (57%) | 144/215 (67%) | NA | NA |
| 12–17 | 99/192 (52%) | 143/213 (67%) | NA | NA |
| 18–23 | 147/197 (75%) | 190/243 (78%) | NA | NA |
| 24–29 | 173/188 (92%) | 215/233 (92%) | NA | NA |
| 30–35 | 195/201 (97%) | 229/245 (93%) | 167/213 (78%) | 185/248 (76%) |
| 36–47 | 367/379 (97%) | 445/455 (98%) | 341/392 (87%) | 416/458 (91%) |
| 48–59 | 413/414 (100%) | 471/472 (100%) | 401/435 (92%) | 461/476 (97%) |
| 60–72 | 414/419 (99%) | 467/469 (100%) | 413/431 (96%) | 460/473 (97%) |
NA = not applicable.
There was no difference in testability between the right eyes and left eyes with either test for any age group. There were no gender-related differences in Retinomax testability overall (P = 0.48, chi-square) or when adjusted for age (P = 0.83). Testability with the Retinomax device was significantly higher (67% vs. 52% success rate; P = 0.001, chi-square) in Hispanic than in African American 12- to 17-month-olds. There was no difference in testability between Hispanic and African American children for other age groups.
For IOLMaster, females in the 36- to 47-month-old group (94% vs. 85%, P<0.0001) and Hispanics in the 48- to 59-month-old group (97% vs. 92%, P = 0.002) had significantly higher testability rates than their male counterparts. There were no gender- or ethnic-related differences in testability for other age groups.
Discussion
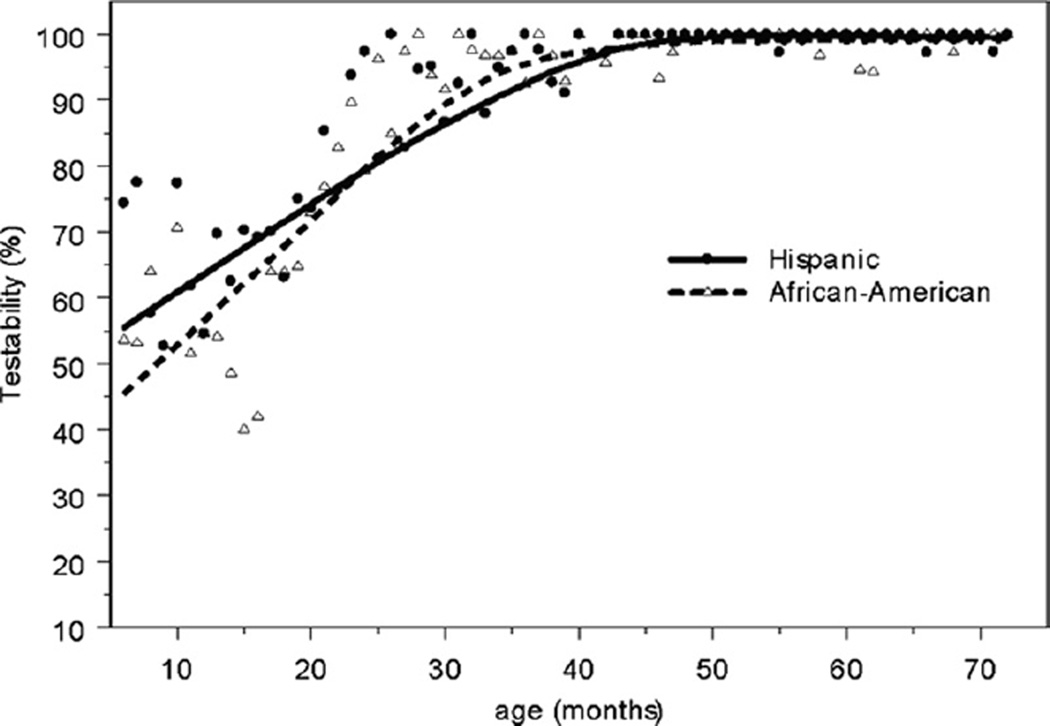
The majority of 6-month-olds, and over 90% of 24-montholds, can be successfully measured with the Retinomax autorefractor. This is considerably younger than documented in previous reports. The Retinomax device used by professionals has previously been demonstrated to be 64% sensitive for detecting highly amblyogenic refractive errors at 90% specificity in ≥36-month-old children.5 Testability in that group was >99%, based on achieving any confidence reading, and 95.4%, based on achieving a confidence reading ≥8 within 3 attempts in either eye without cycloplegia.6 Cycloplegia increases the accuracy of the Retinomax and other autorefractors when used on children.7,8 By 36 months of age, we were able to achieve similar (98%) testability with the Retinomax with cycloplegia with the stricter confidence reading (≥8) recommended by the manufacturer (Fig 1). In fact, 69% of 1-year-olds and 94% of 2-year-olds are testable in both eyes with strict reliability criteria. For comparison to studies that defined testability by either eye, 77% of 1-year-olds and 95% of 2-year-olds were testable in at least one eye. Only 20% of 1-year-olds and 4% of 2-year-olds were unable to perform the test with any confidence reading in both eyes. By 43 months of age, no subjects had a reliability confidence reading of <8 with the Retinomax device if they were able to perform the test at all.
Figure 1.
Testability rates (with trend lines) by months of age with the Retinomax autorefractor are plotted for Hispanic and African American children. Approximately half of children are testable at 6 months of age. Testability rises steeply in both ethnic groups, so that virtually all children are testable by 36 months of age.
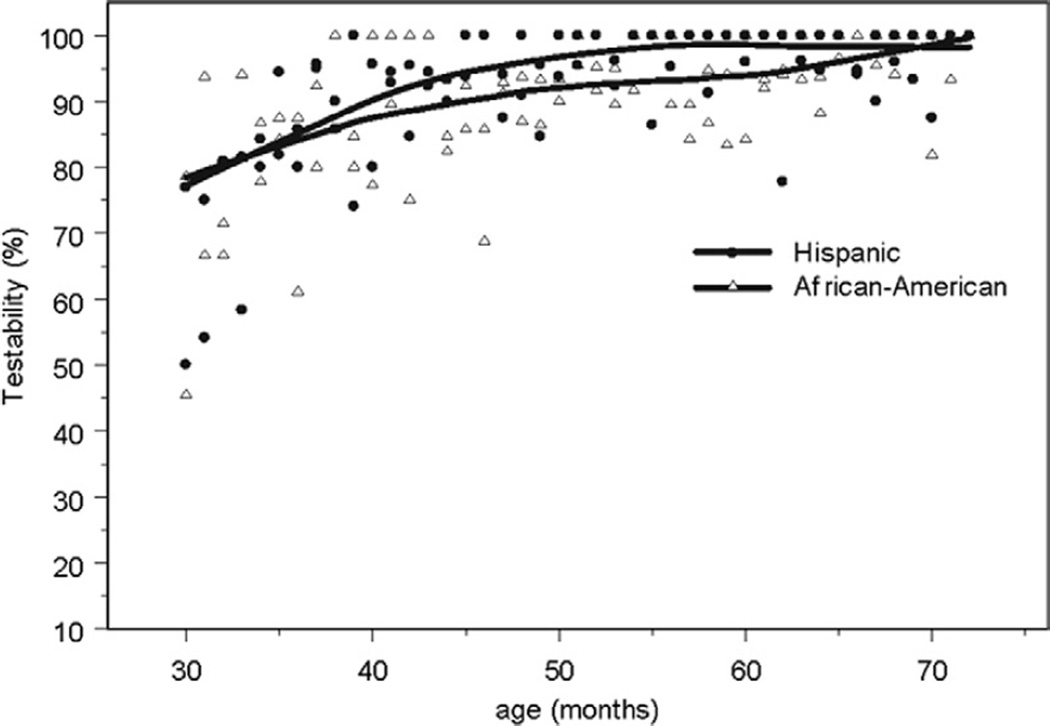
Similarly, IOLMaster testability rates rise in children so that by age 36 months nearly all children can be tested (Fig 2). Partial coherence interferometry has not been previously studied in preschoolers, but it has been demonstrated to be more reproducible than A-scan ultrasonography in measuring axial length in school age children.9,10
Figure 2.
Testability rates (with trend lines) by months of age with the IOLMaster partial coherence interferometer are plotted for Hispanic and African American children. The majority of children are testable at 30 months of age, and nearly all children are testable by age 36 months.
Ophthalmologists and optometrists with pediatric subspecialty training performed all of the tests in this study. Testability of preschoolers with these devices using non-professionals in a screening setting may thus be less. For example, a previous study demonstrated that nurses achieved slightly higher sensitivity for refractive errors when administering Retinomax testing than lay personnel.11 In addition, the sensitivity and specificity of these devices for amblyogenic risk factors have yet to be determined in children younger than 36 months. Nonetheless, the high testability rates of these devices in young children suggest they may be very useful in the hands of physicians for planning treatment with spectacles, contact lenses, or IOLs in addition to cohort studies on biometric development of the eye or refractive error screening.
Acknowledgments
Supported by the National Eye Institute, Bethesda, Maryland (grant nos. EY14472, EY03040), and an unrestricted grant from Research to Prevent Blindness, New York, New York. Dr Varma is a Research to Prevent Blindness Sybil B. Harrington Scholar.
Appendix
The Multi-ethnic Pediatric Eye Disease Study Group
University of Southern California
Rohit Varma, MD, MPH (Principal Investigator), LaVina Abbott (2002–2005), George Ayala (2005–2006), Stanley P. Azen, PhD, Tal Barak, OD, Mark Borchert, MD, Jessica Chang, OD, Felicia K. Chen, OD, Susan Cotter, OD, MS (Co–Principal Investigator), Jennifer Deneen, MPH, Jackie Diaz, Anne DiLauro, MPH (2005–2007), Jill Donofrio, MPH (2003–2005), Claudia Dozal (2003–2004), Athena Foong, James Gardner, Jackson Lau, OD, Jesse Lin, MS, George Martinez, Roberta McKean, PhD, Kisha Milo, Carlos Moya, Sylvia Paz, MS (2002–2005), Ana Penate, Amanda Reiner, MPH, Claudia Salazar, Erin Song, OD, Kristina Tarczy-Hornoch, MD, DPhil, Mina Torres, MS, Natalia Uribe, OD, Ivania Verrico, Ying Wang, MS, Peng Zhao, MS, Amy Zhu.
Battelle Survey Research Center
Charles Aders (2003–2006), Candace Kwong, MPH, Nancy Noedel, Michael Preciado, Karen Tucker, MA.
Footnotes
The authors have no proprietary or commercial interest in any materials discussed in the article.
References
- 1.Donahue SP, Arnold RW, Ruben JB AAPOS Vision Screening Committee. Preschool vision screening: what should we be detecting and how should we report it? Uniform guidelines for reporting results of preschool vision screening studies. J AAPOS. 2003;7:314–316. doi: 10.1016/s1091-8531(03)00182-4. [DOI] [PubMed] [Google Scholar]
- 2.Donahue SP, Baker JD, Scott WE, et al. Lions Clubs International Foundation Core Four photoscreening: results from 17 programs and 400,000 preschool children. J AAPOS. 2006;10:44–48. doi: 10.1016/j.jaapos.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Vision in Preschoolers Study Group. The Electronic Visual Acuity Tester: testability in preschool children. Optom Vis Sci. 2004;81:238–244. doi: 10.1097/00006324-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Varma R, Deneen J, Cotter S, et al. The Multi-Ethnic Pediatric Eye Disease Study: design and methods. Ophthalmic Epidemiol. 2006;13:253–262. doi: 10.1080/09286580600719055. [DOI] [PubMed] [Google Scholar]
- 5.Vision in Preschoolers Study Group. Comparison of preschool vision screening tests as administered by licensed eye care professionals in the Vision in Preschoolers Study. Ophthalmology. 2004;111:637–650. doi: 10.1016/j.ophtha.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Vision in Preschoolers Study Group. Impact of confidence number on the screening accuracy of the Retinomax autorefractor. Optom Vis Sci. 2007;84:181–188. doi: 10.1097/OPX.0b013e3180339f5a. [DOI] [PubMed] [Google Scholar]
- 7.Choong YF, Chen AH, Goh PP. A comparison of autorefraction and subjective refraction with and without cycloplegia in primary school children. Am J Ophthalmol. 2006;142:68–74. doi: 10.1016/j.ajo.2006.01.084. [DOI] [PubMed] [Google Scholar]
- 8.Barry JC, Konig HH. Non-cycloplegic screening for amblyopia via refractive findings with the Nikon Retinomax hand held autorefractor in 3 year old kindergarten children. Br J Ophthalmol. 2001;85:1179–1182. doi: 10.1136/bjo.85.10.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hussin HM, Spry PG, Majid MA, Gouws P. Reliability and validity of the partial coherence interferometry for measurement of ocular axial length in children. Eye. 2006;20:1021–1024. doi: 10.1038/sj.eye.6702069. [DOI] [PubMed] [Google Scholar]
- 10.Carkeet A, Saw SM, Gazzard G, et al. Repeatability of IOLMaster biometry in children. Optom Vis Sci. 2004;81:829–834. doi: 10.1097/01.opx.0000145020.33250.c0. [DOI] [PubMed] [Google Scholar]
- 11.Vision in Preschoolers Study Group. Preschool vision screening tests administered by nurse screeners compared with lay screeners in the vision in preschoolers study. Invest Ophthalmol Vis Sci. 2005;46:2639–2648. doi: 10.1167/iovs.05-0141. [DOI] [PubMed] [Google Scholar]