Abstract
Human respiratory syncytial virus (RSV) is a major cause of acute lower respiratory tract infection in young children, immunocompromized adults and the elderly. The innate immune response plays a pivotal role in host defense against RSV, but whether severe outcomes following RSV infection result from excessive or poor innate immune recognition remains unclear. Recent research suggests a situation in which crosstalk between families of pattern recognition receptors (PRRs) occurs in a cell type-dependent manner. The current challenge to empower novel therapeutic approaches and vaccine development is to confirm the role of the individual receptors in RSV pathogenesis in humans.
Keywords: Respiratory Syncytial Virus, Pathogen Recognition Receptors, Innate immunity, Pathogenesis
The role of innate immune sensing in host responses to respiratory syncytial virus infection
Natural RSV infection induces poor adaptive immunity and consequently, maternal respiratory syncytial virus (RSV)-specific antibodies are insufficiently protective in early life. As such, innate immunity plays a pivotal role during primary infection to restrict viral replication in the airways [1]. Production of antiviral and proinflammatory cytokines, a hallmark of the early innate immune response, is initiated by the recognition of highly conserved ‘molecular signatures’ of microbes by a heterogeneous group of germ-line encoded pattern recognition receptors (PRRs), including the Toll-like receptors (TLRs), retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs) and NOD-like receptors (NLRs) [2, 3] (Figure 1). Opinions diverge whether severe outcomes following RSV infection result from of excessive innate immune activation [4, 5], or alternatively from immune evasion and poor innate immune recognition [6, 7]. RSV primarily infects airway epithelial cells (AECs), but also innate immune cells, e.g. alveolar macrophages (AMs) [8, 9] and dendritic cells (DCs) [10, 11]. The outcome of RSV infection is likely determined by a complex interplay between PRR-dependent mechanisms occurring in non-immune and immune cells. Recent research has generated a consensus view in which the coordinated involvement of different PRRs is clear, but the relative importance of each receptor in a given cell type remains a matter of debate, most likely because of numerous confounding factors in the multiple experimental designs (Box 1). Interestingly, recent advances have highlighted RSV entry and compartmentalization mechanisms, shedding new light on RSV innate immune sensing. Here, we argue that the currently available data regarding PRR-dependent sensing of RSV could be improved by experimental designs that reflect more closely human natural infection. This new knowledge is expected to help develop novel treatment and improve vaccine design.
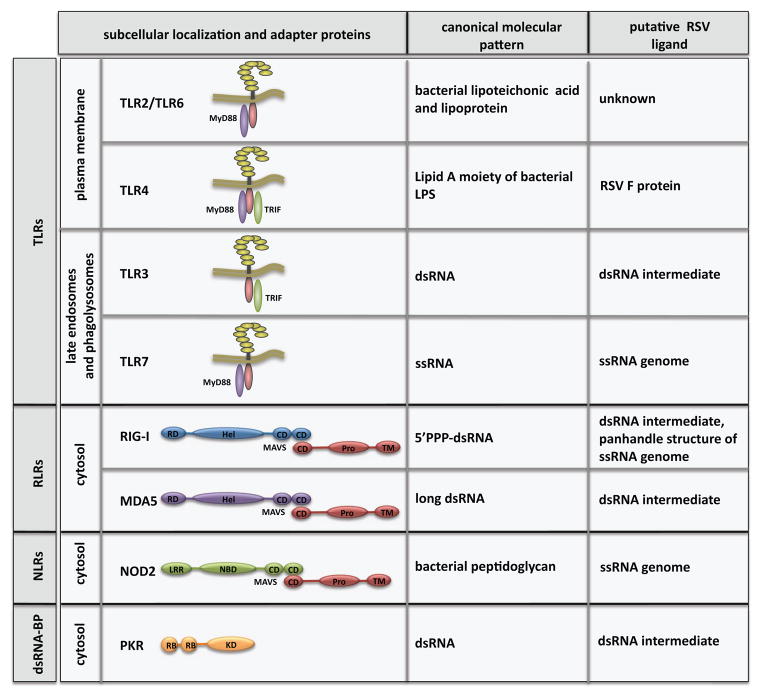
Figure 1.
PRRs implicated in RSV sensing. The families of PRRs include TLRs: Toll-like receptors, RLRs: RIG-I-like receptors, NLRs: NOD-like receptors and dsRNA-BP: double-stranded RNA binding protein. The specific adaptors used by PRRs, including Myd88, TRIF and MAVS are indicated. Abbreviations: RD, regulatory domain; Hel, helicase domain; CD, CARD domain; Pro, proline-rich domain; TM, transmembrane domain; LRR, leucin-rich repeat; NBD, nucleotide binding domain; KD, kinase domain; RB, dsRNA binding domain.
Box 1. Confounding factors in the characterization of PRRs engagement.
It is important to be aware of the variability and/or bias introduced by the different experimental systems used to define the role of various PRRs in RSV sensing. This understanding may explain experimental discrepancies and avoid inappropriate generalization of some conclusions. Importantly, inherent limitations of the different models can also be misleading.
Use of laboratory RSV strains vs clinical isolates. The prototypic laboratory strains A2 and Long or line19 and the various clinical isolates contain significant genetic variability that account for differences in infectivity and associated pathogenesis and immune responses.
Use of crude RSV preparation vs sucrose-purified RSV. Non-purified RSV preparations consist of clarified supernatant of cells harvested at times of obvious cytopathic effects. Clarified supernatant contains cytokines, which may alter PRRs expression and trigger independent immune responses, and by-products of damaged cells that might act as damage-associated molecular pattern capable of activating PRRs.
Cell line used for RSV propagation. RSV amplification is performed in human Hep-2 or monkey Vero cells. Besides differences in envelope composition, RSV propagated in Vero cells appears to express a truncated G protein [50]. Thus, cellular attachment and entry mechanisms are likely different.
Use of infectious RSV virions vs purified RSV F protein. Structural and biochemical studies are needed to confirm that the F protein anchored in the viral envelope of the infectious virions exhibit motifs accessible to PRRs similar to purified RSV F.
Presence of defective interfering (DI) particles in RSV preparation. Culture of RSV by serial passage leads to generation of DI virus-like particles that are competent to stimulate innate immunity. DI particles stain positive for F but negative for N, indicating that they lack a nucleocapsid. RIG-I binds DI genome of ssRNA virus [51] implying that variations in the amount of DI particles in RSV preparations could alter sensing mechanisms.
Human vs mouse models. Human and mice exhibit differences in their PRRs repertoire, ligand specificity, regulation and function. Importantly, while 150–650 viral units are required to establish infection in humans, infection in mice requires 105–107 viral units to observe signs of illness [52, 53].
Use of cell lines vs primary cells. Primary cells and cell lines, cancer-derived or immortalized through virus-related techniques, differ in basal and inducible expression of PRRs. Importantly, nucleolin is overexpressed in highly proliferative cells, a property used as a marker for cancer, likely impacting on RSV entry and permissiveness and subsequent ligand accessibility and innate immune activation.
PRR crosstalk in AEC
Various PRRs have been shown to sense RSV in AECs, highlighting potential crosstalk. The importance of the ubiquitously expressed cytosolic receptor RIG-I in the replication-dependent recognition of RSV was demonstrated by silencing experiments in the A549 (adenocarcinomic human alveolar basal epithelial cells) cell line model. In the absence of RIG-I, RSV fails to activate the NF-κB and IRF-3 transcription factors and the subsequent production of interferon-β (IFN-β), interferon-stimulated genes (ISGs), chemokine (C-C motif) ligand 5 (CCL5) and thymic stromal lymphopoietin (TSLP) [12–15]. The role of RIG-I is also supported in A549 cells by the colocalization of viral genomic RNA with RIG-I at an early time point of infection [12, 13, 16]. A physical interaction between the RSV N transcript and ectopically expressed RIG-I was also identified by UV-crosslinking immunoprecipitation [12]. While it was initially thought that the cytosolic sensors RIG-I and MDA5 recognize selective families of viruses, more recent reports suggest that several viruses, including single-stranded RNA (ssRNA) viruses, are sensed by both receptors [17]. Silencing of MDA5 in A549 cells leads to diminished RSV-induced NF-κB activation. Interestingly, while RIG-I triggers the classical NF-κB activation pathway, MDA5 activates NF-κB by an uncharacterized alternative pathway, suggesting non-redundant functions [13]. As in most, if not all, viral infections, a physical interaction between RSV nucleic acids and MDA5 remains to be demonstrated. Consistent with the role of RLRs, silencing of the adaptor MAVS, which transmits the signal immediately downstream of RLRs, also abrogates NF-κB activation [13]. Studies detailed above were performed in A549 cells infected with the purified strain A2. Confirmation of the role of RLRs in primary human AEC and using a spectrum of RSV clinical isolates have not yet been published (Box 1). Nevertheless, the observation that MAVS is required for IFN-β and inflammatory cytokine induction in primary murine AEC infected ex vivo with the RSV strain line 19 [18] supports a conserved role of RLRs in RSV sensing.
Intriguingly, a 2009 report suggests cytosolic NOD2 contributes to RSV sensing through MAVS in A549 and primary normal human bronchial epithelial (NHBE) cells, triggering both IRF-3 activation and IFNβ induction prior to RLRs engagement [19]. In contrast to the well-documented involvement of RLRs in sensing ssRNA viruses, this is the only study to report a role of NOD2 in sensing ssRNA virus nucleic acids. Further independent studies are required to validate the role of NOD2 in RSV sensing.
Although not considered a typical PRR, the double-stranded RNA (dsRNA)-dependent protein kinase R (PKR) is attracting increasing interest as a dsRNA sensor in viral infections [20]. RSV-induced PKR expression in A549 cells plays an important role in the formation of RSV-induced cytoplasmic inclusion bodies, but has minimal effect on RSV replication [21].
Besides cytoplasmic PRRs, TLR3, which responds to dsRNA in the endosomes, is involved in RSV sensing, but at late time points following infection. Knockdown of TLR3 impaired IFN-β, interferon-γ induced protein 10 (IP-10), ISG15 and CCL5 responses following infection with either RSV A2 or a RSV clinical isolate in A549 cells. The observation that TLR3 is involved at a later time compared to RLRs [12, 22] is consistent with the inducible expression of TLR3 during infection.
PRR-dependent sensing of RSV in innate immune cells
It is increasingly recognized that innate immune cells, including AMs and DCs, are infected by RSV and play a pivotal role in the early host response [1]. Immune cells exert cell type-specific immune responses distinct from AEC and express a broad repertoire of PRRs, but their role in RSV infection has been the subject of far less experimental scrutiny. The initial investigations were limited to the role of TLR4 in human monocytes or murine peritoneal macrophages. In these cells, the RSV F protein solvent-purified from RSV A2 was found to induce proinflammatory interleukin 6 (IL6) expression in a TLR4- and CD14-dependent manner [4]. This function was recently challenged by a report indicating a role of TLR4 in the resolution of RSV-induced inflammation. Infection of murine peritoneal macrophages and AMs with non-purified RSV Long induced TLR4-dependent expression of markers specific for alternatively activated macrophages (arginase-1, FIZZ1, or MR) [23]. These conflicting results raise the question of the physiological relevance of the results obtained using purified RSV F protein (Box 1). In line with the observed RSV F/TLR4 proinflammatory function, the RSV F protein purified from RSV Long was shown to activate NF-κB in a TLR4-dependent manner through a direct interaction with MD2 in HEK293 cells expressing the TLR4 MD2 CD14 complex [24]. Again, the physiological relevance of this study is challenged by the lack of NF-κB activation in HEK293 cells upon infection with purified RSV A2 and Long strains [7]. Only recently a role of the cell surface-expressed TLR2/6 heterodimer in the replication-independent induction of proinflammatory cytokines was reported in murine peritoneal macrophages infected ex vivo with non-purified RSV A2 [5].
In summary, TLRs crosstalk might be involved in the regulation of AM functions during RSV infection, but further studies are required to clarify their role in primary human AMs during natural infection with purified RSV (Box 1). Importantly, how RSV components activate TLR4 or TLR2/6 complexes during natural infection remains unknown. It will be of importance to confirm the direct interactions with additional structural and biochemical studies (Box 1).
Only very few studies have reported the involvement of PRRs other than TLRs in RSV sensing in AMs. Similar to what was observed in AEC, NOD2 was found to be essential for RSV A2-induced IFN-β production in murine AMs [19]. Whether the observation that MAVS is required for RSV-induced IFN-β production in isolated AMs and intestinal macrophages [18] reflects the involvement of NOD2 or other RLRs remains to be characterized.
Besides AMs, DCs from MAVS knockout (KO) mice also exhibit greatly reduced production of type I IFNs as well as proinflammatory cytokines and chemokines in response to RSV [18]. Moreover, TLRs constitute a main class of PRRs regulating DC functions. Accordingly, bone marrow-derived DCs isolated from TLR7 KO mice exhibited decreased IL12p35 expression together with increased IL-23 in response to RSV [25]. Human plasmacytoid DCs (pDCs) express high TLR7 levels and are the only DC subtype to produce large amounts of type I IFN in response to RSV Long [26]. Surprisingly, RSV-induced IFN-α production in human pDCs was found to require viral replication and to be independent of endosomal acidification, a necessary step for endosomal TLRs activation, supporting a pivotal role of other PRRs, such as RLRs or NLRs [26]. This is in contrast to the generally stated model in which pDCs sense viral nucleic acids primarily via endosomal TLRs, but not RLRs [27]. Moreover, RIG-I and MAVS deficient pDCs retain their ability to produce IFN-α in response to different RNA viruses, such as Newcastle disease or Sendai virus [28]. Human pDCs were recently shown to respond to DNA viruses via two newly described cytosolic DExD/H-box helicases [29]. Both observations indicate that the repertoire of functional PRRs in pDCs might be broader than initially thought. Better characterization of PRRs in pDCs will help clarify their role in protection from RSV-associated disease.
RSV life cycle: to be or not to be detected by PRRs
The cellular localization of PRRs and the route of RSV entry have important consequences for ligand accessibility [30] (Figure 2). In contrast to other ssRNA viruses, the mechanism of RSV entry and the spatial distribution of RSV proteins and nucleic acids during the infection cycle are still poorly characterized. It was only very recently that surface-expressed nucleolin was identified as a functional receptor for RSV that binds the RSV F protein and is required for RSV entry at the apical side of AECs [31]. Intriguingly, cell-surface nucleolin is part of a multiprotein signaling complex at the cell surface and was found to be involved in lipopolysaccharide (LPS) internalization and signaling [32]. It will be important to clarify the potential functional relationship between nucleolin and TLR4 in order to determine whether the observed TLR4-mediated recognition of RSV rather reflects recognition by nucleolin.
Figure 2.
Mechanisms of PRR activation during RSV replication cycle. RSV entry and replication cycle are described. A. Potential RSV-derived ligands for activation of cytosolic PRRs. B. Potential mechanisms of activation of endosomal TLRs. Solid lines depict the RSV replication cycle. Dashed lines and question marks indicate potential pathways and ligand PRRs interactions that need further investigation. Abbreviations: DI, defective interfering; DAMPs, damage-associated molecular patterns.
It has been appreciated for some time that release of RSV nucleocapsid into the cytoplasm of AECs occurs after fusion with the plasma membrane. Transcription and replication of the genome occurs in the cytoplasm, which is consistent with the capacity of cytosolic PRRs, including RLRs, NLRs and PKR to sense specific replication intermediates that still need to be characterized in the context of natural RSV infection (Figure 1 and 2). Interestingly, the spatiotemporal pattern of RLRs and MAVS colocalization with viral components was found to progress inside different cytoplasmic inclusion bodies during RSV replication cycle [16].
Because RSV fusion does not require an acidified milieu, it was initially proposed that the nucleocapsid delivery into the cytoplasm bypasses the endosomal compartment. However, recent studies have confirmed a more complex mechanism of RSV entry. Initial observations revealed that RSV fusion with the NHBE cell apical surface was dependent on actin polymerization, but was dynamin-independent [33]. The recent observation that RSV transiently activates macropinocytosis in 16HBE14o-(SV40 large T antigen-transformed human bronchial epithelial cell line) and A549 cells provide a likely mechanism for the clathrin-independent endocytic requirement exhibited by RSV [34]. Macropinosomes form from cell surface ruffles and mature in parallel to classical endocytic vesicles before merging at the level of fusion with lysosomes [35]. However, RSV fusion occurs before macropinosomes merge with endolysosomal compartments, thus likely preventing exposure of RSV to endosomal PRRs.
The mechanism of entry of RSV in immune cells is not known and may well be different than in AECs. Autophagy is one of the mechanisms allowing endosomal TLR activation through delivery of cytoplasmic components to the lysosomal compartment. A recent study has established a relationship between RSV-induced autophagy and enhanced type I IFN in murine DCs, thereby providing a possible mechanism for TLR7 activation in murine DCs [36]. However, this mechanism is most likely not conserved between species or DC subtypes as RSV-induced IFN-α production in human pDCs is replication-dependent and cannot be blocked with chloroquine, which abolishes endosomal acidification and autophagy [26]. RSV-induced autophagy has not yet been described in non-immune cells, but it would be interesting to determine whether it permits the delayed engagement of TLR3 in AECs [12, 22].
Little is known about the role of RSV-specific antibody-Fc receptor mediated uptake in immune cells, and the consequences to downstream signaling events. RSV vaccine-enhanced disease observed during the failed vaccine trials had been linked to poor TLR stimulation by the formalin-inactivated (FI) vaccine, and the subsequent production of RSV-specific antibody with poor affinity that promoted RSV entry and immunopathology during subsequent infection [6]. In contrast, high affinity antibody, such as palivizumab used for RSV immunoprophylaxis, leads to RSV neutralization. It remains an open question how RSV-specific antibodies produced upon natural infection (e.g. maternal antibody in infants) affect the route of entry, and whether this affects ligand accessibility and PRR signaling.
PRR function: moving from experimental models to natural human infections
Studies aimed at defining the function of individual PRRs in specific cell types have taken advantage of cell lines and primary cells of human and murine origins. These models have proved to be very useful in generating a mechanistic understanding of RSV sensing. However, inherent differences between cell lines and primary cells and between human and mouse cells [37] in terms of PRR usage (Box 1) run the risk of generating results irrelevant to human innate immune responses. Despite the importance of mouse models in the characterization of the broad picture of the immune response to RSV in the presence or absence of a particular PRR, it is important to validate the results with human experimental approaches. To date important discrepancies have been observed between results obtained in the various TLR KO mice and human polymorphism studies [38]. However, it is also important to be aware that human genetic association studies have led to contradicting results, because of diverging inclusion criteria for the cohorts, limited sample size and ethnic diversity [39, 40]. An important question is how accurately the available animal models reflect the role of PRRs during natural infection in humans, and more specifically in infants and young children who are the most vulnerable for severe RSV disease. A powerful example for the discrepancy between animal models and natural infection are children with autosomal recessive MyD88 deficiency, who suffer exclusively from recurrent pyogenic bacterial infections, while MyD88 deficient mice are susceptible to a broad range of pathogens [41]. Using primary cells from human adults also has its limitations, as it is increasingly recognized that innate immune responses in early life are different from that during adulthood [42], but how differences in PRRs expression and function contributes to these differences remains to be assessed. Bronchial cells isolated from children by bronchial brushing and fully differentiated in ALI culture were shown to model many of the histopathological characteristics observed in severe RSV-associated disease when infected ex vivo [43, 44]. However, studies of the role of various PRRs in this model will likely be highly challenging. First, the availability of these samples is very limited. Second, the number of cells obtained by the culture in ALI makes mechanistic biochemical studies difficult. The molecular tools currently available to perform functional studies in fully differentiated ciliated bronchial epithelial cells are still very limited. However, silencing of PRRs using lentivirus-mediated short hairpin RNA (shRNA) transduction has been described without apparent artefactual activation of the innate immune responses [45], opening the path to studies of PRR function in this model. Although restricted to healthy adult volunteers, controlled human challenge studies, which consist of carefully monitored and controlled infection, are beginning to provide unique insights into RSV pathogenesis in humans [46]. It will be of great interest to collect data about PRRs in this model to accelerate development of novel therapeutic approaches and an RSV vaccine.
Knowledge of PRR-dependent RSV sensing: a path to improve vaccine design ?
Despite the recent progress in our understanding of RSV biology and pathogenesis, and the success in reducing the risk of severe RSV disease among high-risk infants by immunoprophylaxis with palivizumab, the development of an RSV vaccine has remained unsuccessful. Obviously, adequate innate immune activation plays an important role for vaccines to be efficacious, and this is generally achieved either by combining purified vaccine antigens with adjuvants that serve as potent innate immune activators (e.g. alum or monophosphoryl lipid A), or by use of live, attenuated organisms that are capable of autonomously driving innate immune activation. One important lesson from vaccine studies is that activation of multiple innate receptors appears more effective than activation of a single pathway [47]. This raises the question why does natural RSV infection fail to protect against reinfection, given that multiple PRRs play a role in innate immune recognition of RSV? Indeed, the lack of protection from natural RSV infection rather supports the notion that RSV is a poor activator of innate immune defences, and consequently fails to induce long-lived immunological memory. Moreover, FI-RSV vaccine-enhanced diseases has been linked to poor TLR activation [6], and there is a body of evidence suggesting that inclusion of adjuvants, such as the TLR4 agonist MPL [6, 48, 49], abolishes the immunopathology associated with the FI-RSV, and therefore enhances vaccine safety. It will be important to elucidate the contribution of individual PRRs in immunity and immunopathology during RSV infection, particularly during primary infection in infants, but also during re-infection among the elderly. Based upon the existing evidence, a protective RSV vaccine will likely require an adjuvant formulation, which stimulates multiple PRRs to ensure adequate safety, and sufficient immunogenicity.
Concluding remarks
The past 20 years have identified an extended array of PRRs, many of which are capable of sensing RSV in a cell-type dependent manner. Although the cooperation and non-redundant functions of the diverse PRRs is clear, many questions about their respective contribution to the development of severe RSV disease remain to be answered. Due to broad repertoire of PRRs that RSV can engage, a simplistic assumption could be that RSV induces excessive innate immune activation. However, it cannot be ruled out that RSV pathogenesis could in fact be a result of poor innate immune activation due to the multiple evasion mechanisms that RSV uses to counteract PRR-dependent signaling. This could negatively impact the subsequent development of the adaptive immune response, and thereby prevent protection against reinfection. New knowledge of PRRs in human models, including comparative studies between adults and children, is expected to help understanding about why some individuals only develop mild illness, while some infants and young children suffer from severe disease (Box 2). Answers to these questions will empower novel approaches to treat and prevent RSV.
Box 2. Outstanding questions.
How do different PRRs expressed in a single cell type cross-talk to mount an efficient immune response? Are they redundant or do they control distinct characteristics of the immune response, such as duration, strength, etc.?
What is the status of PRR-dependent pathways (e.g. expression and function of PRRs) in infants and young children who are most vulnerable to severe outcome following RSV infection as compared to adults?
Does primary RSV infection, the lung and/or gut microbiome, other infectious diseases, or environmental factors alter the basal and inducible expression of PRRs and/or downstream signalling components?
How does the genetic background, i.e. single nucleotide polymorphisms in genes encoding PRRs and downstream signaling molecules, contribute to the severity of RSV disease?
Does severe illness associated with RSV infection result from poor or excessive innate immune activation?
Highlights.
Crosstalk of multiple pattern recognition receptors (PRRs) in respiratory syncytial virus (RSV) sensing is cell-type specific.
RSV entry route and compartmentalization determine PRR ligand accessibility.
RSV-derived components that bind PRRs during natural RSV infection remain to be identified.
Confirmation of PRR function in children is essential for vaccine design.
Acknowledgments
Research in our laboratories is funded by the Canadian Institutes of Health Research (CIHR) Team Grant in Mutagenesis and Infectious Diseases (to SET) and CIHR operating grants (to NG). SET holds the Aubrey J. Tingle Professorship in Pediatric Immunology and is a clinical scholar of the Michael Smith Foundation for Health Research. NG is recipient of a Tier II Canada Research Chair.
References
- 1.Collins PL, Melero JA. Progress in understanding and controlling respiratory syncytial virus: Still crazy after all these years. Virus Res. 2011;162:80–99. doi: 10.1016/j.virusres.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takeuchi O, Akira S. Innate immunity to virus infection. Immunol Rev. 2009;227:75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 4.Kurt-Jones EA, et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 5.Murawski MR, et al. Respiratory syncytial virus activates innate immunity through Toll-like receptor 2. J Virol. 2009;83:1492–1500. doi: 10.1128/JVI.00671-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delgado MF, et al. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med. 2009;15:34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marr N, Turvey SE. Role of human TLR4 in respiratory syncytial virus-induced NF-kappaB activation, viral entry and replication. Innate Immun. 2012;18:856–865. doi: 10.1177/1753425912444479. [DOI] [PubMed] [Google Scholar]
- 8.Dakhama A, et al. Permissiveness of guinea pig alveolar macrophage subpopulations to acute respiratory syncytial virus infection in vitro. Chest. 1998;114:1681–1688. doi: 10.1378/chest.114.6.1681. [DOI] [PubMed] [Google Scholar]
- 9.Rivera-Toledo E, Gomez B. Respiratory syncytial virus persistence in macrophages alters the profile of cellular gene expression. Viruses. 2012;4:3270–3280. doi: 10.3390/v4123270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson TR, et al. Primary human mDC1, mDC2, and pDC dendritic cells are differentially infected and activated by respiratory syncytial virus. PLoS One. 2011;6:e16458. doi: 10.1371/journal.pone.0016458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guerrero-Plata A, et al. Differential response of dendritic cells to human metapneumovirus and respiratory syncytial virus. Am J Respir Cell Mol Biol. 2006;34:320–329. doi: 10.1165/rcmb.2005-0287OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu P, et al. Retinoic acid-inducible gene I mediates early antiviral response and Toll-like receptor 3 expression in respiratory syncytial virus-infected airway epithelial cells. J Virol. 2007;81:1401–1411. doi: 10.1128/JVI.01740-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoboua F, et al. Respiratory syncytial virus-mediated NF-kappa B p65 phosphorylation at serine 536 is dependent on RIG-I, TRAF6, and IKK beta. J Virol. 2010;84:7267–7277. doi: 10.1128/JVI.00142-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu P, et al. Respiratory syncytial virus induces RelA release from cytoplasmic 100-kDa NF-kappa B2 complexes via a novel retinoic acid-inducible gene-I{middle dot}NF-kappa B-inducing kinase signaling pathway. J Biol Chem. 2008;283:23169–23178. doi: 10.1074/jbc.M802729200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee HC, et al. Thymic stromal lymphopoietin is induced by respiratory syncytial virus-infected airway epithelial cells and promotes a type 2 response to infection. J Allergy Clin Immunol. 2012;130:1187–1196. doi: 10.1016/j.jaci.2012.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lifland AW, et al. Human respiratory syncytial virus nucleoprotein and inclusion bodies antagonize the innate immune response mediated by MDA5 and MAVS. J Virol. 2012;86:8245–8258. doi: 10.1128/JVI.00215-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loo YM, Gale M., Jr Immune signaling by RIG-I-like receptors. Immunity. 2011;34:680–692. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demoor T, et al. IPS-1 signaling has a nonredundant role in mediating antiviral responses and the clearance of respiratory syncytial virus. J Immunol. 2012;189:5942–5953. doi: 10.4049/jimmunol.1201763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabbah A, et al. Activation of innate immune antiviral responses by Nod2. Nat Immunol. 2009;10:1073–1080. doi: 10.1038/ni.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munir M, Berg M. The multiple faces of proteinkinase R in antiviral defense. Virulence. 2013;4:85–89. doi: 10.4161/viru.23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groskreutz DJ, et al. Respiratory syncytial virus limits alpha subunit of eukaryotic translation initiation factor 2 (eIF2alpha) phosphorylation to maintain translation and viral replication. J Biol Chem. 2010;285:24023–24031. doi: 10.1074/jbc.M109.077321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rudd BD, et al. Differential role for TLR3 in respiratory syncytial virus-induced chemokine expression. J Virol. 2005;79:3350–3357. doi: 10.1128/JVI.79.6.3350-3357.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shirey KA, et al. Control of RSV-induced lung injury by alternatively activated macrophages is IL-4R alpha-, TLR4-, and IFN-beta-dependent. Mucosal Immunol. 2010;3:291–300. doi: 10.1038/mi.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rallabhandi P, et al. Respiratory syncytial virus fusion protein-induced toll-like receptor 4 (TLR4) signaling is inhibited by the TLR4 antagonists Rhodobacter sphaeroides lipopolysaccharide and eritoran (E5564) and requires direct interaction with MD-2. MBio. 2012;3:e00218–12. doi: 10.1128/mBio.00218-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lukacs NW, et al. Respiratory virus-induced TLR7 activation controls IL-17-associated increased mucus via IL-23 regulation. J Immunol. 2010;185:2231–2239. doi: 10.4049/jimmunol.1000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hornung V, et al. Replication-dependent potent IFN-alpha induction in human plasmacytoid dendritic cells by a single-stranded RNA virus. J Immunol. 2004;173:5935–5943. doi: 10.4049/jimmunol.173.10.5935. [DOI] [PubMed] [Google Scholar]
- 27.Kato H, et al. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23:19–28. doi: 10.1016/j.immuni.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Gilliet M, et al. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 29.Kim T, et al. Aspartate-glutamate-alanine-histidine box motif (DEAH)/RNA helicase A helicases sense microbial DNA in human plasmacytoid dendritic cells. Proc Natl Acad Sci U S A. 2010;107:15181–15186. doi: 10.1073/pnas.1006539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barton GM, Kagan JC. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nat Rev Immunol. 2009;9:535–542. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tayyari F, et al. Identification of nucleolin as a cellular receptor for human respiratory syncytial virus. Nat Med. 2011;17:1132–1135. doi: 10.1038/nm.2444. [DOI] [PubMed] [Google Scholar]
- 32.Allen KL, et al. A novel pathway for human endothelial cell activation by antiphospholipid/anti-beta2 glycoprotein I antibodies. Blood. 2012;119:884–893. doi: 10.1182/blood-2011-03-344671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.San-Juan-Vergara H, et al. Cholesterol-rich microdomains as docking platforms for respiratory syncytial virus in normal human bronchial epithelial cells. J Virol. 2012;86:1832–1843. doi: 10.1128/JVI.06274-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krzyzaniak MA, et al. Host cell entry of respiratory syncytial virus involves macropinocytosis followed by proteolytic activation of the F protein. PLoS Pathog. 2013;9:e1003309. doi: 10.1371/journal.ppat.1003309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mercer J, Greber UF. Virus interactions with endocytic pathways in macrophages and dendritic cells. Trends Microbiol. 2013 doi: 10.1016/j.tim.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Morris S, et al. Autophagy-mediated dendritic cell activation is essential for innate cytokine production and APC function with respiratory syncytial virus responses. J Immunol. 2011;187:3953–3961. doi: 10.4049/jimmunol.1100524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ariffin JK, Sweet MJ. Differences in the repertoire, regulation and function of Toll-like Receptors and inflammasome-forming Nod-like Receptors between human and mouse. Curr Opin Microbiol. 2013 doi: 10.1016/j.mib.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Klein Klouwenberg P, et al. The role of Toll-like receptors in regulating the immune response against respiratory syncytial virus. Crit Rev Immunol. 2009;29:531–550. doi: 10.1615/critrevimmunol.v29.i6.40. [DOI] [PubMed] [Google Scholar]
- 39.Paulus SC, et al. Common human Toll-like receptor 4 polymorphisms--role in susceptibility to respiratory syncytial virus infection and functional immunological relevance. Clin Immunol. 2007;123:252–257. doi: 10.1016/j.clim.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Awomoyi AA, et al. Association of TLR4 polymorphisms with symptomatic respiratory syncytial virus infection in high-risk infants and young children. J Immunol. 2007;179:3171–3177. doi: 10.4049/jimmunol.179.5.3171. [DOI] [PubMed] [Google Scholar]
- 41.von Bernuth H, et al. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008;321:691–696. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kollmann TR, et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol. 2009;183:7150–7160. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Villenave R, et al. In vitro modeling of respiratory syncytial virus infection of pediatric bronchial epithelium, the primary target of infection in vivo. Proc Natl Acad Sci U S A. 2012;109:5040–5045. doi: 10.1073/pnas.1110203109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fonceca AM, et al. Primary airway epithelial cultures from children are highly permissive to respiratory syncytial virus infection. Thorax. 2012;67:42–48. doi: 10.1136/thoraxjnl-2011-200131. [DOI] [PubMed] [Google Scholar]
- 45.Aarbiou J, et al. Lentiviral small hairpin RNA delivery reduces apical sodium channel activity in differentiated human airway epithelial cells. J Gene Med. 2012;14:733–745. doi: 10.1002/jgm.2672. [DOI] [PubMed] [Google Scholar]
- 46.Pollard AJ, et al. Human microbial challenge: the ultimate animal model. Lancet Infect Dis. 2012;12:903–905. doi: 10.1016/S1473-3099(12)70292-X. [DOI] [PubMed] [Google Scholar]
- 47.Querec TD, Pulendran B. Understanding the role of innate immunity in the mechanism of action of the live attenuated Yellow Fever Vaccine 17D. Adv Exp Med Biol. 2007;590:43–53. doi: 10.1007/978-0-387-34814-8_3. [DOI] [PubMed] [Google Scholar]
- 48.Prince GA, et al. Monophosphoryl lipid A adjuvant reverses a principal histologic parameter of formalin-inactivated respiratory syncytial virus vaccine-induced disease. Vaccine. 2001;19:2048–2054. doi: 10.1016/s0264-410x(00)00417-5. [DOI] [PubMed] [Google Scholar]
- 49.Boukhvalova MS, et al. The TLR4 agonist, monophosphoryl lipid A, attenuates the cytokine storm associated with respiratory syncytial virus vaccine-enhanced disease. Vaccine. 2006;24:5027–5035. doi: 10.1016/j.vaccine.2006.03.064. [DOI] [PubMed] [Google Scholar]
- 50.Kwilas S, et al. Respiratory syncytial virus grown in Vero cells contains a truncated attachment protein that alters its infectivity and dependence on glycosaminoglycans. J Virol. 2009;83:10710–10718. doi: 10.1128/JVI.00986-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baum A, et al. Preference of RIG-I for short viral RNA molecules in infected cells revealed by next-generation sequencing. Proc Natl Acad Sci U S A. 2010;107:16303–16308. doi: 10.1073/pnas.1005077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Collins CH, Kennedy DA. Laboratory-acquired Infection: History, incidence, causes and preventions. 4. Butterworth-Heinemann; 1999. Exposure, sources and route of infection; pp. 38–53. [Google Scholar]
- 53.Bem RA, et al. Animal models of human respiratory syncytial virus disease. Am J Physiol Lung Cell Mol Physiol. 2011;301:L148–156. doi: 10.1152/ajplung.00065.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]