Abstract
Induced Pluripotent Stem (iPS) cells can be generated using retroviral vectors expressing Oct4, Klf4, Sox2 and cMyc. Most prior studies have required multiple retroviral vectors for reprogramming, resulting in high numbers of genomic integrations in iPS cells and limiting their use for therapeutic applications. Here we describe the use of a single lentiviral vector expressing a ‘stem cell cassette’ comprised of the four transcription factors and a combination of 2A peptide and IRES technology, generating iPS cells from post-natal fibroblasts. iPS cells generated in this manner display ES cell-like morphology, express stem cell markers and exhibit in vivo pluripotency as evidenced by their ability to differentiate in teratoma assays and their robust contribution to mouse chimeras. Combining all factors into a single transcript achieves the most efficient reprogramming system to date, and allows derivation of iPS cells with a single viral integration. The use of a single lentiviral vector for reprogramming represents a powerful laboratory tool and a significant step toward the application of iPS technology for clinical purposes.
Keywords: induced pluripotent stem (iPS) cells, stem cell, reprogramming, single lentiviral vector, stem cell cassette
INTRODUCTION
The capacity of embryonic stem cells (ESCs) to give rise to all somatic cells together with their ability to grow indefinitely in culture underscores their potential for in vivo therapeutic applications [1]. However, the generation of patient-specific autologous ESCs is technically challenging and further complicated by ethical concerns, significantly limiting their potential for clinical transplantation. The reprogramming of fibroblasts to an ESC-like state, pioneered by Yamanaka and colleagues, has advanced stem cell research [2] by circumventing these obstacles. These so called ‘induced Pluripotent Stem (iPS) cells’ derived from mouse [3–5] or human fibroblasts [6, 7] have demonstrated that an entire organism can be derived from readily accessible post-natal somatic cells. iPS cells provide a powerful in vitro model system for the study of the molecular mechanisms of reprogramming [8–12] and have been successfully employed in proof-of-principle cell-based therapies in mouse models of disease [13, 14]. However, the derivation of iPS cells has typically required multiple individual viral vectors to deliver the constellation of transcription factors (typically Oct4, Sox2, Klf4, and cMyc) needed to induce reprogramming. The application of sufficient quantities of each virus to deliver 4 factors simultaneously to each target cell results in high numbers of genomic integrations in successfully reprogrammed progeny. This presence of multiple viral integrations across the genome prohibits their genetic elimination to produce safer iPS cells [2]. Moreover, many cells will receive only one, two or three factors, making it difficult to study the biochemistry of reprogramming on a homogeneous population of cells. Most recently, studies have shown that iPS cells may be generated without integrations, however, the efficiency of iPS cell derivation was significantly reduced and the reprogrammed cell types limited [15, 16]. Here, we describe the generation of a single lentiviral vector expressing the four transcription factors, Oct4, Klf4, Sox2 and cMyc from a single multicistronic transcript. Expression of this ‘stem cell cassette’ accomplishes the most efficient derivation of iPS cells to date. Constitutive or inducible expression of the cassette generates iPS cells able to differentiate into all three primary germ layers. We believe that our approach represents a powerful laboratory tool and a significant advance toward the removal of a single viral genome by loxp/Cre technology or the development of non-integrating systems for the potential application of iPS technology in human clinical trials.
MATERIALS AND METHODS
Construction of lentiviral vectors
We designed a multiple expression system based on the pHAGE lentiviral vector. pHAGE is a 3rd generation lentiviral vector previously described [17, 18]. We re-engineered pHAGE for multicistronic gene expression to accomplish the production of the proteins Oct4, Klf4, Sox2 and cMyc from a single transcript. First, two DNA fragments were generated by overlapping PCR using Pfu Turbo® DNA polymerase (Stratagene, La Jolla, CA, http://www.stratagene.com); one fragment consisting of the complementary DNAs (cDNAs) of murine Oct4 and Klf4 separated by an intervening sequence encoding the F2A peptide, the second fragment containing the cDNAs of murine Sox2 and cMyc, separated by an intervening sequence encoding the E2A peptide. To obtain the Oct4-F2A-Klf4 fragment, two PCR reactions were carried out using the primer pairs Oct4 5′ NotI/Oct4-F2A 3′ and F2A-Klf4 5′/Klf4 3′ BamHI (see Table 1) under the following conditions: initial denaturation at 94°C for 2 min followed by 35 cycles of 45 s at 94°C, 45 s at 60°C and 2 min at 72°C. Aliquots of the two purified amplicons were then mixed in a 1:1 ratio and used in a second PCR round with the primers Oct4 5′ NotI and Klf4 3′ BamHI under the following conditions: initial denaturation at 94°C for 2 min, 5 cycles of 45 s at 94°C, 45 s at 58°C and 2 min at 72°C, and 30 cycles of 45 s at 94°C, 45 s at 62°C and 2 min at 72°C. The resulting fragment (Oct4-F2A-Klf4) was gel-purified and inserted by directional cloning into the Not I- and BamH I-digested pHAGE2 lentiviral vector backbone upstream of an IRES element. Similarly, a DNA fragment corresponding to Sox2-E2A-cMyc was obtained by PCR using the conditions described above and the primer pairs Sox2 5′ NdeI/Sox2-E2A 3′ and E2A-cMyc 5′/cMyc 3′ ClaI (first round of amplification) and Sox2 5′ NdeI/c-Myc 3′ ClaI (second round of amplification). This fragment (Sox2-E2A-cMyc) was then inserted between the NdeI and ClaI sites, downstream of the IRES element of the pHAGE2-Oct4-F2A-Klf4 vector. Finally, the human EF1α promoter or the TetO/miniCMV promoter was cloned into SpeI and NotI sites of the recombinant vector to generate pHAGE-EF1α-STEMCCA and pHAGE-Tet-STEMCCA vectors, respectively. Sequence identity was confirmed by sequencing.
Table 1.
Primers used for vector construction
| Oct4 5′ NotI | CACCGGCGGCCGCCATGGATCCTCGAACCTGGCTAAGCTTCCAAG |
| Oct4-F2A 3′ | CTTGAGAAGGTCAAAATTCAAAGTCTGTTTCACGCCACTTCCGTTTG AATGCATGGGAGAGCCCAGAGCAG |
| F2A-Klf4 5′ | AAACAGACTTTGAATTTTGACCTTCTCAAGTTGGCGGGAGACGTGGA GTCCAACCCAGGGCCCATGGCTAGCGACGCTCTGCTCCC |
| Klf4 3′ BamHI | TTTGGATCCTTAAAAGTGCCTCTTCATGTGTAAGGCAAG |
| Sox2 5′ NdeI | GGTTTCTTACATATGATGTATAACATGATGGAGACGGAGCTGAAG |
| Sox2-E2A 3′ | TTTCAACATCGCCAGCGAGTTTCAACAAAGCGTAGTTAGTACATTGCC CACTACCCATGTGCGACAGGGGCAGTGTGCCGTTAATGGCCG |
| E2A-cMyc 5′ | CTTTGTTGAAACTCGCTGGCGATGTTGAAAGTAACCCCGGTCCTATGC CCCTCAACGTGAACTTCACCAACAGGAACTATG |
| cMyc 3′ ClaI | GGTTTATCGATTTATGCACCAGAGTTTCGAAGCTGTTC |
Cell culture
Tail-tip fibroblasts (TTFs) were derived from Sox2-GFP/R26-M2rtTA double knock-in mice [12]. These cells carry an M2rtTA gene encoding a reverse tetracycline transactivator targeted to the constitutively active ROSA26 locus as well as a reporter cDNA targeted to the Sox2 locus. Tail snips from 3–4 day old mice were cultured according to standard methods to expand TTFs in fibroblast growth media (DMEM 10% FBS, L-Glutamine, penicillin/streptomycin). TTFs were infected at passage 3 for generation of iPS cells. Efficiency of iPS cell generation was calculated based on transduction efficiency and number of GFP+ colonies. Specifically, to determine transduction efficiency, MEFs were transduced with 15 μl of pHAGE-STEMCCA virus and four days later stained with DAPI and a specific antibody against mouse Oct4. Transduction efficiency was calculated by the following formula: # Oct4+ / # DAPI+. Efficiency of iPS cell generation was then calculated as: (# GFP+ colonies generated/100,000 fibroblasts exposed to virus) x 1/transduction efficiency. Reported results are averaged from experiments performed in triplicate.
Lentivirus production and infection
Lentiviruses were produced using a five plasmid transfection system in 293T packaging cells as previously described [17]. Supernatants were collected every 12 hours during two consecutive days starting 48 hours after transfection and viral particles were concentrated by centrifugation at 16,500 rpm for 1.5 hours at 4°C. Approximately 100,000 fibroblasts were seeded on plastic in 35-mm culture plates and infected with 15 μl of concentrated virus in the presence of polybrene (5 μg/ml). The media was replaced after 16 hours with mouse ES cell media (DMEM supplemented with 15% FBS, L-glutamine, penicillin/streptomycin, nonessential amino acids, β-mercaptoethanol and 1000 U/ml LIF) and changed every 2–3 days. Doxycycline (Sigma-Aldrich, St. Louis, http://www.sigmaaldrich.com) was added at a final concentration of 1 μg/ml, where indicated, and removed at day 10 post-infection. iPS colonies were picked 20 to 25 days post-infection based on morphology and GFP expression and expanded by plating on Mitomycin C treated MEFs in ES cell media.
Antibodies
Immunofluorescence and western blot assays were performed using routine methods. The following primary antibodies were used: rabbit anti-Oct4 (Abcam, Cambridge, MA, http://www.abcam.com), goat anti-Klf4 (R&D Systems, Minneapolis, MN, www.rndsystems.com), mouse anti-Sox2 (R&D Systems), mouse anti-cMyc (NeoMarkers, Fremont, CA, http://www.labvision.com), mouse anti-GAPDH (Millipore, Bedford, MA, http://www.millipore.com) and mouse anti-SSEA-1 (Santa Cruz Biotechnology, Santa Cruz, CA, http://www.scbt.com). The following fluorochrome-conjugated secondary antibodies were applied: Alexa Fluor 488 donkey anti-goat, Texas Red goat anti-rabbit and Cy3 goat anti-mouse (Molecular Probes, Eugene, OR, http://probes.invitrogen.com). Alkaline phosphatase staining was performed with the Vector Red Substrate Kit (Vector Laboratories, Burlingame, CA, http://www.vectorlabs.com) according to the manufacturer’s instructions. Flow cytometry was performed using standard procedures. All flow cytometric data were acquired using equipment maintained by the Boston University Medical Campus Flow Cytometry Core Facility.
RT-PCR of marker genes
Total RNA was purified with TriPure Isolation Reagent (Roche Applied Sciences Co., Mannheim, Germany, https://www.roche-applied-science.com). One microgram of RNA was reverse-transcribed using ImProm-II Reverse Transcriptase (Promega, Madison, WI, http://www.promega.com) according to the manufacturer’s instructions. Primers for ES cell marker genes are described elsewhere [2].
Quantitative RT-PCR
qRT-PCR was carried out in a StepOnePlus real-time PCR system (Applied Biosystems, Foster City, CA, http://www.appliedbiosystems.com). The viral transcript was amplified using Taqman custom primers and probe designed to amplify a cMyc to WPRE fragment within the pHAGE-STEMCCA vector. Amplification of the endogenous control β-actin was performed according to the manufacturer’s instructions (Applied Biosystems). Reactions were performed in triplicate using 1/20 of the cDNA obtained as described above. The expression level of the viral transcript in each sample was normalized to β-actin and relative quantification of expression was estimated using the comparative Ct method.
Teratoma formation
One million iPS cells were injected subcutaneously into each flank of recipient NOD/SCID mice (Jackson Laboratory, Bar Harbor, ME, http://www.jax.org). Paraffin sections of formalin-fixed teratoma specimens were prepared 3–5 weeks after injection, and analysis of H and E stained tissue sections was performed for each specimen. All animal experiments were performed in accordance with institutional guidelines.
Southern Blot
Southern blot analysis using standard methods was performed on DNA digested with BglII (New England Biolabs, Beverly, MA, http://www.neb.com) that cuts once in each of the two viral LTRs, in order to estimate the proviral copy number per genome. In the Tet-STEMCCA vector a 8.3 kb band is expected. Because the EF1αpromoter contains a BglII site, a smaller band of 6.7 Kb is expected. A WPRE fragment that recognizes all our constructs was used as a probe. As a confirmatory method, Southern blot analysis was also performed on DNA digested with BamHI that cuts only once within the viral sequence, and therefore each viral integration is detected as a distinctive band.
Chimera generation and histological analysis
iPS cells were injected into mouse blastocysts and implanted into pseudopregnant foster mothers using routine methods. Pregnant mice were sacrificed at day E11.5 and whole embryos were photographed with an inverted fluorescence microscope. Histological analysis was performed on 5 μm thick sections after DAPI staining in order to visualize GFP fluorescence and cell nuclei.
RESULTS
A single lentiviral vector for the expression of a stem cell cassette
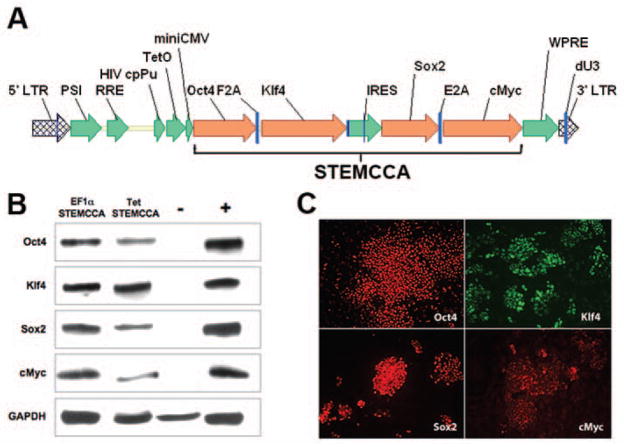
Previous studies have developed multicistronic lentiviral vectors based on a combination of an IRES element and 2A peptide sequences [19] to express multiple genes simultaneously from a single lentiviral vector [20]. Using a similar approach we designed a single lentiviral vector expressing a “STEM-Cell CAssette”, (hereafter pHAGE-STEMCCA). This cassette is comprised of a single multicistronic mRNA containing an IRES element separating two fusion cistrons. The two cistrons consist of Oct4 and Sox2 coding sequences fused to Klf4 and cMyc, respectively, through the use of intervening sequences encoding ‘self-cleaving’ 2A peptides (Fig. 1A). We generated two forms of pHAGE-STEMCCA in which the multicistronic transcript is driven by either a constitutive EF1α promoter or a doxycycline (dox)-inducible TetO-miniCMV promoter. Both vectors resulted in the expression of all four individual proteins (Oct4, Klf4, Sox2, and cMyc) as detected by western blot analysis and immunohistochemistry (Fig. 1B,C).
Figure 1. Generation of a single lentiviral vector expressing a stem cell cassette.
(A) Schematic representation of pHAGE-STEMCCA (inducible version). The engineered stem cell cassette consists of a single multicistronic mRNA transcribed under the control of a doxycycline-inducible TetO-miniCMV promoter. The mRNA contains an IRES element separating two fusion cistrons. The two cistrons consist of Oct4 and Sox2 coding sequences fused to Klf4 and cMyc, respectively, through the use of intervening sequences encoding ‘self-cleaving’ 2A peptides (F2A and E2A). LTR: long terminal repeat; PSI: packaging signal; RRE: rev responsive element; cpPu: central polypuryne tract; WPRE: Woodchuck hepatitis virus post-transcriptional regulatory element; dU3: deleted U3. (B) Western blot analysis of lysates from 293T transfected cells. Cells were co-transfected with pHAGE-Tet-STEMCCA and a vector expressing the rtTA protein and maintained in doxycycline for 72 h before lysate preparation. Cells transfected with either mock vectors or four monocistronic pHAGE vectors encoding the four individual transcription factors were used as negative (-) or positive (+) controls, respectively. (C) Immunofluorescence microscopy of MEFs infected 4 days earlier with pHAGE-EF1α-STEMCCA shows expression of all four transcription factors. Uninfected MEFs or secondary antibody only staining control showed no detectable staining.
Generation of iPS cells with a single lentiviral vector
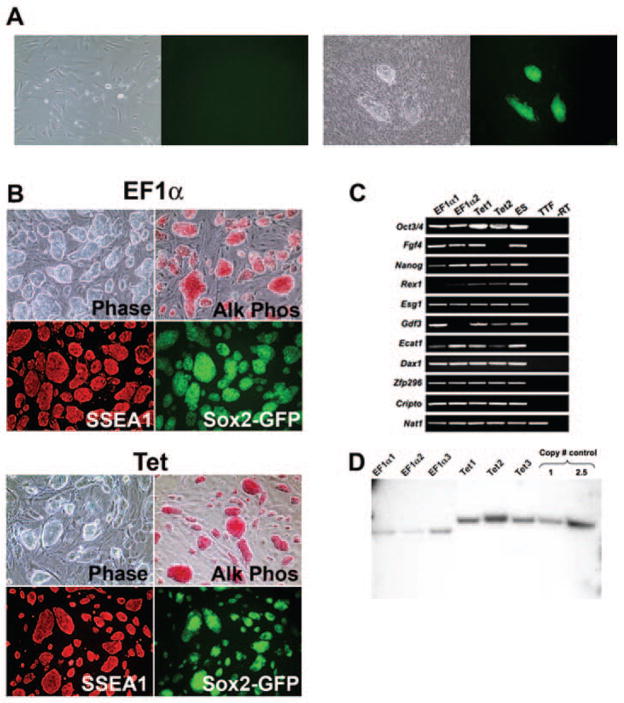
Next, we sought to test the capacity of pHAGE-STEMCCA to derive iPS clones from mouse embryonic or post-natal fibroblasts. As expected from the large size of the proviral genome of these vectors (>9Kb), pHAGE-STEMCCA viral titers (2–3×108/ml) were lower than those obtained using monocistronic pHAGE vectors (5×109/ml). Nevertheless, mouse embryonic fibroblasts (MEFs) and tail-tip fibroblasts (TTFs) transduced with the constitutive EF1α STEMCCA construct showed a dramatic change in morphology already evident 6 days post-infection and formed colonies that were clonally expanded and displayed the typical morphology of ES cell colonies (Fig. 2B).
Figure 2. Characterization of iPS cells generated using a single lentiviral vector.
(A) Representative pictures of TTFs from a Sox2-GFP M2rtTA mouse transduced with the inducible STEMCCA vector, in the absence (left panel) or presence (right panel) of doxycycline induction. Images were acquired on day 14 post-infection. (B) Representative pictures of iPS cells derived using the constitutive pHAGE-EF1α-STEMCCA (EF1α) or the inducible pHAGE-Tet-STEMCCA vector (Tet) show colony morphology (Phase), high alkaline phosphatase activity (Alk Phos), SSEA1 immunostaining, and Sox2-GFP reporter gene expression. (C) Expression of ESC ‘marker’ genes detected by RT-PCR in four representative iPS cell clones generated by the constitutive (EF1α) or inducible (Tet) STEMCCA vector. Nat1 is a constitutively expressed gene and serves as a control for loading. Representative samples from unmanipulated tail tip fibroblasts (TTF) and mouse ES cells are also shown. An iPS cell sample prepared without RT was used as negative control (-RT). (D) Southern blot analysis of genomic DNA (gDNA) purified from 6 representative iPS clones produced with the constitutive (EF1α) or inducible (Tet) vector. gDNA was digested with BglII to obtain a band of 6.7Kb in the EF1α colonies or 8.3Kb in the Tet colonies, representing most of the proviral genome. For control, pHAGE-Tet-STEMCCA plasmid DNA representing 1 or 2.5 copies of the insert was digested with BglII. A single band of the expected size of the proviral gene insertion is present in all clones. The density of each band indicates between 1–3 proviral integrations in each clone.
For the generation of iPS cells with the dox-inducible construct, we transduced TTFs from a Sox2-GFP Rosa26-M2rtTA double knock-in mouse in which the rtTA is constitutively expressed but the Sox2-GFP allele is largely repressed. TTFs transduced with the inducible pHAGE-STEMCCA were exposed to doxycycline and changes in cell morphology were evident 6–8 days post induction with colonies appearing at day 12–14 (Fig. 2A). Transduced TTFs that were not exposed to doxycycline showed no morphological changes and no detectable GFP expression (Fig. 2A). iPS colonies derived using either the constitutive (EF1α) or inducible (Tet) pHAGE-STEMCCA vector showed comparable alkaline phosphatase (AP) and SSEA1 staining as well as consistent and strong GFP expression from the Sox2 locus, indicating reactivation of a crucial ES cell marker (Fig. 2B). In addition, iPS clones generated with either vector expressed a variety of other classic ES cell marker genes (Fig. 2C) while these genes were not expressed in fibroblasts prior to reprogramming. Furthermore, each iPS clone showed the correct transmission of the full lentiviral vector genome as analyzed by Southern blot and contained only 1–3 integrated viral copies (Fig. 2D). These results were confirmed using a second restriction digest strategy where each integration appears as a discrete band (data not shown). Supplemental online Table 1 summarizes the number of viral integrations in iPS clones generated with the pHAGE-STEMCCA vector. An average of 1.5 or 2.8 proviral copies using either the constitutive or inducible pHAGE-STEMCCA vector, respectively, was observed.
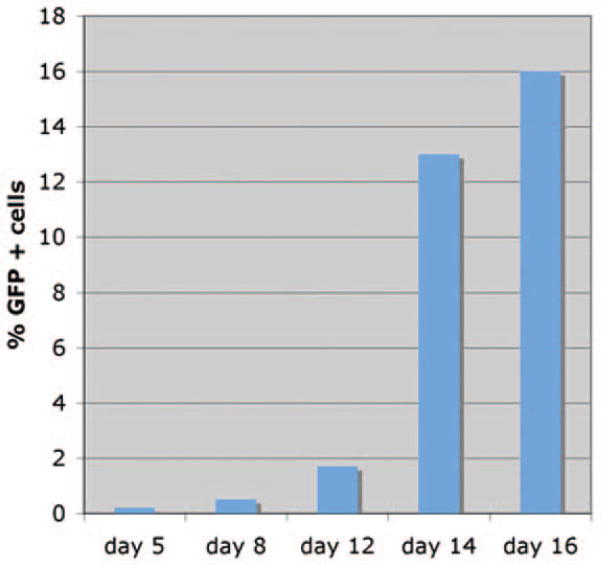
Expression of STEMCCA mRNA transcript was determined in vitro using qRT-PCR in transduced fibroblasts (under doxycycline induction) or in established iPS clones in the absence of doxycycline (Supplemental online Fig. 1). As expected, transcript expression was found to be downregulated in iPS clones generated with the inducible vector (56-fold reduction) in the absence of doxycycline. In contrast, iPS clones generated with the constitutive vector continue to show high level transgene expression. We found that the pHAGE-STEMCCA vector reprograms fibroblasts with similar kinetics but higher efficiency compared to prior systems [4, 5]. Robust expression of GFP from the Sox2 locus in TTFs following dox-induction of reprogramming factors was detectable (by FACS and microscopy) at days 8–9 of induction, similar to previous observations [12]. Cells exposed to doxycycline for <8 days did not give rise to stable iPS colonies (data not shown). By day 16, approximately 15% of total cells were GFP+ (Fig. 3). We obtained 50±8 (average +/− SD) GFP positive colonies out of 100,000 TTFs exposed to pHAGE-STEMCCA lentiviruses. Taking into consideration the low viral transduction efficiency in our experiments (10%) (See Methods), which is likely due to the low viral titers used, the effective reprogramming efficiency is approximately 0.5%, 10-fold higher than that observed in prior reports (0.01–0.05%) [4, 5].
Figure 3. Dynamics of reprogramming using a single lentiviral stem cell cassette.
Analysis of GFP expression over time in TTFs purified from Sox2-GFP M2rtTA double knock-in mice infected with pHAGE-Tet-STEMCCA vector. Transduced cells from independent wells were collected at each time point following doxycycline exposure. GFP expression was analyzed using a FACScan machine. The Day 5 result was indistinguishable from background GFP expression.
iPS cells generated with pHAGE-STEMCCA are pluripotent
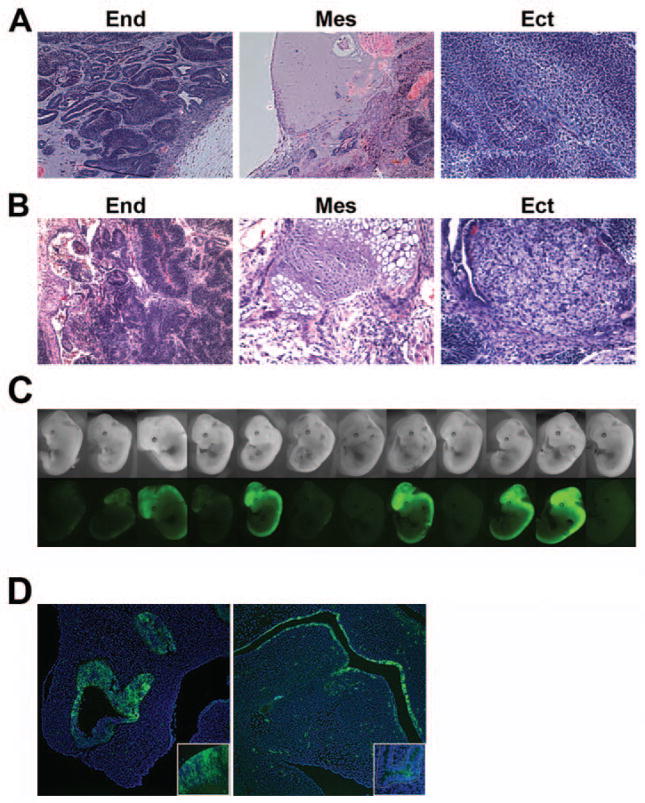
We sought to assess the capacity of iPS clones derived with constitutive and inducible STEMCCA vectors to differentiate into the three germ layers in teratomas. When injected into NOD/SCID mice, iPS cells derived from both vectors were capable of inducing teratoma formation with the generation of derivatives from all three germ layers (Fig. 4A, 4B). Next we performed blastocyst injections in order to further confirm the pluripotency of iPS cells generated using pHAGE-STEMCCA. iPS cells derived from the TTFs of Sox2-GFP Rosa26-M2rtTA mice using the inducible pHAGE-STEMCCA contributed to embryo development when injected into blastocysts (Fig. 4C), as evidenced by Sox2-GFP expression in neural crest-derived tissues. Out of 14 implanted blastocysts, 12 developed into mid-term embryos and 9 showed easily detectable GFP+ iPS-derived cells contributing to the chimeric embryos (Fig. 4C). Histological analysis of serial sections from these embryos confirmed neuroectodermal contribution in the brain and spinal cord. Furthermore, contribution to developing endodermal tissues known to express Sox2 was also evident by GFP expression (Fig. 4D). iPS cell contribution to mesoderm was demonstrated by deriving STEMCCA-tagged MEFs from these embryos (see below).
Figure 4. iPS cells generated with a single lentiviral vector are pluripotent.
(A and B) Teratomas derived from iPS cell lines produced with pHAGE-EF1α-STEMCCA (A) or pHAGE-Tet-STEMCCA (B) vector, showing differentiation into cell types of all three germ layers: endoderm (end), mesoderm (mes) and ectoderm (ect). Images are representative of two independent experiments testing three individual iPS clones for each construct. (C) iPS cells generated with pHAGE-Tet-STEMCCA vector from TTFs of a Sox2-GFP Rosa26-M2rtTA mouse show high levels of embryonic contribution following injections into blastocysts. Chimerism is evidenced by Sox2-GFP expression in neural crest-derived tissues in 9 of 12 mid-term embryos. (D) Contribution to neuroectoderm (left panel: olphactory region; inset: developing brain) and endoderm (right panel: oropharynx; inset: proximal lung epithelium) is evidenced by Sox2-GFP expression in histological sections of mid-term chimeric embryos.
Secondary iPS generation by reactivation of STEMCCA
We utilized MEFs derived from the mid-gestation chimeric embryos in order to assess the reinducibility of Tet-STEMCCA. Because these MEFs derive from a single iPS clone, they represent an ideal tool for assessing the kinetics of reprogramming [21]. After exposure to doxycycline in culture for varying durations (0–16 days), we observed emergence of stable iPS colonies with reactivated Sox2-GFP expression from MEFs exposed to doxycycline for as little as 4 days (Fig. 5A, 5B). These results indicated: a) the inducible Tet-STEMCCA construct can be reactivated with doxycycline re-exposure following the completion of reprogramming; and b) STEMCCA reinduction for a remarkably brief period (4 days) allows rapid and self-perpetuating reprogramming of MEFs into stable iPS colonies.
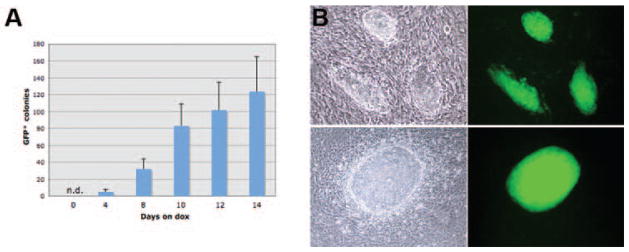
Figure 5. Reactivation of STEMCCA.
(A) MEFs were obtained from E11.5 chimeric embryos generated by iPS cells derived from TTFs of Sox2-GFP mice transduced with pHAGE-Tet-STEMCCA. MEFs were exposed to doxycycline (dox) for varying times and GFP colonies were counted on day 22. Stable iPS clones expressing Sox2-GFP were derived from cells exposed to docycycline for as little as 4 days. Experiments were done in duplicates. n.d.= not detected. (B) Colony formation and Sox2-GFP expression in 2 representative fields is shown. Parallel samples cultured in the absence of dox showed no GFP expression and no morphological changes.
DISCUSSION
Reprogramming mediated by the pHAGE-STEMCCA vector offers several advances over existing multi-vector approaches. The major advantage of a single vector-based approach is the possibility of inducing reprogramming with limited numbers of viral integrations. Indeed, in some experiments, we were able to derive iPS clones with only a single integrated viral copy (Fig. 2D and supplemental online Table 1). This is in marked contrast to previous reports using multiple vectors which required >15 viral integrations [2, 5]. While this manuscript was in preparation, Okita et al demonstrated the use of a two plasmid system to derive iPS cells free of integrations [15]. Notably, reprogramming efficiency using this system was low and was demonstrated solely in pre-natal mouse cells. It will be interesting to assess whether combining all four reprogramming factors in a single cassette, such as pHAGE-STEMCCA, will now allow efficient iPS derivation from post-natal mouse and human cells using a similar non-integrating approach. Our results showing that the use of STEMCCA enables high efficiency of reprogramming, together with rapid reactivation kinetics, suggest that this may be the case.
Prior reports using multiple viruses have found a relatively consistent ratio of integrations of each gene [5, 21]. Based on these data, some investigators have suggested that successful reprogramming requires a specific stoichiometric relationship of gene expression between the four transcription factors. This is consistent with results in ESCs where the levels of Oct4 and Sox2 are critical for maintaining pluripotency vs. inducing differentiation [22, 23]. Our studies using a single vector in which expression of the four proteins derives from a single multicistronic message suggests that the stoichiometry of the four factors may not be absolutely critical for successful reprogramming. However, it remains possible that the iPS cells generated with the STEMCCA vector are the product of a specific level of each protein achieved during the translation of the multicistronic message. In this regard, the recent work of Okita et al. showed that indeed the order in which genes are expressed from multicistronic vectors significantly affects reprogramming efficiency [15].
Previous studies have suggested that iPS clones derived using vectors with constitutively active promoters fail to properly differentiate, likely due to their inability to silence the expression of the four pluripotency-inducing transcription factors [8]. However, these results are controversial as different studies found that mouse and human iPS cells produced with constitutive lentiviruses are pluripotent [7, 24]. Our results showed that when using a constitutive promoter, high levels of transgene expression were still evident in established iPS clones (Supplemental Figure 1), a finding that did not preclude expression of endogenous stem cell genes (as evidenced by the RT-PCR data shown in Figure 2C and the reprogramming of the Sox2 endogenous locus shown in Figure 3). Moreover, in our hands, iPS clones generated using the constitutive STEMCCA vector underwent pluripotent differentiation in teratoma assays. We did not, however, assess for persistent expression of the viral transcript in the teratomas, and thus it remains unclear whether transgene silencing in vivo is necessary for in vivo differentiation.
CONCLUSION
We believe that the use of a single lentiviral vector for the derivation of iPS cells will help reduce the variability in efficiency that has been observed between different laboratories, thus enabling more consistent genetic and biochemical characterizations of iPS cells and the reprogramming process. From the safety perspective, we have shown that iPS cells can be produced with minimal numbers of viral integrations, significantly reducing the risks of insertional mutagenesis and viral reactivation. Furthermore, a single vector encoding a stem-cell cassette represents an important step toward the removal of the viral genome by loxp/Cre technology or ideally the development of non-integrating versions of the vector, a necessary step for the application of iPS technology in human clinical trials.
Supplementary Material
Acknowledgments
The authors are grateful to Raul Mostoslavsky for critical reading of the manuscript and technical advice. We thank Alejandro Balazs and Wellington Cardoso for insightful discussions, Andreia Gianotti Sommer and Constantina Christodoulou for their technical support, and Linda Neville for administrative assistance. Support to K.H. came from the NIH Director’s Innovator Award, The Harvard Stem Cell Institute, the Kimmel Foundation and the V Foundation. Support to D.N.K. came from NIH PO1 HL047049-16A1.
Footnotes
Author contributions: C.A.S.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; M.S. and G.J.M.: collection and assembly of data; K.H.: conception and design, data analysis and interpretation; D.N.K.: conception and design, data analysis and interpretation, manuscript writing; G.M.: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript.
References
- 1.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Maherali N, Sridharan R, Xie W, et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70. doi: 10.1016/j.stem.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 5.Wernig M, Meissner A, Foreman R, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 8.Brambrink T, Foreman R, Welstead GG, et al. Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell. 2008;2:151–159. doi: 10.1016/j.stem.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meissner A, Mikkelsen TS, Gu H, et al. Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature. 2008;454:766–770. doi: 10.1038/nature07107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mikkelsen TS, Ku M, Jaffe DB, et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448:553–560. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mikkelsen TS, Hanna J, Zhang X, et al. Dissecting direct reprogramming through integrative genomic analysis. Nature. 2008;454:49–55. doi: 10.1038/nature07056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stadtfeld M, Maherali N, Breault DT, et al. Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell Stem Cell. 2008;2:230–240. doi: 10.1016/j.stem.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanna J, Wernig M, Markoulaki S, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- 14.Wernig M, Zhao JP, Pruszak J, et al. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proc Natl Acad Sci U S A. 2008;105:5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okita K, Nakagawa M, Hyenjong H, et al. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- 16.Stadtfeld M, Nagaya M, Utikal J, et al. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mostoslavsky G, Fabian AJ, Rooney S, et al. Complete correction of murine Artemis immunodeficiency by lentiviral vector-mediated gene transfer. Proc Natl Acad Sci U S A. 2006;103:16406–16411. doi: 10.1073/pnas.0608130103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan H, Mostoslavsky G, Eruslanov E, et al. Dual-promoter lentiviral system allows inducible expression of noxious proteins in macrophages. J Immunol Methods. 2008;329:31–44. doi: 10.1016/j.jim.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szymczak AL, Workman CJ, Wang Y, et al. Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat Biotechnol. 2004;22:589–594. doi: 10.1038/nbt957. [DOI] [PubMed] [Google Scholar]
- 20.Chinnasamy D, Milsom MD, Shaffer J, et al. Multicistronic lentiviral vectors containing the FMDV 2A cleavage factor demonstrate robust expression of encoded genes at limiting MOI. Virol J. 2006;3:14. doi: 10.1186/1743-422X-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wernig M, Lengner CJ, Hanna J, et al. A drug-inducible transgenic system for direct reprogramming of multiple somatic cell types. Nat Biotechnol. 2008;26:916–924. doi: 10.1038/nbt1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kopp JL, Ormsbee BD, Desler M, et al. Small increases in the level of Sox2 trigger the differentiation of mouse embryonic stem cells. Stem Cells. 2008;26:903–911. doi: 10.1634/stemcells.2007-0951. [DOI] [PubMed] [Google Scholar]
- 23.Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 24.Blelloch R, Venere M, Yen J, et al. Generation of induced pluripotent stem cells in the absence of drug selection. Cell Stem Cell. 2007;1:245–247. doi: 10.1016/j.stem.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.