Abstract
Many commercially available electroencephalography (EEG) sensors, including conventional wet and dry sensors, can cause skin irritation and user discomfort owing to the foreign material. The EEG products, especially sensors, highly prioritize the comfort level during devices wear. To overcome these drawbacks for EEG sensors, this paper designs Societe Generale de Surveillance S  A
A  (SGS)-certified, silicon-based dry-contact EEG sensors (SBDSs) for EEG signal measurements. According to the SGS testing report, SBDSs extract does not irritate skin or induce noncytotoxic effects on L929 cells according to ISO10993-5. The SBDS is also lightweight, flexible, and nonirritating to the skin, as well as capable of easily fitting to scalps without any skin preparation or use of a conductive gel. For forehead and hairy sites, EEG signals can be measured reliably with the designed SBDSs. In particular, for EEG signal measurements at hairy sites, the acicular and flexible design of SBDS can push the hair aside to achieve satisfactory scalp contact, as well as maintain low skin-electrode interface impedance. Results of this paper demonstrate that the proposed sensors perform well in the EEG measurements and are feasible for practical applications.
(SGS)-certified, silicon-based dry-contact EEG sensors (SBDSs) for EEG signal measurements. According to the SGS testing report, SBDSs extract does not irritate skin or induce noncytotoxic effects on L929 cells according to ISO10993-5. The SBDS is also lightweight, flexible, and nonirritating to the skin, as well as capable of easily fitting to scalps without any skin preparation or use of a conductive gel. For forehead and hairy sites, EEG signals can be measured reliably with the designed SBDSs. In particular, for EEG signal measurements at hairy sites, the acicular and flexible design of SBDS can push the hair aside to achieve satisfactory scalp contact, as well as maintain low skin-electrode interface impedance. Results of this paper demonstrate that the proposed sensors perform well in the EEG measurements and are feasible for practical applications.
Keywords: Non-skin irritation, silicon-based dry sensors, electroencephalography (EEG), Societe Generale de Surveillance S  A
A (SGS), brain-computer interface
(SGS), brain-computer interface
Many electroencephalography (EEG) sensors, including conventional wet and dry sensors, use materials that can cause skin irritation and discomfort. To overcome this drawback, this study reports on the design and human testing of a SGS-certified, silicon-based dry-contact EEG sensor. In addition to being non-irritating, the acicular sensors are lightweight, flexible, and capable of easily fitting to the scalp, forehead, or hairy sites without any skin preparation or conductive gel. The sensors also maintain low skin-electrode interface impedance. Results of this study demonstrate that the proposed sensors perform well in EEG measurements and are feasible for both medical and entertainment applications.
I. Introduction
Electroencephalography (EEG) evaluates brain activity by placing electrodes on skin of the scalp [1]–[3]. EEG measurement equipment is produced for medical diagnostics and neurobiological research [4]–[6], owing to its high temporal resolution and portability [7], [8]. EEG measurements are not limited to cognitive experiments at lab or medical facilities [8]. The major requirements of EEG products significantly differ from each other in the entertainment and medical markets [9], [10].
Medical EEG products use wet EEG sensors to achieve highly accurate brain dynamics; these EEG measurement locations may include hairy sites. Despite their inconvenience, wet EEG sensors overcome interference from hair. A few biomedical products using EEG signal measurements have become commercially available for entertainment purposes [5], [9]. For measuring forehead EEG signals; dry EEG sensors are generally used in consumer EEG product, owing to their easy attachment to forehead sites in order to acquire brain dynamics without hair interference [4]. Increasing the popularity of consumer EEG products depends on fulfilling demands of ease, comfort and improved methods of attachment. The Zeo-Mobile (Zeo product) uses three fabric electrodes to measure forehead EEG signals [11], and the Necomimi (brainwave cat ears) has one forehead EEG sensor [11]. Both of these sensors use dry EEG electrodes to provide comfort and ease of wear. In hairy sites EEG signals measurements, some dry contact EEG sensors use metal-pins to contact the scalp. Because the sensors contact skin directly, applied force on the sensors will cause user discomfort to different degrees [12]. For example, the g.SAHARA electrode system (the g.tec product) consists of an 8 pin electrode made of a unique golden alloy [6]. The pins are hard and solid. Despite their sufficient length in reaching through the hair to the skin, the electrodes are uncomfortable for long term wear. New EEG sensors must consider not only the signal quality but also their potential applications [13]. An EEG sensor that fulfills medical and consumer EEG product requirements is highly attractive in both markets.
Several dry biosensors are available [14]. For instance, Beckmann et al. tailored their fabric electrodes with different fabric specifications to record biopotential signals [7], [15]. Additional fabric-based sensors have been used to measure biopotential signals [6], [7], [15]. However, many conductive fabric-based materials may contain metals, and long-term skin contact with metals may cause allergic reactions. A hybrid dry electrode is an alternative EEG sensor [11]. Many hybrid dry electrodes contain hard substrates. Users wearing these sensors may experience discomfort, leading to harm if not used carefully [7], [16], [17].
This study presents a multipurpose, dry EEG sensor that addresses the above concerns. The proposed silicon-based dry-contact sensors (SBDSs) are lightweight and flexible, without irritating the skin. The sensors can also measure EEG signals with good quality, even at sites with hair. While made of a biocompatible material that prevents skin irritations, SBDS has a flexible sensor design, explaining why there is no hard, painful substrate. Moreover, the proposed sensor is lightweight, thereby improving comfort. Furthermore, different sensor shapes are available for EEG measurements at different brain locations. Experimental results demonstrate that these SBDSs perform well in EEG measurements.
II. Materials and Methods
A. Design of SBDS
To develop a comfortable wearing SBDS, selection of the SBDS material involves avoiding the use a pure conductive metal (Cu and Fe) for the electrode, Owing to that metals are hard and solid, wearing a metal electrode can cause discomfort [13], [16]. To fulfill the requirements of being conductive, flexible and non-irritating to skin, the sensors in this study are constructed from a silicon-based conductive material. Their major components include silicon and silver-based, thick-film pastes. Analysis results verify the effectiveness of using this material as electrodes.
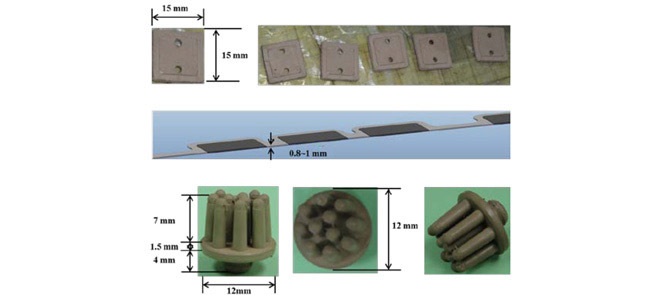
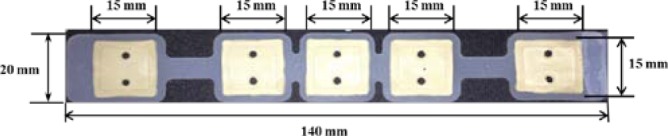
To satisfy comfort requirements, SBDS has a lightweight design and uses limitary material to optimize its sensitivity. Substrate material of the proposed sensor is another major consider. Electrodes with hard substrates may cause discomfort or pain at the scalp surface when force is applied. SBDSs have two design types: forehead-SBDS and acicular-SBDS. Figure 1(A) shows the forehead-SBDS was developed for forehead EEG measurements. The electrode thickness is 0.8 mm, which follows the design goals of the electrode (i.e., as thin as possible, not easily broken in manufacturing and comfortable to users). For the forehead EEG measurements and convenient wear requirement, the forehead-SBDS includes four electrodes for the EEG channels and one electrode for the ground. The electrodes are embedded in a biocompatible silicon substrate. In terms of forehead fit, the contact area was optimized to provide the proper skin-contact impedance. The pattern design of each electrode is a square. Length and width of the electrode are 15 mm each. The sensor substrate is made of flexible biocompatible silicon, which satisfies the requirement for a flexible substrate design. Dimensions of the integrated forehead-SBDS are a thickness of 1 mm, a width of 20 mm and a length of 140 mm (Fig. 1(B)). Figure 1(C) shows the acicular-SBDS, which was designed for use in hairy sites. There are eleven probes with a height of 7 mm, and the substrate has a radius of 12 mm and a height of 1.5 mm. The bottom of the sensor has a male button pattern design with a height of 4 mm for connections to recording devices. The electrode and the substrate of this acicular-SBDS are integrated with each other; they are made of a silicon-based conductive material. This design of the acicular-SBDS allows for flexibility by using several soft flexible probes as the electrodes. When direct pressure is applied to this sensor, the probes spread and push the hairs aside to achieve acceptable scalp contact and low skin-contact impedance. Specifications (i.e., length, radius and springiness) of the acicular-SBDSs can be customized to provide effective scalp contact for different hairy sites with variations in the density and length of the hair. Specific lengths may be more effective for certain conditions, such as shorter and longer probes for temporal and parietal lobe EEG measurements, respectively.
FIGURE 1.
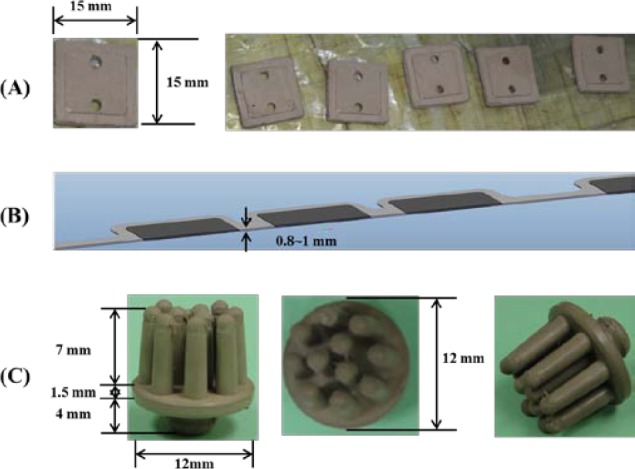
(A) Length and width of the forehead-SBDS electrode are 15 mm in each dimension. Five electrodes are embedded in the forehead-SBDS substrate. (B) Thickness of the forehead-SBDS substrate is approximately 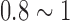 mm. (C) Dimensions of the acicular-SBDS.
mm. (C) Dimensions of the acicular-SBDS.
B. Manufacturing of SBDS
To produce different shapes of SBDSs, various of molds are made. The procedure includes high-temperature and high-pressure treatments. For example, manufacturing the forehead-SBDS, first involves shaping the electrodes. The electrode is then placed in a bio-silicon material to bind. During this step, the manufacturing conditions (i.e., temperature, flow and pressure) must be controlled since the materials have different expansion coefficients. Additionally, the process requires high precision to combine the electrode with the substrate. Figure 2 illustrates the constructed device. Mass of the thin, lightweight and flexible SBDS is approximately 3 grams.
FIGURE 2.
Formation of the forehead-SBDS after the binding procedure.
III. Results
A. Electrochemical Performance of Proposed SBDS
Prior to the biopotential measurements, whether the sensor has sufficient conductivity and supports low, stable impedance must be determined. In particular, the typical potential requirement for EEG signal measurements is approximately 10-6 volts. Accurate impedance measurements were taken by using an Autolab N Series Potentiostat/Galvanostat rather than a general multi-meter for the micro-impedance measurements (Fig. 3(A)). In this test, the poles were placed in contact with positive and negative surfaces to measure the impedance. After several replicates, the averaged results indicated that the impedance was low and stable. The impedances were between 1.3 ohms and 1.6 ohms during 120 seconds of measurements for each forehead-SBDS electrode (Fig. 3(B)). Impedance of the acicular-SBDS electrode was slightly higher than that of the forehead-SBDS electrode. Impedance of the acicular-SBDS electrode was approximately 2 ohms. Experimental results demonstrate SBDSs have sufficient conductivity, low and stable impedance.
FIGURE 3.
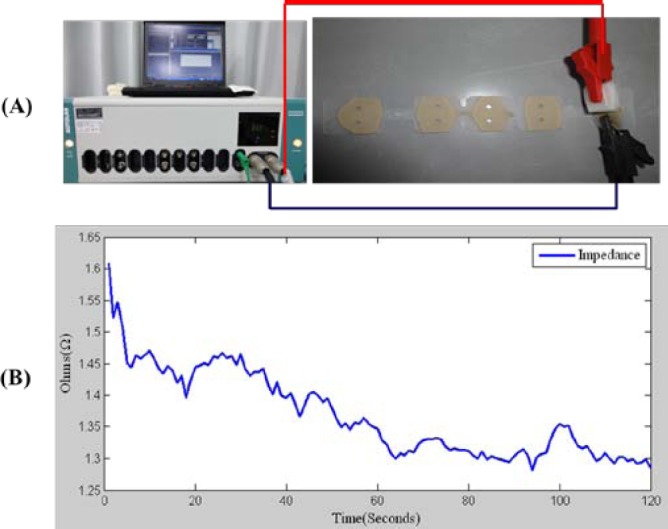
(A) Autolab N Series Potentiostat/Galvanostat for the impedance measurements. (B) Plot of the average impedance measurement. Average impedance (1.4 Ohms) of the forehead-SBDS electrode is low and stable over a measurement period of 120 seconds.
B. Brain Skin-Contact Impedance Quality and Comparison
Brain skin-contact impedance is an important reference in EEG signal measurements [16]. In this study, the brain skin-contact impedances of the forehead-SBDS and a conventional wet sensor were compared [6], [12]. Because the following EEG signals experiments used the Neuroscan system to collect raw data, the Neuroscan system was also used to measure the brain skin-contact impedance (Fig. 4(A)). The detection range of the system was from 0.0 to 20.0 k ohms [18]. The conventional wet sensor and forehead-SBDS were located near the Fp1 location on the subject. The forehead-SBDS were taped to the skin to prevent movement artifact. The data were recorded every 10 minutes. Subjects can drink water or go to the toilet freely during the experiment. The results indicate that the brain skin-contact impedances are stable for both sensors. The blue line corresponds to the skin-contact impedance of the conventional wet electrode, and the red curve is the forehead-SBDS skin-contact impedance. The forehead-SBDS electrodes and the wet electrodes had similar impedance levels during the six-hour experiments. The skin-contact impedance of the forehead-SBDS was approximately 10.0 k ohms, and the skin-contact impedance of the wet sensor was approximately 4.3 k ohms (Fig. 4(B)). Although the contact impedance of the wet sensor was lower than that of the SBDS, the performance of SBDS (an impedance of 10.0 k ohms) is sufficient for general EEG device applications. Conventional wet sensors must be applied with conductive gels. When the wet electrode and the SBDS electrode were applied at room temperature and the contact-impedance measurements were repeated after several days, the SBDS skin-contact impedance remained below 100 k ohm. However, the wet sensor had become too dry to be used. These skin-contact impedance measurements demonstrated that SBDS can provide low, stable skin-contact impedances for long-term EEG measurements.
FIGURE 4.
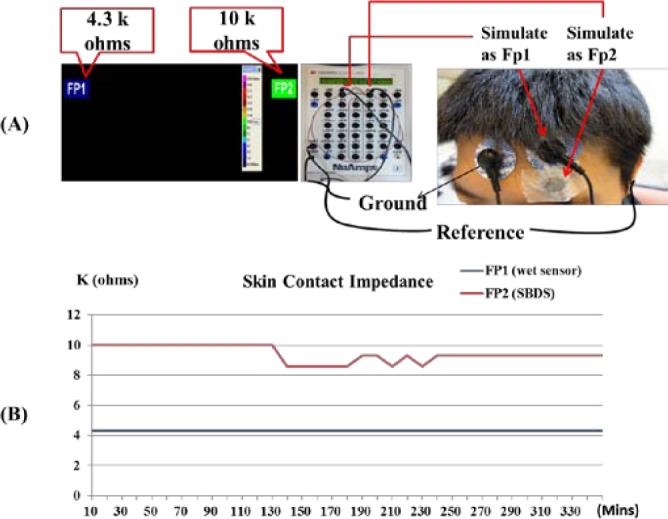
(A) A Neuroscan system was used for the skin-contact impedance measurements. (B) The results indicate that the average skin-contact impedance was approximately 4.3 k ohms (blue line) and 10 k ohms (red line) at the fp1 location for the conventional wet sensor and the SBDS, respectively, based on the impedance measurements with the Neuroscan system. The skin-contact impedance measurement lasted 330 minutes. The skin-contact impedances of the wet electrodes and the SBDS electrodes are stable.
C. EEG Signal Comparisons
The EEG signal quality was defined by comparing the correlation values between the forehead-SBDS and the conventional wet sensor [6], [19]. In this experiment, the EEG signals were collected using the Neuroscan system. The conventional wet electrode and the forehead-SBDS electrode were then placed near the Fp1 location on the subject’s forehead (Fig 4(A)) [20]. Next, the difference in the signal quality of the EEG measurements was evaluated using the linear correlation coefficient function in MATLAB (R2007a, The MathWorks, Natick, MA). EEG raw data was preprocessed by 50 Hz high-pass filter, 1 Hz low-pass filter and 250 Hz down sampling. This test acquired 100 seconds of EEG data, and a sample of the EEG recorded data (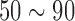 seconds) was taken for analysis. Figure 5(A) compare the EEG signals that were recorded with a conventional wet electrode (red curve) and the forehead-SBDS electrode (blue curve). The green curve shows the correlation and the black curve shows the Root Mean Square Error (RMSE) of these two signals. The peaks in the blue and red curves correspond to eye-blinking signals. The average correlation between the blue and red lines is high (
seconds) was taken for analysis. Figure 5(A) compare the EEG signals that were recorded with a conventional wet electrode (red curve) and the forehead-SBDS electrode (blue curve). The green curve shows the correlation and the black curve shows the Root Mean Square Error (RMSE) of these two signals. The peaks in the blue and red curves correspond to eye-blinking signals. The average correlation between the blue and red lines is high (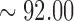 %), the maximum of the RMSE is 2.0050 (
%), the maximum of the RMSE is 2.0050 (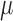 v) and the average of RMSE is 0.4669 (
v) and the average of RMSE is 0.4669 (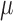 v). The red asterisks located in the bottom portion of the figure indicated significant differences between red curve/blue curve. (p < 0.01, the paired sample Wilcoxon signed-rank test per seconds (250 points)). Analysis results indicate that the signal quality of the forehead-SDBS is similar to that of the conventional wet sensor [6].
v). The red asterisks located in the bottom portion of the figure indicated significant differences between red curve/blue curve. (p < 0.01, the paired sample Wilcoxon signed-rank test per seconds (250 points)). Analysis results indicate that the signal quality of the forehead-SDBS is similar to that of the conventional wet sensor [6].
FIGURE 5.
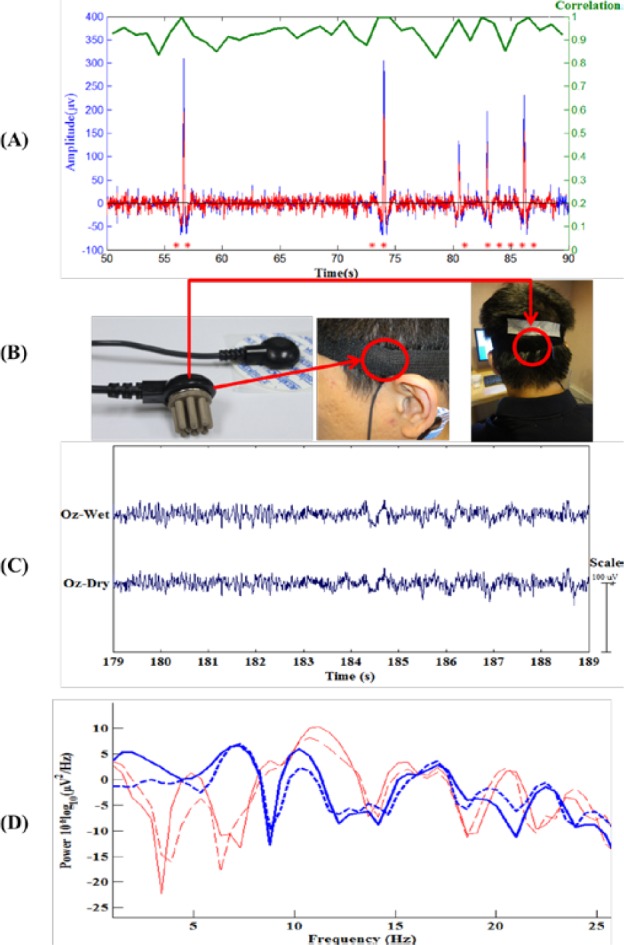
(A). Comparison of the EEG signals recorded by a conventional wet electrode (red curve) and the forehead-SBDS electrode (blue curve). The green curve shows the correlation of these two signals and the black curve shows the RMSE (Root Mean Square Error) of these two signals. The peaks in the blue and red curves correspond to eye-blinking signals. The average correlation between the blue and red lines is high (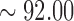 %), the maximum of the RMSE is 2.0050 (
%), the maximum of the RMSE is 2.0050 (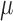 v) and the average of RMSE is 0.4669 (
v) and the average of RMSE is 0.4669 (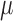 v). The red asterisks, which are in the bottom portion of the figure, indicate the significant differences between red curve/blue curve. (p < 0.01, the paired sample Wilcoxon signed-rank test per seconds (250 points)). This test acquired 100 seconds of EEG data, and a sample of the EEG recorded data (50
v). The red asterisks, which are in the bottom portion of the figure, indicate the significant differences between red curve/blue curve. (p < 0.01, the paired sample Wilcoxon signed-rank test per seconds (250 points)). This test acquired 100 seconds of EEG data, and a sample of the EEG recorded data (50 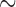 90 seconds) was taken for analysis. EEG raw data is preprocessed by 50 Hz high-pass filter, 1 Hz low-pass filter and 250Hz down sampling. (B) For hairy sites EEG signal measurements, the acicular-SBDS was located at brain by a tire. (C) The resting state EEG signals during the eyes closed within 10s window. (D) Power spectral density showing alpha wave re-placement.
90 seconds) was taken for analysis. EEG raw data is preprocessed by 50 Hz high-pass filter, 1 Hz low-pass filter and 250Hz down sampling. (B) For hairy sites EEG signal measurements, the acicular-SBDS was located at brain by a tire. (C) The resting state EEG signals during the eyes closed within 10s window. (D) Power spectral density showing alpha wave re-placement.
The EEG signal can also be collected from hairy sites by acicular-SBDS. The signal quality of acicular-SBDS was evaluated a typical alpha rhythm testing [21]–[23] was performed. The wet sensor with gel and acicular-SBDS were located at the Occipital (Oz) position nearby. A tire was used to enhance the acicular-SBDS stationary (Fig. 5(B)). Figure 5(C) shows the resting state EEG signals during the eyes closed within 10s window. Figure 5(D) compares the alpha power (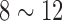 Hz) between eyes open (blue curves) and eyes closed (red curves) cases. The alpha phenomena were observed in both EEG signals recorded by the wet sensor (bold curves) and the acicular-SBDS (dash curves).
Hz) between eyes open (blue curves) and eyes closed (red curves) cases. The alpha phenomena were observed in both EEG signals recorded by the wet sensor (bold curves) and the acicular-SBDS (dash curves).
D. EEG Oddball Experiment for Quality Verification
P200 deficits in auditory oddball tasks are common EEG experiments to verify whether the EEG signal quality is sufficient [11], [13]. The P200 component is a positive deflection with a typical peak latency of approximately 150-250 ms elicited by auditory stimuli [24], [25]. In this experiment, the EEG signals were recorded using the Neuroscan System. The event-related potential (ERP) maps were evaluated using EEGLAB (v10.2.5.5b) and MATLAB [11], [26], [27]. For the forehead EEG signal measurements, the conventional wet sensor and forehead-SBDS were placed near the Fp1 location on the forehead. For hairy sites EEG signal measurements, the wet sensor and acicular-SBDS were placed near the temporal (T3) position. An EEG pre-processing procedure included artifact removals (removal of trials with extraordinary movement and muscle artifact) was performed before evaluating the ERP maps (Fig 6(A)). After pre-processing, the ERP maps were drawn based on the EEG channel ERP image function of EEGLAB. Figures 6(B-C) show the EEG results for the conventional wet sensor and the forehead-SBDS, respectively. Clear P200 components (i.e., the peaks indicated as p200) were detected by the conventional wet sensor and forehead-SBDS while acquiring the ERP maps. Figures 6(D-E) show the EEG results for the wet sensor and the acicular-SBDS located at the temporal (T3) position nearby also detected P200 components. The acicular-SBDS was less effective in this test. These findings validate the use SBDSs for EEG measurements [17].
FIGURE 6.
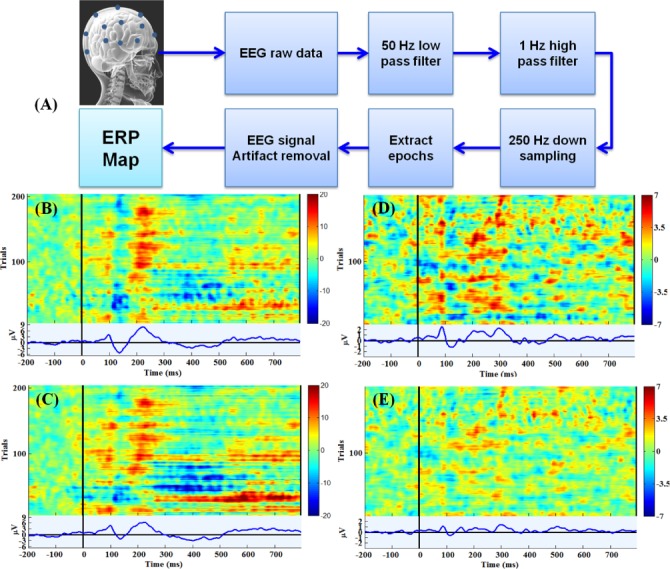
(A) Schematic of the EEG signals pre-processing routine for the ERP test. (B) An ERP map with a conventional wet sensor located at Fp1. (C) An ERP map with a forehead-SBDS located near Fp1. (D) An ERP map with a wet sensor located at T3. (E) An ERP map with acicular-SBDS located near T3. Plots B-C and D-E display the same trials in the same order, at the respective electrodes. Comparatively, SBDSs’ effects were weaker than the wet sensors, but the P200 phenomena were present in the ERPs from both wet and dry channels.
E. SGS Certification
To determine whether the SBDSs are biocompatible sensors that do irritate skin, SGS Taiwan Ltd. was commissioned to perform a series of tests for the prototype SBDSs (the whole sensor was made of the same silicon-based material as the acicular-SBDS). There were no significant findings of erythema or edema in either the control or the treatment group, and there was no mortality. This result indicates that a single topical application of 0.5 ml of the silicon-based electrode extract will not cause skin irritation.
Additionally, cytotoxicity tests were conducted, such as morphological evaluation, observation of monolayer confluence and relative inhibition of cell viability. SGS test results can be summarized as follows. The silicon-silver-based dry-contact EEG sensors (conductive poly rubber with silver particles) extract did not irritate skin or induce non-cytotoxic effects on L929 cells according to ISO10993-5.
F. Mechanical Testing
The life cycles of SBDSs are difficult to identify exactly. According to previous experiences, if the surface of SBDS was kept clean, the repetition frequency of use was over ten times (5 hours/times). The responses to artifact and recovery time of SBDS and wet sensors are almost the same. In this test, the signals were recorded using the Neuroscan system. Figure 7 shows the recovery and eventual shave of artifacts for jaw artifact recorded by wet sensor and acicular-SBDS at Oz location. The subject ground his teeth three times within 3 to 6 seconds. The average recovery time is around 1 second.
FIGURE 7.
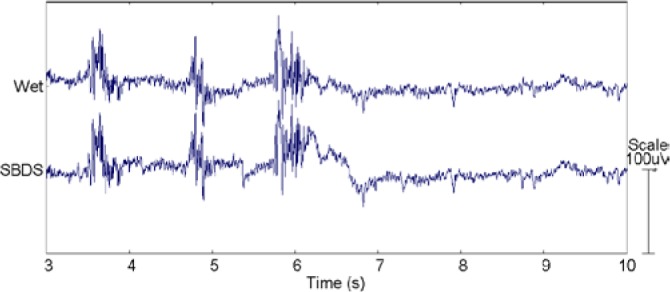
Resting state EEG signals with 3 times of grinding.
IV. Conclusion
This study describes a novel, lightweight, flexible and non-skin irritating SBDS, including its design, fabrication and testing. The proposed SBDSs have the following advantages. First, the sensor material has passed the standard SGS certifications, showing that it was biocompatible and did not irritate skin or induce cytotoxic effects in the test. Second, SBDSs have an ergonomic design with a flexible base and can be used in non-gel applications. The proposed SBDBs are also comfortable to wear without causing pain. Third, the proposed SBDSs perform similarly to wet conventional sensors in the EEG measurements. The proposed SBDSs are superior to wet sensors in their use in long-term EEG measurements. Fourth, the proposed SBDSs are made for two different brain sites for the EEG measurements, especially for the hairy sites. Finally, the proposed SBDSs can achieve the necessary skin-sensor interface impedance to provide EEG signals of sufficient quality for brain dynamics research. Experimental results indicate that the designed sensors are applicable for EEG signals measurement in the human brain.
Despite the above advantages of SBDSs, we recommend that future research attempt to improve their life cycle, since major components of SBDSs include silver-based and SiO2. After several uses, SBDSs may oxidize and reduce conductivity. Although the proposed sensors cannot be abraded or chemically treated to reveal fresh material, they can be washed with soap and sterilized by alcohol for reuse.
Supplementary Material
KB
Windows
Many electroencephalography (EEG) sensors, including conventional wet and dry sensors, use materials that can cause skin irritation and discomfort. To overcome this drawback, this study reports on the design and human testing of a SGS-certified, silicon-based dry-contact EEG sensor. In addition to being non-irritating, the acicular sensors are lightweight, flexible, and capable of easily fitting to the scalp, forehead, or hairy sites without any skin preparation or conductive gel. The sensors also maintain low skin-electrode interface impedance. Results of this study demonstrate that the proposed sensors perform well in EEG measurements and are feasible for both medical and entertainment applications.

Biographies

Yi-Hsin Yu received the B.S. degree in computer science and information engineering from Tunghai University, Taichung, Taiwan, in 1995, and the M.S. degree in computer science and information engineering from National Dong Hwa University, Hualien, Taiwan, in 1998. He is currently pursuing the Ph.D. degree with the Brain Research Center, Institute of Electrical Control Engineering, National Chiao Tung University, Hsinchu, Taiwan. His research interests include brain research, biomedical signal processing, brain-computer interfaces, and biomedical circuits and systems.

Shao-Wei Lu received the B.S. degree in physics and the M.S. degree from the Institute of Molecular Medicine, National Tsing Hua University, Hsinchu, Taiwan, in 2007. He is currently an Assistant Researcher with the Brain Research Center, National Chiao Tung University, Hsinchu, where he is involved in electroneurophysiology and biomedical signal processing.

Lun-De Liao received the Ph.D. degree in electrical engineering from National Chiao Tung University (NCTU), Hsinchu, Taiwan, in 2012, where he was a Post-Doctoral Researcher with the Brain Research Center (BRC). He proposed the world first bioinspired dry EEG sensors and their corresponding circuit to the intelligent image human brain under the guidance of Prof. C.-T. Lin at BRC. He is currently a Senior Research Scientist and the Head of the Neurovascular Imaging Laboratory with the Singapore Institute for Neurotechnology, National University of Singapore, Singapore. He has authored over 45 peer-reviewed SCI journal papers, including the Nature: Journal of Cerebral Blood Flow and Metabolism, the PROCEEDINGS OF THE IEEE, and Neuroimage journals, and holds 10 issued patents. He has selected/nominated for over 30 international awards since 2004. He was a recipient of the Young Investigator’s Awards from the World Association for Chinese Biomedical Engineers for his contributions on medical imaging and bioelectronics domain, in 2011, and an Outstanding Research Award from NCTU, in 2012, for his outstanding research performance. He served on Organization Committee and Technical Program Committee of many flagship international conferences and workshops. He serves as the Co-Editors-in-Chief of Journal of Neuroscience and Neuroengineering and also an Associate Editor of four SCI-index journals. His research interests include neuroimaging, cerebral neuroscience, cognitive neuroscience, in vivo optical microscopy, advanced sensing techniques, and design of optical system.

Chin-Teng Lin (S’88–M’91–SM’99–F’05) received the B.S. degree from National Chiao Tung University (NCTU), Hsinchu, Taiwan, in 1986, and the M.Sc. and Ph.D. degrees in electrical engineering from Purdue University, West Lafayette, IN, USA, in 1989 and 1992, respectively. He is currently the Provost, the Chair Professor of Electrical and Computer Engineering, a Professor with the Institute of Imaging and Biomedical Photonics, and the Director of the Brain Research Center, NCTU. He is currently the Editor-in-Chief of the IEEE Transactions on Fuzzy Systems.
Funding Statement
This work was supported in part by the University System of Taiwan—University of California at San Diego International Center of Excellence in Advanced Bio-Engineering through the Ministry of Science and Technology (MOST), Taiwan, within the I-RiCE Program under Contract NSC-102-2911-I-009-101, the Aiming for the Top University Plan through the MOST, National Chiao Tung University, Hsinchu, Taiwan, and the U.S. Army Research Laboratory, Adelphi, MD, USA, under Grant W911NF-10-2-0022.
References
- [1].Nunez P. L., Electric Fields of the Brain: The Neurophysics of EEG. New York, NY, USA: Oxford Univ. Press, 1981. [Google Scholar]
- [2].Thakor N. V., “Biopotentials and electro-physiology measurement,” in The Measurement, Instrumentation and Sensors Handbook. Boca Raton, FL, USA: CRC Press, 1999. [Google Scholar]
- [3].Thakor N. V., “Translating the brain-machine interface,” Sci. Transl. Med., vol. 5, p. 210–217, Nov. 2013. [DOI] [PubMed] [Google Scholar]
- [4].Lin C.-T., et al. , “Noninvasive neural prostheses using mobile and wireless EEG,” Proc. IEEE, vol. 96, no. , pp. 1167–1183, Jul. 2008. [Google Scholar]
- [5].Lin C.-T., Lin F.-C., Chen S.-A., Lu S.-W., Chen T.-C., and Ko L.-W., “EEG-based brain-computer interface for smart living environmental auto-adjustment,” J. Med. Biol. Eng., vol. 30, no. 4, pp. 237–245, Aug. 2010. [Google Scholar]
- [6].Lin C.-T., Liao L.-D., Liu Y.-H., Wang I.-J., Lin B.-S., and Chang J.-Y., “Novel dry polymer foam electrodes for long-term EEG measurement,” IEEE Trans. Biomed. Eng., vol. 58, no. 5, pp. 1200–1207, May 2011. [DOI] [PubMed] [Google Scholar]
- [7].Liao L.-D., Wang I.-J., Chen S.-F., Chang J.-Y., and Lin C.-T., “Design, fabrication and experimental validation of a novel dry-contact sensor for measuring electroencephalography signals without skin preparation,” Sensors, vol. 11, no. 6, pp. 5819–5834, Jun. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Huang T.-H., Huang H.-P., Liu Y.-H., Kang Z.-H., and Kuan J.-Y., “Development of a brain-controlled rehabilitation system (BCRS),” J. Neurosci. Neuroeng., vol. 2, no. 2, pp. 79–89, 2013. [Google Scholar]
- [9].Lance B. J., Kerick S. E., Ries A. J., Oie K. S., and McDowell K., “Brain-computer interface technologies in the coming decades,” Proc. IEEE, vol. 100, no. 5, pp. 1585–1599, May 2012. [Google Scholar]
- [10].Liao L.-D., et al. , “Gaming control using a wearable and wireless EEG-based brain-computer interface device with novel dry foam-based sensors,” J. NeuroEng. Rehabil., vol. 9, pp. 1–12, Jan. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Liao L.-D., et al. , “Biosensor technologies for augmented brain-computer interfaces in the next decades,” Proc. IEEE, vol. 100, no. 5, pp. 1553–1566, May 2012. [Google Scholar]
- [12].Merletti R., “The electrode-skin interface and optimal detection of bioelectric signals,” Physiol. Meas., vol. 31, no. 10, p. preceding S157, Oct. 2010. [DOI] [PubMed] [Google Scholar]
- [13].Liao L.-D. and Lin C.-T., “Novel trends in biosensors used for electroencephalography measurements in neurocognitive engineering applications,” J. Neurosci. Neuroeng., vol. 1, no. 1, pp. 32–41, 2012. [Google Scholar]
- [14].Grozea C., Voinescu C. D., and Fazli S., “Bristle-sensors-low-cost flexible passive dry EEG electrodes for neurofeedback and BCI applications,” J. Neural Eng., vol. 8, no. 2, p. 025008, Apr. 2011. [DOI] [PubMed] [Google Scholar]
- [15].Beckmann L., et al. , “Characterization of textile electrodes and conductors using standardized measurement setups,” Physiol. Meas., vol. 31, no. 2, pp. 233–247, Feb. 2010. [DOI] [PubMed] [Google Scholar]
- [16].Searle A. and Kirkup L., “A direct comparison of wet, dry and insulating bioelectric recording electrodes,” Physiol. Meas., vol. 21, no. 2, pp. 271–283, May 2000. [DOI] [PubMed] [Google Scholar]
- [17].Gramann K., et al. , “Cognition in action: Imaging brain/body dynamics in mobile humans,” Rev. Neurosci., vol. 22, no. 6, pp. 593–608, 2011. [DOI] [PubMed] [Google Scholar]
- [18].Ferree T. C., Luu P., Russell G. S., and Tucker D. M., “Scalp electrode impedance, infection risk, and EEG data quality,” Clin. Neurophysiol., vol. 112, no. 3, pp. 536–544, Mar. 2001. [DOI] [PubMed] [Google Scholar]
- [19].Estepp J. R., Christensen J. C., Monnin J. W., Davis I. M., and Wilson G. F., “Validation of a dry electrode system for EEG,” Proc. Human Factors Ergonom. Soc. Annu. Meeting, vol. 53, no. 18, pp. 1171–1175, Oct. 2009. [Google Scholar]
- [20].Iguchi H., Watanabe K., Kozato A., and Ishii N., “Wearable electroencephalograph system with preamplified electrodes,” Med. Biol. Eng. Comput., vol. 32, no. 4, pp. 459–461, Jul. 1994. [DOI] [PubMed] [Google Scholar]
- [21].Gargiulo G., et al. , “A new EEG recording system for passive dry electrodes,” Clin. Neurophysiol., vol. 121, no. 5, pp. 686–693, 2010. [DOI] [PubMed] [Google Scholar]
- [22].Gargiulo G. D., Bifulco P., Cesarelli M., Fratini A., and Romano M., “Problems in assessment of novel biopotential front-end with dry electrode: A brief review,” Machines, vol. 2, no. 1, pp. 87–98, 2014. [Google Scholar]
- [23].Mueller-Putz G., Scherer R., Brunner C., Leeb R., and Pfurtscheller G., “Better than random: A closer look on BCI results,” Int. J. Bioelectromagn., vol. 10, no. 1, pp. 52–55, 2008. [Google Scholar]
- [24].Brett-Green B. A., Miller L. J., Gavin W. J., and Davies P. L., “Multisensory integration in children: A preliminary ERP study,” Brain Res., vol. 1242, pp. 283–290, Nov. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ferreira-Santos F., Silveira C., Almeida P. R., Palha A., Barbosa F., and Marques-Teixeira J., “The auditory P200 is both increased and reduced in schizophrenia? A meta-analytic dissociation of the effect for standard and target stimuli in the oddball task,” Clin. Neurophysiol., vol. 123, no. 7, pp. 1300–1308, Jul. 2012. [DOI] [PubMed] [Google Scholar]
- [26].Sellers E. W., Krusienski D. J., McFarland D. J., Vaughan T. M., and Wolpaw J. R., “A P300 event-related potential brain-computer interface (BCI): The effects of matrix size and inter stimulus interval on performance,” Biol. Psychol., vol. 73, no. 3, pp. 242–252, Oct. 2006. [DOI] [PubMed] [Google Scholar]
- [27].Zhang H., Guan C., and Wang C., “Asynchronous P300-based brain-computer interfaces: A computational approach with statistical models,” IEEE Trans. Biomed. Eng., vol. 55, no. 6, pp. 1754–1763, Jun. 2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
KB
Windows
Many electroencephalography (EEG) sensors, including conventional wet and dry sensors, use materials that can cause skin irritation and discomfort. To overcome this drawback, this study reports on the design and human testing of a SGS-certified, silicon-based dry-contact EEG sensor. In addition to being non-irritating, the acicular sensors are lightweight, flexible, and capable of easily fitting to the scalp, forehead, or hairy sites without any skin preparation or conductive gel. The sensors also maintain low skin-electrode interface impedance. Results of this study demonstrate that the proposed sensors perform well in EEG measurements and are feasible for both medical and entertainment applications.



