Abstract
Objective
Our aim was to examine the association between anti-interferon inducible protein 16 (IFI16) antibodies and clinical features of scleroderma.
Methods
Sera from a discovery sample of 94 scleroderma patients and 47 healthy controls were assayed for anti-IFI16 antibodies by ELISA, and associations were examined using regression analyses. As anti-IFI16 autoantibodies strongly associated with digital gangrene in the discovery sample, a subsequent 1:1 disease-duration matched case-control study was designed for further exploration. Cases were patients with scleroderma and digital gangrene, while controls had scleroderma and Raynaud’s alone (n=39 pairs). Nonparametric unadjusted matched-pair analyses, univariate and multivariable conditional logistic regression were performed.
Results
Anti-IFI16 antibodies in the discovery sample were more prevalent in scleroderma than controls (18% vs. 2%; p=0.01). Patients with anti-IFI16 antibodies were more likely to have limited scleroderma (77% vs. 46%; p=0.03), longer disease duration (15.2 [10.6–18.3] vs. 6.0 [3.4–13.8] years; p <0.01), digital gangrene (24% vs. 4%; p=0.02), and a low DLCO (p <0.01). In the case-control study, 35/78 (45%) were anti-IFI16 positive. Anti-IFI16 antibody levels were significantly higher in cases than matched controls (p=0.02). Adjusted for age, cutaneous subtype, smoking, and DLCO, high anti-IFI16 antibody levels associated with digital gangrene (adjusted OR 2.3; 95% CI 1.0, 5.6; p = 0.05). The odds of digital gangrene increased with higher antibody titers in a dose dependent manner.
Conclusion
Anti-IFI16 antibodies are associated with digital gangrene in scleroderma. Longitudinal prospective studies exploring anti-IFI16 antibodies as a disease biomarker, and biological studies investigating the pathogenicity of these antibodies, are warranted.
INTRODUCTION
Scleroderma is an autoimmune disease that is often associated with a systemic vasculopathy, dermal fibrosis, and visceral organ failure. While the etiology of scleroderma is unknown, many have hypothesized that it is primarily a vascular disease mediated by autoimmunity. Evidence for this hypothesis arises from several clinical and biological observations: (i) the onset of Raynaud’s phenomenon is typically one of the earliest signs of disease and may manifest years before the diagnosis of scleroderma is appreciated, (ii) the presence of other vascular complications including pulmonary hypertension, scleroderma renal crisis, cardiac disease, and striking microvascular changes in the capillaries are prominent, and (iii) these clinical features suggest that vascular injury occurs early in scleroderma, preceding fibrosis, and progresses over time ultimately resulting in both macro and microvascular disease in multiple organs (1–3).
IFI16 is a member of the interferon-inducible p-200 protein family (4). Accumulated experimental evidence has attributed a wide array of functions to the p-200 family proteins, including roles in activities such as cell differentiation, apoptosis, cell growth regulation, and autoimmunity (4). Anti-IFI16 antibodies are present in 20–30% of patients with scleroderma, particularly in those with limited skin disease (5, 6). Patients with limited skin disease are at greater risk than other scleroderma subtypes for severe vascular manifestations of scleroderma, including pulmonary arterial hypertension, and severe digital ischemia (7, 8). In this regard, it is interesting that IFI16 levels are constitutively enriched in vascular endothelial cells (9). A prior study reported a significant association between anti-IFI16 antibodies and cardiac disease as measured by the presence of pericarditis, congestive heart failure, arrhythmias requiring treatment, conduction system abnormalities or diastolic dysfunction; however, there is no indication that an association between anti-IFI16 antibody positivity and severity of Raynaud’s or digital loss was ever evaluated (5, 6).
In this study, we examined whether patients with scleroderma who test positive for anti-IFI16 antibodies have any associated clinical features or complications of scleroderma. Findings made in our initial discovery sample showed that patients with anti-IFI16 antibodies are at a greater risk for digital gangrene. These observations were confirmed in a subsequent case-control study. We also showed that anti-IFI16 antibody levels are highest close to the ischemic event. These findings highlight the potential utility of anti-IFI16 antibodies as a biomarker of peripheral vascular disease manifesting as digital gangrene in patients with scleroderma.
PATIENTS AND METHODS
Patients
The discovery sample included 94 patients randomly selected from the Johns Hopkins Scleroderma Center cohort database who met either 1980 American College of Rheumatology (ACR) criteria or at least three of five features of the CREST (calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, telangiectasia) syndrome for scleroderma (all of these met ACR 2013 criteria); all study patients were evaluated as part of routine clinical care at the Johns Hopkins Scleroderma Center and had an available serum sample. Patients were classified as having diffuse or limited cutaneous scleroderma based on the extent of skin involvement (10, 11). For a pilot longitudinal study, we subsequently selected 5 individuals from the discovery sample with anti-IFI16 antibodies for whom a minimum of 5 serum samples, taken at various times, were banked and available for study. All serum samples were obtained during routine clinical visits at the Johns Hopkins Scleroderma Center and were stored at −80°C. Forty-seven healthy controls without a history of scleroderma or other autoimmune disease were selected as a comparator group.
As our initial discovery sample analyses suggested an association between anti-IFI16 autoantibodies and digital gangrene, we performed a matched case-control study to enrich for the event of interest and confirm these findings. Cases and controls again met either 1980 American College of Rheumatology (ACR) criteria or at least three of five features of the CREST (calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, telangiectasia) syndrome for scleroderma (all but one of these met ACR 2013 criteria). Cases were seen between the dates March 25, 1991 to August, 26, 2014 and were selected based on a history of digital gangrene and the presence of a blood sample drawn within 6 months, preceding or following the ischemic event. Thirty-nine cases meeting these criteria were available in our cohort and were matched to 39 scleroderma controls characterized by having Raynaud’s alone without a history of any digital ischemic events (maximum Raynaud’s Medsger severity scale score of 1)(12). Cases and controls were matched on disease duration because it was a potential confounder in the initial cross-sectional study. For cases, disease duration was defined as the interval from the time of the first scleroderma symptom (Raynaud’s or non-Raynaud’s) to the time of the serum draw closest to the clinic visit for digital gangrene. Control serum samples were identified to represent comparable disease duration at the time of the sample draw.
We sought to determine whether anti-IFI16 antibody levels were highest immediately around the time of the digital gangrene event. We therefore compared anti-IFI16 antibody levels between 2 groups of patients with gangrene: i) those with blood drawn close (within 6 months) to the ischemic event who had positive IFI16 antibodies (N=20/39 original cases) and (ii) those with blood drawn farther from the ischemic event (6–24 months) who had positive IFI16 antibodies (N=10 of 23 patients tested).
Written informed consent was obtained from all patients. The present study was approved by the Johns Hopkins Institutional Review Board.
Clinical Phenotyping
Demographic and clinical data, including age, sex, race, smoking status, disease duration, scleroderma subtype, specific organ involvement, and autoantibody status, were previously obtained on each patient at the time of the clinical visit. Digital gangrene was defined using the Medsger’s severity score for Raynaud’s phenomenon (RP) (0 = no RP, 1 = RP with or without vasodilator required, 2 = digital pitting scars, 3 = digital tip ulcerations, 4 = digital gangrene). This requires demarcated ischemic territory on a digit (usually at the tip) which is dark, cool and often intensely painful. This gangrenous area is clearly demarcated from viable painless flesh at the proximal border. Gangrene does not include non-digital vascular lesions. Also excluded are palpable purpura, erythema nodosum, oral or nasal ulcers, rheumatoid nodules, genital ulcers, traumatic ulcers and stasis ulcers due to venous insufficiency. (12). This definition includes both wet and dry types of gangrene. The presence of non-scleroderma vascular disease (peripheral arterial disease) was evaluated by chart review of cases with lower extremity gangrenous lesions. Additional measures of RP severity were also determined using the Raynaud’s Medsger severity score (12). Skin involvement was scored according to the modified Rodnan skin thickness score (MRSS [range 0–51]). Internal organ involvement was assessed using previously published criteria (12). Specific measures of pulmonary function were determined based on findings on pulmonary function tests (PFTs) (forced vital capacity [FVC] and single breath diffusing capacity for carbon monoxide [DLCO], measured as the absolute value as well as the percent predicted value for sex and age). A low DLCO was defined as a value that was <70% predicted. For this study, patients were considered to have evidence of pulmonary arterial hypertension (PAH) with a measured mean pulmonary arterial pressure (mPAP) ≥ 25 mmHg and a pulmonary capillary wedge pressure (pcwp) ≤ 15mmHg. Cardiac, pulmonary, gastrointestinal, or renal involvement was considered present when the relative Medsger severity score was ≥ 1. Evidence of Sicca complex was determined by clinical criteria. Anti-centromere, anti-RNA polymerase III, anti-ribonucleoprotein (RNP), and anti-topoisomerase-1 antibody status were obtained from clinical test results.
IFI16 ELISA
Serum levels of anti-IFI16 antibodies were quantitated using an ELISA assay as follows. Recombinant full-length human IFI16, expressed and purified as described (13), was used to coat 96-well ELISA plates (100 ng/well, overnight at 4°C). Washes were included between this and all subsequent steps, and all further incubations were carried out at room temperature for 1 hour. Wells were blocked with 5% BSA in PBS/0.05% Tween 20 (PBST), followed by incubation with human sera diluted 1:400 in 1% BSA/PBST. Horseradish peroxidase-labeled goat anti-human antibody (1:10,000 dilution, Jackson Immunoresearch) was added to each well, and color development was subsequently performed with SureBlue peroxidase reagent (KPL) before reading absorbances at 450 nm. A positive reference serum (1:400 dilution with an OD in the linear range) was included in every IFI16 ELISA assay; all absorbances were calibrated relative to this reference absorbance. Sera were considered to be positive for anti-IFI16 antibodies if their log-transformed calibrated OD value was >2 SD above the mean log-transformed OD of the 47 healthy controls.
Centromere ELISA
This was performed using a commercially available kit, per the manufacturer’s protocol (Inova Diagnostics).
Statistical analysis
Variables were log transformed when a non-normal distribution was apparent. In the first cross-sectional analysis (discovery cohort), the evaluation for associations between dichotomous variables was done using Chi-square or Fischer’s exact tests. T-tests were used for parametric data to evaluate for significant differences in the means of continuous variables between two groups. In a subset of scleroderma patients with the highest anti-IFI16 antibody titers who had longitudinal sera available, we evaluated whether IFI16 autoantibody levels over time associated with Raynaud’s severity as assessed by the patient-reported scleroderma Health Assessment Questionnaire (sHAQ) using regression analysis (14). The correlation of repeated measurements in the same patient was taken into account.
In the matched case-control analysis, we determined the association of anti-IFI16 antibodies with severity of scleroderma complications using a 1:1 case:control matched study design. For cases, we included all patients with scleroderma from our database who had a history of digital gangrene based on the Medsger Raynaud’s severity score of 4 and an available serum sample drawn +/− 6 months from the ischemic event. Anti-IFI16 antibody positivity and levels were compared in cases and disease control sera (patients with scleroderma and a maximum Medsger Raynaud’s severity score of 1). The control samples were matched to those of cases based on disease duration (defined by earliest symptom, either RP or non RP) at the time of the serum draw using Stata, version 11 (StataCorp, College Station, TX). For every case sample, one control sample with the nearest disease duration was chosen. To examine whether anti-IFI16 antibody levels may peak at the time of the digital gangrene event, a second group of patients with scleroderma and digital gangrene was selected based on availability of serum drawn within 6–24 months of the ischemic event.
The associations between two dichotomous variables in the matched case-control study were analyzed using the McNemar’s test. Paired t-tests were used to evaluate the differences between means of parametric continuous variables in cases and controls. The non-parametric sign test was used to evaluate the difference between two groups for non-normally distributed continuous variables. The dependence of digital gangrene on the risk factors considered was evaluated using univariate conditional logistic regression. Significance was tested using the regression coefficients, and the association between risk factors and outcome was expressed as the odds ratio (OR) and the corresponding 95% confidence interval (95% CI), or as a P value (considered significant when ≤ 0.05). A multivariable conditional logistic regression model was then constructed based on the use of statistically significant covariates from the simple conditional logistic regression as well as clinically relevant covariates which were fixed in the model. We evaluated the final model for collinearity and no variables were removed. We then compared antibody levels in anti-IFI16 positive patients with digital gangrene from the case-control study (who had blood drawn +/− 6 month from the event (n=20/39)) to antibody levels from anti-IFI16 positive patients who had blood drawn within 6–24 months of the event (n=10/23) using the Wilcoxon-Mann-Whitney test.
In our initial analysis, we did not detect an association between digital gangrene and anti-centromere antibody status. Because this may reflect variability in assay methodology from clinically obtained test results, we systematically performed anti-centromere antibody ELISA’s as detailed above. We then looked at the association between digital gangrene (dependent variable) and the presence of anti-centromere autoantibodies using univariate and multivariate conditional logistic regression. Furthermore, we examined whether the association between digital gangrene and IFI16 antibody status varied by anti-centromere antibody status by modeling the interaction between these two antibodies.
RESULTS
Association of anti-IFI16 antibodies with scleroderma disease characteristics
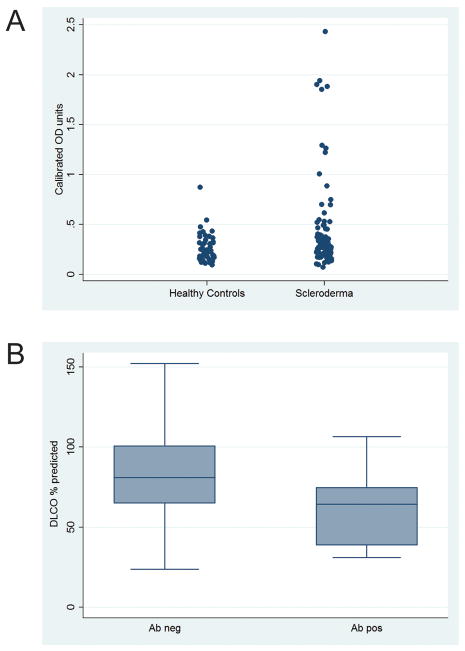
Sera from 94 patients with scleroderma who met ACR or CREST criteria and 47 healthy controls were assayed for anti-IFI16 antibodies by ELISA. Anti-IFI16 antibodies were detected in 17/94 (18%) patients with scleroderma compared to 1/47 (2%) healthy controls (p=0.01) (Figure 1A). Table 1 summarizes the associations between anti-IFI16 antibody positivity and the variables studied in the scleroderma group. Of the 17 anti-IFI16 antibody positive patients, 14/17 (82%) were female, 11/17 (65%) were Caucasian, and 6/17 (35%) were African American. Seven out of seventeen (41%) had smoked at some point in their lifetime. Compared to patients who were anti-IFI16 negative, patients with anti-IFI16 antibodies were more likely to have limited scleroderma (77% vs. 46%; p = 0.03), a longer disease duration (15.2 [10.6–18.3] vs. 6.0 [3.4–13.8] years; p <0.01), severe Raynaud’s as manifested by digital gangrene (24% vs 4%; p=0.02), and a low DLCO (60 ± 23 vs. 81 ±27; p <0.01) (Figure 1B). Forced vital capacity was similar in both groups (75% vs. 81%; p=0.33), and pulmonary involvement as measured by the Medsger severity scale was also comparable between groups (94% vs. 80%; p=0.29). Patients with anti-IFI16 autoantibodies did not have a significantly increased risk of pulmonary hypertension defined by right heart catheterization, though the number of patients with PAH in this group was small (Table 1).
Figure 1. Anti-IFI16 antibody levels are significantly higher in scleroderma patients compared to healthy controls, and associate with a lower DLCO.
(A) Anti-IFI16 antibody levels in patients with scleroderma and healthy controls were assayed by ELISA. Calibrated optical density levels are significantly higher (p=0.01) in patients with scleroderma than healthy controls (n=94 scleroderma, n=47 controls). (B) Amongst scleroderma patients, DLCO is significantly lower (p<0.01) in anti-IFI16 antibody positive patients compared to those without this specificity (antibody positive, n = 17; antibody negative, n = 77).
Table 1.
Demographic and disease characteristics of the 94 scleroderma patients in the discovery study. Patients are grouped according to the presence or absence of anti-IFI16 antibodies.
| Variable | Negative (n=77) | Positive (n=17) | P-value |
|---|---|---|---|
| Age, year (range 30–90 ) | 55.8 ± 12.4 | 61.0 ± 15.1 | 0.13 |
| Female, % | 61 (79.2) | 14 (82.4) | 1.00 |
| Race, % | |||
| Caucasian | 64 (83.1) | 11 (64.7) | 0.13 |
| African American | 11(14.3) | 6 (35.3) | |
| Other | 2 (2.6) | 0 (0) | |
| Ever smoker, % | 31 (40.3) | 7 (41.2) | 0.94 |
| Scleroderma type, limited, % | 35 (45.5) | 13 (76.5) | 0.03 |
| Disease duration, yrs (range 0–51) *┼ | 6.0 (3.4–13.8) | 15.2 (10.6–18.3) | <0.01 |
| Rodnan skin score, modified (range 2–45) ┼ | 13.0 (5.0–26.0) | 7.0 (5.0–12.0) | 0.14 |
| Severe GI (≥ 3)^, % | 12 (15.6) | 1 (5.9) | 0.45 |
| Severe Raynaud’s (> 3)^, % | 3 (3.9) | 4 (23.5) | 0.02 |
| Lung involvement (≥1)^, % | 62 (80.5) | 16 (94.1) | 0.29 |
| Cardiac involvement (≥1)^, % | 27 (35.1) | 9 (52.9) | 0.17 |
| Tendon friction rub, % | 26 (33.8) | 4 (23.5) | 0.57 |
| Kidney involvement, % | 18 (23.4) | 5 (29.4) | 0.60 |
| Sicca complex, % | 54 (70.1) | 15 (88.2) | 0.22 |
| Pulmonary function | |||
| FVC, %predicted | 80.7 ± 20.8 (n=76) | 75.2 ± 21.6 (n=17) | 0.33 |
| DLCO, %predicted | 81.4 ± 26.5 (n=71) | 60.3 ± 22.7 (n=16) | <0.01 |
| RVSP, mmHg┼ | 32.5 (28.0–40.0) (n=58) | 36 (30.5–57.0) (n=12) | 0.23 |
| Pulmonary arterial hypertension, % | 5/19 (26.3) | 5/8 (62.5) | 0.10 |
| Autoantibodies, % | |||
| Anti-topoisomerase-1 | 20/76 (26.3) | 5/16 (31.3) | 0.69 |
| Anti-centromere | 15 (19.5) | 3 (17.7) | 1.00 |
| Anti-RNP | 8/74 (10.8) | 0/16 (0) | 0.34 |
| Anti-RNA polymerase 3 | 21/70 (30) | 4/13 (30.8) | 1.00 |
Time since first symptom (Raynaud’s or non-Raynaud’s);
Median (IQR);
cutoffs as per the Medsger severity scale
Chi-squared or Fischer’s exact used on categorical data; ttest used on parametric continuous data;
Wilcoxin-Mann-Whitney test is used on non-parametric continuous data
Of the anti-IFI16 positive scleroderma patients, 5/16 (31%) also had anti-topoisomerase-1 antibodies, 3/17 (18%) were anti-centromere positive, 4/13 (31%) were anti-RNA polymerase III positive, and none were positive for anti-RNP. The distribution of coexisting antibodies between those with and without anti-IFI16 antibodies was not significantly different.
Because there was an association between anti-IFI16 antibodies and severe digital ischemia, we performed a small pilot study to explore whether anti-IFI16 antibody levels changed over time and associated with patient-reported Raynaud’s severity as assessed by the sHAQ. Banked longitudinal serum samples were available for 5 high titer anti-IFI16 antibody positive patients (>5 samples/patient). In the longitudinal analysis of sera from these 5 patients, we found a significant association between antibody levels and Raynaud’s severity (β coeff 0.16, 95%CI 0.02, 0.3; p = 0.02).
IFI16 antibodies are associated with digital gangrene
Since the association between anti-IFI16 antibody positivity and digital gangrene in the initial cross-sectional analysis was made on a small number of patients with digital gangrene (n=7), we subsequently designed a matched case-control study to enrich for the outcome of interest (digital gangrene) while controlling for scleroderma disease duration to confirm this association. In addition, as the longitudinal analysis from the first cross-sectional study suggested that high anti-IFI16 antibody levels may correlate with clinically worsening Raynaud’s, we considered this element in the study design. After matching, the disease duration in cases and controls was not significantly different (7.81 yrs [3.9–17.0] vs 7.79 yrs [2.8 – 16.5] (p= 0.85). The difference in disease duration among pairs was a median of 0.09 years (−4.0 – 1.10) (see Table 2).
Table 2.
Clinical characteristics of cases and controls in the confirmation study
| Variable | Digital gangrene (n=39) | Raynaud’s alone (n=39) | P-value |
|---|---|---|---|
| Age, year (range 30–90 ), mean ± SD | 54.6 ± 15.0 | 56.3 ±12.2 | 0.58 |
| Female, % | 72 | 79 | 0.61 |
| Race/Ethnicity, % | |||
| White | 64 | 79 | 0.21 |
| Ever Smoker, % | 50 | 39 | 0.35 |
| Scleroderma type, % | |||
| Limited | 69 | 67 | 1.00 |
| Disease duration from Raynaud’s, yrs (0–66); Median (IQR) | 9.88 (5.8–22.4) | 10.4 (4.6–21.4) | 0.87 |
| Disease duration from 1st yrs symptom, *α(0–56); Median (IQR) | 7.81 (3.9–17.0) | 7.79 (2.8–16.5) | 0.85 |
| Difference in disease duration among pairs (yrs) | Reference | 0.09 (−4.0 to 1.10) | n/a |
| Modified Rodnan skin score (range 0–45);Median (IQR) | 9 (4–17) | 5 (2–16) | 0.18 |
| Severe GI (≥3)^, % | 56 | 44 | 0.23 |
| Lung involvement (≥1)^, % | 85 | 77 | 0.61 |
| Cardiac involvement (≥1)^, % | 41 | 28 | 0.33 |
| Kidney involvement, % | 21 | 18 | 1.00 |
| Sicca complex, % | 62 | 72 | 0.39 |
| Pulmonary function | |||
| FVC, %predicted | 63.6 ± 20.8 (n=34) | 74.9 ± 19.4 (n=34) | 0.05 |
| DLCO, %predicted | 52.2 ± 20.1 (n=31) | 66.6 ± 22.7 (n=31) | |
| Autoantibodies, % | |||
| Anti-topoisomerase-1, n (%) | 11/30 (37) | 5/37 (14) | 0.09 |
| Anti-centromere, n (%) | 11/33 (33) | 12/37 (32) | 1.00 |
| Anti-RNP, n (%) | 4/29 (14) | 4/32 (13) | 1.00 |
| IFI16 antibody level, median (IQR) | 0.59 (0.30–1.07) | 0.47 (0.29–0.77) | 0.02 |
| % positive | 51 | 38 | 0.49 |
Time since first symptom (Raynaud’s or non-Raynaud’s);
cutoffs as per the Medsger severity scale;
patients were matched on this variable
McNemar’s test used on categorical data; paired ttest used on parametric continuous data;
The sign test is used on non-parametric continuous data
All demographic characteristics including age, gender, race, smoking status, disease subtype, and antibody status were not different between those with and without digital gangrene in the confirmatory study (Table 2). Presence of lung, cardiac, renal involvement and sicca complex also did not differ between groups. Mortality was greater in patients with digital gangrene than controls (46% vs. 13%, p=0.01). Though the numbers were small, there was no significant difference in the distribution of cardiac disease in cases (p=0.09) and controls (p = 0.51) that were alive vs. dead. Among patients with digital gangrene with available data on coexisting antibodies, 11/33(33%) were anti-centromere positive, 11/30 (37%) were positive for anti-topoisomerase-1, 4/29 (14%) were positive for anti-RNP antibodies, and none had anti-RNA polymerase III antibodies. Seventeen out of 39 patients (44%) had documented digital ulcers preceding the gangrenous event. DLCO was significantly lower in cases with digital gangrene relative to controls (52% vs. 67 % predicted; p = 0.02).
The prevalence of scleroderma patients with anti-IFI16 antibodies was significantly higher in the confirmatory study (45%) compared to the discovery sample (18%) (p<0.01). This was consistent with an enrichment of patients with scleroderma having an outcome of digital gangrene in the confirmatory study (39/78 (50%) compared to 7/94 (7%) of the discovery sample). A comparison of anti-IFI16 antibody levels in cases and controls using the nonparametric sign test confirmed significantly higher anti-IFI16 antibody levels in cases compared to matched controls (p=0.02) (Figure 2). To further evaluate the association of digital gangrene and other relevant scleroderma characteristics that were normally distributed, we used univariate conditional logistic regression. Every 10% lower percent predicted DLCO was associated with a 32% higher odds of having digital gangrene (OR 1.32; 95% CI 1.0, 1.7; p=0.03). There were no significant associations between any other clinical or demographic variables and digital gangrene. We subsequently constructed a multivariable model examining the relationship between digital gangrene and anti-IFI16 antibody levels, adjusted for DLCO and other clinically relevant covariates (age, smoking, and limited vs diffuse cutaneous subtype). In this model, there was a 2.3 times higher odds of having digital gangrene for each unit increase in anti-IFI16 antibodies (adjusted OR 2.3, 95% CI 1.0, 5.6; p = 0.05) (Supplemental Table 1). To further examine the relationship between anti-IFI16 antibody levels and digital gangrene, we studied three different cutoffs for defining anti-IFI16 positivity: 2, 3 and 4 standard deviations (SD) from the mean OD of normal controls (Table 3). When using 2 SD as the cutoff for anti-IFI16 antibody positivity, the adjusted odds ratio of developing digital gangrene was 4.2 (95% CI 1.0, 18.0; p = 0.06). Using 3 SD as the cutoff for anti-IFI16 antibody positivity, the adjusted odds ratio of developing digital gangrene was 7.7 (95% CI 1.2, 48.3; p= 0.03) and when using 4 SD, the adjusted OR was 10.5 (95% CI 1.0, 108.7; p= 0.05). Thus, patients with higher levels of anti-IFI16 antibodies are more likely to have digital gangrene.
Figure 2. Anti-IFI16 antibody levels in cases and controls.
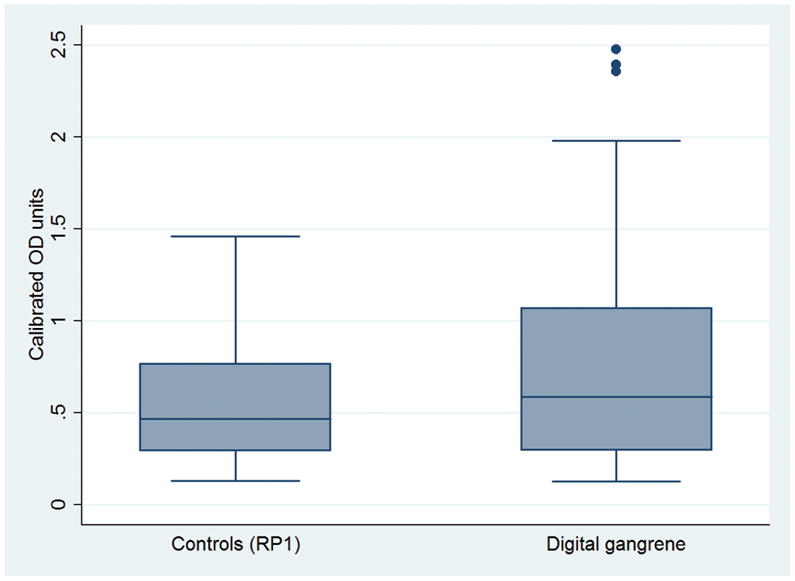
Anti-IFI16 antibody levels are significantly higher in patients with scleroderma and digital gangrene (n=39) than in scleroderma controls with Raynaud’s and no history of digital ischemic events (maximum Raynaud’s Medsger severity score of 1) (n=39) (p=0.02).
Table 3.
Higher anti-IFI16 antibody level cutoffs are associated with higher odds of digital gangrene
| Anti-IFI16 antibody positive status cutoffs using increasing SD from the mean of normal controls | Adjusted OR* | p-value | 95% CI |
|---|---|---|---|
| 2 | 4.2 | 0.06 | 1.0, 18.0 |
| 3 | 7.7 | 0.03 | 1.2, 48.3 |
| 4 | 10.5 | 0.05 | 1.0, 108.7 |
multivariable model adjusted for age, cutaneous subtype, DLCO, and smoking status; matched on disease duration; different standard deviations (SD) are used to define anti-IFI16 antibody positivity in each model
Anti-IFI16 antibody levels are highest within 6 months of the ischemic event
To determine whether anti-IFI16 antibody levels are highest in patients with blood drawn nearest the event, we selected additional patients with scleroderma and associated digital gangrene who had blood drawn 6–24 months from the time of the ischemic event (n=23). Anti-IFI16 antibody levels in positive patients from this group (n=10/23) were compared to those in anti-IFI16 positive patients with digital gangrene (from the case-control study) who had blood drawn within 6 months of the event (n=20/39). We found that anti-IFI16 antibody levels were significantly higher closer to the ischemic event (z= −2.2, p = 0.03) (Figure 3).
Figure 3. Anti-IFI16 antibody levels are highest in patient sera drawn within 6 months of the event.
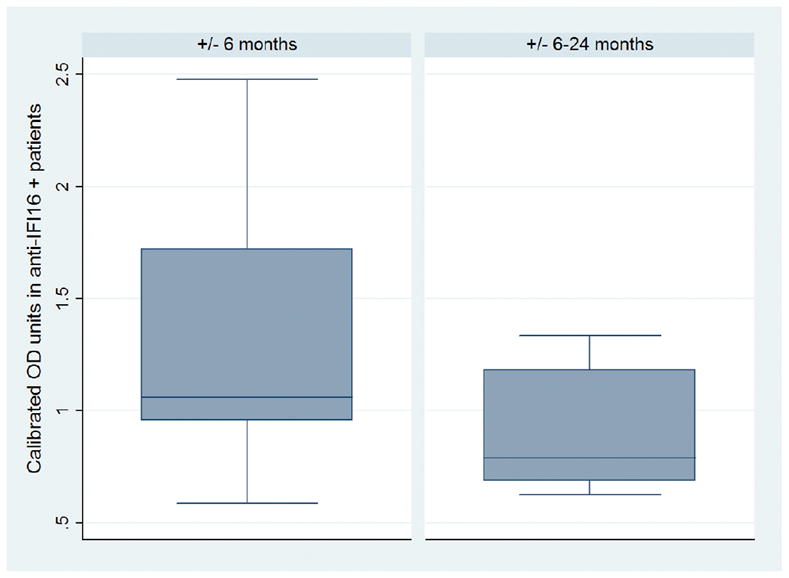
Mean anti-IFI16 antibody levels in patients with digital gangrene who are positive for anti-IFI16 antibodies (n=30/62) are shown. Patients were binned by the time interval between serum draw and clinical visit for digital gangrene. Anti-IFI16 antibody levels are higher in scleroderma patients with digital gangrene and blood drawn within 6 months of the event (n=20/39) compared to levels in patients who had blood drawn within 6–24 months of the event (n= 10/23).
Sensitivity Analyses
Because it is well established in the literature that anti-centromere antibodies associate with digital loss in scleroderma (15, 16), we were surprised to find that there was no association in our data sets between the presence of anti-centromere autoantibodies and digital gangrene. As the anti-centromere antibody status in our database was obtained from patient clinical records, we attributed this discrepancy to variations in testing across different laboratories. We therefore reassessed anti-centromere antibody status in all of the cases and controls by performing anti-centromere ELISA assays. When examining for anti-centromere positivity, we found a significant association with the outcome of digital gangrene in the univariate model (OR 3.0, 95% CI 1.3, 7.1; p = 0.01), and a significant association in the multivariable model (OR 4.8, 95% CI 1.1, 21.3; p = 0.04). However, the association between digital gangrene and anti-IFI16 antibody status did not vary by anti-centromere antibody status (data not shown). It was interesting, however, that the prevalence of patients positive for both antibodies was higher in cases (n=20/62) than in controls (n=4/62) (p <0.01).
We then did a second sensitivity analysis to evaluate whether the association between anti-IFI16 antibodies and digital gangrene, was influenced by patients with co-existing risk factors for macrovascular disease. In this analysis we excluded 5 pairs with lower extremity gangrene who had coexisting macrovascular disease. We found that excluding this patient subset had no effect on the statistically significant association between anti-IFI16 antibody levels and digital gangrene (p=0.02).
DISCUSSION
This study evaluated the association of anti-IFI16 antibodies with clinical features of scleroderma. Our initial exploratory study assessed anti-IFI16 antibodies in 94 patients with scleroderma and revealed an association between anti-IFI16 antibodies and digital gangrene. In a small longitudinal analysis performed on sera from 5 anti-IFI16 positive patients identified in the exploratory group, we found that anti-IFI16 antibody levels correlated temporally with symptoms of worsening Raynaud’s. We therefore designed a confirmatory study using a case-control study design with 78 patients, 39 of whom had digital gangrene with a banked serum sample available that was taken within 6 months of the ischemic event. In this second group, we confirmed the association between anti-IFI16 antibodies and digital gangrene, after adjusting for confounders. We also determined that anti-IFI16 antibody positive patients with scleroderma have a significantly lower DLCO (a potential measure of pulmonary vascular disease), but no significant difference in FVC, than those without this specificity (Table 1). In aggregate, these findings suggest that anti-IFI16 antibodies may be markers of systemic vascular disease in scleroderma.
The association of anti-IFI16 antibody levels with digital gangrene, combined with the observation that those levels are highest in patient samples drawn near the time of the ischemic event, suggests that the anti-IFI16 immune response and digital ischemia might be related mechanistically, either proximally or distally. For example, IFI16 is known to be prominently expressed in vascular endothelial cells (9, 17), and is upregulated by oxidative stress (a likely feature of severe vasospasm in Raynaud’s phenomenon (18)). It is therefore possible that during vascular injury, IFI16 becomes available to drive the immune response, and higher antibody levels in the serum reflect this injury. Alternatively, anti-IFI16 antibodies or other IFI16-directed immune effector pathways (e.g. cytotoxic T lymphocytes) may themselves participate in injuring endothelial cells. A growing body of data suggests that some autoantibodies can penetrate the cell membrane of live cells, translocate into the subcellular compartments containing the associated autoantigen, and induce cellular dysfunction or death (19, 20). It will be of interest to determine whether anti-IFI16 autoantibodies have this capacity, as well as whether IFI16 reactive CD8 cells have cytotoxic effects against endothelial cells.
Our study suggests that anti-IFI16 antibody levels may be more clinically relevant than the anti-IFI16 antibody positive/negative status. While the percentage of patients who tested positive for anti-IFI16 antibodies was higher in the patients with severe digital ischemia than in controls, the significant difference between groups was observed when comparing autoantibody levels. This difference was likely driven by the patients with particularly high antibody titers in the group with digital gangrene, as represented by the higher interquartile range of antibody levels in cases with digital gangrene relative to controls (see Table 2). Indeed, patients with the highest antibody levels, which were 3 and 4 standard deviations above the means of normal controls, appear to have the highest odds of digital gangrene, even after adjusting for other clinically relevant covariates (eg, age, limited versus diffuse disease subtype, smoking, and DLCO) (Table 3). It will be interesting to determine in future longitudinal studies whether absolute autoantibody levels or the change in antibody levels over time have utility to predict incipient digital ischemia.
Importantly, only a subset of patients with scleroderma develops severe digital ischemia in the form of digital gangrene during the course of their disease. A survey of the Johns Hopkins Scleroderma Center database showed that 337/3224 (~10%) of patients with scleroderma had a history of digital gangrene. Others have reported digital loss in patients with scleroderma at a prevalence of 16–20% (16, 21). Digital gangrene in scleroderma has significant consequences for patients including pain, tissue loss, risk for hospitalization, and long term disability (3, 22). It is widely accepted that a subset of patients with autoantibodies targeting centromere are at particularly high risk for digital loss (16). Our data, and data from prior studies demonstrate that a significant proportion of patients with anti-centromere antibodies have coexisting anti-IFI16 autoantibodies (31% in the discovery cohort)(6). While we did not see an association between digital gangrene and anti-IFI16 autoantibodies change by anti-centromere antibody status, the high prevalence of patients positive for both antibodies in the cases suggests that further exploration of this association may be warranted.
Studies to date have not demonstrated an association between anti-centromere autoantibody titers and measures of disease activity, greatly limiting their use in real-time to predict incipient digital vascular events (23). However, our data does suggest that anti-IFI16 antibodies may have a role as a novel longitudinal biomarker if levels do indeed change over time and correlate with disease activity. Additional studies exploring the clinical utility of assessing longitudinal anti-IFI16 antibody titers within individuals and across populations will be important.
Anti-IFI16 autoantibodies have been previously described in scleroderma. Other investigators (5, 6) have reported a 20–30% prevalence of this specificity among patients, and our findings in the discovery sample are consistent with this. Associations between anti-IFI16 antibodies and Raynaud’s-associated complications of scleroderma have not been previously reported (5, 6), perhaps because digital gangrene is a rare event, and a cohort of scleroderma all-comers might be underpowered to detect such an association. Additionally, our data suggests that anti-IFI16 antibody levels may change over time, peaking around the time of the digital gangrene event. By seeking to determine whether the relationship between anti-IFI16 antibodies and digital gangrene was more striking at the time nearest to the event, we were able to detect this association, whereas it would otherwise have been difficult.
These findings are limited by the retrospective study design, which inevitably led to some missing data, including data related to other coexisting antibodies. As noted above, the cross-sectional nature of this study limits our ability to definitively determine longitudinal anti-IFI16 antibody trends in an individual or across the population. Confirming these findings in a longitudinal prospective study will be an important next step in understanding the utility of anti-IFI16 antibodies as markers of systemic vascular disease in scleroderma.
Supplementary Material
Acknowledgments
Sources of Financial support: Dr. McMahan’s funding for this project was provided by the Rheumatology Research Foundation, Scientist Development Award, by the Maryland Stem Cell Research Fund, and by the Jerome L. Greene Foundation Scholar Award. Dr. Casciola-Rosen’s work was supported by the NIH (R56-AR-062615 and P30-AR-053503). Drs Rosen and Casciola-Rosen are supported by NIH grant DE-12354. Dr. Shah’s work was supported by the NIH/NIAMS (K23 AR061439). Dr. Wigley receives support from the Scleroderma Research Foundation and the Martha McCrory Professorship. Dr. Vaidya is supported by the Johns Hopkins Center for Child and Community Health Research.
We thank Adrianne Woods and Margaret Sampedro for their involvement in the dataset generation and sample acquisition.
Footnotes
Conflicts of interest: DV declares a conflict of interest as he is a consultant for Consumable Science, Inc. None of the other authors has received any financial support or other benefits from commercial sources for the work reported in this manuscript, nor do any of the other authors have any financial interests which could create a potential conflict of interest, or the appearance thereof.
References
- 1.Wigley FM. Clinical practice. Raynaud's Phenomenon. N Engl J Med. 2002 Sep 26;347(13):1001–8. doi: 10.1056/NEJMcp013013. [DOI] [PubMed] [Google Scholar]
- 2.Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med. 2009 May 7;360(19):1989–2003. doi: 10.1056/NEJMra0806188. [DOI] [PubMed] [Google Scholar]
- 3.Steen V, Denton CP, Pope JE, Matucci-Cerinic M. Digital ulcers: overt vascular disease in systemic sclerosis. Rheumatology (Oxford) 2009 Jun;48(Suppl 3):iii19–24. doi: 10.1093/rheumatology/kep105. [DOI] [PubMed] [Google Scholar]
- 4.Veeranki S, Choubey D. Interferon-inducible p200-family protein IFI16, an innate immune sensor for cytosolic and nuclear double-stranded DNA: regulation of subcellular localization. Mol Immunol. 2012 Jan;49(4):567–71. doi: 10.1016/j.molimm.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mondini M, Vidali M, De Andrea M, Azzimonti B, Airo P, D'Ambrosio R, et al. A novel autoantigen to differentiate limited cutaneous systemic sclerosis from diffuse cutaneous systemic sclerosis: the interferon-inducible gene IFI16. Arthritis Rheum. 2006 Dec;54(12):3939–44. doi: 10.1002/art.22266. [DOI] [PubMed] [Google Scholar]
- 6.Costa S, Mondini M, Caneparo V, Afeltra A, Airo P, Bellisai F, et al. Detection of anti-IFI16 antibodies by ELISA: clinical and serological associations in systemic sclerosis. Rheumatology (Oxford) 2011 Apr;50(4):674–81. doi: 10.1093/rheumatology/keq372. [DOI] [PubMed] [Google Scholar]
- 7.Wigley FM, Wise RA, Miller R, Needleman BW, Spence RJ. Anticentromere antibody as a predictor of digital ischemic loss in patients with systemic sclerosis. Arthritis Rheum. 1992 Jun;35(6):688–93. doi: 10.1002/art.1780350614. [DOI] [PubMed] [Google Scholar]
- 8.Steen V, Medsger TA., Jr Predictors of isolated pulmonary hypertension in patients with systemic sclerosis and limited cutaneous involvement. Arthritis Rheum. 2003 Feb;48(2):516–22. doi: 10.1002/art.10775. [DOI] [PubMed] [Google Scholar]
- 9.Gariglio M, Azzimonti B, Pagano M, Palestro G, De Andrea M, Valente G, et al. Immunohistochemical expression analysis of the human interferon-inducible gene IFI16, a member of the HIN200 family, not restricted to hematopoietic cells. J Interferon Cytokine Res. 2002 Jul;22(7):815–21. doi: 10.1089/107999002320271413. [DOI] [PubMed] [Google Scholar]
- 10.LeRoy EC, Medsger TA., Jr Criteria for the classification of early systemic sclerosis. J Rheumatol. 2001 Jul;28(7):1573–6. [PubMed] [Google Scholar]
- 11.Preliminary criteria for the classification of systemic sclerosis (scleroderma) Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980 May;23(5):581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 12.Medsger TA, Jr, Silman AJ, Steen VD, Black CM, Akesson A, Bacon PA, et al. A disease severity scale for systemic sclerosis: development and testing. J Rheumatol. 1999 Oct;26(10):2159–67. [PubMed] [Google Scholar]
- 13.Morrone SR, Wang T, Constantoulakis LM, Hooy RM, Delannoy MJ, Sohn J. Cooperative assembly of IFI16 filaments on dsDNA provides insights into host defense strategy. Proc Natl Acad Sci U S A. 2014 Jan 7;111(1):E62–71. doi: 10.1073/pnas.1313577111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steen VD, Medsger TA., Jr The value of the Health Assessment Questionnaire and special patient-generated scales to demonstrate change in systemic sclerosis patients over time. Arthritis Rheum. 1997 Nov;40(11):1984–91. doi: 10.1002/art.1780401110. [DOI] [PubMed] [Google Scholar]
- 15.Herrick AL, Heaney M, Hollis S, Jayson MI. Anticardiolipin, anticentromere and anti-Scl-70 antibodies in patients with systemic sclerosis and severe digital ischaemia. Ann Rheum Dis. 1994 Aug;53(8):540–2. doi: 10.1136/ard.53.8.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wigley FM, Wise RA, Miller R, Needleman BW, Spence RJ. Anticentromere antibody as a predictor of digital ischemic loss in patients with systemic sclerosis. Arthritis Rheum. 1992 Jun;35(6):688–93. doi: 10.1002/art.1780350614. [DOI] [PubMed] [Google Scholar]
- 17.Wei W, Clarke CJ, Somers GR, Cresswell KS, Loveland KA, Trapani JA, et al. Expression of IFI 16 in epithelial cells and lymphoid tissues. Histochem Cell Biol. 2003 Jan;119(1):45–54. doi: 10.1007/s00418-002-0485-0. [DOI] [PubMed] [Google Scholar]
- 18.Gugliesi F, Mondini M, Ravera R, Robotti A, de Andrea M, Gribaudo G, et al. Up-regulation of the interferon-inducible IFI16 gene by oxidative stress triggers p53 transcriptional activity in endothelial cells. J Leukoc Biol. 2005 May;77(5):820–9. doi: 10.1189/jlb.0904507. [DOI] [PubMed] [Google Scholar]
- 19.Deng SX, Hanson E, Sanz I. In vivo cell penetration and intracellular transport of anti-Sm and anti-La autoantibodies. Int Immunol. 2000 Apr;12(4):415–23. doi: 10.1093/intimm/12.4.415. [DOI] [PubMed] [Google Scholar]
- 20.Douglas J, Gardner L, Levin M. Antibodies to an Intracellular Antigen Penetrate Neuronal Cells and Cause Deleterious Effects. J Clin Cell Immunol. 2013;4(1) [Google Scholar]
- 21.Nihtyanova SI, Brough GM, Black CM, Denton CP. Clinical burden of digital vasculopathy in limited and diffuse cutaneous systemic sclerosis. Ann Rheum Dis. 2008 Jan;67(1):120–3. doi: 10.1136/ard.2007.072686. [DOI] [PubMed] [Google Scholar]
- 22.Silva I, Almeida J, Vasconcelos C. A PRISMA-driven systematic review for predictive risk factors of digital ulcers in systemic sclerosis patients. Autoimmun Rev. 2015 Feb;14(2):140–52. doi: 10.1016/j.autrev.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Tramposch HD, Smith CD, Senecal JL, Rothfield N. A long-term longitudinal study of anticentromere antibodies. Arthritis Rheum. 1984 Feb;27(2):121–4. doi: 10.1002/art.1780270201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.