Abstract
Numerous cross-sectional studies have used diffusion tensor imaging (DTI) to link age-related differences in white matter (WM) anisotropy and concomitant decrements in cognitive ability. Due to a dearth of longitudinal evidence, the relationship between changes in diffusion properties of WM and cognitive performance remains unclear. Here we examine the relationship between two-year changes in WM organization and cognitive performance in healthy adults (N = 96, age range at baseline = 18–79 years). We used latent change score models (LCSM) to evaluate changes in age-sensitive cognitive abilities - fluid intelligence and associative memory. WM changes were assessed by fractional anisotropy (FA), axial diffusivity (AD), and radial diffusivity (RD) in WM regions that are considered part of established memory networks and exhibited individual differences in change. In modeling change, we postulated reciprocal paths between baseline measures and change factors, within and between WM and cognition domains, and accounted for individual differences in baseline age. Although baseline cross-sectional memory performance was positively associated with FA and negatively with RD, longitudinal effects told an altogether different story. Independent of age, longitudinal improvements in associative memory were significantly associated with linear reductions in FA and increases in RD. The present findings demonstrate the sensitivity of DTI-derived indices to changes in the brain and cognition and affirm the importance of longitudinal models for evaluating brain-cognition relations.
Keywords: aging, DTI, longitudinal, episodic memory, practice effect
1. Introduction
Cerebral white matter (WM), which constitutes the bulk of brain tissue, exhibits substantial age differences in its volume and structure (Bartzokis 2004; Flechsig, 1901; Kaes, 1907; Paus et al., 2014; Peters, 2002). Alterations in WM structure have been proposed as a neuroanatomical substrate of differences in cognitive performance, and WM deterioration is considered a major contributor to age-related cognitive declines (Bartzokis, 2004; Bennett & Madden, 2014; Gunning-Dixon & Raz, 2000; Rao, 1996; Walhovd et al., 2014). Recent evidence indicates that WM changes can occur over a very short period and can be linked to gains in cognitive and motor performance (Engvig et al., 2012, Hofstetter et al., 2013; Wang et al., 2013). Thus, in vivo evaluation of WM changes and their relation to behavioral measures is of substantial interest for students of lifespan development.
Thanks to the development of magnetic resonance imaging (MRI) many properties of WM can be assessed in vivo, and current MRI methods provide a wealth of information about regional WM volumes, gross structural alterations of WM appearance (WM hyperintensities, WMH), and microstructural properties of water diffusion in cerebral WM. WMH reflect multiple modifications of WM that are largely detrimental to brain function and include ischemic lesions, gliosis, demyelination, and breaches of blood-brain barrier. Because WMH are rarely found in younger persons and have been linked to brain diseases, their interpretation as pathological markers is a matter of consensus (Erten-Lyons, 2013; Fazekas et al., 1998; Kim et al., 2008). The volume and number of WMH increases with age (Raz et al., 2012; Sachdev et al., 2007; Schmidt et al., 2000) and extant literature links elevated WMH burden to age-related cognitive declines (Gunning-Dixon & Raz, 2000; Kloppenborg et al., 2014). As WMH are relatively rare in healthy brains and show relatively slow change over time (Fazekas et al., 1998), they are of limited use for assessment of normative development. Diffusion tensor imaging (DTI) is a more promising approach for the study of healthy brains as it provides several indices that exhibit significant variations in the normal population. DTI yields several indicators of WM properties - fractional anisotropy (FA), mean (MD), axial (AD) and radial (RD) diffusivity – that are computed from the diffusion tensor eigenvalues. The magnitude of DTI-derived indices may be affected by multiple microstructural entities that restrict or hinder water diffusion in WM: axonal size and density, myelin content, size of extracellular spaces, integrity of axonal cytoskeleton as well as fiber geometry and spatial distribution (Beaulieu, 2012; Jones et al., 2013a).
Although the extant literature on age differences in WM diffusion properties is quite sizeable, the understanding of relationships between WM and cognition in healthy persons remains unclear. A prominent reason for such lack of knowledge is the paucity of studies that have examined changes in WM over time, individual differences therein, and associations between alterations in WM microstructural properties and cognition along the adult lifespan. Recent longitudinal DTI studies of WM change have shown differential patterns of decline across fiber tracts and pronounced individual differences in change among multiple WM regions (Bender & Raz, 2015; Lövdén et al., 2014; Pfefferbaum et al., 2015; Sexton et al., 2014; Vik et al., 2015). An important question that has received relatively little consideration is whether individual variations in WM change are related to individual differences in rate of change in cognitive performance. Although longitudinal study designs are optimal for assessing relationships between WM and cognition in adult development, with few exceptions (Charlton et al., 2010; Lövdén et al., 2014; Ritchie et al., 2015), most DTI studies of WM and cognition have relied on cross-sectional designs (see Bennett & Madden, 2014; Madden et al., 2012 for reviews). Thus, elucidating individual differences in relationships between temporal dynamics in WM and cognition – i.e., measures of intra-individual change – may aid in clarifying constraints on adult cognitive and brain plasticity (Brehmer et al., 2014).
Advanced age is associated with decrements across multiple cognitive domains, including speed of perceptual processing, working memory and executive abilities, fluid reasoning and episodic memory (Horn & Cattell, 1967; Lindenberger, 2014; Salthouse, 1996). In the episodic memory domain, age differences maybe particularly strong in cognitive tasks that call for binding information from more than one source (Old & Naveh-Benjamin, 2008; Spencer & Raz, 1995). Although investigations of age-related changes in associative memory are limited by cross-sectional designs, the state of affairs is improving with introduction of latent change score models (LCSM; McArdle & Nesselroade, 1994). LCSMs have been applied to evaluating the relationship between changes in the brain (Bender & Raz, 2015; McArdle et al., 2004; Persson et al., 2014; Raz et al., 2005, 2010) and cognition (Schmiedek et al., 2010; Ghisletta & Lindenberger, 2004) over time. This approach allows examining change, individual differences in change, and associations between rates of change in multiple domains at the latent factor (construct) level, after accounting for measurement error. For example, in a recent study of very old adults, Lövdén et al. (2014) used a bivariate-LCSM approach to demonstrate that changes in perceptual speed in were associated with FA changes in corticospinal tracts. A particularly useful feature of the LCSM is quantification of individual differences in change, an essential requirement for modeling associations with other factors. In contrast, traditional methods for modeling change can only assume that variance in change differs from zero. Thus, the bivariate-LCSM approach can assess whether individual differences in change exist in WM and cognitive factors, and test the relationship between such changes.
One of the most important aspects of research on lifespan changes in brain and behavior is the strong commonality of calendar age and longitudinal trajectories of neural and cognitive change (Salthouse, 2011). Moreover, although longitudinal investigations of aging offer the only means by which to study individual differences in change over time (Raz & Lindenberger, 2011), change in cognitive performance is contaminated by retest effects (Salthouse, 2010). Repeated testing may boost performance by mere exposure to test format and content, and may obscure age-related declines (Salthouse & Tucker-Drob, 2008). In addition, repeated testing of cognitive abilities with the same instruments may impose the homogeneity of change within a sample. Such forced uniformity precludes evaluating the determinants of individual variance in change, including age, sex, and health characteristics, such as vascular risk (VR) factors (Raz et al., 2008; Bender et al., 2013).
Just as chronological age often contains variance that is common with brain and cognitive measures, so too may VR markers, such as hypertension and various indices associated with metabolic syndrome (Bender & Raz, 2013, 2015; Raz et al., 2005; 2008; Qui & Fratiglioni, 2015). Thus, modeling relations between WM properties and cognition in healthy adults should account for the influences of such markers. An additional complication in modeling age-related change in brain and behavior is the link between VR factors and WMH burden, which in turn affects WM indices such as FA via partial voluming (Vos et al., 2011; Zhan et al., 2009), and may influence the associations between the brain properties and cognitive performance (Maillard et al., 2013; Maniega et al., 2015; Vernooij et al., 2008). Therefore, in studies of the aging brain and cognition, it is desirable not only to take into account VR factors but also to limit WM sampling to regions without WMH or cerebrospinal fluid (CSF).
Formulating hypotheses linking diffusion properties of specific WM regions to specific cognitive functions is particularly challenging in light of the substantial heterogeneity in methods and findings across DTI studies of aging. Considerable evidence suggests that multiple anatomically distinct WM regions may be best described by a single common latent factor (Lövdén et al., 2013; Penke et al., 2010; Ritchie et al., 2015; see Bennett & Madden, 2014; Madden et al., 2012 for reviews). Executive function, speeded performance, fluid reasoning, and episodic memory all appear associated with diffusion properties of multiple WM pathways, although association and projection fiber tracts connecting prefrontal with parietal, temporal, and subcortical regions appear particularly strongly implicated in working memory and episodic memory (Kennedy & Raz, 2009; Lockhart et al., 2012; see Bennett & Madden, 2014; Madden et al., 2012 for reviews). Unfortunately, cross-sectional studies are of limited use in examining mediators of age-related change in brain and cognition (Maxwell & Cole, 2007), and the dearth of longitudinal findings limits the confidence in formulating highly specific a priori hypotheses regarding the role of regional WM changes in alterations in specific cognitive abilities.
The present study was designed to address most of the outlined limitations of the extant literature by examining bi-directional influences between normal-appearing WM microstructure and cognitive performance in a sample of healthy participants covering the adult lifespan. We used a latent variable modeling approach to examine the relationship between DTI indices of WM microstructure (FA, RD, and AD) and change in cognitive abilities. Our goals were to determine whether two-year changes in WM were related to concurrent changes in cognition, and to gauge the associations between cognition and WM properties at baseline, as well as their connection to change in both domains over time. We hypothesized that the WM regions that have been linked to the cognitive constructs assessed in this study and have shown significant variability in change in this sample (Bender & Raz, 2015) would be associated with changes in cognitive performance.
2. Methods
2.1 Participants
The present study was based on the same sample as Bender & Raz (2015), where detailed information on longitudinal attrition and missingness analysis, as well as specific reasons for exclusions based on findings at either wave can be found. In accordance with the guidelines for human subject research established by the University Institutional Review Board and the Declaration of Helsinki, all participants provided written informed consent at each occasion of measurement. At both assessment occasions, the participants were screened via a questionnaire to rule out depressed state (CES-D; Radloff, 1977; cut-off = 15). At each wave, an experimenter administered the Mini Mental Status Examination (MMSE; Folstein, Folstein, & McHugh, 1975; cut-off = 26) to screen for cognitive impairment. The experimenters screened all participants for near, far, and color vision problems (Optec 2000 Vision Tester, Stereo Optical Co., Inc., Chicago, IL) and speech-range hearing deficits (MA27 Screening Audiometer, Maico Diagnostics, Eden Prairie, MN). All participants scored above 75% on the Edinburgh Handedness Questionnaire (Oldfield, 1975), indicating right-hand dominance across the sample. A trained experimenter measured each participant’s blood pressure on three separate visits for each of the two measurement occasions. Blood pressure was measured from the left arm using an auscultatory method using diastole phase V for identification of diastolic pressure (Pickering et al., 2004), and measurements were averaged across days for each wave of the study. Participants who reported any history of neurological and psychiatric disorders, cardiovascular disease other than physician-diagnosed and medically treated essential hypertension, diagnosis or treatment for metabolic or endocrine disorders, head injury accompanied by loss of consciousness for more than five min, use of anxiolytic, antidepressant, or antiepileptic medications, or consumption of more than three alcoholic beverages per day at either wave were excluded from the study.
The sample consisted of 96 participants, including 66 women and 30 men, whose age at baseline assessment ranged from 19 to 78 years (Table 1). Men and women did not differ with regard to mean age, MMSE scores, self-reported years of education, self-reported engagement in regular exercise and frequency of exercise, or body mass index (BMI). However, men had significantly higher diastolic blood pressure, greater proportion of persons with treated hypertension, and marginally higher systolic blood pressure than women. In addition, women had one month longer delay on average between MRI scans than men. In addition to both occasions of DTI data, all participants had at least one occasion of each cognitive and VR measurement. At baseline, the sample included seven participants aged 18 to 30 years, four participants from 31 to 40 years of age, 24 participants aged 41 to 50 years, 27 participants aged 51 to 60 years, 19 participants 61 to 70 years old, and 15 participants from 71 to 78 years of age (Figure S1).
Table 1.
Participant characteristics
| Variable | Women | Men | t or χ2 a | p |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| Age (years) | 54.59 (13.44) | 55.3 (14.12) | −0.236 | .814 |
| Delay (months) | 25.44 (2.13) | 24.54 (2.12) | 1.932 | .056 |
| MMSE | 29.03 (0.99) | 28.87 (0.97) | 0.754 | .453 |
| Education | 15.73 (2.23) | 15.47 (2.58) | 0.505 | .615 |
| Systolic BP | 120.24 (12.75) | 125.41 (11.20) | −1.912 | .059 |
| Diastolic BP | 73.86 (6.64) | 77.81 (8.56) | −2.461 | .016 |
| % Exercise | 78.8% | 83.3% | 0.268a | .604 |
| Days Exercise | 3.17 (2.14) | 3.83 (2.19) | −1.406 | .163 |
| BMI | 3.26 (0.21) | 3.33 (0.16) | −1.689 | .095 |
| % HBP Dx | 15.15% | 30.00% | 4.134 a | .042 |
Notes: BP = blood pressure; BMI = body mass index; Dx = diagnosis.
a single degree of freedom chi-square test.
% Exercise = proportion of sample reporting regular exercise. BMI = body mass index; % HBP Dx = proportion of sample reporting physician diagnosed and treated hypertension at baseline.
Vascular and Metabolic risk (VR) Assessment
The Detroit Medical Center hospital laboratory analyzed blood samples collected by a trained phlebotomist from participants following a 12-hour overnight fast. Hospital laboratory staff employed a direct cholesterol oxidase/cholesterol esterase method to measure triglyceride levels. Laboratory staff measured whole blood glucose levels by the standard enzymatic glucose oxidase method. Blood glucose levels cut-offs were: 70 mg/dl (3.9 mmol/L) > 126 mg/dl (7.0 mmol/L). At the time of blood sampling a trained research staff member measured participant waist and hip circumference using a fabric measuring tape, and these values were used to calculate waist-to-hip ratio.
2.2 MRI imaging
2.2.1 DTI Acquisition
MRI images were originally acquired as part of a longer protocol, using a Bruker MedSpec 4T scanner equipped with an 8-channel head coil. A 2-D echo planar diffusion-weighted sequence acquired images with the following parameters: TR = 4900 ms; TE = 79 ms; 41 slices; slice thickness = 3 mm; distance factor = 0; FOV = 256 mm; matrix = 128 × 128; voxel size = 2.0 × 2.0 × 3.0 mm; generalized autocalibrating partially parallel acquisition acceleration (GRAPPA) factor = 2; number of excitations = 10. Diffusion weighted data were collected in six orthogonal gradient directions using a diffusion weighting of 800 s/mm2; an additional T2-weighted image was collected without diffusion weighting (b0 = 0 s/mm2).
2.2.2 DTI processing
A custom DTI processing pipeline included pre-processing, longitudinal registration, masking of WMH and cerebrospinal fluid (CSF), WM skeletonization, atlas-based, skeletonized region of interest (ROI) mask creation, native space deprojection and sampling. The pipeline was implemented using software tools available in the FMRIB Software Library (FSL; Jenkinson et al., 2012) v5.0.2 (Analysis Group, FMRIB, Oxford, UK). A complete description of the pipeline is provided in the supplemental material published with Bender & Raz (2015). Here, we provide an overview of the procedures employed for DTI processing and sampling. The pipeline combined traditional DTI pre-processing, diffeomorphic registration, tract-based spatial statistics (TBSS; Smith et al., 2006) procedures for skeletonisation, and probabilistic WM atlases to generate regions of interest (ROIs) on the WM skeleton.
We first extracted and linearly registered the non-diffusion-weighted b0 images from the two occasions, and calculated the intermediate space between the two images for each subject. Native space diffusion tensor estimation retained the tensor components, which were subsequently rotated and refitted in the halfway space between occasions. The resulting FA maps were subsequently used in the TBSS processing framework to create a group-wise WM skeleton mask in standard space. TBSS processing employed the FMRIB58_FA standard space image as the target for non-linear registration, and the final TBSS processing step used a threshold of 0.3 to limit inclusion of regions with poor reliability. Following skeletonization, the tbss_deproject routine was applied to the mean WM skeleton mask, as well as to the JHU-ICBM white matter atlas labels at 1mm, and the JHU-ICBM white matter tractography atlas (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases; Mori et al., 2005; Wakana et al., 2007) to nonlinearly transform the mean skeletonized mask and skeletonized WM atlases to native space.
We extracted separate, subject-specific masks from the atlases deprojected to native space, with separate masks for left and right and combined hemispheres. The data in the present study were based on WM regions previously shown to exhibit significant variance in two-year change (Bender & Raz, 2015): anterior limb of internal capsule (ALIC), body of the corpus callosum (CC body), dorsal cingulum bundle (CBd), forceps minor (FMin), inferior frontal-occipital fasciculus (IFOF), and uncinate fasciculus (UF). We sampled mean values and standard deviations for non-zero voxels for masks from left and right hemisphere ROI masks, restricted to exclude WMH/CSF.
2.3 Cognitive Tests
2.3.1 Speed of Perceptual-Motor Processing (Speed)
An experimenter administered the Letter Comparison and Pattern Comparison tests (Salthouse, 1996) to gauge speed of perceptual processing. The performance index for each test is the total correct, divided by time for completion (# correct/60 s). Reliabilities for letter and pattern comparison are estimated to be .77 and .87, respectively (Salthouse & Meinz, 1995). See Bender and Raz (2012) for a complete description of testing procedures.
2.3.2 Executive function (EF)
2.3.2.1 EF – Inhibition
Participants completed a paper version of the Stroop task (Stroop, 1935; Salthouse & Meinz, 1995). An experimenter first presented the participant with a sheet containing 20 items organized in two columns; each item was surrounded by a 13.5 × 19 mm rectangle. In each test, experimenter instructed the participant to respond as quickly as possible, starting with the left column, before continuing with the right column. In the color neutral (CN) subtest participants named the color of the ink of strings of six Xs printed in red, green, yellow, and blue ink. In the color incompatible (CI) subtest, the words ‘red,’ ‘green,’ ‘blue,’ and ‘yellow’ were presented but in incongruently colored ink; participants were instructed to name the color of the ink, rather than the word. Participants completed two versions of each subtest using alternate forms. Interference scores were calculated as the difference between mean response times for CI and CN subtests. This task has an estimated split-half reliability of .72 (Salthouse & Meinz, 1995).
2.3.2.2 EF – Working Memory
The tests are briefly described below. For a complete description of all testing procedures for working memory tasks, see Bender and Raz (2012).
2.3.2.2.1 EF – Working Memory: Size Judgment Span
Originally described by Cherry and Park (1993), the task requires participant to maintain representations in working memory, compare them based on semantic features, and re-order the items in ascending physical size for verbal report. The performance index was total number of correct responses. The test-retest reliability coefficient for the task is r = .79, p < .001 (Cherry & Park, 1993).
2.3.2.2.2 EF – Working Memory: Spatial Recall
Working memory was assessed with a modified, computerized version of the task described in the literature (Salthouse, 1974, 1975; Salthouse et al., 1988). This task requires participants to study briefly presented 5×5 matrices, memorize the locations of target cells, and subsequently recall those locations immediately following presentation. The index of performance was the average number correct across the 25 test trials. Cronbach’s alpha was α = .89, as computed across the 25 test trials.
2.3.2.2.3 EF – Working Memory: n-Back Test
Working memory storage and maintenance was assessed with a computerized n-back test using non-verbal materials (modeled after Dobbs & Rule, 1989; Hultsch et al., 1990). The non-verbal test presented abstract shapes on a 17-inch monitor. Following presentation of all items in each trial, participants selected the item presented in the position specified by the given subtest. The performance index tasks is the number of correct responses (out of 20). The task’s estimated reliability coefficient is .88 (Salthouse et al., 1996).
2.3.2.5 EF – Task Switching
We used a computerized test (Salthouse et al., 1998) to assess participants’ ability to switch between stimuli and between tasks. The program instructed participants to associate specific computer keys with different stimulus properties; the software serially presented participants with stimuli (digits), and participants made the appropriate keyboard response based on the changing stimulus property. A dual switching task required participants to switch between indicating if a number was more or less than 5 and whether the digit was odd or even. The costs due to switching were calculated as the difference in accuracy (total errors) between switch and non-switch trials.
2.3.3 Episodic Memory (EM)
2.3.3.1 EM – Recognition: Picture-Name Associations
Participants completed the Memory for Names subtest of the Woodcock-Johnson Psychoeducational Battery-Revised (WJ-r; Woodcock & Johnson, 1989). Participants serially viewed novel visual stimuli, cartoons depicting ‘space creatures,’ and listened as the experimenter stated the creature’s name consisting of one- and two-syllable nonsense stimuli. Following each new item presentation, participants viewed a page containing multiple pictures and pointed to each previously studied item after the experimenter stated its name. The experimenter provided the correct answer for incorrect responses during the immediate testing phase. There were 72 possible correct responses. Following a 20-minute delay, the experimenter showed the participant 12 pages, each with 12 space creatures; using its previously learned name, the experimenter asked the participant to point to each space creature. Unlike the immediate testing phase, only a single recognition judgment was requested for each presentation of a given page, and the experimenter provided no feedback on performance accuracy. The maximum score possible in the delayed testing phase was 36. The total number of correct responses for immediate and delayed cued associative memory tests formed the two performance indices for the task. Both immediate and delayed tests have estimated reliabilities of .91 (Woodcock & Mather, 1989).
2.3.3.2 EM – Recognition: Word pairs
An experimenter administered a computerized recognition test for associative recognition using word pairs (Naveh-Benjamin, 2000; for details, see Bender et al., 2010) implemented in Visual Basic. The task used an intentional encoding condition in which all participants received instructions to study and remember both the individual words and the pairs, and that both would be tested. Each participant viewed 26 pairs of unrelated words, presented at a rate of 5.5 s per pair with a 200 ms inter-stimulus interval. To minimize rehearsal following the study phase, the task instructed participants to audibly count backwards by threes from a randomly generated 900 number for 60 s. Participants then completed separate single item, yes/no recognition tests for items (individual words) and associations (word pairs); test order was counterbalanced across the sample. Both item and associative tests included 16 trials. Item test trials presented 16 individual words (8 targets, 8 foils), and the associative test presented 16 pairs (8 intact pairs, 8 recombined pairs). For each trial, participants indicated via keyboard button press if a word had been presented at study or not or if pairs were intact or recombined. After completing both tests the process was repeated with a second list of 26 new word pairs. The lists for each participant were randomly assigned out of six possible lists. At the second wave of testing, approximately two years later, one of the lists was repeated from the initial administration (repeated list) and the other was a new list containing novel stimuli (non-repeated list).
2.3.4 Fluid reasoning (Gf)
Participants completed two tests of fluid reasoning previously used in studies of aging (Raz et al., 1998, 2008; Rabbitt & Lowe 2000; Schretlen et al., 2000), the Cattell Culture Fair Intelligence Test, form 3B (CFIT, 3B; Cattell & Cattell, 1973) and Letter Sets Test (parts 1 and 2) from the Educational Testing Service Factor--Referenced Test Kit (Ekstrom et al. 1976). The CFIT is a test of nonverbal reasoning, and includes four subtests (see Raz et al., 2008 for a complete description of the task). The total number correct on forms 1–3 for the CFIT (Cattell & Cattell, 1973) served as performance indices. The Letter Sets Test included two pages, each with 15 items. Each item consists of a row of five sets of 4-letter strings; the task instructed participants to identify the rule common to four out of the five sets, and mark the set that does not match the rule. The task provided participants seven minutes to complete each page. For each incorrect response, 0.25 point is deducted from the total number correct to yield the performance index. Each page was scored separately to yield two performance measures for each participant.
2.4 Data Conditioning
For the word-pair tests of recognition memory, we used hit rate and false alarm rate data to compute A′, a nonparametric index of discriminability (Pollack & Norman, 1964; Stanislaw & Todorov, 1999; Stewart, 2002), and applied an arcsine transformation to A′ scores to correct for significant skewness in their distributions. Similarly, we corrected skewness in the distributions of several cognitive variables by applying a log-transformation. These included the letter and pattern comparison speed, and switching costs index. We similarly applied a log-transformation to vascular risk variables (fasting blood glucose and triglycerides) to eliminate skewness in the distributions of those variables. All data were standardized to z-scores for analysis, and z-scores for the second measurement occasion were standardized to the first occasion in order to calculate latent difference scores. For MRI analyses, cases in which the number of voxels in any WM mask was > 3 standard deviations below the mean were dropped, resulting in the exclusion of CB dorsal values in one participant and uncinate fasciculus (UF) in three cases.
2.5 Data Analysis
We tested the cross-sectional and longitudinal association of WM and cognitive constructs: associative memory, executive functioning, fluid reasoning, and perceptual speed. We sought to examine whether cognitive ability at baseline was associated with changes in WM structure, whether the baseline microstructural properties of WM were related to changes in any of the cognitive domains, and whether longitudinal change in WM structure was associated with longitudinal change in cognition.
To test the mutual relations between changes in WM and cognition, we employed latent change score models (LCSM; McArdle & Nesselroade, 1994), a structural equation modeling (SEM) framework that permits evaluation of changes and their mutual influences at the latent factor level (Lövdén et al., 2014) after accounting for measurement error. The SEM-based LCSM analyses were implemented in Mplus 7 (Muthén & Muthén, 2012), and treated missing data as missing at random (MAR) with full information maximum likelihood (FIML) estimation. The WM structure was represented by two latent factors denoting baseline WM diffusion properties and a change gradient thereof. DTI derived indicators from multiple WM regions that were shown to exhibit variance in two-year FA change (Bender & Raz, 2015) were selected to form the WM factor. Separate models were evaluated for each cognitive domain and the ones that exhibited significant variance in change were retained. To address our primary questions, we tested whether there were significant covariances between cognitive change factors and the baseline WM structure, whether there were significant covariances between change in WM structure and cognitive baseline factors, and whether there was significant covariance between the change factor in WM structure and the change factors of each of the cognitive domains.
The final model was obtained by incremental model building and evaluation of goodness-of-fit in two major steps. First, independent univariate models of latent changes were constructed in both the cognitive and the WM domains. Second, in each domain we retained only constructs exhibiting significant variance in change. Finally, we specified a composite model of change in cognition and WM in which the domains were coupled to test for mutual associations of baseline and change within and between domains, particularly including an association of change scores across domains.
2.5.1 Models of change in DTI indices
We employed the same framework for analysis of DTI data with regard to change in the three DTI indices (FA, AD, RD) as reported in Bender and Raz (2015) and used two-occasion LCSMs (McArdle & Nesselroade, 1994) to evaluate concurrent and associated WM changes. Six brain regions were previously shown in this sample to exhibit significant variance in two-year FA change (Bender and Raz, 2015): ALIC, CBd, CC body, FMin, IFOF, and UF. The corresponding models were assumed to be time-invariant within brain area (i.e., equal factor loadings and allowing for correlated residuals across time). Unlike previous reports that used separate mean values for left and right hemisphere to form a latent WM factor (Bender & Raz, 2015; Lövdén et al., 2014; Ritchie et al., 2015), bilaterally sampled mean values for each region served as indicators for the multi-region LCSMs. Finally, we extended the multi-region LCSMs for each DTI index by regressing age, centered at the sample mean, onto each baseline and change score factor.
2.5.2 Models of Cognitive Change
We used the same LCSM framework to model latent change in multiple cognitive domains. The scores from the Letter Comparison and Pattern Comparison tests served as observed indicators for the model of Speed. A separate EF-working memory LCSM included five indicators: spatial recall score, number of correct responses on the size judgment span task, number of correct responses on the non-verbal 3-back task, the switching cost index and the interference score from the Stroop color word task. We multiplied the Stroop interference scores by −1 to bring its scale in line with the other variables. Manifest variables for the LCSM of Gf included the three Cattell’s CFIT-3B subtest scores and the scores from the two forms of the Letter Sets Test. Observed indicators for the model on Memory included the scores on the WJ-R memory for names immediate and delayed subtests, and the A′ scores for associations from the word pair recognition task from on the lists that were repeated from baseline (i.e., repeated list). Initial univariate models for each cognitive outcome were fit, also assuming time invariance (i.e., equal factor loadings, residuals, and allowing for correlated residuals across time).
2.5.3 Coupled WM Change-Cognitive Change Models
We used a bivariate LCSM modeling approach similar to that reported by Lövdén et al. (2014) to evaluate associations between levels and change in WM and cognition (Fig. 1). Here, we specified bivariate LCSMs that included the univariate models for Gf or Memory and WM, separately for FA and RD. In addition to the specifications from the age-partialed univariate models, each model specified covariances between the baseline WM factor and the cognitive latent change factor, the baseline cognition factor and the WM change factor, as well as covariances between the change factors for cognition and WM (Fig. 2).
Figure 1.
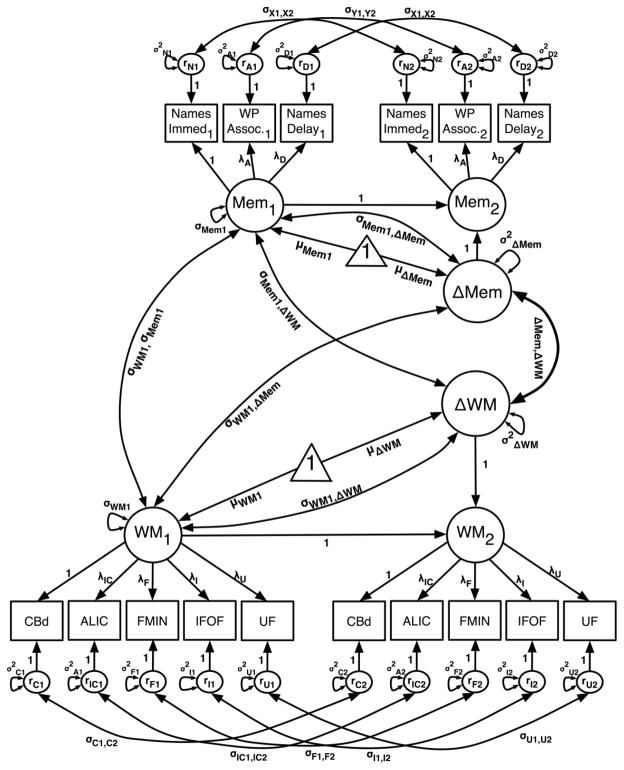
Schematic diagram of bivariate LDM measurement model used in combined analysis of DTI and cognitive data. The model comprises separate univariate change score models for cognition (memory) and white matter (WM) that are joined in a cross-lagged fashion. Squares represent observed variables and the larger circles represent latent variables; Mem1 and Mem2 represent the latent memory variable at Time 1 and Time 2, respectively, whereas WM1 and WM2 represent the WM latent variable (i.e., FA, RD) at Time 1 and Time 2, respectively. The triangles signify means and intercepts for the latent change factors for each constituent univariate model. Observed Memory factor indicators included performance indices from three separate tests of associative memory: Names Immed- WJ-r Memory for Names subtest, immediate performance; Names Delay- WJ-r Memory for Names subtest delayed performance; WP Assoc- associative recognition (A′ scores) from the word pairs task. The observed WM indicators refer to bilaterally sampled mean values from five anatomical regions. CBd: dorsal cingulum bundle; ALIC: anterior limb of internal capsule; FMIN: forceps minor; IFOF: inferior frontal-occipital fasciculus; UF: uncinate fasciculus. The model includes estimated covariances between the following factors: Time 1 (T1) WM–WM change (Δ), T1 Memory–ΔMemory, T1 WM–ΔMemory, T1 Memory– ΔWM, and ΔMemory–ΔWM. Auto-correlated residuals for observed variables are kept equivalent between occasions. Equivalency is imposed on the factor loadings for both Mem and WM factors to maintain factorial invariance across occasions. For additional information on two-occasion latent difference score models, see McArdle and Nesselroade (1994).
Figure 2.
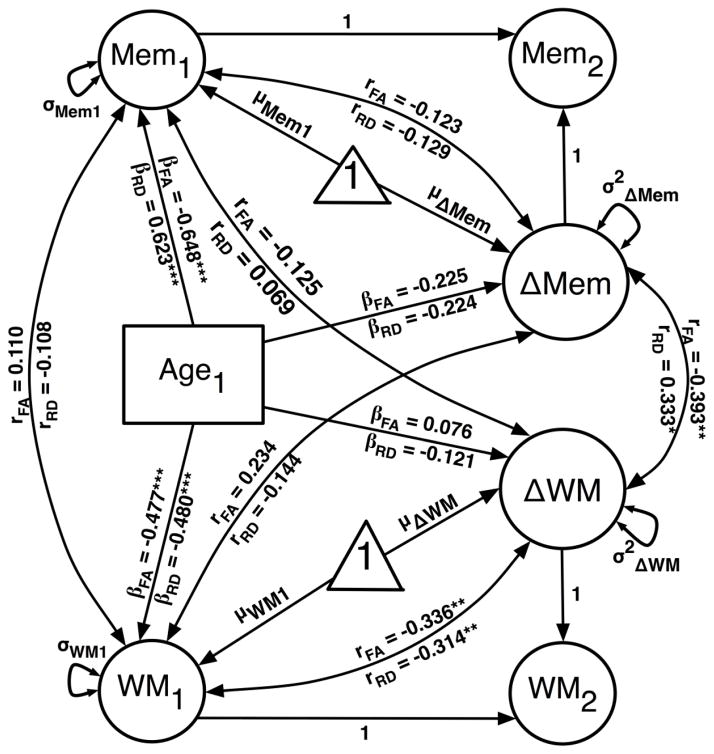
Diagram of the age-partialed bivariate change score model for FA and RD, with parameter estimates for each model indicated as subscripts. Covariances between baseline WM and memory latent factors were estimated, as were covariances between baseline factors and change. Regressions on baseline Age were specified for the following factors: baseline WM and Memory, WM change, and Memory change. Indicator level variables (see Figure 1) are omitted for model simplicity.
We used the LCSM described in Bender and Raz (2015), to fit a two occasion univariate model for metabolic syndrome risk (Met) factor that included indicators systolic blood pressure, fasting blood glucose, triglycerides, and waist-to-hip ratio (WHR). Because of its stability across measurement occasions (Bender & Raz, 2015), the baseline Met factor was used as a covariate in the DTI-Cognition models described below. We also evaluated additional models that included reported or observed hypertension as a dichotomous predictor of level and change in both WM and cognition.
2.5.4 Model fit
Model fit was assessed by multiple goodness-of-fit indices. The comparative fit index (CFI) compares model fit to that of a null model, and values of .95 were cutoffs for the CFI. For chi-square (χ2) tests of model fit, smaller χ2 value indicates precision of fit in comparison to a null model. A related, more informative fit statistic, χ2 divided by degrees of freedom (Jöreskog & Sörbom, 1993) used a cut-off value of ≤ 2.0 (Mueller, 1996). Root mean square error of approximation (RMSEA), a measure of model misspecification, served as an additional goodness-of-fit index, and acceptable fit was indicated by values of .08 and below.
3. Results
3.1 Univariate Models
We fit univariate LCSMs for each cognitive and DTI index. Final univariate cognitive models were all two-occasion models with significant change score variances. As we did not find significant change variance in Speed and Executive Function (Table 2), only Gf and Memory were retained for the bivariate WM-cognition LCSMs described below.
Table 2.
Univariate LCSM Fit Indices and Change Parameter Statistics for Cognitive and WM Models
| Cognitive Models | WM Models | ||||||
|---|---|---|---|---|---|---|---|
| EF | Gf | Memory | Speed | AD∘ | RD | FA | |
| χ2 | 52.576 | 46.577 | 20.24 | 10.41 | 33.265 | 43.383 | 48.365 |
| df | 44 | 42 | 14 | 6 | 22 | 39 | 39 |
| χ2/df | 1.195 | 1.109 | 1.446 | 1.735 | 1.512 | 1.112 | 1.240 |
| CFI | 0.981 | 0.989 | 0.988 | 0.979 | 0.970 | 0.995 | 0.988 |
| RMSEA | 0.045 | 0.034 | 0.068 | 0.088 | 0.073 | 0.034 | 0.050 |
| Mean Δ | 3.689* | 0.266 | 7.062* | −2.155* | −2.825* | −1.179 | −0.616 |
| Δ Variance | – | 1.995* | 3.514* | – | 1.853 | 3.419* | 3.505* |
Notes: Mean Δ=estimate/std. error for mean change score parameter;
p < .05;
– = parameter constrained to zero due to model error from negative variance estimate;
model without forceps minor;
EF = executive function; Gf = fluid reasoning; AD = axial diffusivity; RD = radial diffusivity; FA = fractional anisotropy.
The initial multi-region model for FA change included six bilaterally sampled WM regions: ALIC, CBd, CC body, FMin, IFOF, and UF. The model, however, did not fit well: χ2 = 162.492, df = 84, χ2/df = 1.931, CFI = 0.931, RMSEA = 0.099. Although all included regions had previously exhibited significant change variance in FA (Bender & Raz, 2015), the mean FA change in CC body was the opposite of the other regions included in the WM factor. Thus, this region was excluded from the remainder of analyses, and following this re-specification, model fit was substantially improved (see Table 2), and became acceptable by the common criteria outlined in the Method section. The initial model of AD change did not fit well (χ2 = 67.331, df = 39, χ2/df = 1.726, CFI = 0.933, RMSEA = 0.087). Because FMin had shown significant AD increases, whereas the other four regions evidenced linear declines (Bender & Raz, 2015), FMin was excluded as an indicator from the model for AD, and the model fit improved. Although the univariate of AD change showed significant linear declines, the change variance for AD was not significant. Therefore, because the bivariate change score model assumes significant change variance, the coupled WM-cognition models were only estimated for FA and RD.
3.2 Bivariate Change Score Models
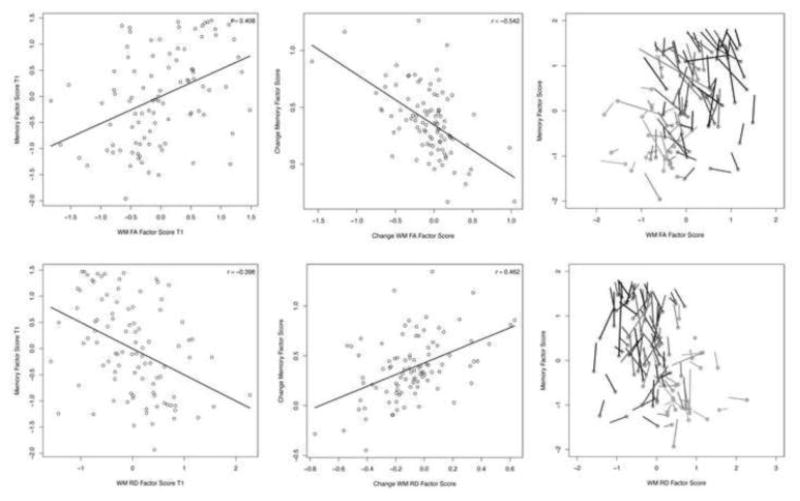
The coupled WM-memory change score models showed acceptable fit for both FA (χ2 = 180.656, df = 121, χ2/df = 1.493, CFI = 0.959, RMSEA = 0.072) and RD (χ2 = 158.630, df = 122, χ2/df = 1.300, CFI = 0.974, RMSEA = 0.056). All loadings were significant for WM and Memory factors (Table 3). As shown in Table 4, the change-change covariance parameter estimate was significant in both FA (r = −0.402; p < .01; bias-corrected bootstrap 95% CI = −0.121 to −0.012) and RD models (r = 0.333; p < .05; bias-corrected bootstrap 95% CI = 0.001 to 0.079; see Fig. 3). Greater two-year decreases in FA and increases in RD were associated with greater improvement on the associative memory factor. In addition, baseline age was negatively associated with baseline FA and positively associated with baseline RD. Although at baseline older age was associated with poorer memory performance, it was unrelated to differences in change of FA, RD, or memory. For FA, a significant negative baseline-change covariance indicated that higher baseline FA was associated with smaller FA change (r = −0.337; p < .01). For RD, a negative baseline-change covariance reflected lower baseline RD association with greater two-year RD increase (r = −0.314; p < .01).
Table 3.
Cognitive and WM factor loadings from bivariate change score models.
| Model/Factor | Variable | Estimate | SE | p value | Model/Factor | Variable | Estimate | SE | p value |
|---|---|---|---|---|---|---|---|---|---|
| FA-Memory Model | RD-Memory Model | ||||||||
| FA | CBd | 1.000 | – | – | RD | CBd | 1.000 | – | – |
| ALIC | 0.956 | 0.112 | 0.000 | ALIC | 0.863 | 0.118 | 0.000 | ||
| FMin | 1.167 | 0.107 | 0.000 | FMin | 1.186 | 0.115 | 0.000 | ||
| IFOF | 1.221 | 0.112 | 0.000 | IFOF | 1.270 | 0.116 | 0.000 | ||
| UF | 0.915 | 0.121 | 0.000 | UF | 0.882 | 0.124 | 0.000 | ||
| Memory | NAME | 1.000 | – | – | Memory | NAME | 1.000 | – | – |
| ASSOC | 0.632 | 0.079 | 0.000 | ASSOC | 0.629 | 0.079 | 0.000 | ||
| NAMED | 0.980 | 0.036 | 0.000 | NAMED | 0.983 | 0.038 | 0.000 | ||
| FA-Gf Model | RD-Gf Model | ||||||||
| FA | CBd | 1.000 | – | – | RD | CBd | 1.000 | – | – |
| ALIC | 0.960 | 0.113 | 0.000 | ALIC | 0.859 | 0.118 | 0.000 | ||
| FMin | 1.175 | 0.107 | 0.000 | FMin | 1.193 | 0.115 | 0.000 | ||
| IFOF | 1.221 | 0.113 | 0.000 | IFOF | 1.273 | 0.117 | 0.000 | ||
| UF | 0.922 | 0.122 | 0.000 | UF | 0.881 | 0.124 | 0.000 | ||
| Gf | LS1 | 1.000 | – | – | Gf | LS1 | 1.000 | – | – |
| LS2 | 1.024 | 0.089 | 0.000 | LS2 | 1.026 | 0.089 | 0.000 | ||
| CAT1 | 0.717 | 0.097 | 0.000 | CAT1 | 0.720 | 0.097 | 0.000 | ||
| CAT2 | 0.579 | 0.098 | 0.000 | CAT2 | 0.584 | 0.098 | 0.000 | ||
| CAT3 | 0.448 | 0.098 | 0.000 | CAT3 | 0.448 | 0.098 | 0.000 | ||
Notes: Factor loadings are based on unstandardized parameter estimates. – represents missing statistics for constant values; White matter abbreviations: CBd: dorsal cingulum bundle; ALIC: anterior limb of internal capsule; FMin: forceps minor; IFOF: inferior frontal-occipital fasciculus; UF: uncinate fasciculus. Cognitive abbreviations: NAME, NAMED: WJ-r memory for names subtests, immediate and delayed performance, respectively. ASSOC: A′ scores on associative recognition from the word pair task using repeated forms between testing occasions; LS1, LS2: Letter sets forms 1 and 2; CAT1-3: Cattell Culture Fair Intelligence tests, forms 1–3.
Table 4.
Latent associations of bivariate change score models for WM with Memory and Gf, for FA and RD models
| Model/Path type | Path | FA | RD | ||||
|---|---|---|---|---|---|---|---|
| Estimate | S.E. | p value | Estimate | S.E. | p value | ||
| Memory | |||||||
| Level–Level | Memory↔WM | 0.110 | 0.116 | 0.344 | −0.108 | 0.113 | 0.340 |
| Level–Change (Δ) | Memory↔ΔWM | −0.125 | 0.119 | 0.296 | 0.069 | 0.126 | 0.582 |
| Memory↔ΔMemory | −0.123 | 0.149 | 0.407 | −0.129 | 0.147 | 0.382 | |
| WM↔ΔWM | −0.336 | 0.111 | 0.002 | −0.314 | 0.117 | 0.007 | |
| WM↔ΔMemory | 0.234 | 0.143 | 0.103 | −0.144 | 0.141 | 0.307 | |
| Change (Δ) – Change (Δ) | ΔWM↔ΔMemory | −0.393 | 0.141 | 0.005 | 0.333 | 0.150 | 0.026 |
| Age–Level | Age→WM | −0.648 | 0.064 | <0.001 | 0.623 | 0.066 | <0.001 |
| Age→Memory | −0.477 | 0.084 | <0.001 | −0.48 | 0.083 | <0.001 | |
| Age–Change (Δ) | Age→ΔWM | 0.076 | 0.112 | 0.496 | −0.121 | 0.117 | 0.302 |
| Age→ΔMemory | −0.225 | 0.129 | 0.083 | −0.224 | 0.129 | 0.081 | |
|
| |||||||
| Gf | |||||||
| Level–Level | Gf↔WM | −0.088 | 0.120 | 0.464 | 0.110 | 0.117 | 0.347 |
| Level–Change (Δ) | Gf↔ΔWM | −0.029 | 0.123 | 0.812 | −0.153 | 0.127 | 0.230 |
| Gf↔ΔGf | −0.270 | 0.181 | 0.136 | −0.268 | 0.181 | 0.138 | |
| WM↔ΔWM | −0.341 | 0.111 | 0.002 | −0.316 | 0.117 | 0.007 | |
| WM↔ΔGf | 0.099 | 0.189 | 0.602 | −0.082 | 0.183 | 0.655 | |
| Change (Δ) – Change (Δ) | ΔWM↔ΔGf | −0.173 | 0.194 | 0.371 | 0.278 | 0.200 | 0.165 |
| Age–Level | Age→WM | −0.648 | 0.064 | <0.001 | 0.622 | 0.066 | <0.001 |
| Age→Gf | −0.483 | 0.085 | <0.001 | −0.483 | 0.085 | <0.001 | |
| Age–Change (Δ) | Age→ΔWM | 0.078 | 0.112 | 0.488 | −0.121 | 0.117 | 0.302 |
| Age→ΔGf | −0.075 | 0.173 | 0.663 | −0.077 | 0.173 | 0.656 | |
Notes: ↔ = covariance parameter; → = regression path parameter; parameter estimates are standardized.
Abbreviations: FA = fractional anisotropy; RD = radial diffusivity; Gf = fluid reasoning; WM = white matter.
Figure 3.
White matter (WM) and Memory factors at baseline and in change between baseline and follow-up. Depicted in all plots are standardized factor scores estimated from bivariate change scores models. In all plots, a WM property factor (A–C: FA; D–F: RD) is plotted on the x-axis, and Memory factor is plotted on the y-axis. A. Scatter plot with fitted linear regression lines for baseline (T1) FA and T1 Memory factor scores. B. Scatter plot with fitted linear regression lines for estimated change (Δ) score factors for FA and Memory. C. Spaghetti-and-meatball plot of change between two measurement occasions for FA and Memory: Circles represent the first measurement and the line shows the magnitude (line length) and direction of change-change relationships. Lines are coded according to participant age at baseline as a gradient from black (youngest) to light gray (oldest). In this plot, although the direction of differences in FA-memory associations is similar to cross-sectional relationships shown in A., the vectors demonstrate intra-individual change-change trajectories that suggest patterns more like those depicted in B. In addition, this plot shows clear cross-sectional age differences but no effect of age on change. D. Scatter plot with fitted linear regression lines for T1 RD and T1 Memory factor scores. E. Scatter plot with fitted linear regression lines for estimated change (Δ) score factors for RD and Memory. F. Spaghetti-and-meatball plot of change between two measurement occasions for RD and Memory: Circles represent the first measurement and the line shows the magnitude (line length) and direction of change-change relationships.
The fit for the coupled Gf-memory change score models was acceptable for both FA (χ2 = 217.324, df = 195, χ2/df = 1.114, CFI = 0.983, RMSEA = 0.035) and RD (χ2 = 245.898, df = 195, χ2/df = 1.261, CFI = 0.962, RMSEA = 0.052). Loadings were significant for WM and Memory factors (Table 3). Although older age was associated with lower baseline Gf, none of the relationships between Gf and WM were significant (Table 4).
For the four bivariate change score models, the Met factor and a dichotomous variable representing self-reported hypertension at either occasion were entered into the models as covariates on the baseline and change scores for WM and cognition. Although all models exhibited acceptable fit (for all, χ2/df < 2.0, CFI > 0.950, RMSEA < .065), inclusion of these covariates did not affect the significant covariance between the change factors for memory and WM. Moreover, none of the paths from the Met or hypertension factors were significant.
4. Discussion
The main finding of this study is that in healthy adults, two-year changes in white matter diffusion properties in regions linked to established memory networks are coupled with improvement in performance on associative memory tests. Specifically, WM changes (reductions in FA and increases in RD) that suggest reduction in barriers to anisotropic diffusion in tracts connecting frontal and temporal lobes were associated with greater re-test gains. Whereas directions of change in the brain and memory are consistent with the extant literature, the negative association between changes in them was unexpected. Notably, in line with previous cross-sectional findings, higher FA and lower RD were associated with better memory performance at baseline. Thus, their stability or improvement would be expected to augur further improvement in memory scores. The opposite was observed: brain changes that are usually viewed as signs of aging and age-related pathology were coupled with greater positive changes in an age-sensitive cognitive ability.
The reversal of a relationship across units of observations – in our case between cross-sectional and longitudinal analysis – is not uncommon, and may reflect a well-known Simpson’s paradox (Simpson, 1951; Kievit et al., 2013). As pointed out by Simpson in his original article, the decision on selection of the within-unit or between-unit outcome hinges on interpretation rather than on purely statistical grounds (Simpson, 1951). In the case of research described in this article, we argue that seemingly conflicting outcomes of the cross-sectional and longitudinal analyses should be resolved in favor of the latter, as long as one is interested in gauging individual change over time and not individual differences at a given moment.
Because there were no relations between baseline memory scores and subsequent repetition-related gains, it is unlikely that participants with high initial scores had no room for improvement while their less apt peers reaped additional benefits from exposure to tests. To the contrary, persons with better WM indicators at baseline (and better, correlated memory performance) showed lesser WM change. As we do not know what the participants experienced between the two measurement occasions, we can only surmise that those who showed greater gains underwent WM re-organization that resulted in reduced fiber coherence and enhanced diffusivity. These results are in accord with the suggestion that such changes could result from increases in axon diameter or development of crossing fibers in healthy white matter, rather than via pathological mechanisms (Johansen-Berg, 2012). Therefore, it is that possible greater two-year increase in signal from secondary fiber populations reflects increased plastic response of the white matter to repeated testing. Notably, the coupling was observed independent of age and within WM regions associated with learning and memory (Aggleton & Brown, 2006; Charlton et al., 2013). If indeed the observed WM changes can be taken as evidence of re-organization rather than deterioration, their coupling with a positive change in cognitive gains suggests that retest learning effects may serve as valuable indices of adult plasticity (Bender et al., 2013; Salthouse & Tucker-Drob, 2008, Yang, 2011).
One of the difficulties with interpreting these findings and placing them into the context of the extant literature is a relative dearth of longitudinal studies of WM with cognition in general and memory in particular. Furthermore, the existent studies that examined such changes are limited to older adults and frequently focused on either much narrower or wider time windows than the two-year period studied here. Evidence from comparable studies of older (Ritchie et al., 2015) and very old adults (Lövdén et al., 2014) showed mean decline in FA was linked with reduced fluid reasoning and speed of perceptual-motor processing, respectively. Although both studies report strong associations in FA change across evaluated tracts, Lövdén and colleagues (2014) found processing speed reduction associated with FA declines only in the corticospinal tract. Ritchie and colleagues, who used an approach similar to the one employed in the present study, found that more global FA reductions were linked with reduced Gf. Neither study found relationships between WM changes and memory.
Neurobiological underpinnings of the observed changes in DTI-derived parameters and their association with repetition-related gains in memory are unclear. Many cellular and molecular processes as well as fiber geometry determine FA and RD (Jones et al., 2010, 2013b). Studies in rodents and monkeys reveal many age-related differences in WM structure, including disruption of the myelin sheaths, preferential loss of small-diameter axonal fibers, reduced axonal fiber packing density and increased extra-axonal spaces (Marner et al., 2003; Nielsen & Peters, 2000; Peters et al., 1996; Peters, 2002, 2009; Sandell & Peters, 2003; Tang et al., 1997). Yet, the correspondence between these features of the aging WM and DTI indices has never been clearly established. Although some of studies suggested FA reductions and RD increases as proxies for lower fiber density and myelin loss (Makris et al., 2007; Song et al., 2003, 2005; Sun et al., 2006, 2008), the supporting evidence came mainly from evaluating highly coherent and highly myelinated WM regions: optic nerve, spinal tract or corpus callosum. Moreover, the methods used to induce WM change in animal models included targeted ischemia and genetic manipulations that may not accurately reflect slow processes associated with WM changes in many regions of the aging human brain (Guan & Kong, 2015; Hines et al., 2015; Nave, 2010). Furthermore, measures derived from a single-tensor model appear uninformative about myelination (Beaulieu, 2012; De Santis et al., 2014; Jones et al., 2013; Kolind et al., 2008; Mädler et al., 2008).
Short-term training studies examining WM changes over short periods revealed mixed results. In healthy young adults, motor training was accompanied by increased FA in internal capsule, CC body, and corona radiata (Wang et al., 2013). On the other hand, a history of training and acquired expertise are not always associated with greater FA as for example, professional ballet dancers who accumulated many hours of intensive motor practice evidenced lower FA values in sensorimotor regions than did persons who lacked similar training (Hanggi et al., 2010). Our findings are in partial agreement with a report of WM changes after a 10-week memory training program with middle-aged and older adults. In that study, training and control groups both showed increase in MD (an average of AD and RD), whereas FA decreased only in the controls (Engvig et al., 2012). In that study, however, improvement on memory tests after training was associated with increase in peak voxel FA.
FA is negatively related to AD and RD, and within each voxel and region, both AD and RD depend on fiber orientation (Wheeler-Kingshott & Cercignani, 2009). Thus, FA is influenced by the diversity of fiber orientations in the voxel (Budde and Annese, 2013; Pierpaoli and Basser, 1996), and therefore, the FA declines observed in our study may reflect increased heterogeneous axonal directionality rather than myelin loss or changes in axonal size (Billiet et al., 2015). Indeed, orderly arrangement is not a common feature of the cerebral white matter and 60–90% of WM voxels include crossing fibers (Jeurissen et al., 2013; Vos et al., 2012). Thus, it is likely that the preponderance of WM voxels sampled in the present study included multiple, multidirectional fiber populations (Tuch et al., 2002) as the WM factor evaluated in the present study included five regions previously shown to harbor multiple fiber orientations (Jeurissen et al., 2013). ALIC includes thalamo-cortical-striatal, cortico-pontine, and intra-striatal fibers that vary substantially in their orientation (Axer and Keyserlingk, 2000), CBd and FMin contain multiple subregions and crossing fibers (Catheline et al., 2010; Jones et al., 2013a; Malykhin et al., 2011), and inferior association fibers (IFOF) may include multiple functionally and anatomically specific subregions (Sarubbo et al., 2013). Thus, when WM characteristics are inferred from traditional DTI data, interpreting age-associated changes in WM diffusion properties and attributing specific neurobiological mechanisms to their relationships with cognitive performance (see Madden et al., 2012 for a review), is not warranted (Jones & Cercignani, 2010; Jones et al., 2013b; Wheeler-Kingshott & Cercignani, 2009). Consequently, a cautious interpretation of changes in FA of a healthy brain may be reduced intra-voxel fiber coherence (Pfefferbaum et al., 2000) and it is possible that the observed FA declines may represent fiber re-organization related to experiences of the participants in the period of two years. Determining which of these experiences affected white matter organization and cognitive performance is beyond the scope of this investigation. Nonetheless, change in WM diffusion properties may reflect biological processes that differ from those that are represented by cross-sectional differences.
4.1 Limitations and Future directions
In addition to the well-known difficulties in assigning biological meaning to DTI findings, the results reported here should be interpreted in the context of several limitations. First, with only two occasions and a two-year delay between the measurements, the analyses presented here deal with the most rudimentary longitudinal design. Such a design precludes investigation of many critical questions, such as possible nonlinearity of change and leading-lagging effects of the brain and cognitive variables. Future studies, including the one underway in our laboratory must address this limitation.
Second, as frequently happens in longitudinal studies, the data collection is limited by the method applied at baseline, which in the case of DTI meant using sequences that today would be considered less than optimal. Future studies will take advantage of many new developments that may afford better, more nuanced neurobiological interpretation of the data. One can envision using multi-pool models and sequences such as High Angular Resolution Diffusion Imaging (HARDI, Tuch et al., 2002), AxCaliber (Assaf et al., 2008), Composite Hindered and Restricted Model of Diffusion (CHARMED, Assaf & Basser, 2005) in conjunction with traditional single tensor models (with much greater number of gradient directions) to better understand the relationships between the DTI indices reported for the last 15 years and the presence and influence of crossing fibers (De Santis et al, 2014; Douaud et al., 2011; Ennis & Kindlmann, 2006). Furthermore, assessment of microstructural organization of WM should be accompanied by more direct estimation of myelin content via multiecho T2 relaxometry (Mackay et al, 1994).
The third major limitation has to deal with TBSS-derived region labeling. Although the approach to ROI generation on skeletonized WM data that was employed here have been used by other researchers (Foley et al., 2014; Lövdén et al., 2014; Sadeghi et al., 2013; Teipel et al., 2010), we must keep in mind that the TBSS may be less than perfectly reliable (Bach et al., 2014; Madhysatha et al., 2014). Thus, TBSS-derived anatomic labels may have limited correspondence to the underlying WM structures identified in probabilistic atlases, particularly in areas such as the frontal lobes that contain fibers of multiple orientations (Malykhin et al., 2011). Furthermore, the use of TBSS and WM atlases for identification of WM tracts allows only limited anatomically specificity in comparison to DTI tractography. Thus, future longitudinal DTI studies of WM aging should evaluate tractography-derived streamlines.
5. Conclusion
Performance gains after repeated administration of memory tests, two years apart, are coupled with changes in white matter microstructure and organization in brain regions associated with memory-related processing. Although the neurobiological meaning of these observations remains unclear, it is plausible that they reflect experience-related changes in white matter complexity and organization.
Supplementary Material
Highlights.
Healthy adults were followed up for two years with MRI and cognitive assessment.
Latent change score models quantified coupled changes of white matter & cognition.
Baseline anisotropy (FA) & radial diffusivity (RD) were linked to associative memory.
FA reductions and RD increases over time were coupled with memory retest gains.
Acknowledgments
We offer special thanks to Cheryl Dahle, Peng Yuan, members of the Cognitive Neuroscience of Aging lab involved in cognitive testing and MR imaging data collection, and our research participants. This study was supported in part by a grant from the National Institutes of Health: R37-AG-011230 to N. R. This work was based in part on the doctoral dissertation of A. R. B.
Footnotes
The authors have no conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton JP, Brown MW. Interleaving brain systems for episodic and recognition memory. Trends Cogn Sci. 2006;10(10):455–463. doi: 10.1016/j.tics.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Assaf Y, Basser PJ. Composite hindered and restricted model of diffusion (CHARMED) MR imaging of the human brain. NeuroImage. 2005;27(1):48–58. doi: 10.1016/j.neuroimage.2005.03.042. [DOI] [PubMed] [Google Scholar]
- Assaf Y, Blumenfeld-Katzir T, Yovel Y, Basser PJ. Axcaliber: A method for measuring axon diameter distribution from diffusion MRI. Magnetic Resonance in Medicine. 2008;59(6):1347–1354. doi: 10.1002/mrm.21577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axer H, Keyserlingk DG. Mapping of fiber orientation in human internal capsule by means of polarized light and confocal scanning laser microscopy. J Neurosci Methods. 2000;94(2):165–175. doi: 10.1016/s0165-0270(99)00132-6. [DOI] [PubMed] [Google Scholar]
- Bach M, Laun FB, Leemans A, Tax CM, Biessels GJ, Stieltjes B, Maier-Hein KH. Methodological considerations on tract-based spatial statistics (TBSS) Neuroimage. 2014;100:358–369. doi: 10.1016/j.neuroimage.2014.06.021. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Sultzer D, Lu PH, Nuechterlein KH, Mintz J, Cummings JL. Heterogeneous age-related breakdown of white matter structural integrity: implications for cortical “disconnection” in aging and Alzheimer’s disease. Neurobiology of Aging. 2004;25(7):843–851. doi: 10.1016/j.neurobiolaging.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The biological basis of diffusion anisotropy. In: Johansen-Berg H, Behrens TE, editors. Diffusion MRI: From quantitative measurement to In vivo neuroanatomy. Academic Press; 2012. pp. 106–126. [Google Scholar]
- Bender AR, Daugherty AM, Raz N. Vascular risk moderates associations between hippocampal subfield volumes and memory. J Cogn Neurosci. 2013;25(11):1851–1862. doi: 10.1162/jocn_a_00435. [DOI] [PubMed] [Google Scholar]
- Bender AR, Naveh-Benjamin M, Raz N. Associative deficit in recognition memory in a lifespan sample of healthy adults. Psychology and Aging. 2010;25(4):940–948. doi: 10.1037/a0020595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender AR, Raz N. Age-related differences in memory and executive functions in healthy APOE varepsilon4 carriers: The contribution of individual differences in prefrontal volumes and systolic blood pressure. Neuropsychologia. 2012;50(5):704–714. doi: 10.1016/j.neuropsychologia.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender AR, Raz N. Normal-Appearing Cerebral White Matter in Healthy Adults: Mean Change over Two Years and Individual Differences in Change. Neurobiology of Aging. 2015;36(5):1834–1848. doi: 10.1016/j.neurobiolaging.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett IJ, Madden DJ. Disconnected aging: cerebral white matter integrity and age-related differences in cognition. Neuroscience. 2014;276:187–205. doi: 10.1016/j.neuroscience.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiet T, Vandenbulcke M, Madler B, Peeters R, Dhollander T, Zhang H, … Emsell L. Age-related microstructural differences quantified using myelin water imaging and advanced diffusion MRI. Neurobiol Aging. 2015;36(6):2107–2121. doi: 10.1016/j.neurobiolaging.2015.02.029. [DOI] [PubMed] [Google Scholar]
- Brehmer Y, Kalpouzos G, Wenger E, Lovden M. Plasticity of brain and cognition in older adults. Psychol Res. 2014;78(6):790–802. doi: 10.1007/s00426-014-0587-z. [DOI] [PubMed] [Google Scholar]
- Budde MD, Annese J. Quantification of anisotropy and fiber orientation in human brain histological sections. Frontiers in integrative neuroscience. 2013;7 doi: 10.3389/fnint.2013.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catheline G, Periot O, Amirault M, Braun M, Dartigues JF, Auriacombe S, Allard M. Distinctive alterations of the cingulum bundle during aging and Alzheimer’s disease. Neurobiology of Aging. 2010;31(9):1582–1592. doi: 10.1016/j.neurobiolaging.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Cattell RB, Cattell A. Measuring intelligence with the culture fair tests. Institute for Personality and Ability Testing; 1973. [Google Scholar]
- Charlton RA, Barrick TR, Markus HS, Morris RG. Verbal working and long-term episodic memory associations with white matter microstructure in normal aging investigated using tract-based spatial statistics. Psychol Aging. 2013;28:768–777. doi: 10.1037/a0032668. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Schiavone F, Barrick TR, Morris RG, Markus HS. Diffusion tensor imaging detects age related white matter change over a 2 year follow-up which is associated with working memory decline. Journal of Neurology, Neurosurgery & Psychiatry. 2010;81(1):13–19. doi: 10.1136/jnnp.2008.167288. [DOI] [PubMed] [Google Scholar]
- Cherry KE, Park DC. Individual difference and contextual variables influence spatial memory in younger and older adults. Psychology and Aging. 1993;8(4):517–526. doi: 10.1037//0882-7974.8.4.517. [DOI] [PubMed] [Google Scholar]
- De Santis S, Drakesmith M, Bells S, Assaf Y, Jones DK. Why diffusion tensor MRI does well only some of the time: variance and covariance of white matter tissue microstructure attributes in the living human brain. Neuroimage. 2014;89:35–44. doi: 10.1016/j.neuroimage.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs AR, Rule BG. Adult age differences in working memory. Psychology and Aging. 1989;4(4):500–503. doi: 10.1037//0882-7974.4.4.500. [DOI] [PubMed] [Google Scholar]
- Douaud G, Jbabdi S, Behrens TE, Menke RA, Gass A, Monsch AU, … Smith S. DTI measures in crossing-fibre areas: increased diffusion anisotropy reveals early white matter alteration in MCI and mild Alzheimer’s disease. NeuroImage. 2011;55(3):880–890. doi: 10.1016/j.neuroimage.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom RB, French JW, Harmon HH, Derman D. Manual for kit of factor-referenced cognitive tests. 1976. [Google Scholar]
- Engvig A, Fjell AM, Westlye LT, Moberget T, Sundseth O, Larsen VA, Walhovd KB. Memory training impacts short-term changes in aging white matter: A longitudinal diffusion tensor imaging study. Hum Brain Mapp. 2012;33(10):2390–2406. doi: 10.1002/hbm.21370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis DB, Kindlmann G. Orthogonal tensor invariants and the analysis of diffusion tensor magnetic resonance images. Magnetic Resonance in Medicine: Official journal of the Society of Magnetic Resonance in Medicine. 2006;55(1):136–146. doi: 10.1002/mrm.20741. [DOI] [PubMed] [Google Scholar]
- Erten-Lyons D, Woltjer R, Kaye J, Mattek N, Dodge HH, Green S, … Silbert LC. Neuropathologic basis of white matter hyperintensity accumulation with advanced age. Neurology. 2013;81(11):977–983. doi: 10.1212/WNL.0b013e3182a43e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazekas F, Schmidt R, Scheltens P. Pathophysiological mechanisms in the development of age-related white matter changes in the brain. Dementia and Geriatric Cognitive Disorders. 1998;9(Suppl 1):2–5. doi: 10.1159/000051182. [DOI] [PubMed] [Google Scholar]
- Flechsig P. Developmental (myelogenic) localisation of the cerebral cortex in the human subject. Lancet. 1901;158(4077):1027–1030. [Google Scholar]
- Foley JM, Salat DH, Stricker NH, Zink TA, Grande LJ, McGlinchey RE, … Leritz EC. Interactive effects of apolipoprotein E4 and diabetes risk on later myelinating white matter regions in neurologically healthy older aged adults. Am J Alzheimers Dis Other Demen. 2014;29(3):222–235. doi: 10.1177/1533317513517045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Ghisletta P, Lindenberger U. Static and dynamic longitudinal structural analyses of cognitive changes in old age. Gerontology. 2004;50(1):12–16. doi: 10.1159/000074383. [DOI] [PubMed] [Google Scholar]
- Guan T, Kong J. Functional regeneration of the brain: white matter matters. Neural Regen Res. 2015;10(3):355–356. doi: 10.4103/1673-5374.153675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon F, Raz N. The cognitive correlates of white matter abnormalities in normal aging: A Quantitative review. Neuropsychology. 2000;14(2):224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- Hanggi J, Koeneke S, Bezzola L, Jancke L. Structural neuroplasticity in the sensorimotor network of professional female ballet dancers. Hum Brain Mapp. 2010;31(8):1196–1206. doi: 10.1002/hbm.20928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines JH, Ravanelli AM, Schwindt R, Scott EK, Appel B. Neuronal activity biases axon selection for myelination in vivo. Nat Neurosci. 2015;18(5):683–689. doi: 10.1038/nn.3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstetter S, Tavor I, Moryosef ST, Assaf Y. Short-term learning induces white matter plasticity in the fornix. The Journal of Neuroscience. 2013;33(31):12844–12850. doi: 10.1523/JNEUROSCI.4520-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn JL, Cattell RB. Age differences in fluid and crystallized intelligence. Acta Psychologica. 1967;26:107–129. doi: 10.1016/0001-6918(67)90011-x. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, Hertzog C, Dixon RA. Ability correlates of memory performance in adulthood and aging. Psychology and Aging. 1990;5(3):356–368. doi: 10.1037//0882-7974.5.3.356. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Jeurissen B, Leemans A, Tournier JD, Jones DK, Sijbers J. Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Hum Brain Mapp. 2013;34(11):2747–2766. doi: 10.1002/hbm.22099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Cercignani M. Twenty-five pitfalls in the analysis of diffusion MRI data. NMR Biomed. 2010;23(7):803–820. doi: 10.1002/nbm.1543. [DOI] [PubMed] [Google Scholar]
- Jones DK, Christiansen KF, Chapman RJ, Aggleton JP. Distinct subdivisions of the cingulum bundle revealed by diffusion MRI fibre tracking: Implications for neuropsychological investigations. Neuropsychologia. 2013;51(1):67–78. doi: 10.1016/j.neuropsychologia.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Knosche TR, Turner R. White matter integrity, fiber count, and other fallacies: The do’s and don’ts of diffusion MRI. Neuroimage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Jöreskog KG, Sörbom D. LISREL 8: Structural equation modeling with the SIMPLIS command language. Lincolnwood, IL: Scientific Software; 1993. [Google Scholar]
- Kaes T. Die Grosshirnrinde des Menschen in ihren Massen und in ihrem Fasergehalt: ein gehirnanatomischer Atlas, mit erläuterndem Text. Fischer; 1907. [Google Scholar]
- Kennedy KM, Raz N. Aging white matter and cognition: Differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia. 2009;47:916–927. doi: 10.1016/j.neuropsychologia.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kievit RA, Frankenhuis WE, Waldorp LJ, Borsboom D. Simpson’s paradox in psychological science: A practical guide. Frontiers in Psychology. 2013;4:513. doi: 10.3389/fpsyg.2013.00513. http://doi.org/10.3389/fpsyg.2013.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KW, MacFall JR, Payne ME. Classification of white matter lesions on magnetic resonance imaging in elderly persons. Biological psychiatry. 2008;64(4):273–280. doi: 10.1016/j.biopsych.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloppenborg RP, Nederkoorn PJ, Geerlings MI, van den Berg E. Presence and progression of white matter hyperintensities and cognition: A meta-analysis. Neurology. 2014;82(23):2127–2138. doi: 10.1212/WNL.0000000000000505. [DOI] [PubMed] [Google Scholar]
- Kolind SH, Laule C, Vavasour IM, Li DKB, Traboulsee AL, Mädler B, … MacKay AL. Complementary information from multi-exponential T2 relaxation and diffusion tensor imaging reveals differences between multiple sclerosis lesions. NeuroImage. 2008;40(1):77–85. doi: 10.1016/j.neuroimage.2007.11.033. [DOI] [PubMed] [Google Scholar]
- Lindenberger U. Human cognitive aging: Corriger la fortune? Science. 2014;346(6209):572–578. doi: 10.1126/science.1254403. [DOI] [PubMed] [Google Scholar]
- Lockhart SN, Mayda AB, Roach AE, Fletcher E, Carmichael O, Maillard P, Schwarz CG, Yonelinas AP, Ranganath C, Decarli C. Episodic memory function is associated with multiple measures of white matter integrity in cognitive aging. Front Hum Neurosci. 2012;6:56. doi: 10.3389/fnhum.2012.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovden M, Kohncke Y, Laukka EJ, Kalpouzos G, Salami A, Li TQ, … Backman L. Changes in perceptual speed and white matter microstructure in the corticospinal tract are associated in very old age. Neuroimage. 2014;102(Pt 2):520–530. doi: 10.1016/j.neuroimage.2014.08.020. [DOI] [PubMed] [Google Scholar]
- MacKay A, Whittall K, Adler J, Li D, Paty D, Graeb D. In vivo visualization of myelin water in brain by magnetic resonance. Magnetic Resonance in Medicine. 1994;31(6):673–677. doi: 10.1002/mrm.1910310614. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Burzynska A, Potter GG, Chen NK, Song AW. Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochim Biophys Acta. 2012;1822(3):386–400. doi: 10.1016/j.bbadis.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhyastha T, Mérillat S, Hirsiger S, Bezzola L, Liem F, Grabowski T, Jäncke L. Longitudinal reliability of tract-based spatial statistics in diffusion tensor imaging. Human Brain Mapping. 2014 doi: 10.1002/hbm.22493. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mädler B, Drabycz SA, Kolind SH, Whittall KP, MacKay AL. Is diffusion anisotropy an accurate monitor of myelination? Correlation of multicomponent T2 relaxation and diffusion tensor anisotropy in human brain. Magn Reson Imaging. 2008;26(7):874–888. doi: 10.1016/j.mri.2008.01.047. [DOI] [PubMed] [Google Scholar]
- Maillard P, Carmichael O, Harvey D, Fletcher E, Reed B, Mungas D, DeCarli C. FLAIR and diffusion MRI signals are independent predictors of white matter hyperintensities. AJNR Am J Neuroradiol. 2013;34(1):54–61. doi: 10.3174/ajnr.A3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Papadimitriou GM, van der Kouwe A, Kennedy DN, Hodge SM, Dale AM, … Rosene DL. Frontal connections and cognitive changes in normal aging rhesus monkeys: a DTI study. Neurobiology of Aging. 2007;28(10):1556–1567. doi: 10.1016/j.neurobiolaging.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Malykhin N, Vahidy S, Michielse S, Coupland N, Camicioli R, Seres P, Carter R. Structural organization of the prefrontal white matter pathways in the adult and aging brain measured by diffusion tensor imaging. Brain Struct Funct. 2011;216(4):417–431. doi: 10.1007/s00429-011-0321-1. [DOI] [PubMed] [Google Scholar]
- Maniega SM, Valdes Hernandez MC, Clayden JD, Royle NA, Murray C, Morris Z, … Wardlaw JM. White matter hyperintensities and normal-appearing white matter integrity in the aging brain. Neurobiol Aging. 2015;36(2):909–918. doi: 10.1016/j.neurobiolaging.2014.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. The Journal of comparative neurology. 2003;462(2):144–152. doi: 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- Maxwell SE, Cole DA. Bias in cross-sectional analyses of longitudinal mediation. Psychol Methods. 2007;12:23–44. doi: 10.1037/1082-989X.12.1.23. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Hamgami F, Jones K, Jolesz F, Kikinis R, Spiro A, Albert MS. Structural modeling of dynamic changes in memory and brain structure using longitudinal data from the normative aging study. Journals of Gerontology Series B-Psychological Sciences and Social Sciences. 2004;59(6):P294–P304. doi: 10.1093/geronb/59.6.p294. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Nesselroade JR. Structuring data to study development and change. In: Cohen SH, Reese HW, editors. Life-Span Developmental Psychology: Methodological Innovations. Hillsdale, NJ: Erlbaum; 1994. pp. 223–267. [Google Scholar]
- Mori S. MRI Atlas of Human White Matter. Amsterdam, Netherlands: Elsevier; 2005. [Google Scholar]
- Mueller RO. Basic principles of structural equation modeling: An introduction to LISREL and EQS. New York: Springer Verlag; 1996. [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. 7. Los Angeles, CA: Muthén & Muthén; 2012. [Google Scholar]
- Nave KA. Myelination and support of axonal integrity by glia. Nature. 2010;468(7321):244–252. doi: 10.1038/nature09614. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M. Adult age differences in memory performance: Tests of an associative deficit hypothesis. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26(5):1170–1187. doi: 10.1037//0278-7393.26.5.1170. [DOI] [PubMed] [Google Scholar]
- Nielsen K, Peters A. The effects of aging on the frequency of nerve fibers in rhesus monkey striate cortex. Neurobiology of Aging. 2000;21(5):621–628. doi: 10.1016/s0197-4580(00)00169-x. [DOI] [PubMed] [Google Scholar]
- Old SR, Naveh-Benjamin M. Differential effects of age on item and associative measures of memory: A meta-analysis. Psychology and Aging. 2008;23(1):104–118. doi: 10.1037/0882-7974.23.1.104. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paus T, Pesaresi M, French L. White matter as a transport system. Neuroscience. 2014;276:117–125. doi: 10.1016/j.neuroscience.2014.01.055. [DOI] [PubMed] [Google Scholar]
- Persson N, Ghisletta P, Dahle CL, Bender AR, Yang Y, Yuan P, … Raz N. Regional brain shrinkage over two years: Individual differences and effects of pro-inflammatory genetic polymorphisms. Neuroimage. 2014;103C:334–348. doi: 10.1016/j.neuroimage.2014.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A. The effects of normal aging on myelin and nerve fibers: A review. Journal of neurocytology. 2002;31(8):581–593. doi: 10.1023/a:1025731309829. [DOI] [PubMed] [Google Scholar]
- Peters A. The effects of normal aging on myelinated nerve fibers in monkey central nervous system. Frontiers in neuroanatomy. 2009;3:11. doi: 10.3389/neuro.05.011.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Rosene DL, Moss MB, Kemper TL, Abraham CR, Tigges J, Albert MS. Neurobiological bases of age-related cognitive decline in the rhesus monkey. Journal of neuropathology and experimental neurology. 1996;55(8):861. doi: 10.1097/00005072-199608000-00001. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Cross-sectional versus longitudinal estimates of age-related changes in the adult brain: overlaps and discrepancies. Neurobiol Aging. 2015 doi: 10.1016/j.neurobiolaging.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Chu W, Sassoon SA, Rohlfing T, Pohl KM, … Sullivan EV. White matter microstructural recovery with abstinence and decline with relapse in alcohol dependence interacts with normal ageing: a controlled longitudinal DTI study. The Lancet Psychiatry. 2014;1(3):202–212. doi: 10.1016/S2215-0366(14)70301-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, … Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: A statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45(1):142–161. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magnetic Resonance in Medicine. 1996;36(6):893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Pollack I, Norman DA. A non-parametric analysis of recognition experiments. Psychonomic Science. 1964;1:125–126. [Google Scholar]
- Qiu C, Fratiglioni L. A major role for cardiovascular burden in age-related cognitive decline. Nature Reviews Cardiology. 2015;12(5):267–277. doi: 10.1038/nrcardio.2014.223. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Lowe C. Patterns of cognitive ageing. Psychological Research. 2000;63(3):308–316. doi: 10.1007/s004269900009. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Rao SM. White matter disease and dementia. Brain and cognition. 1996;31(2):250–268. doi: 10.1006/brcg.1996.0044. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: Evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12(1):95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U. Only time will tell: Cross-sectional studies offer no solution to the age-brain-cognition triangle: comment on Salthouse (2011) Psychol Bull. 2011;137(5):790–795. doi: 10.1037/a0024503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Ghisletta P, Rodrigue KM, Kennedy KM, Acker JD. Neuroanatomical correlates of fluid intelligence in healthy adults and persons with vascular risk factors. Cerebral Cortex. 2008;18(3):718–726. doi: 10.1093/cercor/bhm108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, … Acker JD. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cerebral Cortex. 2005;15(11):1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Yang YQ, Rodrigue KM, Kennedy KM, Lindenberger U, Ghisletta P. White matter deterioration in 15 months: latent growth curve models in healthy adults. Neurobiol Aging. 2012;33(2):429 e421–425. doi: 10.1016/j.neurobiolaging.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie SJ, Bastin ME, Tucker-Drob EM, Maniega SM, Engelhardt LE, Cox SR, … Pattie A. Coupled changes in brain white matter microstructure and fluid intelligence in later life. The Journal of Neuroscience. 2015;35(22):8672–8682. doi: 10.1523/JNEUROSCI.0862-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev P, Wen W, Chen X, Brodaty H. Progression of white matter hyperintensities in elderly individuals over 3 years. Neurology. 2007;68(3):214–222. doi: 10.1212/01.wnl.0000251302.55202.73. [DOI] [PubMed] [Google Scholar]
- Sadeghi N, Prastawa M, Fletcher PT, Wolff J, Gilmore JH, Gerig G. Regional characterization of longitudinal DT-MRI to study white matter maturation of the early developing brain. Neuroimage. 2013;68:236–247. doi: 10.1016/j.neuroimage.2012.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Using selective interference to investigate spatial memory representations. Memory & Cognition. 1974;2(4):749–757. doi: 10.3758/BF03198151. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Simultaneous processing of verbal and spatial information. Memory & Cognition. 1975;3(2):221–225. doi: 10.3758/BF03212901. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103(3):403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Influence of age on practice effects in longitudinal neurocognitive change. Neuropsychology. 2010;24(5):563–572. doi: 10.1037/a0019026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Neuroanatomical substrates of age-related cognitive decline. Psychological Bulletin. 2011;137(5):753–784. doi: 10.1037/a0023262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Fristoe N, McGuthry KE, Hambrick DZ. Relation of task switching to speed, age, and fluid intelligence. Psychology and aging. 1998;13(3):445. doi: 10.1037/0882-7974.13.3.445. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Hancock HE, Meinz EJ, Hambrick DZ. Interrelations of age, visual acuity, and cognitive functioning. The Journals of Gerontology: Series B, Social and Psychological Sciences. 1996;51(6):P317–330. doi: 10.1093/geronb/51b.6.p317. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Kausler DH, Saults JS. Investigation of student status, background variables, and feasibility of standard tasks in cognitive aging research. Psychology and Aging. 1988;3(1):29–37. doi: 10.1037//0882-7974.3.1.29. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Meinz EJ. Aging, inhibition, working memory, and speed. The Journals of Gerontology: Series B, Social and Psychological Sciences. 1995;50B(6):297–306. doi: 10.1093/geronb/50b.6.p297. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Tucker-Drob EM. Implications of short-term retest effects for the interpretation of longitudinal change. Neuropsychology. 2008;22(6):800–811. doi: 10.1037/a0013091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandell JH, Peters A. Disrupted myelin and axon loss in the anterior commissure of the aged rhesus monkey. The Journal of comparative neurology. 2003;466(1):14–30. doi: 10.1002/cne.10859. [DOI] [PubMed] [Google Scholar]
- Sarubbo S, De Benedictis A, Maldonado IL, Basso G, Duffau H. Frontal terminations for the inferior fronto-occipital fascicle: anatomical dissection, DTI study and functional considerations on a multi-component bundle. Brain Struct Funct. 2013;218(1):21–37. doi: 10.1007/s00429-011-0372-3. [DOI] [PubMed] [Google Scholar]
- Sasson E, Doniger GM, Pasternak O, Tarrasch R, Assaf Y. White matter correlates of cognitive domains in normal aging with diffusion tensor imaging. Front Neurosci. 2013;7 doi: 10.3389/fnins.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Roob G, Kapeller P, Schmidt H, Berghold A, Lechner A, Fazekas F. Longitudinal change of white matter abnormalities. Springer; 2000. [DOI] [PubMed] [Google Scholar]
- Schmiedek F, Lövdén M, Lindenberger U. Hundred days of cognitive training enhance broad cognitive abilities in adulthood: Findings from the COGITO study. Frontiers in aging neuroscience. 2010;2 doi: 10.3389/fnagi.2010.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schretlen D, Pearlson GD, Anthony JC, Aylward EH, Augustine AM, Davis A, Barta P. Elucidating the contributions of processing speed, executive ability, and frontal lobe volume to normal age-related differences in fluid intelligence. Journal of the International Neuropsychological Society. 2000;6(1):52–61. doi: 10.1017/s1355617700611062. [DOI] [PubMed] [Google Scholar]
- Sexton CE, Walhovd KB, Storsve AB, Tamnes CK, Westlye LT, Johansen-Berg H, Fjell AM. Accelerated changes in white matter microstructure during aging: A longitudinal diffusion tensor imaging study. J Neurosci. 2014;34(46):15425–15436. doi: 10.1523/JNEUROSCI.0203-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E. The interpretation of interaction in contingency tables. J Royal Stat Soc, Series B. 1951;13:238–241. [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, … Behrens TE. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. NeuroImage. 2003;20(3):1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26(1):132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Spencer WD, Raz N. Differential effects of aging on memory for content and context: a meta-analysis. Psychology and Aging. 1995;10(4):527–539. doi: 10.1037//0882-7974.10.4.527. [DOI] [PubMed] [Google Scholar]
- Stanislaw H, Todorov N. Calculation of signal detection theory measures. Behavior Research Methods, Instruments, & Computers. 1999;31(1):137–149. doi: 10.3758/bf03207704. [DOI] [PubMed] [Google Scholar]
- Stewart KJ. Exercise training and the cardiovascular consequences of type 2 diabetes and hypertension: plausible mechanisms for improving cardiovascular health. JAMA: The journal of the American Medical Association. 2002;288(13):1622–1631. doi: 10.1001/jama.288.13.1622. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of experimental psychology. 1935;18(6):643. [Google Scholar]
- Sun SW, Liang HF, Cross AH, Song SK. Evolving Wallerian degeneration after transient retinal ischemia in mice characterized by diffusion tensor imaging. NeuroImage. 2008;40(1):1–10. doi: 10.1016/j.neuroimage.2007.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SW, Liang HF, Le TQ, Armstrong RC, Cross AH, Song SK. Differential sensitivity of in vivo and ex vivo diffusion tensor imaging to evolving optic nerve injury in mice with retinal ischemia. NeuroImage. 2006;32(3):1195–1204. doi: 10.1016/j.neuroimage.2006.04.212. [DOI] [PubMed] [Google Scholar]
- Tang Y, Nyengaard JR, Pakkenberg B, Gundersen HJ. Age-induced white matter changes in the human brain: a stereological investigation. Neurobiology of Aging. 1997;18(6):609–615. doi: 10.1016/s0197-4580(97)00155-3. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Bokde ALW, Meindl T, Amaro E, Jr, Soldner J, Reiser MF, … Hampel H. White matter microstructure underlying default mode network connectivity in the human brain. NeuroImage. 2010;49(3):2021–2032. doi: 10.1016/j.neuroimage.2009.10.067. [DOI] [PubMed] [Google Scholar]
- Tseng BY, Gundapuneedi T, Khan MA, Diaz-Arrastia R, Levine BD, Lu H, … Zhang R. White matter integrity in physically fit older adults. Neuroimage. 2013;82:510–516. doi: 10.1016/j.neuroimage.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuch DS, Reese TG, Wiegell MR, Makris N, Belliveau JW, Wedeen VJ. High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn Reson Med. 2002;48(4):577–582. doi: 10.1002/mrm.10268. [DOI] [PubMed] [Google Scholar]
- Vernooij MW, de Groot M, van der Lugt A, Ikram MA, Krestin GP, Hofman A, … Breteler MM. White matter atrophy and lesion formation explain the loss of structural integrity of white matter in aging. NeuroImage. 2008;43(3):470–477. doi: 10.1016/j.neuroimage.2008.07.052. [DOI] [PubMed] [Google Scholar]
- Vik A, Hodneland E, Haasz J, Ystad M, Lundervold AJ, Lundervold A. Fractional anisotropy shows differential reduction in frontal-subcortical fiber bundles - A longitudinal MRI study of 76 middle-aged and older adults. Front Aging Neurosci. 2015;7:81. doi: 10.3389/fnagi.2015.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos SB, Jones DK, Viergever MA, Leemans A. Partial volume effect as a hidden covariate in DTI analyses. Neuroimage. 2011;55(4):1566–1576. doi: 10.1016/J.Neuroimage.2011.01.048. [DOI] [PubMed] [Google Scholar]
- Vos SB, Jones DK, Jeurissen B, Viergever MA, Leemans A. The influence of complex white matter architecture on the mean diffusivity in diffusion tensor MRI of the human brain. Neuroimage. 2012;59(3):2208–2216. doi: 10.1016/j.neuroimage.2011.09.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, … Dubey P. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36(3):630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Johansen-Berg H, Karadottir RT. Unraveling the secrets of white matter–Bridging the gap between cellular, animal and human imaging studies. Neuroscience. 2014;276:2–13. doi: 10.1016/j.neuroscience.2014.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Casadio M, Weber KA, 2nd, Mussa-Ivaldi FA, Parrish TB. White matter microstructure changes induced by motor skill learning utilizing a body machine interface. Neuroimage. 2013;88C:32–40. doi: 10.1016/j.neuroimage.2013.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler-Kingshott CAM, Cercignani M. About “axial” and “radial” diffusivities. Magn Reson Med. 2009;61(5):1255–1260. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- Woodcock R, Johnson M, Battery-Revised WJPE. Tests of cognitive ability. DLM Teaching Resources; Allen, TX: 1989. [Google Scholar]
- Woodcock RW, Mather N. WJ-R tests of achievement: Examiner’s manual. In: Woodcock RW, Johnson MB, editors. Woodcock-Johnson Psycho-Educational Battery–Revised. Allen, TX: DLM Teaching Resources; 1989. [Google Scholar]
- Yang L. Practice-oriented retest learning as the basic form of cognitive plasticity of the aging brain. J Aging Res. 2011;2011:407074. doi: 10.4061/2011/407074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan W, Zhang Y, Mueller SG, Lorenzen P, Hadjidemetriou S, Schuff N, Weiner MW. Characterization of white matter degeneration in elderly subjects by magnetic resonance diffusion and FLAIR imaging correlation. NeuroImage. 2009;47(Suppl 2):T58–65. doi: 10.1016/j.neuroimage.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.