Abstract
Unrecognized in-hospital cardiorespiratory instability (CRI) risks adverse patient outcomes. Although step down unit (SDU) patients have continuous non-invasive physiologic monitoring of vital signs and a ratio of 1 nurse to 4–6 patients, detection of CRI is still suboptimal. Telemedicine provides additional surveillance but, due to high costs and unclear investment returns, is not routinely used in SDUs. Rapid response teams have been tested as possible approaches to support CRI patients outside the intensive care unit with mixed outcomes. Technology-enabled early warning scores, though rigorously studied, may not detect subtle instability. Efforts to utilize nursing intuition as a means to promote early identification of CRI have been explored, but the problem still persists. Monitoring systems hold promise, but nursing surveillance remains the key to reliable early detection and recognition. Research directed toward improving nursing surveillance and facilitating decision-making is needed to ensure safe patient outcomes and prevent CRI.
Keywords: Vital signs, Physiologic Monitoring, cardiorespiratory instability, early warning scores, rapid response systems, medical emergency team, nursing surveillance
Introduction
Unrecognized cardiorespiratory instability (CRI) risks adverse patient outcomes (Schien, Hazday, Pena, Ruben, & Sprung, 1990; Hillman et al., 2001; Franklin & Mathew, 1994). CRI, defined as abnormalities in heart rate (HR), respiratory rate (RR), blood pressure (BP), and peripheral oxygenation by pulse oximetry (SpO2) that precede an adverse event, may not be detected until late in the instability course or until cardiopulmonary arrest. Nursing surveillance alone has not been successful in detecting CRI early enough to prevent negative outcomes (Henneman, Gawlinski, & Giuliano, 2012). Telemedicine and rapid response systems have been advocated as possible approaches to prevent adverse events, with mixed outcomes (Kerlin et al., 2013; Venkataraman & Ramakrishnan, 2015; Winters et al., 2013). Utilization of early warning scoring systems, with or without automated calculation and notification, has resulted in improvement, but these systems also have deficiencies (Alam et al., 2014). Even mature rapid response systems, with track-and-trigger systems based on intermittent patient evaluation, miss avoidable cardiopulmonary arrest (Trinkle & Flabouris, 2011). The purpose of this review is to use the literature to analyze current approaches for early detection of CRI, identify why early detection is problematic, and make recommendations that may lead to improved detection.
Methods
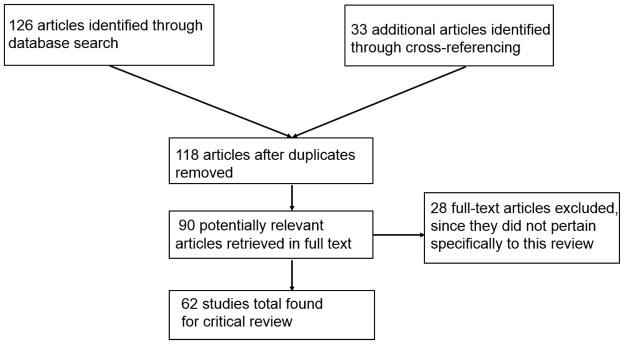
This narrative review was guided by the flowchart described by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement (Moher, Liberati, Tetzlaff, & Altman, 2009). A computerized search of literature was conducted using CINAHL via EBSCO host. Subject headings used in the search included “cardiac arrest,” “nursing surveillance,” “telemedicine,” “rapid response systems” and “early warning scores.” Additional relevant articles for review were found through cross-referencing. The review was not limited by study design but was restricted to full text articles published only in English, on adults (19 and above) and within the past 10 years. Of the 159 articles returned, 62 were chosen for inclusion in the review while the rest were not specific to the review purpose as stated (Figure 1).
Figure 1.
Literature search trail using PRISMA flowchart.
Outcomes of in-hospital cardiac arrest and evolving CRI
Once a patient experiences in-hospital cardiac arrest (IHCA), patient outcomes are poor. In a study that reported outcomes following IHCA in 732 patients, only 6.6% lived to discharge, 5.2% for 1 year, and 3% for 3 years (Bloom et al., 2007). There is some evidence to suggest that CRI prior to its progression to IHCA is missed. In an international multi-center study of 634 patients with IHCA by Kause et al. 79.4% had CRI evolving between 4 and 24 hours prior to the event (Kause et al., 2004). In another study by Rozen et al., all IHCA patients exhibited signs of evolving CRI in the preceding 12 hours, and > 50% experienced in-hospital mortality (Rozen et al., 2014). Another report examining the records of 14,720 adult patients before IHCA reported that few (44%) had return of spontaneous circulation and only 17% survived to discharge (Perberdy et al., 2003). These reports demonstrate that survival is poor once IHCA has occurred and suggest there may be missed opportunity to recognize instability and apply supportive interventions preceding IHCA.
Factors that impact poor detection of CRI
Nursing surveillance, a process wherein nurses assess patients on a routine or as-needed basis to evaluate and act on emerging indicators of a status change is a central nursing function that directly impacts patient outcomes (Bulechek, Butcher, Dochterman, & Wagner, 2012). Although technology can improve assessment sensitivity, failure to notice subtle changes in VS over time prevents nurses from intervening to reverse the process.
Several reasons have been posed to explain why subtle status changes may go undetected. Patient mortality has been shown to be higher as a nurse’s patient caseload increases (Aiken et al., 2011; Beaudoin & Edgar, 2003; Ebright, Patterson, Chalko, & Render, 2003; Needleman et al., 2011; Patrician et al., 2011). However, staffing level alone does not appear to influence CRI detection but, rather, staffing in combination with other nurse characteristics, such as education level and familiarity with the care required (Aiken et al., 2012; Blegen, Goode, Park, Vaughn, & Spetz, 2013). In one study, CRI was less likely to be detected when patients were admitted to units where nurses were less familiar with their care, or when cared for by agency or “pulled” staff (Schmid-Mazzoccoli, Hoffman, Wolf, Happ, & DeVita, 2008). Frequent interruption of nurses during care provision also negatively impacts ability to recognize a patient’s changing condition (Page, 2004; Potter et al., 2005).
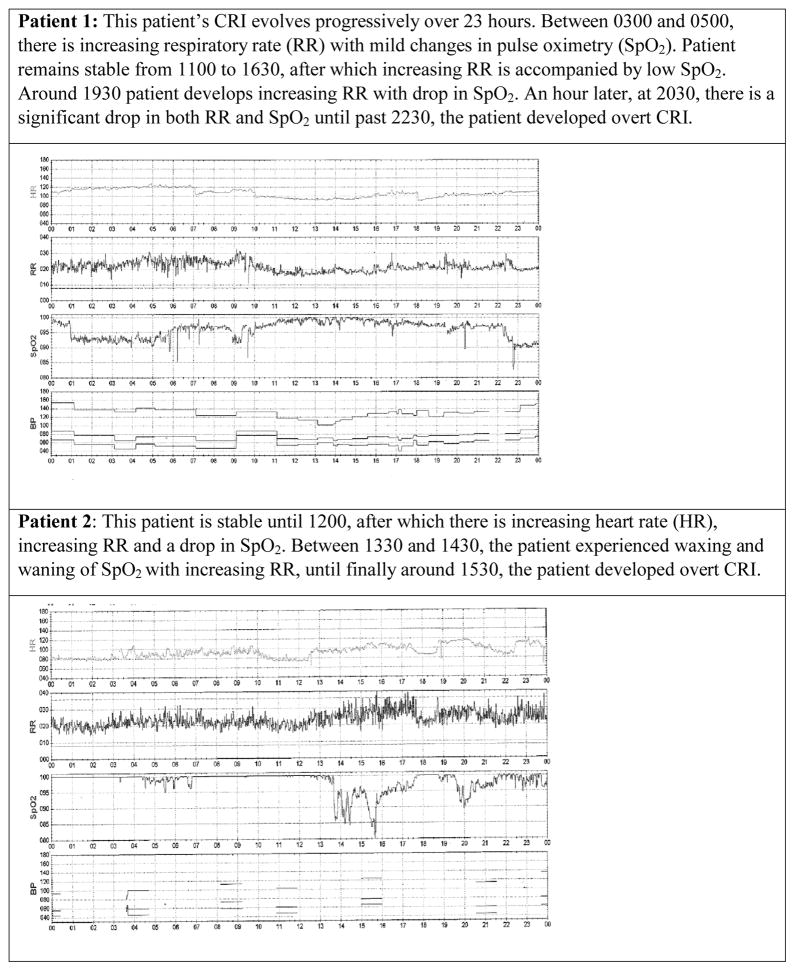
An additional factor that may contribute to unrecognized CRI lies within the very nature of the evolving CRI process. Unless due to an abrupt catastrophic event such as pulmonary embolism, CRI is more commonly due to processes that evolve slowly over time (Hravnak et al., 2008; Hravnak et al., 2011). Also, VS changes may wax and wane between normal and abnormal (Figure 2). With only intermittent evaluation, patients may be seen by a nurse during periods of seeming stability when, in fact, CRI is present and progressing. This waxing and waning may contribute to nurses feeling unsure that the patient is truly unstable and in need of care escalation.
Figure 2.
Waxing and waning of vital signs over time between normal and abnormal as patients proceed to overt cardiorespiratory instability (CRI).
Telemedicine and Rapid Response Teams
Attempts to provide earlier treatment of CRI focus on two approaches. The first--telemedicine--provides continuous monitoring, teleconferencing, quick-consultation and advice from experienced physicians around the clock to sites without specialized resources, with the goal of more promptly and appropriately managing any acute patient status change. At present, this model of surveillance is most commonly deployed in intensive care units (ICUs). Hospitals adopting this system are typically large (> 400 beds), teaching and urban (Kahn, Cicero, Wallace, & Iwashyna, 2014). Telemedicine is expensive, with start-up costs ranging from $45,000–$50,000 per bed and annual costs between $28,000–$32,000 per bed (Ries, 2009) that may not be reimbursed by insurance (Alverson et al., 2004; Kumar et al., 2013). Benefits of ICU telemedicine are conflicting (Wootton, 2012) with some experts positing that expenditures are regained by reductions in ICU length of stay and in-hospital complications (Breslow, 2007), while others report no cost offset (Franzini, Sail, Thomas, & Wueste, 2011; Morrison et al., 2010). Additional concerns relate to medical liability (Hoffman & Rowthorn, 2011; Sao, Gupta, & Gantz, 2012; Whitten & Mackert, 2005), loss of privacy (Sao et al., 2012), lack of control over remote physician practices (Alverson et al., 2004), and unclear investment return (Moehr et al., 2006). In addition to ICUs, telemedicine has been used in emergency department based evaluation of acute stroke (Schwamm et al., 2004) and for paramedics in the field to communicate with coronary care units (Keane, 2009). Telemedicine has also been implemented in psychiatric settings to evaluate patients who live in rural areas (Norman, 2006; Holden & Dew, 2008). In acute care settings, such as patient wards or units designated for transitional care, e.g., step down units (SDUs), telemedicine is generally not used even though patient to nurse ratios are higher than in ICUs, and instability is frequently unrecognized (Hravnak et al., 2008; Nangalia, Prytherch, & Smith, 2010).
A second option involves utilizing a rapid response system (RRS) and medical emergency team (MET) to address needs of unstable patients outside the ICU (DeVita et al., 2010). The MET is usually a multidisciplinary team composed of medical, nursing, and respiratory therapy staff who can be summoned immediately to the patient’s bedside at any time for prompt evaluation, triage, and treatment of clinical deterioration (Chan, Jain, Nallamothu, Berg, & Sasson, 2010). The RRS is comprised of an “afferent arm” consisting of some system for “track and trigger,” meaning an established set of conditions or rules whereby unstable patients can be identified (tracked) and then an organized system whereby the clinician (or family member) can place a call for help (trigger) as shown in Table 1. This component is accompanied by the RRS “efferent arm”, i.e., MET deployment, organization, membership, systems, equipment, and protocols. The MET can assess and treat patients in whom respiratory, neurologic, or cardiac deterioration is in process (Jones, DeVita, & Bellomo, 2011).
Table 1.
Example of thresholds of vital sign abnormalities of changes in patient condition use to “track” for instability and the “trigger” deployment of the Medical Emergency Team (Hravnak et al., 2008).
| Cardiorespiratory system |
| Respirations < 8/min or > 36/min |
| New onset of breathing difficulty |
| New pulse oximeter reading < 85% for > 5 minutes, unless patient is known to have chronic hypoxemia |
| Heart rate < 40 beats/min or >140 beats/min with new symptoms or any rate > 160 beats/min |
| Blood pressure: systolic < 80 or >200 mm Hg or diastolic 110 mm Hg with symptoms (neurologic change, chest pain, or breathing difficulty) |
|
|
| Neurologic system |
| Acute loss of consciousness |
| New onset of lethargy or difficulty in waking |
| Sudden collapse |
| Seizure (outside of seizure monitoring unit) |
| Sudden loss of mobility (or weakness) of face, arm, or leg |
|
|
| Other criteria |
| More than 1 stat page required to assemble MET needed to respond to a crisis |
| Patient report of (cardiac) chest pain (unresponsive to nitroglycerine or physician unavailable) |
| Color change in patient or extremity to pale, dusky, gray or blue |
| Unexplained agitation for > 10 min |
| Suicide attempt |
| Uncontrolled bleeding |
| Bleeding into airway |
| Naloxone hydrochloride use without immediate response |
| Large volume of short-term blood loss |
| Crash cart must be used for rapid delivery of medications |
In the most comprehensive evaluation of benefit, a multicenter cluster-randomized controlled trial called the Medical Early Response Intervention and Therapy (MERIT) study failed to demonstrate a reduction in mortality (Hillman et al., 2005) with MET use. However, post-hoc analysis (Chen et al., 2009) showed that the study reported all deaths associated with RRS calls, some of which occurred after IHCA had already developed. When these patients were excluded, and only unstable patients prior to an arrest state were included, there was a benefit.
Nevertheless, a MET team can only be deployed when called to help. In one setting with a longstanding RRS and clear call guidelines, of 108 MET calls on medical-surgical units, 44% were delayed (documented evidence that pre-established criteria for a MET call were present for > 30 minutes) (Schmid-Mazzoccoli et al., 2008). Several reasons have been posed for failure to recognize CRI, including episodic or abbreviated recording of VS, e.g., RR monitored for 15 seconds and multiplied by 4 (Ludikhuize, DeRooij, Sophia, & DeJonge, 2012), failure to recognize the significance of VS changes, and the belief that the problem can be managed without additional support (Galhotra, DeVita, Simmons, & Dew, 2007; Ludikhuize et al., 2012; Trinkle & Flabouris, 2011).
Several recent studies provide findings which expand understanding of factors that prompt or delay response to CRI. Wynn et al. reported that nurses prepared at the baccalaureate level or with less than 3 years of experience were less likely to self-initiate a MET call (Wynn, Engelke, & Swanson, 2009). Leach et al. identified traditional hierarchies and relationships with physicians and supervisors as factors that could delay the decision to initiate a MET call (Leach, Mayo, & O’Rourke, 2010). Astroth et al. reported that support and encouragement from nursing unit colleagues and leaders were facilitators to MET activation, whereas the decision to call a physician first delayed activation (Astroth, Woith, Stapleton, Degitz, & Jenkins, 2013). Conversely, sharing of concerns with colleagues who provided verification of the problem led to prompt notification of the RRT and prevented adverse events (Leach, Mayo, & O’Rourke, 2010).
Technology-enabled early warning scores
Early warning scoring (EWS) systems and modified early warning scores (MEWS) have been developed and utilized to calculate a single composite score from multiple noninvasively acquired VS parameters. EWS objectively quantify concern to intervene or request additional support and are therefore less influenced by individual opinion (DeVita et al., 2010; Gardner-Thorpe, Love, Wrightson, Walsh, & Keeling, 2006; Subbe, Gao, & Harrison, 2007). EWS are typically designed to be bedside scoring systems for use by nurses or other clinicians who collect data via handwritten score cards or, more recently, personal digital assistants (PDAs). Technologically assisted EWS, also known as aggregate weighted track and trigger systems (AWTTS), are available from a variety of manufacturers, e.g. VitalPAC™, The Learning Clinic (http://www.thelearningclinic.co.uk/vitalpac.html). PDA-based applications allow VS data to be linked by a wireless network to the hospital’s information system. When this system is used, data is integrated with other patient information, e.g., lab results, and programmed to make direct contact with the MET team through an automated alerting system (Smith et al., 2006).
Since 2012, the National Health Service in the United Kingdom has been mandated to use the National Early Warning Score (NEWS), a commercially available EWS, nationally. In one report, data (RR, HR, SpO2, supplemental oxygen [Yes/No], temperature [°C], systolic blood pressure [mmHg] and level of consciousness using the Alert-Verbal-Painful-Unresponsive [AVPU] system), were entered whereupon VitalPAC® software automatically computed the score as shown in Table 2. The NEWS was then assessed to determine its ability to derive scores that identified patients with or without cardiac arrest. In order to perform this comparison, they used a measurement process termed area under receiving operator characteristics (AUROC) curves. In terms of discrimination, an AUROC value between 0.7 and 0.8 indicates fair, a value between 0.8 and 0.9 indicates good and a value > 0.9 is excellent. When assessed in this manner, NEWS achieved an AUROC value of 0.722 (95% CI 0.685–0.759) for IHCA discrimination which was better than any other EWS system evaluated (Smith, Prytherch, Meredith, Schmidt, & Featherstone, 2013).
Table 2.
The National Early Warning Score (NEWS) (adapted from Smith et al., 2013)
| Physiological Parameters | 3 | 2 | 1 | 0 | 1 | 2 | 3 |
|---|---|---|---|---|---|---|---|
| Respiration Rate (bpm) | ≤ 8 | 9–11 | 12–20 | 21–24 | ≥ 25 | ||
| Oxygen Saturation (%) | ≤ 91 | 92–93 | 94–95 | ≥96 | |||
| Any Supplemental Oxygen | Yes | No | |||||
| Temperature (°C) | < 35.0 | 35.1–36.0 | 36.1–38.0 | 38.1–39.0 | ≥ 39.1 | ||
| Systolic BP (mm Hg) | ≤ 90 | 91–100 | 101–110 | 111–219 | ≥ 220 | ||
| Heart Rate | ≤ 40 | 41–50 | 51–90 | 91–110 | 111–130 | ≥ 131 | |
| Level of Consciousness | A | V,P,U |
Definition of abbreviations: A = alert; V = responds to voice; P = responds to pain; U = unresponsive
Another EWS--VitalPAC™ EWS—(ViEWS) has been tested in a similar manner. ViEWS is used to enter data that includes HR, RR, temperature, systolic BP, SpO2, fraction of inspired oxygen (FiO2), and level of consciousness (AVPU scale), as recommended by the National Institute for Health and Clinical Excellence (Armitage, Eddelston, & Stokes, 2007). Using ViEWS, the authors were able to predict in-hospital mortality within 24 h of a given observation with a much higher AUROC (0.888) compared to 33 other AWTTS whose AUROCs ranged from 0.803 to 0.850 (Prytherch, Smith, Schmidt, & Featherstone, 2010). However, in a randomized controlled trial, Kollef et al. reported that alerts calculated by EWS and sent directly to the RRS did not reduce in-hospital mortality (odds ratio = 0.944, 95% CI: 0.509–1.764) (Kollef et al., 2014). This literature continues to evolve. Churpek et al. recently reported that a cardiac arrest risk triage score (CART) was more accurate than ViEWS for predicting cardiac arrest (CART AUROC 0.88 vs. ViEWS 0.78, p<0.001) (Churpek, Yuen, Park, Gibbons, & Edelson, 2014).
Although EWS tools can amalgamate intermittently determined VS data and determine when a threshold value is reached, such systems do not interface with continuous monitoring and therefore may not detect instability that waxes and wanes, a factor that may explain conflicting study findings. Romero-Brufau et al. concluded that the most widely used AWTTS do not offer good predictive capabilities and called for better criteria to be developed and validated (Romero-Brufau et al., 2014). A more ideal goal would be a system that could continuously and in “real-time” predict patients likely to become unstable in the future allowing for preemptive, rather than reactive, intervention.
Nursing Intuition
In addition to using objective measures, nurses often recognize a deteriorating patient based on intuition (Boniatti et al., 2010; Odell, Victor, & Oliver, 2009) or “gut-feeling” (Hams, 2000), described by Benner et al. as ‘judgment without a rationale’(Benner, Tanner, & Chesla, 2009). A concept analysis on nursing intuition by Robert et al. noted that intuition develops as a consequence of experiencing and, in turn, developing the ability to recognize antecedents to a situation (Robert, Tilley, & Petersen, 2014). The ability to identify antecedents is gained from knowledge, experience and pattern recognition skills. Pattern recognition can include constellations of quantitative data such as vital signs or mental status scales, but also more subjective information, such as subtle change in appearance or mentation and communication. This information is then used to envision a consequence. Thus, nursing intuition may not be well developed until experience progresses—perhaps at the expense of unrecognized instability as this skill evolves.
One approach to incorporating nursing intuition into CRI recognition entails adding the term “worry” or “concern” to MET trigger criteria to facilitate activation when deterioration is believed to be present, but signs are vague (Chen et al., 2009; Douw et al., 2015). A second approach involves insuring a supportive environment to report concerns. Nurses often validate intuitive observations by first asking colleagues’ opinion and, if they encounter criticism may hesitate to share concerns. It has therefore been suggested that nursing intuition can be optimized through initiatives designed to promote a positive environment for early sharing of concerns. It is also important to note that insights gained from nursing intuition can only be applied when information becomes known to the nurse—during a patient encounter or review of incoming information. Thus, the same constraints on nurse-to-patient encounters which apply to the previous discussion on nursing surveillance. Multiple factors, e.g., intermittent evaluation, seeming stability, familiarity with the patient, impact nurses’ ability to apply intuition even though they have enough experience to develop this sense.
Clinical Implications
Newly developed technology and EWS offer the promise of better detection and recognition of CRI, but are imperfect. MET activation presents the advantage of bringing a skilled team immediately to the bedside to assist in care triage, but can only act when summoned. Despite literature devoted to identifying ways to detect CRI sooner, and extensive resources dedicated to developing technology designed to reliably detect CRI and institute a support structure to bring needed care to the bedside of deteriorating patients, problems persist. In the absence of a “perfect” solution, nurse surveillance continues to be a critical factor. To encourage best practices in recognizing CRI at the bedside, the following are recommended:
Establish clear guidelines for initiating a MET call
METs have been driven by the belief that they make hospitals safer by preventing adverse events due to CRI. To fully embrace this system, nurses need clear and established unit-based guidelines for MET activation—both the circumstances triggering a call, and unimpeded means to place the call.
Provide education regarding adverse outcomes
Staff education about the importance of accurate VS measurements and the adverse outcomes of unrecognized CRI and IHCA is vital. There must be proper training and strict adherence to standards especially by care technicians and early notification of the primary nurse or other care providers (or MET activation) when CRI is detected.
Avoid behaviors that discourage prompt notification
Since MET activation is vital to support deteriorating patients, barriers to MET activation by nurses, such as criticism of their actions or expertise should be avoided. Nurses who believe their actions positively impact patient outcomes are more likely to implement them.
Share positive outcomes following CRI detection with the team as a way of promoting best practices
Staff nurses need validation of the positive consequences of their actions. Too often, the focus is on what did not succeed, rather than success.
Regardless of the approach used, nursing surveillance is critically important in detecting CRI. Research directed towards improving and enhancing nurses’ ability nurses to monitor caseloads of patients and recognize evolving CRI earlier in SDUs will ensure better patient outcomes.
Implications for Clinical Practice.
Establish clear guidelines for initiating a medical emergency team (MET) call
Provide education regarding adverse outcomes
Avoid behaviors that discourage prompt notification
Share positive outcomes following cardiorespiratory instability detection with the team as a way of promoting best practices.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Eliezer Bose, Email: eliezerbose@gmail.com, elb93@pitt.edu, School of Nursing, University of Pittsburgh, 3500 Victoria St., 336 Victoria Building, Pittsburgh, PA 15261, USA.
Leslie Hoffman, Email: lhof@pitt.edu, School of Nursing, University of Pittsburgh, 3500 Victoria St., 336 Victoria Building, Pittsburgh, PA 15261, USA.
Marilyn Hravnak, Email: mhra@pitt.edu, School of Nursing, University of Pittsburgh, 3500 Victoria St., 336 Victoria Building, Pittsburgh, PA 15261, USA.
References
- Aiken LH, Cimiotti JP, Sloan DM, Smith HL, Flynn L, Neff DF. Effects of nurse staffing and nurse education on patient deaths in hospitals with different nurse work environments. Journal of Nursing Administration. 2012;42(10 Suppl):S10–16. doi: 10.1097/01.NNA.0000420390.87789.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiken LH, Cimiotti JP, Sloane DM, Smith HL, Flynn L, Neff DF. The effects of nurse staffing and nurse education on patient deaths in hospitals with different nurse work environments. Medical Care. 2011;49(12):1047–1053. doi: 10.1097/MLR.0b013e3182330b6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam N, Hobbelink EL, Van Tienhoven AJ, Van de Ven PM, Jansma EP, Nanayakkara PW. The impact of the use of Early Warning Score (EWS) on patient outcomes: A Systematic Review. Resuscitation. 2014;85(5):587–594. doi: 10.1016/j.resuscitation.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Alverson DC, Shannon S, Sullivan E, Prill A, Effertz G, Helitzer D, Preston A. Telehealth in the trenches: reporting back from the frontlines in rural America. Telemedicine Journal & e-Health. 2004;10(Suppl 2):S95–S109. [PubMed] [Google Scholar]
- Armitage M, Eddelston J, Stokes T. NICE guidelines: Recognizing and responding to acute illness in adults in hospital: Summary of NICE guidelines. British Medical Journal. 2007;335(7613):258. doi: 10.1136/bmj.39272.679688.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astroth KS, Woith WM, Stapleton SJ, Degitz RJ, Jenkins SH. Qualitative exploration of nurses’ decisions to activate rapid response teams. Journal of Clinical Nursing. 2013;22:2876–2882. doi: 10.1111/jocn.12067. [DOI] [PubMed] [Google Scholar]
- Beaudoin LE, Edgar L. Hassles: their importance to nurses’ quality of work life. Nurs Econ. 2003;21(3):106–113. [PubMed] [Google Scholar]
- Benner PE, Tanner CA, Chesla CA. Expertise in Nursing Practice: Caring, Clinical Judgment and ethics. 2. New York: Springer Publishing Company; 2009. [Google Scholar]
- Blegen MA, Goode CJ, Park SH, Vaughn T, Spetz J. Baccalaureate education in nursing and patient outcomes. Journal of Nursing Administration. 2013;43(2):89–94. doi: 10.1097/NNA.0b013e31827f2028. [DOI] [PubMed] [Google Scholar]
- Bloom HL, Shukrullah I, Cuellar JR, Lloyd MS, Dudley SC, Samuel C, Zafari AM. Long-term survival after successful inhospital cardiac arrest resuscitation. American Heart Journal. 2007;153(5):831–836. doi: 10.1016/j.ahj.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boniatti MM, Azzolini N, DaFonseca DL, Ribeiro BS, DeOliveira VM, Castilho RK, Edison M. Prognostic value of the calling criteria in patients receiving a medical emergency team review. Resuscitation. 2010;81(6):667–670. doi: 10.1016/j.resuscitation.2010.01.025. [DOI] [PubMed] [Google Scholar]
- Breslow MJ. Remote ICU care programs: current status. Journal of Critical Care. 2007;22(1):66–76. doi: 10.1016/j.jcrc.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Buist M, Bernard S, Nguyen TV, Moore G, Anderson J. Association between clinically abnormal observations and subsequent in-hospital mortality: a prospective study. Resuscitation. 2004;62(2):137–141. doi: 10.1016/j.resuscitation.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Bulechek GM, Butcher HK, Dochterman JM, Wagner C. Nursing Interventions Classification (NIC) 6. St. Louis, MO: Elsevier Health Sciences; 2012. [Google Scholar]
- Chan PS, Jain R, Nallamothu BK, Berg RA, Sasson C. Rapid response systems: a systematic review and meta-analysis. Archives of Internal Medicine. 2010;17(1):18–26. doi: 10.1001/archinternmed.2009.424. [DOI] [PubMed] [Google Scholar]
- Chen J, Flabouris A, Bellomo R, Hillman K, Finfer S. The relationship between early emergency team calls and serious adverse events. Critical Care Medicine. 2009;37(1):148–153. doi: 10.1097/CCM.0b013e3181928ce3. [DOI] [PubMed] [Google Scholar]
- Churpek MM, Yuen TC, Park SY, Gibbons R, Edelson DP. Using electronic health record data to develop and validate a prediction model for adverse outcomes in the wards. Critical Care Medicine. 2014;42(4):841–848. doi: 10.1097/CCM.0000000000000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVita MA, Smith GB, Adam SK, Adams-Pizarro I, Buist M, Bellomo R, Winters B. Identifying the hospitalized patient in crisis--a consensus conference on the afferent limb of rapid response systems. Resuscitation. 2010;81(4):375–382. doi: 10.1016/j.resuscitation.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Douw G, Schoonhoven L, Holwerda T, VanZanten AR, VanAchterberg T, Van Der Hoeven JG. Nurses’ worry or concern and early recognition of deteriorating patients on general wards in acute care hospitals: a systematic review. Critical Care. 2015;19(1):230–234. doi: 10.1186/s13054-015-0950-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresser S. The role of nursing surveillance in keeping patients safe. Journal of Nursing Administration. 2012;42(7):361–368. doi: 10.1097/NNA.0b013e3182619377. [DOI] [PubMed] [Google Scholar]
- Ebright PR, Patterson ES, Chalko BA, Render ML. Understanding the complexity of registered nurse work in acute care settings. Journal of Nursing Administration. 2003;33(12):630–638. doi: 10.1097/00005110-200312000-00004. [DOI] [PubMed] [Google Scholar]
- Franklin C, Mathew J. Developing strategies to prevent inhospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event. Critical Care Medicine. 1994;22(2):244–247. [PubMed] [Google Scholar]
- Franzini L, Sail KR, Thomas EJ, Wueste L. Costs and cost effectiveness of a telemedicine intensive care unit program in 6 intensive care units in a large health care system. Journal of Critical Care. 2011;26(3):e321–e329. doi: 10.1016/j.jcrc.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galhotra S, DeVita MA, Simmons RL, Dew MA. Mature rapid response system and potentially avoidable cardiopulmonary arrests in hospital. Quality and Safety in Health Care. 2007;16(4):260–265. doi: 10.1136/qshc.2007.022210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner-Thorpe J, Love N, Wrightson J, Walsh S, Keeling N. The value of Modified Early Warning Score (MEWS) in surgical in-patients: a prospective observational study. Annals of The Royal College of Surgeons of England. 2006;88(6):571–576. doi: 10.1308/003588406X130615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hams SP. A gut feeling? Intuition and critical care nursing. Intensive and Critical Care Nursing. 2000;16(5):310–318. doi: 10.1054/iccn.2000.1500. [DOI] [PubMed] [Google Scholar]
- Henneman EA, Gawlinski A, Giuliano KK. Surveillance: a strategy for improving patient safety in acute and critical care units. Critical Care Nurse. 2012;32(2):e9–e18. doi: 10.4037/ccn2012166. [DOI] [PubMed] [Google Scholar]
- Hillman K, Chen J, Cretikos M, Bellomo R, Brown D, Doig G. Introduction of the medical emergency team (MET) system: a cluster randomized controlled trial. Lancet. 2005;365(9477):2091–2097. doi: 10.1016/S0140-6736(05)66733-5. [DOI] [PubMed] [Google Scholar]
- Hillman KM, Bristow PJ, Chey T, Daffurn K, Jacques T, Norman SL, Simmons G. Antecedants to hospital deaths. Internal Medicine Journal. 2001;31(6):343–8. doi: 10.1046/j.1445-5994.2001.00077.x. [DOI] [PubMed] [Google Scholar]
- Hoffman D, Rowthorn V. Legal impediments to the diffusion of telemedicine. Journal of Health Care Law & Policy. 2011;14:1–53. [Google Scholar]
- Holden D, Dew E. Telemedicine in a rural gero-psychiatric inpatient unit: comparison of perception/satisfaction to onsite psychiatric care. Telemedicine and e-health. 2008;14(4):381–384. doi: 10.1089/tmj.2007.0054. [DOI] [PubMed] [Google Scholar]
- Hravnak M, DeVita MA, Clontz A, Edwards L, Valenta C, Pinsky MR. Cardiorespiratory instability before and after implementing an integrated monitoring system. Critical Care Medicine. 2011;39(1):65–73. doi: 10.1097/CCM.0b013e3181fb7b1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hravnak M, Edwards L, Clontz A, Valenta C, DeVita MA, Pinsky MR. Defining the incidence of cardiorespiratory instability in patients in step-down units using an integrated electronic monitoring system. Archives of Internal Medicine. 2008;168(12):1300–1308. doi: 10.1001/archinte.168.12.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D, DeVita MA, Bellomo R. Rapid-response teams. New England Journal of Medicine. 2011;365(2):139–146. doi: 10.1056/NEJMra0910926. [DOI] [PubMed] [Google Scholar]
- Kahn JM, Cicero BD, Wallace DJ, Iwashyna TJ. Adoption of ICU telemedicine in the United States. Critical Care Medicine. 2014;42(2):362–368. doi: 10.1097/CCM.0b013e3182a6419f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kause J, Smith G, Prytherch D, Parr M, Flabouris A, Hillman K. A comparison of Antecedents to Cardiac Arrests, Deaths and EMergency Intensive care Admissions in Australia and New Zealand, and the United Kingdom—the ACADEMIA study. Resuscitation. 2004;62:275–282. doi: 10.1016/j.resuscitation.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Keane MG. A review of the role of telemedicine in the accident and emergency department. Journal of Telemedicine and Telecare. 2009;15(3):132–134. doi: 10.1258/jtt.2009.003008. [DOI] [PubMed] [Google Scholar]
- Kerlin MP, Small DS, Cooney E, Fuchs BD, Bellini LM, Mikkelsen ME, Harhay MO. A randomized trial of nightime physician staffing in an intensive care unit. New England Journal of Medicine. 2013;368(23):2201–2209. doi: 10.1056/NEJMoa1302854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollef MH, Chen Y, Heard K, LaRossa GN, Lu C, Martin NR, Bailey T. A randomized trial of real-time automated clinical deterioration alerts sent to a rapid response team. Journal of Hospital Medicine. 2014;9(7):424–429. doi: 10.1002/jhm.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar G, Falk DM, Bonello RS, Kahn JM, Perencevich E, Cram P. The costs of critical care telemedicine programs: a systematic review and analysis. Chest. 2013;143(1):19–29. doi: 10.1378/chest.11-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach LS, Mayo A, O’Rourke M. How RNs rescue patients: a qualitative study of RN’s perceived involvement in rapid response teams. Quality and Safety in Health Care. 2010;19:1–4. doi: 10.1136/qshc.2008.030494. http://dx.doi.org/doi:10.1136/qshc.2008.030494. [DOI] [PubMed] [Google Scholar]
- Ludikhuize S, DeRooij SM, Sophia E, DeJonge E. Identification of deteriorating patients on general wards; a measurement of vital parameters and potential effectiveness of the Modified Early Warning Score. Journal of Critical Care. 2012;27(4):e424–e427. doi: 10.1016/j.jcrc.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Ludikhuize S, Dongelmans DA, Smorenberg SM, Gans-Langelaar M, DeJonge E, DeRooij SE. How nurses and physicians judge their own quality of care for deteriorating patients on medical wards: Self-assessment of quality of care is suboptimal. Critical Care Medicine. 2012;40(11):2982–2986. doi: 10.1097/CCM.0b013e31825fe2cb. [DOI] [PubMed] [Google Scholar]
- Moehr JR, Schaafsma J, Anglin C, Pantazi SV, Grimm NA, Anglin S. Success factors for telehealth--a case study. International Journal of Medical Informatics. 2006;75(10):755–763. doi: 10.1016/j.ijmedinf.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- Morrison JL, Cai Q, Davis N, Yan Y, Berbaum ML, Ries M, Solomon G. Clinical and economic outcomes of the electronic intensive care unit: Results from two community hospitals. Critical Care Medicine. 2010;38(1):2–8. doi: 10.1097/CCM.0b013e3181b78fa8. [DOI] [PubMed] [Google Scholar]
- Nangalia V, Prytherch DR, Smith GB. Health technology assessment review: remote monitoring of vital signs--current status and future challenges. Critical Care. 2010;14(5):233–237. doi: 10.1186/cc9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman J, Buerhaus P, Pankratz VS, Leibson CL, Stevens SR, Harris M. Nurse staffing and inpatient hospital mortality. New England Journal of Medicine. 2011;364(11):1037–1045. doi: 10.1056/NEJMsa1001025. [DOI] [PubMed] [Google Scholar]
- Norman S. The use of telemedicine in psychiatry. Journal of Psychiatric and Mental health nursing. 2006;13(6):771–777. doi: 10.1111/j.1365-2850.2006.01033.x. [DOI] [PubMed] [Google Scholar]
- Odell M, Victor C, Oliver D. Nurses’ role in detecting deterioration in ward patients: systematic literature review. Journal of Advanced Nursing. 2009;65(10):1992–2006. doi: 10.1111/j.1365-2648.2009.05109.x. [DOI] [PubMed] [Google Scholar]
- Page A. Keeping Patients Safe: Transforming the Work Environment of Nurses. 1. Washington, D.C: National Academies Press; 2004. [PubMed] [Google Scholar]
- Parker CG. Decision-Making Models Used by Medical-Surgical Nurses to Activate Rapid Response Teams. MedSurg Nursing. 2014;23(3):159–164. [PubMed] [Google Scholar]
- Patrician PA, Loan L, McCarthy M, Fridman M, Donaldson N, Bingham M, Brosch LR. The association of shift-level nurse staffing with adverse patient events. Journal of Nursing Administration. 2011;41(2):64–70. doi: 10.1097/NNA.0b013e31820594bf. [DOI] [PubMed] [Google Scholar]
- Perberdy MA, Kaye W, Ornato JP, Larkin GL, Nadakarni V, Mancini ME, Lane-Truitt T. Cardiopulmonary resuscitation of adults in the hospital: a report of 14720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003;58(3):297–308. doi: 10.1016/s0300-9572(03)00215-6. [DOI] [PubMed] [Google Scholar]
- Potter P, Wolf L, Boxerman S, Grayson D, Sledge J, Dunagan C, Evanoff B. Understanding the cognitive work of nursing in the acute care environment. Journal of Nursing Administration. 2005;35(7):327–335. [PubMed] [Google Scholar]
- Prytherch DR, Smith GB, Schmidt PE, Featherstone PI. ViEWS--towards a national early warning score for detecting adult inpatient deterioration. Resuscitation. 2010;81(8):932–937. doi: 10.1016/j.resuscitation.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Ries M. Tele-ICU: a new paradigm in critical care. International Anesthesiology Clinics. 2009;47(1):153–170. doi: 10.1097/AIA.0b013e3181950078. [DOI] [PubMed] [Google Scholar]
- Robert RR, Tilley DS, Petersen S. A power in clinical nursing practice: Concept Analysis on Nursing Intuition. Med Surg Nursing. 2014;23(5) [PubMed] [Google Scholar]
- Romero-Brufau S, Huddleston JM, Naessens MM, Johnson MG, Hickman J, Morlan BW, Santrach PJ. Widely used track and trigger scores: are they ready for automation in practice? Resuscitation. 2014;85(4):549–552. doi: 10.1016/j.resuscitation.2013.12.017. [DOI] [PubMed] [Google Scholar]
- Rozen TH, Mullane S, Kaufman M, Hsiao YF, Warrillow S, Bellomo R, Jones DA. Antecedents to cardiac arrests in a teaching hospital intensive care unit. Resuscitation. 2014;85(3):411–417. doi: 10.1016/j.resuscitation.2013.11.018. [DOI] [PubMed] [Google Scholar]
- Sao D, Gupta A, Gantz DA. The Globalization of Health Care: Legal and Ethical Issues. Oxford, United Kingdom: Oxford University Press; 2012. Legal and Regulatory barriers to telemedicine in the United States: public and private approaches toward health care reform. [Google Scholar]
- Schien RM, Hazday N, Pena M, Ruben BH, Sprung CL. Clinical antecedants to in-hospital cardiopulmonary arrest. Chest. 1990;98(6):1388–92. doi: 10.1378/chest.98.6.1388. [DOI] [PubMed] [Google Scholar]
- Schmid-Mazzoccoli A, Hoffman LA, Wolf GA, Happ MB, DeVita MA. The use of medical emergency teams in medical and surgical patients: impact of patient, nurse and organizational characteristics. Quality and Safety in Health Care. 2008;17(5):377–381. doi: 10.1136/qshc.2006.020438. [DOI] [PubMed] [Google Scholar]
- Schwamm LH, Rosenthal ES, Hirshberg A, Schaefer PW, Little EA, Kvedar JC, Levine SR. Virtual TeleStroke Support for the Emergency Department Evaluation of Acute Stroke. Academic Emergency Medicine. 2004;11(11):1193–1197. doi: 10.1197/j.aem.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Smith GB, Prytherch DR, Meredith P, Schmidt PE, Featherstone PI. The ability of the National Early Warning Score (NEWS) to discriminate patients at risk of early cardiac arrest, unanticipated intensive care unit admission, and death. Resuscitation. 2013;84(4):465–470. doi: 10.1016/j.resuscitation.2012.12.016. [DOI] [PubMed] [Google Scholar]
- Smith GB, Prytherch DR, Schmidt P, Featherstone PI, Knight D, Clements G, Mohammed MA. Hospital-wide physiological surveillance-a new approach to the early identification and management of the sick patient. Resuscitation. 2006;71(1):19–28. doi: 10.1016/j.resuscitation.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Subbe CP, Gao H, Harrison DA. Reproducibility of physiological track-and-trigger warning systems for identifying at-risk patients on the ward. Intensive Care Medicine. 2007;33(4):619–624. doi: 10.1007/s00134-006-0516-8. [DOI] [PubMed] [Google Scholar]
- Tarassenko L, Hann A, Young D. Integrated Monitoring and analysis for early warning of patient deterioration. British Journal of Anaesthesia. 2006;97(1):64–68. doi: 10.1093/bja/ael113. [DOI] [PubMed] [Google Scholar]
- Trinkle RM, Flabouris A. Documenting Rapid Response System afferent limb failure and associated patient outcomes. Resuscitation. 2011;82(7):810–814. doi: 10.1016/j.resuscitation.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Venkataraman R, Ramakrishnan N. Outcomes Related to Telemedicine in the Intensive Care Unit: What we know and would like to know. Critical Care Clinics. 2015;31(2):225–37. doi: 10.1016/j.ccc.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Whitten PS, Mackert MS. Addressing telehealth’s foremost barrier: provider as initial gatekeeper. International Journal of Technology Assessment in Health Care. 2005;21(4):517–521. doi: 10.1017/S0266462305050725. [DOI] [PubMed] [Google Scholar]
- Winters BD, Weaver SJ, Pfoh ER, Yang T, Pham JC, Dy SM. Rapid-response systems as a patient safety strategy: a systematic review. Annals of Internal Medicine. 2013;158(5):417–425. doi: 10.7326/0003-4819-158-5-201303051-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootton R. Twenty years of telemedicine in chronic disease management-an evidence synthesis. Journal of Telemedicine and Telecare. 2012;18(4):211–220. doi: 10.1258/jtt.2012.120219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn JD, Engelke MK, Swanson M. The Front Line of Patient Safety: Staff Nurses and Rapid Response Team Calls. Quality Management in Health Care. 2009;18(1):40–47. doi: 10.1097/01.QMH.0000344592.63757.51. [DOI] [PubMed] [Google Scholar]