Abstract
We profiled the humoral response in the penis, an area that has been minimally explored but may be relevant for protecting insertive men against HIV and other sexually-acquired infections. Comparing paired tissue samples from 20 men at risk of HIV infection, foreskin contains less IgA and more IgG2 than colon. Using foreskin dermal and epidermal explants and paired plasma from 17 men, we examined Ig accumulation by normalizing Ig to human serum albumin (HSA) transudation. Dermal IgM, IgG2, IgA, and IgE ratios were greater than in plasma, suggesting there is local antibody secretion at the dermis. Local Ig transcription was concentrated at the inner rather than the outer foreskin, and inner foreskin Ig ratios did not correlate with blood, indicating that localized production can contribute to the foreskin response. IgM, IgG1, IgG3, and IgA have preferential access to the foreskin epidermis, whereas IgG2, IgG4, and IgE are restricted to the dermis. Lastly, Ad5-specific IgA was selectively in the colon; whereas foreskin Ad5 IgG was mainly derived from blood, and reached the inner epidermis at higher ratios than the outer (p<0.002). In summary, the foreskin antibody response combines local and systemic sources and there is selective isotype accumulation in the epidermis.
INTRODUCTION
Substantial evidence has emerged over the years in non-human primate (NHP) models that potent antibodies can mediate protective effects against SIV and SHIV infection1. In humans, the RV144 clinical trial demonstrated 31% protection among Thai volunteers2 where infection risk was directly associated with blood IgA titers against the C1 region of HIV Env, and inversely associated with high titers of anti-Env V1V2 IgG33–5. However, it remains to be determined whether these vaccine correlates can protect at the rectal, vaginal, and penile surfaces, where HIV is predominantly transmitted6,7. Whether passively infused antibodies or HIV vaccine candidates can reach human genital and rectal sites at sufficient concentrations is also unclear.
Quantitative methods to measure vaginal and rectal antibody responses have been developed8, but penile Ig assessments are limited. In uncircumcised heterosexual men14–16 and men who have insertive sex with men17,18, the foreskin is an important site of HIV exposure9, as three independent randomized controlled trials showed that circumcision reduced HIV infection risk by 51–60%14–16. However, 70% of the world’s men remain uncircumcised10 and the uptake of circumcision faces logistical11,12 and cultural barriers13, so it remains important to investigate whether the foreskin can be armed with humoral responses that can prevent HIV infection. In fact a recent study demonstrated that SIV can preferentially persist in proximity to target cells at the macaque inner foreskin and glands, but not the outer foreskin,9 indicating that immune control at these sites may be most useful for protection.
Distinct Ig isotypes predominate in various mucosal surfaces, suggesting that antibody restrictions are present at the sites of host-pathogen interactions. IgM is first induced during the immune response to a new antigen and has C1q and complement activation functions; however, it is present in low concentrations in female genital and intestinal surfaces, and is undetectable in seminal fluid of most healthy men19–21. IgA isotypes dominate in the intestine and can inactivate pathogens by neutralization and exclusion21–23. Both intestinal and genital IgA rely on local production, with minor components transudating from blood24–28. Compared to other isotypes, IgG is most abundant in blood, semen, cervical, and vaginal compartments29. There are four subclasses arranged by their abundance in serum: IgG1, IgG2, IgG3, and IgG4. They have remarkable differences in complement activation, phagocytosis, antibody dependent cell mediated cytotoxicity (ADCC), and Fc-Receptor binding, with a general order of activating capacity being IgG3 > IgG1 ≫ IgG2 > IgG430.
In addition to the isotypes, the specificity of the antibody response can also be compartmentalized. Exposure to oral Salmonella typhi or intranasal adenovirus can lead to IgG antibody responses that concentrate in the nose and mouth, as well as vaginal IgA31,32; whereas rectal exposure can lead to antigen-specific IgG in tears and IgA that dominates in rectal secretions31. Deltoid delivery of a canarypox HIV vaccine can generate both IgG and IgA in rectal secretions, but this is limited after inguinal immunizations, which drain the genitals33. Thus, the immunization strategies and natural infections that trigger penile antibody responses may not match those that successfully generate responses at mucosal surfaces.
To better understand the antibody profile that may play a role in controlling infections at the foreskin, we evaluated the humoral responses in the foreskin of sexually active young men who have sex with men (MSM) at high risk of HIV infection, and compared these with colonic and systemic B-cell responses. Our results indicate that some foreskin Ig isotypes transudate directly from blood, whereas others are locally produced. These findings have important implications for the development of strategies to induce relevant Ig responses against sexually transmitted infections (STI) so that immune responses reach this important site of pathogen exposure.
METHODS
Tissue and Blood Donation
We evaluated foreskin and colon biopsy samples collected in Lima, Peru during HVTN 914. They correspond to 20 HIV negative, 21–30 year-old MSM at high risk of HIV acquisition34. All study participants provided written informed consent prior to HVTN 914 participation, and met safety criteria to undergo elective circumcision and sigmoidoscopy procedures, as approved by the relevant institutional review boards.
Participants received two Ringer’s Lactate enemas to cleanse the intestine previous to collection of 29 sigmoid biopsies using a 3.3 mm diameter cup forceps. Two weeks later, circumcisions were performed using the sleeve procedure after dorsal penile nerve block anesthesia. The inner foreskin was identified based on the relative decrease in melanocytes compared to the outer. Procedures were carried out after confirming the absence of signs and symptoms of infection, but samples collected at time of procedures indicated that ten of these individuals were STI-positive, when tested for HSV-2 serology (n=5), C. trachomatis in rectal swabs (n=3) and urine (n=1), and/or N. gonorrhoeae PCR in rectal swabs (n=3).
Protein Lysate Preparation
Colonic biopsies or foreskin pieces were frozen at −80°C within two hours of surgery. Tissue was homogenized in lysate buffer35, and normalized to 200 μg/ml of protein as measured by bicinchoninic acid assay (Pierce)34.
Plasma Isolation
Paired blood samples were collected by venipuncture into acid citrate dextrose tubes a few hours previous to the procedures. Plasma was isolated by consecutive centrifugations: first at 300×g to separate plasma from cells, and then at 800–1,000×g to remove any heme-containing contaminant.
Foreskin Explant Cultures
Epidermal sheets from foreskin were separated from dermis after dispase digestion, then washed and cultured for 48h34. As only the dermis is vascularized, hemoglobin ELISA (ICL) in epidermal explant supernatants was used to assess dermal contamination34.
Multiplex Bead Array (MBA)
Tissue lysates, explant culture supernatants, and plasma were assayed for Ig content using a multiplex human Ig isotyping kit, single-plex HSA kit, and single-plex human IgE kit, which used plastic beads measured in a Luminex platform (all from Millipore). Samples were diluted to be within the quantitative ranges of the standard curves. Tissue lysates were assayed at 1:2, 1:10 and 1:100 dilutions in 0.5× lysate buffer, which was also used to prepare standard curves, positive controls, and background controls. Explant supernatants were assayed undiluted, 1:2, 1:200 and 1:400 in RPMI 20% FBS; plasma samples were assayed at 1:30, 1:15,000 and 1:150,000 in RPMI 20% FBS, along with standard curves, positive controls, and background controls diluted in the same buffer.
Curve fitting for MBA used nCal R package36. A conservative lower limit of detection was defined as the estimated concentration at which the lower confidence interval of the estimate equaled the lowest standard used in the calibration curve. All Ig values smaller than this limit were replaced by 0. All samples contained HSA values within the standard curve limits of detection so there was no replacement for HSA.
Concentrations of antibody in tissue lysates were normalized to protein content to permit comparisons among foreskin and colon. Because explant cultures contained FBS, normalization was not carried out with respect to protein concentration, but rather with HSA as it is synthesized in the liver and transported from blood through the skin via convection37. This accounted for anticoagulant dilution in the plasma collections, and explant tissue size in the cultures.
RNA Isolation, Reverse Transcription and Quantitative PCR
Inner and outer foreskin pieces and colon biopsies were preserved in RNAlater (Life Technologies) and stored at −80°C. RNA isolations were carried out by placing the thawed tissue in 1ml of TRI Reagent (Life Technologies) and homogenizing it with a homogenizer with wide probe (Omni). Nucleic acid separation used 1-bromo-3-chloropropane (Sigma-Aldrich) and Ribopure Kit (Life Technologies) columns. Blood was collected in Tempus tubes, with RNA isolated according to the Tempus Spin RNA Isolation Kit (Life Technologies).
RNA integrity was measured with an Agilent 2100 Bioanalyzer and all samples had a median RNA integrity number of 8.9 (Interquartile range [IQR] 8.3–9.3), well above the necessary quality threshold to conduct these studies. 1μg of RNA was used in reverse transcriptions using Superscript VILO (Life Technologies).
RT-PCR samples containing 1ng of the original RNA input were quantitated using Taqman Gene Expression assays (Life Technologies) designed to expand across exons within TATA-box binding protein (TBBP) as an endogenous control, and a primer designed to amplify shared sequences in IgM, IgG1, IgG3, and IgG4 (IgMG134), or IgA. A 7900HT real-time PCR machine with 384 well plate handling was used to run each of the samples. A standard curve, containing 108 to 5.65 copies of the specific PCR product cloned in a TOPO pCR2.1 plasmid (Life Tech) was used to extrapolate absolute transcript copy numbers using an nCal R package36. Three different blood samples from each of the procedure visits, three colonic samples from two sigmoidoscopy procedures, and 3–5 inner or outer foreskin samples were each run in duplicate and summarized by tissue for each participant using a trimmed mean to remove extreme outliers. The IgMG134 and IgA copies were normalized to TBBP expression, which was the least variable endogenous control when compared to β-actin, β-glucoronidase, and cyclophilin A among the different tissues.
Immunofluorescence Microscopy
Foreskin pieces were fixed in buffered formalin for a maximum of 7 days, dehydrated in 70% ethanol, and paraffin embedded. Four micron thick sections were deparaffinized and rehydrated through graded alcohols to distilled water for staining. Sections were incubated 20 min in pH6.0 Target Retrieval Solution (DakoCytomation), cooled and loaded onto the DakoCytomation Autostainer. Endogenous peroxidases and biotin were blocked with 3% H2O2, followed by a Avidin/Biotin block (Biocare) following manufacturer instructions, and a 0.25% casein TBS, 0.1% Tween-20 (Sigma) solution. Anti-CD138 (1:25 from Cell Marque) or mouse IgG isotype (0.2μg/ml) was added to the slide for 60 min, followed by biotinylated anti-mouse IgG (1:200; Vector), and streptavidin Ax568 (Invitrogen) for 30 min each. For IgM/IgG staining, goat anti-human IgG and IgM (1:5,000; Jackson Immunoresearch) or goat isotype (3.4μg/ml) was added for 60 min followed by CSA II amplification for readout on FITC. For IgA, rabbit anti-human IgA (1:5,000) or isotype (4.8μg/ml) was added for 60 min, washed, and stained with goat anti-rabbit Alexa Fluor 647 (1:50, Invitrogen) for 30 min.
Samples were counterstained with DAPI, coverslipped with ProLong Gold (Life Technologies), cured, and then stored at 4°C until image acquisition. Slides were scanned on an Aperio FL (Leica Microsystems) using single-band filter cubes (Semrock) for each fluorochrome. Exposure times were determined from stained control tissue and remained constant on experimental samples. The validity of the staining was confirmed on colon, abdominal skin and lymph node sections (data not shown).
Adenovirus 5 (Ad5) Neutralization Assay and ELISA
An Ad5 neutralization assay was carried out using participant plasma obtained one week previous to sigmoidoscopy as previously described38.
For ELISA, 96 well plates (Thermo Scientific) were coated overnight with 1×109 viral particles of Ad5 purified by double-banding, CsCl gradients, and dialysis, and diluted in 100μl of coating buffer (Immunochemistry Technologies). Plates were then blocked for 2h with 5% non-fat milk (Fisher), 3% Inactivated Goat Serum (Invitrogen), and 0.2% Tween-20 (Sigma) in 1× PBS (Life Tech). Explant samples were assayed undiluted and at 1:10 and 1:100 dilutions in blocking buffer, and negative controls included RPMI with 20% FBS. Plasma samples were diluted in six 1:5 dilutions starting with 1:500. Samples, standard curves, and controls were incubated for 2h at 37°C.
After washing, goat anti-human IgG-HRP (Southern Biotech) or mouse anti-human IgA-HRP (Abcam) at 1:1,000 was incubated for 1h at 37°C, then detected with addition of BM Blue substrate (Roche) for 15 min. The reaction was stopped by adding 50μl of 1N H2SO4 and read within 20min using a SpectraMax M2 ELISA Plate Reader (Molecular Devices) at 450nm absorbance. Standard curves used human IgG (Bethyl) or human IgA (Sigma), at 20.0 to 0.009ng/well, and the linear ranges were used for extrapolation of the concentrations.
Statistical Analysis
Statistical comparisons were carried out using Prism v6 (GraphPad Software). Nonparametric comparisons among multiple tissues were carried out using the Friedman test39. If significant, a Wilcoxon signed ranked post-test was used to identify differences between two specific tissues. Unless specifically described, all p values described are Wilcoxon p values corrected for multiple comparisons using Bonferroni. Spearman’s correlation was used to assess the association between dermal antibody HSA ratios and those in paired blood. Only comparisons with r>0.5 and p<0.05 were considered significant.
RESULTS
Foreskin tissue has different Ig isotype distributions than colon tissue
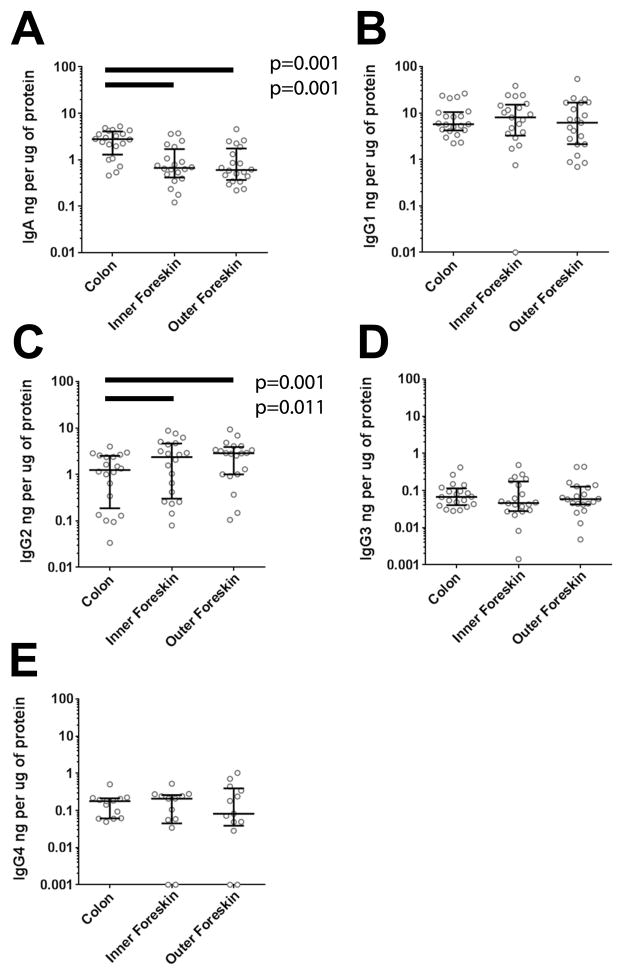
To explore the tissue Ig isotype distributions in sexually active men, we generated protein lysates and compared Ig concentrations from 20 matched colonic and foreskin tissues (including inner and outer foreskin), normalized for protein content (Figure 1). No differences were detected in total IgA or IgGs when men with STIs were compared to men without STIs (data not shown), so they were studied as a single cohort. The median IgA concentration was at least four-fold greater in the colon (3.13ng/μg, IQR 1.34–4.93) than in the inner (0.72ng/μg, IQR 0.45–2.17; p=0.001) and outer foreskin tissue (0.65ng/μg, IQR 0.38–2.82; p=0.001; Figure 1A), and at least two-fold greater than in blood (p=0.001; data not shown).
Figure 1. The foreskin contains more IgG2 and less IgA than colonic mucosa.
Twenty paired foreskin and colonic tissue samples were homogenized and assayed by multiplex bead array for A) IgA, B) IgG1, C) IgG2, D) IgG3, and E) IgG4. All Ig concentrations were normalized to μg of protein in the tissue biopsy. Bars indicate median with IQR for each tissue. Lysate buffer designed for homogenization of skin samples prevented detection of IgM and IgE standards, and thus their quantitation was not included in the figure. Only significant differences in Wilcoxon post-test after Bonferroni correction for three comparisons are reported in the figure.
IgG1 had the highest overall median concentration in all tissues and there were no significant differences among them (Friedman Test p=0.387; Figure 1B). Interestingly, colonic tissue median IgG2 concentrations (1.24ng/μg, IQR 0.19–2.51) were lower compared to inner (2.36ng/μg, IQR 0.30–4.73; p=0.011) and outer foreskin (2.85ng/μg, IQR 1.00–3.88; p=0.001 (Figure 1C). IgG3 and IgG4 were less abundant, and comparable among the different tissues (Friedman p=0.951 and p=0.981 respectively; Figure 1D and 1E). These colon and foreskin tissue IgG to protein ratios are 5.6%–11.8% of those expected in plasma (data not shown). The results indicated that, similar to the female genital tract26,40, foreskin has a unique distribution of antibody isotypes that are distinct from intestinal tissues.
Foreskin sources of antibody combine transudation from blood and local production
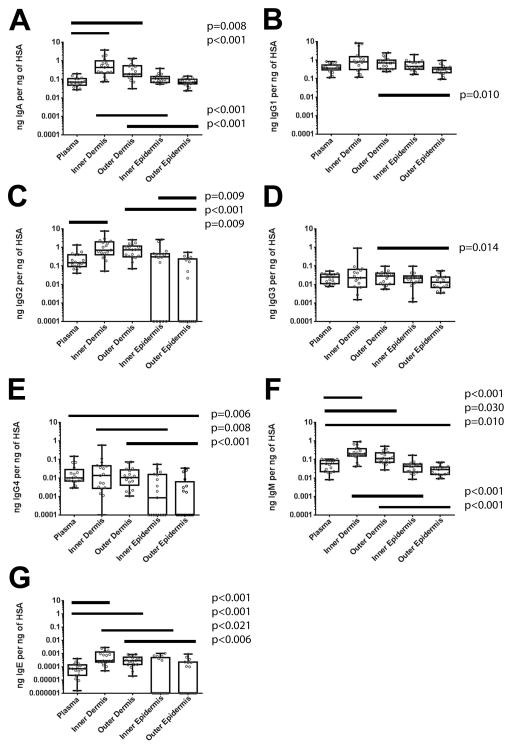
Antibodies might reach the foreskin through convection/transudation from blood, local production, or both. To define the sources of Igs in the foreskin of sexually active men, we cultured separately dermal and epidermal explants from 20 donors, measured Ig/HSA ratios released into 48h culture supernatants, and compared levels of released Igs to those in paired blood taken prior to the procedure. Three participants’ tissues were excluded because of inefficient dermal-epidermal separation, as indicated by hemoglobin contamination (data not shown). In the remaining 17 individuals, dermal Ig/HSA ratios were comparable to or higher than plasma (Figure 2). Foreskin IgA/HSA ratios at the inner dermis and the outer dermis were increased six-fold (p<0.001) and three-fold (p=0.008), respectively, when compared to plasma (Figure 2A). Inner foreskin IgG2/HSA ratios were five-fold larger than blood ratios (p=0.009; Figure 2C). Foreskin inner dermis IgM/HSA ratios and outer dermis IgM/HSA ratios were increased by three-fold (p<0.001) and two-fold (p=0.030) compared to plasma (Figure 2F). Similarly, inner (p<0.001) and outer dermis IgE/HSA ratios (p<0.001) were greater than in plasma (Figure 2G). Thus, the results suggested local accumulation or production of most Ig isotypes at the dermal foreskin, after 48h of culture.
Figure 2. Comparison of dermal and epidermal foreskin Igs to blood Igs.
Foreskin and plasma samples from 17 participants were compared by calculating the ratios of each antibody concentration normalized to Human Serum Albumin (HSA) in each sample. HSA, A) IgA, B) IgG1, C) IgG2, D) IgG3, E) IgG4, F) IgM, and G) IgE were measured by multiplex bead array (MBA). Samples under the level of detection are graphed at the bottom of the axis. Only significant differences in Wilcoxon post-test after Bonferroni correction for eight comparisons are reported in the figure.
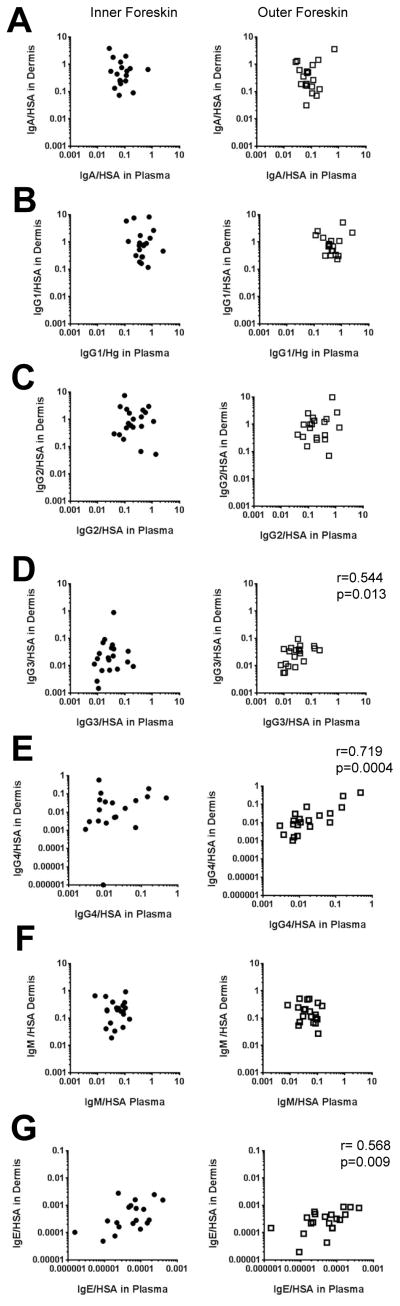
To determine whether Ig transudation from blood is a primary contributor to humoral responses at the foreskin dermis, we examined the correlation of plasma Ig/HSA ratios with inner and outer foreskin dermis ratios (Figure 3). No correlation was identified among the inner dermal isotypes and blood (Figure 3, black circles), supporting that antibody is locally produced/accumulated at the inner dermis. Similar results were found for outer foreskin dermal IgA, IgG1, IgG2, and IgM and their blood counterparts (Figure 3A, 3B, 3C, 3F), suggesting dominant isotypes have local production/accumulation at the foreskin. However, the concentrations of less frequent Ig isolates – IgG3, IgG4 and IgE – were directly correlated between outer foreskin and blood (IgG3: r=0.544, p=0.013 [Figure 3D]; IgG4: r=0.719, p=0.0004 [Figure 3E]; IgE: r=0.568, p=0.009 [Figure 3G]). Thus, these results suggest blood sources are the major contributors of outer foreskin IgG3, IgG4, and IgE antibodies, whereas other Igs have localized foreskin accumulation or production that contributes to the total response.
Figure 3. Most Ig/HSA ratios in the foreskin dermis do not correlate with those in blood.
Correlation between 17 foreskin dermal antibody HSA ratios and those in paired blood were analyzed using Spearman’s Correlation. HSA, A) IgA, B) IgG1, C) IgG2, D) IgG3, E) IgG4, F) IgM, and G) IgE were measured via multiplex bead array (MBA). Inner dermis samples are labeled with filled black circles; outer dermis samples are labeled with open black squares. Spearman’s p values reported in the figure are shown after Bonferroni correction for seven comparisons.
Foreskin dermis contains IgM, IgG, and IgA secreting cells, but no organized B cell follicles
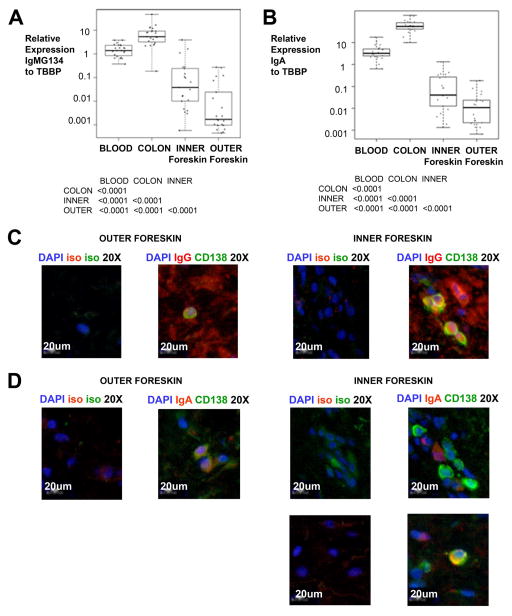
To determine whether local antibody secreting cells (ASCs) could be producing foreskin Igs, we first investigated evidence for local transcription of Ig genes, comparing inner and outer foreskin RNA samples to matched samples of blood and colon. A primer capable of amplifying IgM, IgG1, IgG3, and IgG4 transcripts (IgMG134; Figure 4A) or IgA (Figure 4B) using real time PCR identified ASCs in all tissues in different frequencies. The results were normalized to an endogenous control, TBBP. Median relative expression of colonic IgMG134 (3.189, IQR 3.189-5.348) was higher compared to blood (1.381, IQR 0.846-2.315; p=0.0001), inner (0.038, IQR 0.010-0.240; p<0.0001), and outer foreskin (0.002, IQR 0.001-0.024; p<0.0001). Median relative expression of colon IgA (51.26, IQR 33.00-97.21) was also increased compared to blood (3.23, IQR 2.45-5.15; p=0.0001), inner (0.041, IQR 0.013-0.273; p<0.0001), and outer foreskin (0.011, IQR 0.002-0.023; p<0.0001). Blood IgMG134/TBBP and IgA/TBBP ratios were also significantly higher than both foreskin areas (p<0.0001 each). The results indicated that foreskin Ig transcription is low, but detectable.
Figure 4. Foreskin tissue contains antibody secreting cells.
A–B) Real-time PCR comparing 20 paired colonic biopsies, blood, and inner and outer foreskin was used to quantitate mRNA transcription with a primer that amplifies A) IgM, IgG1, IgG3, and IgG4 (IgMG134); and B) IgA; normalized to TATA-box binding protein (TBBP). Only significant differences in Wilcoxon post-test after Bonferroni correction for six comparisons are reported beneath each graph. Individual measurements are represented by circles. Box plots show the median and IQR, with whiskers extending to the range of the data for each tissue. C) Representative 20× magnification immunofluorescence microscopy images of paired inner and outer foreskin sections stained with DAPI nuclear counterstain (pseudocolored blue), CD138 (pseudocolored green) and IgG or their isotype control (both pseudocolored red). D) Selected 20× magnification immunofluorescence microscopy images of paired inner and outer foreskin sections stained with DAPI nuclear counterstain (pseudocolored blue), CD138 (pseudocolored green) and IgA or their isotype control (pseudocolored red). In C) and D), we analyzed ~2.6mm2 sections from 18 individuals, but photos represent only 1/200 of the area used to quantitate ASCs.
No differences in Ig transcription in colon or foreskin were detected among men with STIs and men without STIs in our cohort (data not shown). Interestingly, there appeared to be compartmentalization of the Ig transcription at the foreskin, with inner foreskin levels being consistently higher than outer (Figure 4A; p<0.0001). Thus, the inner foreskin appears to especially accumulate ASCs capable of local Ig transcription.
To test for the presence of ASCs in the foreskin dermis, we also conducted immunofluorescence microscopy staining of inner and outer foreskin tissues. In healthy men, plasma cells were identified by their co-staining for CD138 and intracellular IgA or IgG. The cells were rare, most often located in dermal areas, and not organized in follicular structures (Figure 4C). Inner foreskin (median 101.2 IgG+ or IgA+ cells per mm2 IQR 55.5-153.5) contained more cells than the outer (median 67.4 IgG+ or IgA+ cells per mm2 IQR 25.8-91.5; p=0.0002). CD138− IgA+ and CD138− IgG+ cells were also identified. The presence of ASCs could explain the observed Ig of dermal explant cultures (Figure 2).
The foreskin epidermis permits the passage of selected antibody isotypes
Ideally, vaccine-induced protection via HIV-1 specific antibodies would rely on antibodies present at sites of exposure and early infection, to prevent rapid initiation of local infection and spread41–44. Thus, we explored the extent to which antibodies can permeate the foreskin epithelial barrier (Figure 2). All antibody isotypes had significantly lower Ig/HSA ratios in the outer epidermis than in the outer dermis (p<0.014; Figure 2). For example, median IgM in the outer epidermis was four-fold lower compared to the outer dermis (p<0.001; Figure 3F), median IgA was three-fold lower (p<0.001; Figure 2A), and median IgG1 was two-fold lower (p<0.010; Figure 2B). Even more dramatic examples of reduced antibody transport across the epithelium were IgG2, IgG4, and IgE, as these isotypes were often undetectable at the epidermis (Figure 2C, 2E, 2G). The results indicated that the outer epidermal barrier restricts the passage of antibody isotypes to the most outer layers.
The inner foreskin epidermis, however, showed differences in permeability for the different isotypes (Figure 2). IgA, IgM, IgE, and IgG4 showed significant reductions in inner epidermis Ig/HSA ratios when compared to those in the inner dermis. For example, median IgM/HSA ratios in the inner epidermis were five-fold lower than those of the inner dermis; (p<0.001; Figure 3F). IgA/HSA ratios in the inner epidermis were four-fold reduced compared to the outer dermis (p<0.001; Figure 2A). This indicated that the epidermal barrier limits the passage of some isotypes.
Interestingly, inner foreskin epidermis IgG1, IgG2, and IgG3 ratios were not distinguishable from inner foreskin dermal levels (Figure 2C–E). These results suggested some preferential Ig deposition at the inner epidermis.
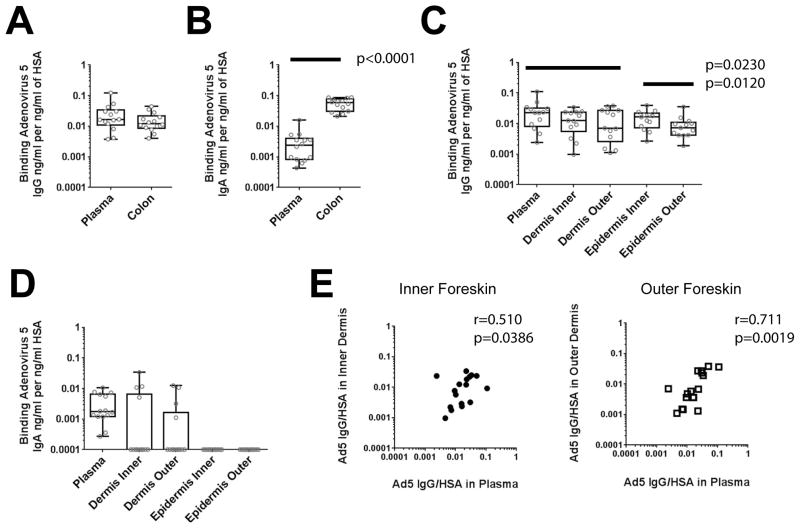
Adenovirus 5 (Ad5) antibody responses at the foreskin come primarily from blood and can reach the inner foreskin epidermis
To determine if our methods would be useful to elucidate the distribution of antigen-specific responses at the foreskin, we used an Ad5 ELISA to assess the IgG/HSA and IgA/HSA ratios in plasma, colon and foreskin explant supernatants using samples from the 14 Ad5-seropositive participants (Ad5 neutralization titer >18).
In colonic biopsies, Ad5-specific IgG responses were not distinguishable from those in plasma taken previous to the sigmoidoscopy (p=0.0590; Figure 5A). However, colonic Ad5-specific IgA responses were 22-fold higher than those in plasma (p<0.0001; Figure 5B). Thus, natural infection with adenovirus can generate abundant antibody responses in the colonic mucosa.
Figure 5. Foreskin Ad5 IgG transudates from blood and preferentially reaches the inner epidermis, whereas Ad5 IgA is mainly in the colon.
A–D) After exclusion of 6 samples due to dermal contamination or Ad5 seronegative status, colon (A,B), foreskin (C,D), and corresponding plasma samples from 14 participants were compared by calculating the ratios of binding Ad5 IgG (A,C) or IgA (B,D) normalized to HSA in each sample. HSA was measured by multiplex bead array (MBA); Ad5 IgG and IgA was assessed by ELISA. Only significant differences in Wilcoxon post-test after Bonferroni correction for multiple comparisons are reported in the figures. E) Correlation between 14 foreskin dermal Ad5 IgG/HSA ratios and those in paired blood were analyzed using Spearman’s rho. Inner dermis samples are labeled with filled black circles; outer dermis samples are labeled with open black squares.
Contrary to our findings for total foreskin Ig (Figure 2), Ad5 IgG responses in plasma were higher than those at the outer foreskin dermis (p=0.0230; Figure 5C). Ad5 IgG in plasma also trended higher than in inner dermis, but was not significantly different after adjustment for multiple comparisons. A similar pattern was observed when Ad5 IgA was compared (Figure 5D). These results indicated that local adenovirus IgG and IgA responses did not accumulate at the foreskin.
We also examined the correlation between dermal and plasma Ad5 IgG/HSA ratios (Figure 5E). Ad5 IgA ratios were not correlated, because many foreskin samples were below the limit of detection of the assay (Figure 5D). Both inner (r=0.510, Spearman’s p=0.0386) and outer (r=0.711, Spearman’s p=0.0019) dermal Ad5 IgG ratios were moderately correlated to plasma, indicating that Ad5 IgG antibodies at the foreskin mainly transudate from blood.
Lastly, we assessed differences among inner and outer foreskin epidermis Ad5 IgG/HSA ratios, to evaluate our findings of differential antibody permeability in these surfaces (Figure 5C). Inner epidermis (median 0.016, IQR 0.007-0.022) had two-fold more Ad5 IgG than outer epidermis (median 0.007, IQR 0.004-0.011; p=0.0120). This indicated that the inner foreskin can allow for increased penetration of Ad5 IgG antibodies.
DISCUSSION
We investigated the antibody isotypes present in different areas of the human foreskin and compared them to colonic mucosa and blood. Our results indicate that foreskin humoral responses differ from intestinal humoral responses in critical aspects such as the lack of inductive follicles, relatively few ASCs, and the relative frequency of specific Ig isotypes (such as increased IgG2 and reduced IgA). After 48h of culture, IgA, IgG1, IgG3, and IgM Ig/HSA ratios at the foreskin epidermis were indistinguishable from blood ratios, but IgG2, IgG4, and IgE were rare. Thus, vaccine-induced immunity against HIV in males may need to meet distinct requirements in order to provide protection at systemic, rectal, and penile infection sites.
We explored whether the humoral immune response at the foreskin relies on diffusion/convection of systemic antibodies. In addition to blood sources, we found evidence that the foreskin contains local ASCs that may contribute to local IgM, IgA, IgG2, and IgE responses, as shown by increased dermal total Ig/HSA ratios compared to blood, the presence of Ig transcripts at the foreskin, the lack of correlation between inner dermal responses and blood, and the presence of dermal cells with intracellular IgA or IgG. Our results indicate that the foreskin combines roles for systemic responses and resident ASCs, similar to what has previously been shown for the intestine, the female reproductive tract, and seminal fluid24–27. Thus, examining localized responses could reveal new mucosal and genital correlates of exposure and protection, such as the newly identified neutralizing anti-HIV IgA in sub-preputial swabs 45,46..
Examining Ig/HSA ratios also may aid understanding of the location of pathogen-specific responses, such as for adenovirus. Previous reports have indicated adenoviruses could be shed rectally in HIV infected individuals47; and uncircumcised Ad5-seropositive MSM vaccinated in the Step study were found to be at increased risk of HIV infection48. We show that Ad5-seropositive MSM have Ad5 IgG and IgA localized in the colonic mucosa, whereas only Ad5 IgG reaches the foreskin. These results suggest that natural adenovirus exposure does not attract Ad5 ASCs to this genital surface, but Ad5 IgG from plasma can reach dermal and epidermal compartments. Detecting antigen-specific antibodies in explant cultures may serve to evaluate whether adoptive transfer therapies and HIV vaccinations can reach the foreskin in humans, and provide a picture of the effector response at male genital sites.
Our data indicates that the foreskin has selective barriers for antibody transport at the epidermis. We demonstrate that the foreskin epithelium is relatively impermeable to IgG2, IgG4, and IgE transport, whereas IgA, IgG1, IgG3, and IgM are consistently detected at the foreskin epidermal layers. This could suggest differential turnover of the isotypes in the epithelium, or selective receptor-mediated transport. The polymeric Ig receptor (pIgR) could play a role in IgA and IgM movement across the epithelium, like it does in the gut and female genital compartments 49,50. IgG1 and IgG3 have higher affinity for the neonatal Fc receptor (FcRn) than IgG2 and IgG4, and it has been shown to modify IgG transport at the penile urethra and human endocervix 51. Future work will aim to elucidate the transport mechanisms, and define whether they can aid in control of infections at the foreskin.
Additionally, our study is consistent with NHP studies, where the foreskin-localized B-cell response was concentrated at the inner foreskin52. Compared to outer layers, we observed increased transcription of local Igs at the inner foreskin. Blood and dermal Ig/HSA ratios for less dominant isotypes were directly correlated only at the outer foreskin, suggesting local responses play a more important role at the inner foreskin This might correspond to a localized response to the inner foreskin microbiota 53, and may include localized antibody deposition from penile secretions, which can collect in the subpreputial space.
Given the enrolment restrictions of our study population, it is unclear whether these findings are common to all uncircumcised men, or if it only represents young men at high HIV risk (with or without asymptomatic infections). One report has identified CD138+ IgA+ cells in the foreskin of one low risk Kenyan man, but they were lacking in another one 45. Further work will determine if humoral vaccine-induced responses can reach these sites of host-pathogen interaction and potentially provide protection for all uncircumcised males.
Curiously, our data suggests that both foreskin IgG3 and IgG4 antibodies are derived primarily from blood, but only IgG3 can reach the foreskin epithelium. This finding is interesting in the context of the RV144 study, where circulating anti-Env IgG3 antibodies correlated with reduced risk of infection, while the anti-Env antibodies in the non-protective VAX003 study utilizing a similar regimen were predominantly IgG44,5. In addition to the functional consequences associated with each isotype5,30, their ability to reach genital surfaces in sufficient concentration and to find appropriate mucosal cells for effector mechanisms may contribute to vaccine efficacy.
In summary, we have demonstrated that both local and systemic antibodies contribute to the foreskin immune response. We show selective antibody isotype penetration to the foreskin epidermis, especially at the inner foreskin. The assays presented in this paper, and the recently reported collection of subprepucial swabs45,46, may provide valuable information to assess penile antibody responses in vaccine studies protecting against STIs.
Acknowledgments
Thanks to the study volunteers for their participation; to Dr. V.M. Barrientos, Dr. H. Agurto and the clinical staff at IMPACTA for follow up of study participants; to Matt Perez, Tracy Goodpaster, Jane Vasylyeva, Greg Spies, Maria E. Gamero, Sonia Minaya, Richard Negrón, Jesus Jurupe, Monica Nieto, Nilda Gadea, Elisa Ahern, and McKenzie Momany for laboratory support. Thanks to the FHCRC Genomics, Imaging Laboratory, and Experimental Histopathology Cores for immunofluorescence microscopy assistance and Agilent measurements. Thanks to Florian Hladik, Georgia Tomaras, and Julie Overbaugh for helpful discussion, and Stephen Voght for critical reading of the manuscript. Thanks to Gina Escamilla and Cheryl de Boer for help with data management, and to Sarah Holte for consultations at the CFAR Biostatistical Core. We thank the James B. Pendleton Charitable Trust for their generous equipment donation.
Footnotes
DISCLOSURE AND COPYRIGHT STATEMENT
Research reported in this publication was supported by the National Institute of Allergy and Infectious Disease of the National Institutes of Health under awards numbers UM1AI068618, UM1AI068614, U01AI069438 and a New Investigator Award from the University of Washington Center for AIDS Research (CFAR) P30AI027757. The authors disclose no conflict of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views, policy or position of the National Institutes of Health, Department of the Navy, Department of Defense, or U.S. Government. Dr. Montano is an employee of the U.S. Government who contributed to this work as part of her official duties. No copyright protection is available for her work as defined by Titles 17 U.S.C. §105 and §101.
References
- 1.Lynch RM, Yamamoto T, McDermott AB. HIV vaccine research and discovery in the nonhuman primates model: a unified theory in acquisition prevention and control of SIV infection. Current opinion in HIV and AIDS. 2013;8:288–294. doi: 10.1097/COH.0b013e328361cfe8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rerks-Ngarm S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. The New England journal of medicine. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 3.Haynes BF, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. The New England journal of medicine. 2012;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yates NL, et al. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Science translational medicine. 2014;6:228ra239. doi: 10.1126/scitranslmed.3007730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung AW, et al. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Science translational medicine. 2014;6:228ra238. doi: 10.1126/scitranslmed.3007736. [DOI] [PubMed] [Google Scholar]
- 6.Goedert JJ, et al. Determinants of retrovirus (HTLV-III) antibody and immunodeficiency conditions in homosexual men. Lancet. 1984;2:711–716. doi: 10.1016/s0140-6736(84)92624-2. [DOI] [PubMed] [Google Scholar]
- 7.Johnson AM, et al. Transmission of HIV to heterosexual partners of infected men and women. AIDS. 1989;3:367–372. doi: 10.1097/00002030-198906000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Mestecky J. Humoral immune responses to the human immunodeficiency virus type-1 (HIV-1) in the genital tract compared to other mucosal sites. Journal of reproductive immunology. 2007;73:86–97. doi: 10.1016/j.jri.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Dinh MH, et al. Visualization of HIV-1 interactions with penile and foreskin epithelia: clues for female-to-male HIV transmission. PLoS pathogens. 2015;11:e1004729. doi: 10.1371/journal.ppat.1004729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.UNAIDS, W. a. New Data on Male Circumcision and HIV Prevention: Research Implications for Policy and Programming. WHO and UNAIDS; Montreux, Switzerland: 2007. [Google Scholar]
- 11.Auvert B, et al. Estimating the resources needed and savings anticipated from roll-out of adult male circumcision in Sub-Saharan Africa. PloS one. 2008;3:e2679. doi: 10.1371/journal.pone.0002679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallett TB, et al. Understanding the impact of male circumcision interventions on the spread of HIV in southern Africa. PloS one. 2008;3:e2212. doi: 10.1371/journal.pone.0002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herman-Roloff A, Otieno N, Agot K, Ndinya-Achola J, Bailey RC. Acceptability of medical male circumcision among uncircumcised men in Kenya one year after the launch of the national male circumcision program. PloS one. 2011;6:e19814. doi: 10.1371/journal.pone.0019814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Auvert B, et al. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS medicine. 2005;2:e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray RH, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369:657–666. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 16.Bailey RC, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369:643–656. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez J, et al. Male circumcision and risk of HIV acquisition among MSM. AIDS. 2011;25:519–523. doi: 10.1097/QAD.0b013e328340fd81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodreau SM, et al. What drives the US and Peruvian HIV epidemics in men who have sex with men (MSM)? PloS one. 2012;7:e50522. doi: 10.1371/journal.pone.0050522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tauber PF, Zaneveld LJ, Propping D, Schumacher GF. Components of human split ejaculates. I. Spermatozoa, fructose, immunoglobulins, albumin, lactoferrin, transferrin and other plasma proteins. Journal of reproduction and fertility. 1975;43:249–267. doi: 10.1530/jrf.0.0430249. [DOI] [PubMed] [Google Scholar]
- 20.Rumke P. The origin of immunoglobulins in semen. Clinical and experimental immunology. 1974;17:287–297. [PMC free article] [PubMed] [Google Scholar]
- 21.Gelzayd EA, Kraft SC, Kirsner JB. Distribution of immunoglobulins in human rectal mucosa. I. Normal control subjects. Gastroenterology. 1968;54:334–340. [PubMed] [Google Scholar]
- 22.Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal immunology. 2008;1:11–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- 23.Brandtzaeg P, Baklien K, Fausa O, Hoel PS. Immunohistochemical characterization of local immunoglobulin formation in ulcerative colitis. Gastroenterology. 1974;66:1123–1136. [PubMed] [Google Scholar]
- 24.Crago SS, et al. Distribution of IgA1-, IgA2-, and J chain-containing cells in human tissues. J Immunol. 1984;132:16–18. [PubMed] [Google Scholar]
- 25.Kutteh WH, Prince SJ, Hammond KR, Kutteh CC, Mestecky J. Variations in immunoglobulins and IgA subclasses of human uterine cervical secretions around the time of ovulation. Clinical and experimental immunology. 1996;104:538–542. doi: 10.1046/j.1365-2249.1996.36742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moldoveanu Z, Huang WQ, Kulhavy R, Pate MS, Mestecky J. Human male genital tract secretions: both mucosal and systemic immune compartments contribute to the humoral immunity. J Immunol. 2005;175:4127–4136. doi: 10.4049/jimmunol.175.6.4127. [DOI] [PubMed] [Google Scholar]
- 27.Pudney J, Anderson DJ. Immunobiology of the human penile urethra. The American journal of pathology. 1995;147:155–165. [PMC free article] [PubMed] [Google Scholar]
- 28.Bjercke S, Brandtzaeg P. Glandular distribution of immunoglobulins, J chain, secretory component, and HLA-DR in the human endometrium throughout the menstrual cycle. Hum Reprod. 1993;8:1420–1425. doi: 10.1093/oxfordjournals.humrep.a138271. [DOI] [PubMed] [Google Scholar]
- 29.Kutteh WH, Hatch KD, Blackwell RE, Mestecky J. Secretory immune system of the female reproductive tract: I. Immunoglobulin and secretory component-containing cells. Obstetrics and gynecology. 1988;71:56–60. [PubMed] [Google Scholar]
- 30.Bruhns P, et al. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113:3716–3725. doi: 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- 31.Kantele A, et al. Differences in immune responses induced by oral and rectal immunizations with Salmonella typhi Ty21a: evidence for compartmentalization within the common mucosal immune system in humans. Infection and immunity. 1998;66:5630–5635. doi: 10.1128/iai.66.12.5630-5635.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lubeck MD, et al. Immunogenicity of recombinant adenovirus-human immunodeficiency virus vaccines in chimpanzees following intranasal administration. AIDS research and human retroviruses. 1994;10:1443–1449. doi: 10.1089/aid.1994.10.1443. [DOI] [PubMed] [Google Scholar]
- 33.Yang OO, et al. Differential blood and mucosal immune responses against an HIV-1 vaccine administered via inguinal or deltoid injection. PloS one. 2014;9:e88621. doi: 10.1371/journal.pone.0088621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemos MP, et al. The Inner Foreskin of Healthy Males at Risk of HIV Infection Harbors Epithelial CD4+ CCR5+ Cells and Has Features of an Inflamed Epidermal Barrier. PloS one. 2014;9:e108954. doi: 10.1371/journal.pone.0108954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hendrix SW, et al. Optimization of the skin multiple analyte profile bioanalytical method for determination of skin biomarkers from D-Squame tape samples. Skin Res Technol. 2007;13:330–342. doi: 10.1111/j.1600-0846.2007.00235.x. [DOI] [PubMed] [Google Scholar]
- 36.Fong Y, Sebestyen K, Yu X, Gilbert P, Self S. nCal: a R package for nonlinear calibration. Bioinformatics. 2013;29:2653–2654. doi: 10.1093/bioinformatics/btt456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rothschild MA, Bauman A, Yalow RS, Berson SA. Tissue distribution of I131 labeled human serum albumin following intravenous administration. The Journal of clinical investigation. 1955;34:1354–1358. doi: 10.1172/JCI103183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aste-Amezaga M, et al. Quantitative adenovirus neutralization assays based on the secreted alkaline phosphatase reporter gene: application in epidemiologic studies and in the design of adenovector vaccines. Human gene therapy. 2004;15:293–304. doi: 10.1089/104303404322886147. [DOI] [PubMed] [Google Scholar]
- 39.Hollander M, Wolfe DA, Chicken E. Nonparametric Statistical Methods. 3rd. Wiley; Hoboken, NJ: 2013. [Google Scholar]
- 40.Kutteh WH, Mestecky J. Secretory immunity in the female reproductive tract. Am J Reprod Immunol. 1994;31:40–46. doi: 10.1111/j.1600-0897.1994.tb00845.x. [DOI] [PubMed] [Google Scholar]
- 41.Mehandru S, et al. Mechanisms of gastrointestinal CD4+ T-cell depletion during acute and early human immunodeficiency virus type 1 infection. Journal of virology. 2007;81:599–612. doi: 10.1128/JVI.01739-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hel Z, McGhee JR, Mestecky J. HIV infection: first battle decides the war. Trends in immunology. 2006;27:274–281. doi: 10.1016/j.it.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 43.Li Q, et al. Visualizing antigen-specific and infected cells in situ predicts outcomes in early viral infection. Science. 2009;323:1726–1729. doi: 10.1126/science.1168676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hladik F, et al. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity. 2007;26:257–270. doi: 10.1016/j.immuni.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirbod T, et al. HIV acquisition is associated with increased antimicrobial peptides and reduced HIV neutralizing IgA in the foreskin prepuce of uncircumcised men. PLoS pathogens. 2014;10:e1004416. doi: 10.1371/journal.ppat.1004416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prodger JL, et al. Immune correlates of HIV exposure without infection in foreskins of men from Rakai, Uganda. Mucosal immunology. 2014;7:634–644. doi: 10.1038/mi.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Curlin ME, et al. Frequent detection of human adenovirus from the lower gastrointestinal tract in men who have sex with men. PloS one. 2010;5:e11321. doi: 10.1371/journal.pone.0011321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buchbinder SP, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaetzel CS, Robinson JK, Chintalacharuvu KR, Vaerman JP, Lamm ME. The polymeric immunoglobulin receptor (secretory component) mediates transport of immune complexes across epithelial cells: a local defense function for IgA. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:8796–8800. doi: 10.1073/pnas.88.19.8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Natvig IB, Johansen FE, Nordeng TW, Haraldsen G, Brandtzaeg P. Mechanism for enhanced external transfer of dimeric IgA over pentameric IgM: studies of diffusion, binding to the human polymeric Ig receptor, and epithelial transcytosis. J Immunol. 1997;159:4330–4340. [PubMed] [Google Scholar]
- 51.Gupta S, et al. The Neonatal Fc Receptor (FcRn) Enhances Human Immunodeficiency Virus Type 1 (HIV-1) Transcytosis across Epithelial Cells. PLoS pathogens. 2013;9:e1003776. doi: 10.1371/journal.ppat.1003776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rothaeusler K, et al. Antiviral antibodies and T cells are present in the foreskin of simian immunodeficiency virus-infected rhesus macaques. Journal of virology. 2012;86:7098–7106. doi: 10.1128/JVI.00410-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Price LB, et al. The effects of circumcision on the penis microbiome. PloS one. 2010;5:e8422. doi: 10.1371/journal.pone.0008422. [DOI] [PMC free article] [PubMed] [Google Scholar]