Abstract
Objective
To describe patterns and secular trends in use of immunomodulatory agents in pregnant women with systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), psoriatic arthritis (PsA), or ankylosing spondylitis (AS).
Methods
A cohort of women with SLE, RA, PsA, or AS enrolled in public (Medicaid, 2001–2010) or private (OptumClinformatics, 2004–2012) health insurance was identified and women filling prescriptions for immunomodulatory agents- including steroids, non-biologic disease-modifying agents, and biologics- in the 3-month period immediately prior to their pregnancy were included. The proportion of women continuing or discontinuing individual agents during pregnancy was reported. Annual prescription fill rates, estimated after accounting for patient characteristics and random variability from year-to-year in mixed-effect regression models, were used to conduct time-trends analysis.
Results
A total of 2,645 women actively treated with immunomodulatory agents prior to pregnancy were included. More women with PsA or AS stopped filling immunomodulatory prescriptions in pregnancy (61%) than those with SLE (26%) or RA (34.5%). From the first to the third trimester, the proportion of women filling prescriptions for immunomodulatory agents decreased across all indications. Overall, steroids (48.4%) and hydroxychloroquine (27.1%) were the most frequently used agents in pregnancy. The rates (reported per 100 deliveries in our cohort) for steroid prescription fills during pregnancy fell significantly from 54.4 to 42.4; while rates for biologics increased from 5.1 to 16.6 between 2001 and 2012 (p<0.001 for both trends).
Conclusions
Steroids and hydroxychloroquine remain the most widely prescribed treatment options in pregnancy; but the use of biologics is becoming increasingly common.
Background
Systemic inflammatory conditions such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), psoriatic arthritis (PsA), and ankylosing spondylitis (AS) affect many women during their childbearing years. The influence of pregnancy on disease activity of these rheumatologic conditions is variable. For instance, despite widespread belief that disease activity improves in nearly all RA patients in pregnancy based on historical data (1, 2), contemporary research suggest that only 48–65% of patients experience improvement (3, 4). In case of SLE and AS, patients may experience no improvement or even worsening of their disease activity (5, 6). As a result, treatment with immunomodulatory agents may be required during pregnancy to manage active disease in women with these rheumatic conditions.
The absence of conclusive evidence on fetal safety of immunomodulatory medications makes the decision to continue or discontinue these agents at various stages of pregnancy challenging for patients and providers. A recent survey from the UK revealed that fewer than two thirds of rheumatologists and only 39% of obstetricians offered any patient education or advice to women with rheumatologic conditions regarding the use of disease-modifying agents in pregnancy (7). While this uncertainty among providers regarding the management of rheumatologic conditions with immunomodulatory agents during pregnancy is well-recognized, empirical data describing the time-trends and patterns of use of these agents during pregnancy in routine practice are scarce. We therefore designed this study in a large US cohort of publicly or privately insured pregnant women with SLE, RA, PsA, or AS who were actively treated with immunomodulatory agents prior to their pregnancy in order to examine the treatment changes at various stages of pregnancy. We also evaluated secular trends in the use of these agents during pregnancy in this cohort between 2001 and 2012.
Patients and Methods
Data source and study population
Data for this study were drawn from the Medicaid Analytical eXtract (MAX) files for enrollees in 46 US states and Washington, DC for the period of 2001 to 2010 (excluding Arizona, Connecticut, Michigan, and Montana because of incomplete data) and OptumClinformatics files for private insurance enrollees from all 50 states and Washington, DC for the period of 2004 to 2012. These files contain information on demographics, diagnoses and procedures performed during outpatient visits or inpatient stays, and outpatient filled prescription records. The use of these de-identified databases for research was approved by the institutional review board of Brigham and Women’s Hospital.
The study population consisted of women aged 12 to 55 years with completed pregnancies resulting in live born infants. Methods used for linking mothers with their infants in Medicaid files have been described in detail previously (8). In OptumClinformatics files, we used a family identifier to achieve linkage between mothers and infants. In the next step, the date of last menstrual period (LMP) was identified based on the delivery date and codes for preterm birth using a validated algorithm (9). In both databases, we required women to have continuous eligibility in their health insurance plan between 3-months prior to the LMP date and the delivery date in order to ensure the completeness of their healthcare claims. Finally, we identified women with a recorded diagnosis of the systemic inflammatory conditions of interest (RA, SLE, AS, or PsA), who filled at least one outpatient prescription for an immunomodulatory agent 3 months prior to LMP. The list of ICD-9 diagnosis codes and immunomodulatory agents considered for inclusion is provided in Appendix 1.
Definitions of pregnancy periods and drug exposure
Three trimesters were defined based on the estimated LMP date; the first trimester extended from LMP date to day-90 of pregnancy, the following 90 days were defined as the second trimester, and the period between day-181 and the delivery date was defined as the third trimester. Based on the dispensing date of an individual prescription for the immunomodulatory agents of interest, use was classified into one of these three trimesters. For the time-trend analysis, we aggregated utilization data at any time in pregnancy at the class level annually for all non-biologic disease-modifying agents except hydroxychloroquine and for all biologic agents.
Definition of study subgroups
To describe prescription utilization according to the underlying systemic inflammatory condition diagnosis, we divided our study cohort into three subgroups; 1) SLE subgroup, 2) RA subgroup, 3) AS or PsA subgroup. Each study subgroup exclusively contained women with a recorded diagnosis for the respective condition. Accordingly, women who had recorded diagnoses for two disparate conditions, RA and SLE for instance, were not included in these study subgroups; and only included in the main study cohort.
Statistical analysis
Maternal characteristics, including maternal age, race (for Medicaid only), and region of delivery were summarized for each of the three study subgroups and for the whole cohort, separated by the data source. For the five most frequently used immunomodulatory agents at baseline, we reported the proportion of women changing or continuing the treatment.
The time-trend analysis was conducted between 2001 and 2012 using data from everyone in the whole cohort in two steps. First, to account for variation in the demographic characteristics, data source, and case-mix of underlying systemic inflammatory condition diagnoses as well as random variability from year to year, we used mixed effect regression models with a logit link(10) and calculated adjusted annual rates of prescription fills per 100 pregnancies included in our cohort for each of the four classes of immunomodulatory agents (steroids, hydroxychloroquine, non-biologics except hydroxychloroquine, and biologics) considered. In these models, maternal characteristics were modeled as fixed effects, and the year was modeled as a normally distributed random intercept. In the second step, tests for linear trend in the adjusted rates were conducted to check for significance in time-trends.
Results
The study cohort consisted of 2,645 pregnancies actively treated with immunomodulatory medications 3-months prior to conception for SLE, RA, PsA, or AS; that were identified from a population of 2.1 Million eligible pregnancies (0.13%). The study cohort represented women with Medicaid enrollment (62.8%) as well as women with commercial insurance enrollment (37.2%). Overall, 37.7% of the cohort had only SLE, 37.4% had only RA, 13.7% had either AS or PsA, and 11.1% had recorded diagnoses for more than one of these conditions. Table 1 shows key demographic characteristics of the cohort. Notably, women with Medicaid enrollment were younger (Mean (SD) age 27.1 (6) years) than commercially insured women (32.4 (5) years) and a high proportion of Medicaid-enrolled women with SLE were black (45.1%), while 80.3% of Medicaid-enrolled women with PsA or AS were white.
Table 1.
Demographic and delivery characteristics of pregnant women linked to live-born infants and actively treated with immunomodulatory agents 3-months prior to conception for selected rheumatologic conditions
| Medicaid enrollees (2000–2010) | United enrollees (2004–2012) | |||||||
|---|---|---|---|---|---|---|---|---|
| SLE (n=712) | RA (n=596) | PsA/AS (n=178) | SLE, RA, PsA or AS* (n=1,663) | SLE (n=285) | RA (n=394) | PsA/AS (n=185) | SLE, RA, PsA or AS* (n=982) | |
| Maternal age, years (Mean±SD) | 26.3 ± 5.8 | 28.1 ± 6.2 | 26.4 ± 5.1 | 27.1 ± 6.0 | 32.0 ± 5.3 | 32.5 ± 5.0 | 32.9 ± 4.8 | 32.4 ± 5.0 |
| Maternal race** | ||||||||
| White | 194 (27.2) | 270 (45.3) | 143 (80.3) | 672 (40.5) | - | - | - | - |
| Black | 321 (45.1) | 108 (18.1) | 13 (7.3) | 498 (29.9) | - | - | - | - |
| Hispanic | 140 (19.7) | 141 (23.7) | <11 | 328 (19.7) | - | - | - | - |
| Other | 57 (8.0) | 77 (12.9) | 13 (7.3) | 165 (9.9) | - | - | - | - |
| Region | ||||||||
| Midwest | 176 (24.7) | 161 (27.0) | 76 (42.7) | 466 (28.0) | 52 (18.5) | 93 (23.6) | 42 (22.7) | 207 (21.1) |
| Northeast | 157 (22.1) | 104 (17.4) | 27 (15.2) | 319 (19.2) | 40 (14.0) | 61 (15.5) | 18 (9.7) | 150 (15.3) |
| South | 226 (31.7) | 156 (26.2) | 58 (32.6) | 488 (29.3) | 132 (46.3) | 168 (42.6) | 94 (50.8) | 445 (45.3) |
| West | 153 (21.5) | 175 (29.4) | 17 (9.5) | 390 (23.5) | 61 (21.4) | 72 (18.3) | 31 (16.8) | 180 (18.3) |
Total number in the overall cohort is greater than the sum of three individual cohorts because the overall cohort includes women with recorded diagnoses of multiple conditions, who were excluded from individual cohorts.
Race information not recorded in United Healthcare claims.
Abbreviations: AS- Ankylosing spondylitis, PsA- Psoriatic arthritis, RA- Rheumatoid arthritis, SLE- Systematic lupus erythematosus
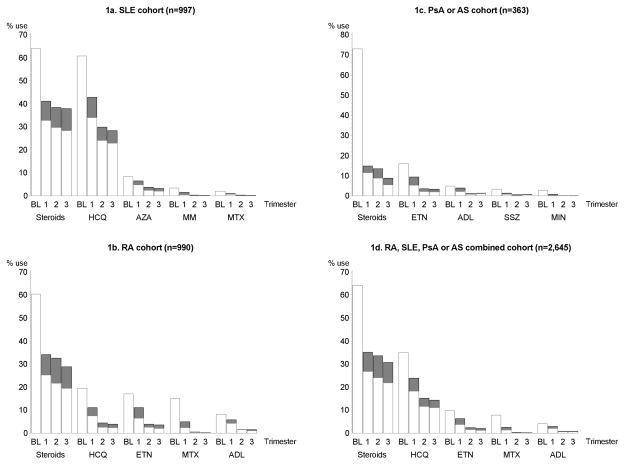
Women with SLE used steroids (64.1%) and hydroxychloroquine (60.9%) most frequently 3 months prior to conception. Approximately half of these women continued filling prescriptions for the same agents in the first trimester (32.8% and 34.0%, respectively for steroids and hydroxychloroquine- Figure 1a). In the second and third trimesters, fewer women continued filling prescriptions for steroids (29.7% and 28.4%, respectively) or hydroxychloroquine (24.1% and 23.0%, respectively). New steroid prescriptions were filled by 8.3%, 8.8%, and 9.5% of the women with SLE who did not use any steroids 3 months prior to conception in the first, second, and third trimesters, respectively. Similarly, new hydroxychloroquine prescriptions were filled by 8.9%, 5.8%, and 5.4% of the women with SLE who did not use any hydroxychloroquine 3 months prior to conception in the first, second, and third trimesters, respectively. Very few women filled new prescriptions of mycophenolate mofetil or methotrexate during pregnancy (<1%). Approximately a fourth of the women in the SLE cohort (26.0%) who were actively treated with an immunomodulatory drug 3 months prior to conception stopped filling prescriptions for these agents in pregnancy.
Figure 1.
Prescription fill patterns at various stages of pregnancy for the five most frequently used immunomodulatory agents in pregnant women with (a) systemic lupus erythematosus; (b) rheumatoid arthritis; (c) ankylosing spondylitis or psoriatic arthritis; and (d) systemic lupus erythematosus, rheumatoid arthritis, ankylosing spondylitis or psoriatic arthritis.
Abbreviations: ADL- Adalimumab, AS- Ankylosing spondylitis, BL- Baseline (3 months prior to conception), ETN- Etanercept, HCQ- Hydroxychloroquine, MIN- Minocycline, MM- Mycophenolate mofetil, MTX- Methotrexate, PsA- Psoriatic arthritis RA- Rheumatoid arthritis, SLE- Systemic lupus erythematosus SSZ- Sulfasalazine.
*White bars indicate the proportion of women continuing that agent from baseline; grey bars indicate the proportion of women initiating that agent during pregnancy. Proportions at any time point may be >100% because women may have used more than one agent at a given time point. Numbers in three subgroups do not add up to the total number in the whole cohort as women who had recorded diagnoses of two disparate conditions, RA and SLE for instance, were not included in these study subgroups; but were included in the main study cohort.
In women with RA, steroids (60.4%), hydroxychloroquine (19.5%), etanercept (17.0%), methotrexate (14.9%), and adalimumab (8.1%) were the five most commonly used agents 3 months prior to conception (Figure 1b) and 25.1%, 7.5%, 6.5%, 2.3%, and 4.2% of women, respectively, continued filling prescriptions for these agents in the first trimester. In the second and third trimesters, steroids were continued by 21.7% and 19.5%, while very few women continued treatment with other agents (2.5% and 2.3% with hydroxychloroquine; 2.5% and 2.0% with etanercept; 0.1% and 0.2% with methotrexate; and 1.4% and 1.0% with adalimumab). Newly filled steroid prescriptions were observed in 9.0%, 10.9%, and 9.3% and newly filled etanercept prescriptions were observed in 4.5%, 1.3%, and 1.5% of the RA cohort in the first, second, and third trimesters, respectively; while new start of other treatments was uncommon. More than a third of the women in the RA cohort (34.5%) did not fill a prescription for an immunomodulatory agent in pregnancy.
In women with PsA or AS, steroids (73.0%) and etanercept (16.0%) were used most frequently 3 months prior to conception (Figure 1c); but only a minority of women continued treatment with these agents in the first trimester (11.6% and 5.2%, respectively). Continued use in the second and third trimesters was even lower for both these agents (8.8% and 5.5% for steroids; 2.2% and 1.9% for etanercept). Other agents including adalimumab, sulfasalazine, and minocycline were seldom used prior to conception and frequently discontinued in pregnancy by women in the PsA or AS cohort. Overall, 61% of the women with PsA or AS stopped treatment with immunomodulatory agents during pregnancy. Appendix Tables 1–3 presents use of immunomodulatory agents in all three cohorts in publicly and privately insured women, separately.
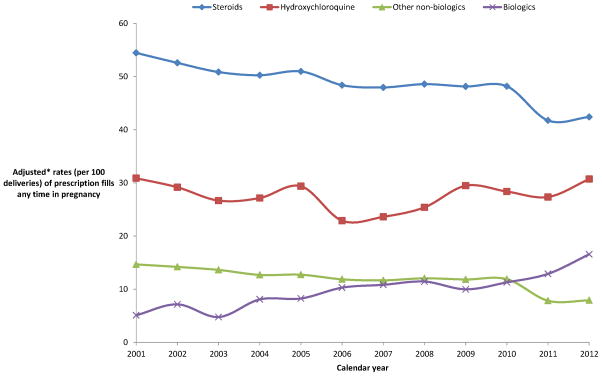
Figure 2 demonstrates the variation in rates of prescription fills (reported per 100 deliveries included in our cohort) in pregnancy for immunomodulatory agents during the 12 years of our study period, after accounting for maternal age, region, insurance type (Medicaid or private), case-mix (underlying treatment indication- SLE, RA, PsA, or AS), and random variability in mixed-effect regression models. While steroids remained the most frequently used drug class in pregnancy; the rates for steroid prescription fills significantly fell from 54.4 in 2001 to 42.4 in 2012 (p<0.001). Among the non-biologic disease modifying agents, hydroxychloroquine was the most commonly used agent during pregnancy for rheumatologic conditions, and its use remained stable during the study period (30.9 in 2001 to 30.7 in 2012). Other non-biologic agents showed decline in use from 14.7 in 2001 to 7.9 in 2012 (p<0.001). Most notably, use of biologics increased three-fold during our study period from 5.1 in 2001 to 16.6 in 2012 (p<0.001). Appendix Table 4 provides yearly numbers for use of all individual immunomodulatory agents included in this study.
Figure 2.
Time-trends in use of immunomodulatory agents during pregnancy in a cohort of women with systemic lupus erythematosus, rheumatoid arthritis, ankylosing spondylitis or psoriatic arthritis.
* Adjusted for maternal characteristics (age, region), insurance type (Medicaid or private), case-mix (underlying treatment indication- SLE, RA, PsA, or AS), and random variability across years using a mixed regression model.
**Other non-biologics include methotrexate, azathioprine, cyclophosphamide, cyclosporine, gold compounds (auranofin, gold, solganol, myochrysine), leflunomide, minocycline, mycophenolate mofetil, penicillamine, and sulfasalazine. Biologics include abatacept, adalimumab, alefacept, anakinra, certolizumab pegol, etanercept, golimumab, infliximab, natalizumab, rituximab, and tocilizumab.
Discussion
In a large cohort of women with rheumatologic conditions, who were actively treated with immunomodulatory agents 3 months prior to pregnancy, approximately one in four with SLE, one in three with RA, and two in three with PsA or AS, stopped filling prescriptions for immunomodulatory agents during pregnancy. Steroids and hydroxychloroquine were the most commonly used immunomodulatory agents during pregnancy over the twelve year study period (2001–2012), however, declining trends in use for steroids were observed accompanied by a compensating increasing trend in biologic use between 2001 and 2012.
Similar to our findings, several prior studies focusing exclusively on women with RA from the US (11), the Netherlands (12), and Norway (4), as well as women with SLE from Canada (13) noted that steroids were the most commonly used immunomodulatory agents for the treatment of RA during pregnancy. For the non-biologics, some interesting differences were seen between this study and prior reports from Europe (4, 12); where sulfasalazine, and not hydroxychloroquine, was the most commonly used agent during pregnancy in cohorts of women with RA. Similar to prior studies, use of potentially teratogenic non-biologics, methotrexate, mycophenolate, and leflunomide, was found to be extremely low during pregnancy in this study, which is reassuring.
Our study has several strengths including a large sample size, availability of nationally representative contemporary data on prescription fills for immunomodulatory agents over a twelve-year period suitable for describing secular trends, and availability of medical claims data to describe the medication use by underlying rheumatologic conditions. We found a significant decrease in use of steroids and non-biologics other than hydroxychloroquine along with a significant increase in use of biologics during pregnancy between 2000 and 2012. Our findings may be reflective of similar trends seen in the general population (14). Our findings may also suggest that with availability of some reassuring data indicating absence of a major fetal adverse event after biologic use in pregnancy (15), physicians have become more comfortable with continuing treatment with these agents during pregnancy in recent years. The use of steroids during pregnancy is considered relatively safe by the European League Against Rheumatism (16). However, data from a multinational survey indicate that both physicians and patients are skeptical of this conclusion (17), potentially due to concerns related to adverse maternal outcomes such as gestational diabetes and hypertension (18). It is possible that an increasing number of treatment options such as biologics as well as accumulating data supporting the safety of older non-biologic agents such as hydroxychloroquine (19) may have contributed to this declining trend of steroid use seen in our study. Nevertheless, as recommended by the American College of Rheumatology Reproductive Health Summit committee (18), more comparative research on the safety of steroids as well as disease-modifying agents used during pregnancy will be critical for providing the necessary evidence to guide treatment decisions in future.
Our study has limitations. First, we only included women with a live-born delivery in this study, which may lead to underestimation in the use of certain non-biologics such as methotrexate and leflunomide as women using these agents are more likely to have had therapeutic abortions and hence may not have been a part of our cohort. We excluded women without a live-born delivery because for these patients, we would not be able to determine the gestational age of the fetus and therefore the LMP estimation and trimester definitions could be invalid. Second, our database only captures prescriptions filled on an outpatient basis and therefore we may have missed any prescriptions filled in hospital, leading to underestimation of actual use of these medications. Further, a filled prescription does not always indicate compliance, so our study may overestimate the actual extent of use if many women fill prescriptions but do not consume it. Next, women with rheumatologic conditions who were not actively treated with immunomodulatory agents 3 months before LMP were not included in this study. It is possible that some of these women may have initiated these medications during pregnancy; and therefore our results may underestimate the overall use of immunomodulatory agents in pregnancy. Finally, we did not have data on disease activity for the women included in our cohort, which precluded us from further investigating the reasons for treatment changes during pregnancy.
In conclusion, despite the decreasing trends seen in recent years, steroids still remain the most widely prescribed treatment option among pregnant women with rheumatologic conditions. Further, the sharp rise seen in biologic use during pregnancy highlights the need for continuing research evaluating the safety of biologics on various maternal and fetal outcomes. Since many women discontinue immunomodulatory treatments during pregnancy, future research should also evaluate the impact of untreated active disease on fetal or maternal health.
Supplementary Material
Acknowledgments
We acknowledge the contributions of Jun Liu, MD, MSc at the Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital in dataset preparation.
Funding:
This study was not funded by any institution.
Footnotes
Conflict of interest/Financial disclosures:
Dr. Huybrechts is supported by a career development award from the National Institute of Mental Health (K01 MH099141). Dr. Bateman is supported by a career development award from the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the NIH (K08HD075831). Dr. Hernandez-Diaz is supported by the NIH grant R01 MH100216 and has consulted for AstraZeneca (London, UK) for unrelated projects. Dr. Kim is supported by the NIH grant K23 AR059677. She reports receiving research support from Pfizer, AstraZeneca and Lilly on unrelated projects. The other authors declare no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Ostensen M, Husby G. A prospective clinical study of the effect of pregnancy on rheumatoid arthritis and ankylosing spondylitis. Arthritis Rheum. 1983;26(9):1155–9. doi: 10.1002/art.1780260915. [DOI] [PubMed] [Google Scholar]
- 2.Ostensen M. The effect of pregnancy on ankylosing spondylitis, psoriatic arthritis, and juvenile rheumatoid arthritis. Am J Reprod Immunol (New York, NY : 1989) 1992;28(3–4):235–7. doi: 10.1111/j.1600-0897.1992.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 3.Barrett JH, Brennan P, Fiddler M, Silman AJ. Does rheumatoid arthritis remit during pregnancy and relapse postpartum? Results from a nationwide study in the United Kingdom performed prospectively from late pregnancy. Arthritis Rheum. 1999;42(6):1219–27. doi: 10.1002/1529-0131(199906)42:6<1219::AID-ANR19>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 4.de Man Y, Hazes JM, van der Heide H, Willemsen S, de Groot C, EAS, et al. Association of higher rheumatoid arthritis disease activity during pregnancy with lower birth weight: results of a national prospective study. Arthritis Rheum. 2009;60(11):3196–206. doi: 10.1002/art.24914. [DOI] [PubMed] [Google Scholar]
- 5.Ostensen M, Fuhrer L, Mathieu R, Seitz M, Villiger PM. A prospective study of pregnant patients with rheumatoid arthritis and ankylosing spondylitis using validated clinical instruments. Ann Rheum Dis. 2004;63(10):1212–7. doi: 10.1136/ard.2003.016881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clowse ME. Lupus activity in pregnancy. Rheum Dis Clin North Am. 2007;33(2):237–52. v. doi: 10.1016/j.rdc.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panchal S, Khare M, Moorthy A, Samanta A. Catch me if you can: a national survey of rheumatologists and obstetricians on the use of DMARDs during pregnancy. Rheumatol Int. 2013;33(2):347–53. doi: 10.1007/s00296-012-2418-0. [DOI] [PubMed] [Google Scholar]
- 8.Palmsten K, Huybrechts KF, Mogun H, Kowal MK, Williams PL, Michels KB, et al. Harnessing the Medicaid Analytic eXtract (MAX) to Evaluate Medications in Pregnancy: Design Considerations. PloS one. 2013;8(6):e67405. doi: 10.1371/journal.pone.0067405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Margulis AV, Setoguchi S, Mittleman MA, Glynn RJ, Dormuth CR, Hernandez-Diaz S. Algorithms to estimate the beginning of pregnancy in administrative databases. Pharmacoepidemiol Drug Saf. 2013;22(1):16–24. doi: 10.1002/pds.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. Springer Science & Business Media; 2009. [Google Scholar]
- 11.Kuriya B, Hernandez-Diaz S, Liu J, Bermas BL, Daniel G, Solomon DH. Patterns of medication use during pregnancy in rheumatoid arthritis. Arthritis Care Res. 2011;63(5):721–8. doi: 10.1002/acr.20422. [DOI] [PubMed] [Google Scholar]
- 12.Viktil KK, Engeland A, Furu K. Use of antirheumatic drugs in mothers and fathers before and during pregnancy-a population-based cohort study. Pharmacoepidemiol Drug Saf. 2009;18(8):737–42. doi: 10.1002/pds.1775. [DOI] [PubMed] [Google Scholar]
- 13.Vinet E, Pineau CA, Scott S, Clarke AE, Platt RW, Bernatsky S. Increased congenital heart defects in children born to women with systemic lupus erythematosus: results from the offspring of Systemic Lupus Erythematosus Mothers Registry Study. Circulation. 2015;131(2):149–56. doi: 10.1161/CIRCULATIONAHA.114.010027. [DOI] [PubMed] [Google Scholar]
- 14.Kim SC, Yelin E, Tonner C, Solomon DH. Changes in Use of Disease-Modifying Antirheumatic Drugs for Rheumatoid Arthritis in the United States During 1983–2009. Arthritis Care Res. 2013;65(9):1529–33. doi: 10.1002/acr.21997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nielsen OH, Loftus EV, Jr, Jess T. Safety of TNF-alpha inhibitors during IBD pregnancy: a systematic review. BMC medicine. 2013;11:174. doi: 10.1186/1741-7015-11-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoes JN, Jacobs JW, Boers M, Boumpas D, Buttgereit F, Caeyers N, et al. EULAR evidence-based recommendations on the management of systemic glucocorticoid therapy in rheumatic diseases. Ann Rheum Dis. 2007;66(12):1560–7. doi: 10.1136/ard.2007.072157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Goes MC, Jacobs JW, Boers M, Andrews T, Blom-Bakkers MA, Buttgereit F, et al. Patient and rheumatologist perspectives on glucocorticoids: an exercise to improve the implementation of the European League Against Rheumatism (EULAR) recommendations on the management of systemic glucocorticoid therapy in rheumatic diseases. Ann Rheum Dis. 2010;69(6):1015–21. doi: 10.1136/ard.2009.114579. [DOI] [PubMed] [Google Scholar]
- 18.Kavanaugh A, Cush JJ, Ahmed MS, Bermas BL, Chakravarty E, Chambers C, et al. Proceedings from the American College of Rheumatology Reproductive Health Summit: the management of fertility, pregnancy, and lactation in women with autoimmune and systemic inflammatory diseases. Arthritis Care Res. 2015;67(3):313–25. doi: 10.1002/acr.22516. [DOI] [PubMed] [Google Scholar]
- 19.Clowse ME, Magder L, Witter F, Petri M. Hydroxychloroquine in lupus pregnancy. Arthritis Rheum. 2006;54(11):3640–7. doi: 10.1002/art.22159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.