Abstract
The impact of topical antiretrovirals for pre-exposure prophylaxis on humoral responses following HIV infection is unknown. Using a binding antibody multiplex assay, we investigated HIV-specific IgG and IgA responses to envelope glycoproteins, p24 Gag and p66 in the genital tract and plasma following HIV acquisition in women assigned to tenofovir gel (n=24) and placebo gel (n=24) in the CAPRISA 004 microbicide trial to assess if this topical antiretroviral had an impact on mucosal and systemic antibody responses. Linear mixed effect modeling and partial least squares discriminant analysis was used to identify multivariate antibody signatures associated with tenofovir use. There were significantly higher response rates to gp120 Env (p=0.03), p24 (p=0.002) and p66 (p=0.009) in plasma and genital tract (GT) in women assigned to tenofovir than placebo gel at multiple time-points post-infection. Notably, p66 IgA titres in the GT and plasma were significantly higher in the tenofovir compared to the placebo arm (p<0.05). Plasma titres for 9 of the ten HIV-IgG specificities predicted genital tract levels. Taken together, these data suggest that humoral immune responses are increased in blood and GT of individuals who acquire HIV infection in the presence of tenofovir gel.
Keywords: Mucosal antibodies, tenofovir, IgG, IgA, HIV-1, immune response
Introduction
Several recent HIV prevention trials have tested the efficacy of tenofovir containing pre-exposure prophylaxis (PrEP) regimens in oral 1–3 or topical form4, with protective effects ranging from 0%-86%1–6. While poor adherence to PrEP has been identified as a major factor limiting efficacy in these trials, the observed disparity in protection urges further investigation into possible biological mechanisms associated with no to incomplete protection. Preclinical studies in non-human primates (NHPs) and in women in the CAPRISA 004 clinical trial have suggested that exposure to PrEP preserves the magnitude of HIV-1-specific CD4 cell responses generated in those experiencing breakthrough HIV infections7,8. Investigations of humoral immunity following breakthrough infections showed slower development of HIV-specific antibody avidity9,10. Additionally decreased titres were shown in HIV-infected individuals on antiretroviral treatment (ART)11–13. The effect of topical tenofovir on the magnitude and kinetics of mucosal and systemic antibody responses remains an important gap in our knowledge.
Antibody responses at the portal of HIV entry, the mucosa of the lower female reproductive tract, are thought to be a key mechanism to block virus dissemination from the GT and to prevent or delay replication and establishment of a productive infection14–16. Vaccine-induced, locally produced gp41 SIV Env IgG in the female macaque GT correlated with protection in animals receiving a high-dose, intra-vaginal challenge 20 weeks post-vaccination16,17. Additionally, in highly-exposed persistently seronegative (HESN) women, the presence of mucosal HIV-specific antibodies has been suggested to correlate with protection18–21. We found gp120 specific IgAs but no HIV-1 specific IgG responses in GT fluid in HESN women recruited into the HPTN 035 microbicide trial21. In HIV-infected women, we showed that both gp41- and gp120-specific IgA and IgG were detected in the GT21,22.
Given these findings, a major goal of HIV prevention research is to induce protective immunity at the genital mucosa. Defining the properties of the earliest antibody responses at the vaginal mucosa following HIV transmission will enable a better understanding of the potential role of tenofovir in modulating protective antibody responses in the female GT. We hypothesised that higher titre antibodies and increased breadth of HIV-specific antibody responses would be seen in plasma as well as in the GT of women enrolled in the CAPRISA004 trial, because of the likely exposure to HIV in the GT in the presence of tenofovir. This is supported by previous observations of preserved HIV-specific CD4 cell responses in the tenofovir compared to the placebo arm8. We compared HIV-1 antibody response rates and titres of IgG and IgA responses in plasma and cervicovaginal lavages (CVLs) to a panel of ten HIV-specific antibody epitopes. Women in the tenofovir arm could be differentiated from the placebo arm by distinct HIV-1 specific antibody signatures, including plasma and CVL IgA responses to p66 during early HIV-1 infection. Elucidation of the effects of microbicides on HIV-1 antibody responses and evolution in those who become infected will assist in the design and development of future combination prevention strategies.
Methods
Study population and specimen collection
The University of KwaZulu-Natal's Biomedical Research Ethics Committee (E111/06), Family Health International's Protection of Human Subjects Committee (#9946) and the South African Medicines Control Council (#20060835) approved the CAPRISA 004 microbicide gel trial (Clinical Trials Number 00441298). Results of the primary analyses have been published previously4. Written informed consent was obtained from all participants. This study was approved by the Institutional Review Board of Duke University and the University of KwaZulu-Natal's Biomedical Research Ethics Committee.
Forty eight women who seroconverted during the CAPRISA 004 1% tenofovir microbicide gel trial were included in this study4. Of these, 24/48 were from the tenofovir arm (cases) and 24/48 were from the placebo arm (controls), with cases and controls matched according to viral loads and CD4 T cell counts at six months post-infection. Recent HIV-1 infection was diagnosed on the basis of two HIV-1 rapid antibody tests and PCR (Roche Amplicor v1.5, New Jersey, USA), and confirmed by enzyme immunoassay. Women were enrolled within 3 months of infection and the timing of infection was estimated either as the midpoint between the last HIV-1-negative test and the first antibody-positive test, or as 14 days before the first PCR positive test for antibody negative participants. CD4 T cell counts were assessed using a FACSCalibur flow cytometer, and viral loads were measured using a Cobas Amplicor HIV-1 Monitor Test, v1.5 (Roche Diagnostics, New Jersey, USA). HIV-infected women were followed for a median of 36 months after enrolment (range 11.5 - 53.5 months), and plasma and CVL samples from 3, 6, 12, 18, 24 and >30 months post-infection were included in this study. All women were naïve to ART for the duration of this sub-study. Plasma collected in EDTA was stored at −80°C until use. CVL was collected from each woman at each study visit under speculum examination using 10mL sterile saline according to the method described by Bebell et al.23.
HIV-specific binding antibody assay
HIV-specific antibodies against HIV-1 specific Env and Gag proteins were measured in plasma (1:50; 1:10,000 and 1:100,000 dilutions) and CVL (1:3 and 1:10 dilutions) using a customized HIV-1 binding antibody multiplex assay (BAMA)22,24–27. IgG was depleted prior to performing HIV-1 specific IgA assays.22 The antigen panel included the following Env proteins: gp70_B.CaseA_V1_V2 (gp 70 V1V2), Transmitted Founder Clade C gp140 (1086 C.gp140)28,29, C.con.env03 140 CF (Consensus Clade C Env gp140), Con6 gp120/B (Consensus Group M gp120), and Con S gp140 CFI (Group M consensus gp140 Env), and were provided by Drs. Haynes and Liao, Duke Human Vaccine Institute, USA30–32. Commercially available reagents included: p24 Gag and p66 RT (Protein Sciences), and gp41 (ImmunoDiagnostics). Consensus Clade B gp41 immunodominant (ID) epitope in tetramer form was kindly provided by Dr. M.A. Moody33, Duke Human Vaccine Institute, USA). The sequences of the immunodominant epitope and the V3 peptide are as follows: (Biotin CRVLAVERYLRDQQLLGIWGCSGKLICTTAVPWNASWSNKSLNKI). Bio-V3.C (Clade C V3 linear epitope, Biotin- CRVLAVERYLRDQQLLGIWGCSGKLICTTAV from JPT Peptide Technologies, Berlin, Germany).
All assays were run under good clinical laboratory practice (GCLP)-compliant conditions, including tracking of positive controls by Levey-Jennings charts. Positivity cut-offs for binding antibody responses in plasma for antibody-antigen pairs were pre-determined from 30 HIV-1 negative individuals (mean fluorescent intensity (MFI) + 3 standard deviations). Positive controls included titrations of HIV-1+ purified IgG (HIVIG), CH58 IgG mAb and 7B2 IgG or IgA mAbs (mAbs from Drs. Liao and Haynes).
Total immunoglobulin quantification and HIV-specific activity in CVL
Total IgG and IgA in CVL were quantified using a 7-plex BioRad Human Isotyping kit on the Bio-Plex 200 multiplex system (Bio-Rad, Hercules, CA), according to the manufacturer's instructions. Ig concentrations were determined by 4-PL logistic regression using the Bioplex Manager 6.0 software (Bio-Rad, Hercules, CA). HIV-1 specific activity (SA) was defined as the antigen-specific MFI (adjusted for dilution factor) divided by the total immunoglobulin amount (antigen-specific MFI*dilution/ng/ml total IgG or IgA) in order to adjust for individual variation in total Ig recovered when performing CVL. HIV-1 antibody responses were considered positive if they met both antigen-specific positivity criteria and specific activity criteria (mean SA + 3 standard deviations) from a set of 30 seronegative CVLs. Samples that did not meet the positivity cutoff for specific activity were set to 1/10 of the specific activity cut-off for statistical analysis and visualization purposes.
Statistical analysis
Fisher's exact test was used to compare the proportion of detectable IgGs at a single time point between the two arms. Linear mixed models were used to assess the impact of CAPRISA 004 gel arm assignment on both plasma and GT IgG and IgA measurements post HIV infection. Linear mixed models were also used to measure the effect of the plasma IgG levels on that of the GT at the same time point, and the effect of CD4 cells and HIV-1 viral load on IgG levels within each arm. β-coefficients were reported as per 50 cells/μl increase in CD4 cells and per 1 log increase in plasma viral load. Odds ratios were determined by fitting a generalized estimating equation (GEE) model with a binomial distribution. IgG values were log transformed to ensure normality and all p<0.05 were considered statistically significant. The false discovery rate method (Benjamini Hochberg) was used to adjust p values for multiple comparisons to prevent a Type 1 error and circumvents over estimating statistical significance. Statistical analysis was performed using SAS version 9.3 (SAS Institute Inc., Cary).
Identification of antibody signatures with partial least squares discriminant analysis
Partial least squares discriminant analysis (PLSDA) and the LASSO method for regression shrinkage and selection34,35 were used to identify antibody signatures across time-points, and in plasma and CVL compartments, that best differentiated women in the placebo arm from those in the tenofovir arm. Due to missing measurements for some time-points, only measurements made at 6 and 12 months were included in this analysis. After eliminating individuals with missing measurements, 31/48 women were included (18 placebo; 13 tenofovir). All analyses were performed using Matlab software (MathWorks, Natick, MA) and PLS_Toolbox for Matlab (Eigenvector Research Incorporated, Wenatchee, WA). LASSO was implemented using K-fold cross-validation to identify the model with the lowest possible mean squared error for prediction and minimum set of antibodies. PLSDA was used to evaluate the classification accuracy of the selected group of antibodies and was compared to classification obtained from individual measurements alone. Loading values indicate the degree of separation of antibody measurements between the tenofovir and placebo arms. Positive or negative loading values on latent variables (LV) identified by the PLSDA model were used to indicate antibody signatures that best separate tenofovir and placebo arms. The more positive or the more negative a loading on LVs in the model, the more strongly that feature contributes to the distinguishing profile. Score plots were used to illustrate the ability of these signatures to distinguish tenofovir and placebo groups. Cross-validation was performed by repeatedly excluding contiguous subsets of data (5-6 data points per set) during model calibration. Excluded data was then used to test model predictions. Raw data was normalized with mean centering and variance scaling prior to analysis.
Results
Study Participants
Overall, 58.3% (28/48) of the women were from rural areas in KwaZulu-Natal, with only 54.2% (26/48) completing high school and most in stable partnerships (Table 1). The median age of the women was 23 years, with a median blood CD4 count of 498 cells/ul during acute HIV infection and plasma viral load of 59,050 copies/ml. No differences were found between the two arms for age, education, hormonal contraceptive use, marital status, number of sexual partners or condom use. Women included in the study were matched for CD4 T cell counts during acute HIV infection since differences in CD4 helper function may have confounded analysis of HIV-antibody responses. There were no differences between the arms for either CD4 cells or viral loads over time (data not shown). After matching, the earliest samples collected from women in this sub-study in the TFV arm, had a significantly longer time from enrolment to acquisition of HIV-1 infection [12.8 months (IQR- 6.6-16.6 months)] than women in placebo arm [7.4 months (IQR 3.3-10.6 months), p=0.02].
Table 1.
Behavioural and clinical characteristics of the women in this study
| Characteristic | All (N=48) | Tenofovir (N=24) | Placebo (N=24) |
|---|---|---|---|
| Rural % (n) | 58.3% (28) | 58.3% (14) | 58.3% (14) |
| Median Age in years (IQR) | 23 (22-25) | 24 (22-28) | 22 (22-23) |
| Median days PI* at enrolment (IQR) | 38 (24-65) | 35 (27-63) | 45 (23-65) |
| Median CD4 count (cells/μl) (IQR) | 498 (434-655) | 468 (444-569) | 515 (433-685) |
| Median viral load (copies/ml) (IQR) | 59050 (17300-135500) | 80600 (22000-130000) | 54800 (13600-148000) |
| Time to HIV-infection from enrolment in months (IQR)◆ | 9.2 (4.9-14.1) | 12.8 (6.6-16.6) | 7.4 (3.3-10.6) |
| Completed high school % (n) | 54.2% (26) | 41.7% (10) | 66.7% (16) |
| Hormonal contraception# (n) | 97.9% (47) | 100% (24) | 95.8% (23) |
| Marital status % (n) | |||
| Stable/married partner | 79.2% (38) | 87.5% (21) | 70.8% (17) |
| > 2 partners | 2.1% (1) | 0.0% (0) | 4.2% (1) |
| Single | 18.7% (9) | 12.5% (3) | 25.0% (6) |
| Number of reported sexual partners in the last 3 months** % (n) | |||
| 0 to 1 | 93.8% (45) | 95.8% (23) | 91.7% (22) |
| 2 to 5 | 6.2% (3) | 4.2% (1) | 8.3% (2) |
| Reported condom used at last sex act % (n) | 68.8% (33) | 75.0% (18) | 62.5% (15) |
PI = post-infection
Two non-rapid progressing women had missing sexual partner data (in previous 3 months) as they refused to answer the question.
Hormonal contraception use included the injectable depot medroxyprogesterone acetate and norethisterone, and oral combined contraception. One woman in the placebo arm of the study was using an intrauterine device.
Time to HIV infection was significantly different between the tenofovir and placebo arms- p= 0.02.
High IgG response rates to multiple HIV antigens in the genital tract
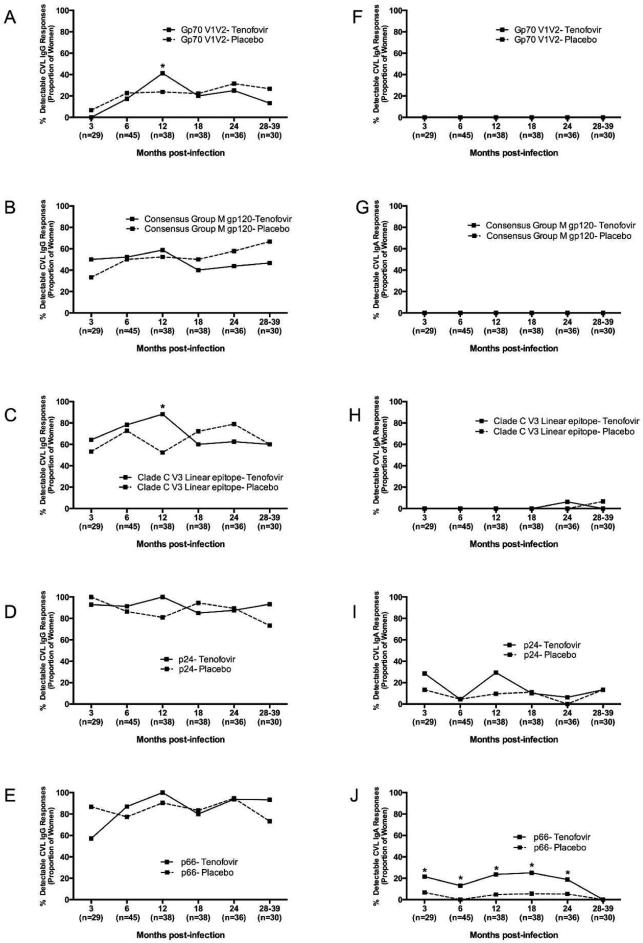
We investigated mucosal IgG and IgA antibody responses (indicated as the number of women responding to any HIV antigen) in the female GT for potential differences in tenofovir vs placebo-exposed women. In the GT, IgG rather than IgA responses, were more readily detected (Fig. 1 and data not shown). The majority of women had GT IgG responses to multiple HIV-1 antigens, compared to more restricted GT IgA responses (Fig. 1 and Suppl. Fig. 1). All of the women had detectable GT IgG responses to p24, p66, gp140 and gp41 (Figs. 1D-E and data not shown). In contrast, GT IgG responses to gp70 V1V2, Consensus Group M gp120, and V3 antigens were seen less frequently and emerged only later after infection (Figs. 1A, 1B and 1C). There was no major difference noted between the study arms except for the rates of GT IgG responses to Clade C V3 linear epitopes were 1.7-times higher in the tenofovir (88.2%) than placebo arm (52.4%) at 12 months post-infection (p=0.03, Fig. 1C).
Figure 1. Mucosal HIV-1-specific IgG but fewer IgA responses in the female genital tract of HIV-1-infected women participating in the CAPRISA 004 microbicide trial.
Cervicovaginal lavage HIV-1 IgG (a–e) and HIV-1 IgA (f–j) to gp70 V1V2, gp120, V3, Gag and p66 antigens were measured in HIV-1-infected CAPRISA 004 participants from 3 to 39 months post infection. Solid lines represent participants in the Tenofovir arm and dotted lines represent participants in the placebo arm. In parentheses below are the sample sizes for each time point. Asterisks indicate significant differences in detection between the arms at respective time points (Fisher’s exact test).
While GT IgA responses to p66 and p24 were detected, gp70 V1V2 and Consensus Group M gp120 IgA responses were not detected (Fig. 1F-G and 1I-J). Women in the tenofovir arm were 3.6-times more likely to have p66 IgA responses than those in the placebo arm (95% CI 1.38 - 9.61; p=0.009; Fig. 1J). No further differences in GT IgA response rates were found between the arms.
Higher prevalence of plasma IgA responses detected in tenofovir compared to placebo arm
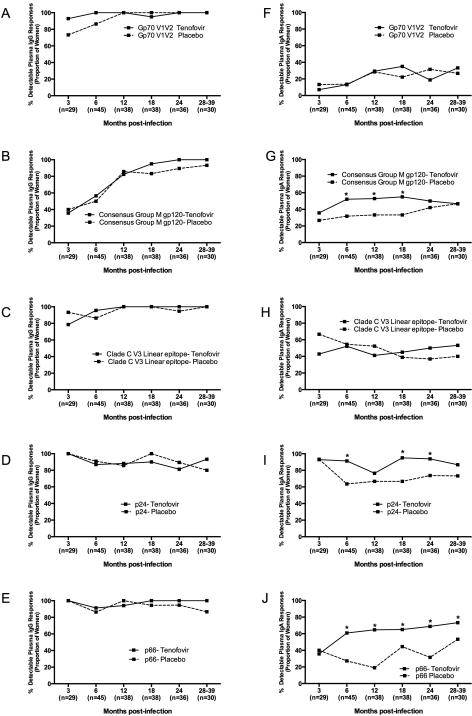
Plasma HIV-1 specific IgG and IgA response rates, indicating the number of women responding to any HIV antigen, were compared to determine whether differences existed between antibody emergence and specificity in those assigned to tenofovir or placebo (Fig. 2). The majority of women had HIV-1 specific IgG responses in plasma that did not differ by treatment arm (Fig. 2A-2E). P24 and p66 were the dominant plasma HIV-1 specific IgG responses in the first three months after infection (Fig. 2D and 2E), with 100% of women in both arms recognizing these antigens. Consensus Group M gp120 IgG responses in plasma emerged more slowly than p24 and p66 responses, with only 35% of women being IgG positive for gp120 at 3 months and 57% being positive at 6 months post-infection (Fig. 2B). Plasma IgG response rates to gp70 V1V2 were generally high throughout the course of infection, ranging from 73-100% response rate in both arms (Fig. 2A). Notably, HIV-infected women from the tenofovir arm had ~4.2-fold higher response rates to gp70 V1V2 than those in the placebo arm (OR=0.8-21.1 95% CI, p=0.08), although this was not significant. In addition, women in the tenofovir arm were 1.8-times more likely to have a Consensus Group M gp120-IgA response from 6-12 months (95% CI 1.06 - 3.11, p=0.08) (Fig. 2G). Plasma IgA response rates to p24 (OR=3.64; 95% CI 1.49 - 6.38; p=0.002) and p66 (OR=2.92; 95% CI 1.68 – 5.07; p=0.0001) were also significantly higher in the tenofovir than in the placebo arm (Fig. 2I and 2J).
Figure 2. Plasma HIV-1-specific IgG and IgA are frequently detected in HIV-1-infected women participating in the CAPRISA 004 microbicide trial.
Plasma HIV-1 IgG (a–e) and HIV-1 IgA (f–j) to gp70 V1V2, gp120, V3, Gag and p66 antigens were measured in HIV-1-infected CAPRISA 004 participants from 3 to 39 months post infection. Solid lines represent participants in the tenofovir arm and dotted lines represent participants in the placebo arm. In parentheses below are the sample sizes for each time point. Asterisks indicate significant differences in detection between the arms at respective timepoints (Fisher’s exact test).
Higher titres of IgG and IgA in plasma and GT in women in the tenofovir arm
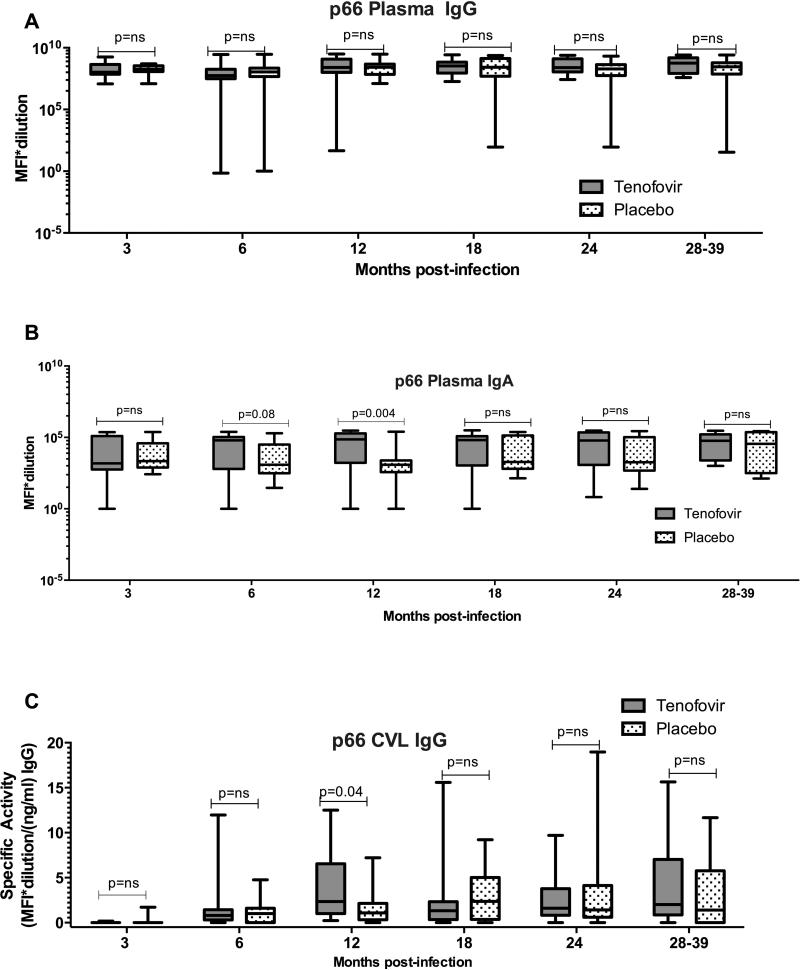
The magnitude of HIV-specific antibody responses, indicative of titres, in plasma and CVL of women in the tenofovir arm were then compared to women in the placebo arm. Similar titres of plasma HIV-specific IgGs were noted in the tenofovir and placebo arms for 9/10 of the antigens tested (Suppl. Fig. 2). Only IgG titres directed against gp70 V1V2 were significantly higher in plasma from women in tenofovir arm compared to women in the placebo arm in the first six months post-infection (p=0.009; adjusted and data not shown). Plasma IgG responses were generally of similar magnitude between the placebo arm and tenofovir arm for most HIV-1 antigens (Fig. 3A; Suppl. Fig. 2). However, mean titres of plasma Consensus Group M gp120 (p=0.05), p24 and p66 IgA responses were higher in the tenofovir treatment arm than the placebo arm. Plasma p24 IgA titres were significantly higher over time and at >6 months post infection in the tenofovir-treated compared to the placebo arm (p=0.016 and p=0.012 respectively- Suppl. Fig. 3) and p66 IgA titres at 6 months trended higher (p=0.08) and was significantly different at 12 months (p=0.004) post HIV-infection (Fig. 3B, Suppl. Fig. 3), although not after adjusting for multiple comparisons. In addition, increased titres of p66 IgGs were found in CVL from tenofovir-treated women compared to those in the placebo arm 12 months (p=0.04) post-infection (Fig. 3C; Suppl. Fig. 4). This trend was also noted for IgA responses in the CVL (Suppl. Fig. 5); although overall IgA response rates and magnitudes were lower in CVL and thus differences could not be compared between groups.
Figure 3. Increased p66 plasma IgA and CVL IgG response magnitude in acute infection with prior tenofovir use.
Plasma response magnitude is presented as MFI*dilution in a (IgG) and b (IgA). CVL response magnitude is presented as specific activity (MFI* dilution factor per ng ml −1) in c (IgG). Boxes denote the 25th and 75th percentile and whiskers denote the minimum and maximum values. The horizontal line represents the median response. Responses with MFI<100 are below the limit of detection for this assay. P<0.05 were statistically significant. Comparisons of MFIs between tenofovir and placebo were done using the Wilcoxon Rank Sums test. CVL, cervicovaginal lavage; MFI, mean fluorescent intensity; NS, not significant.
GT and plasma p66 IgA responses differentiated women in the tenofovir arm
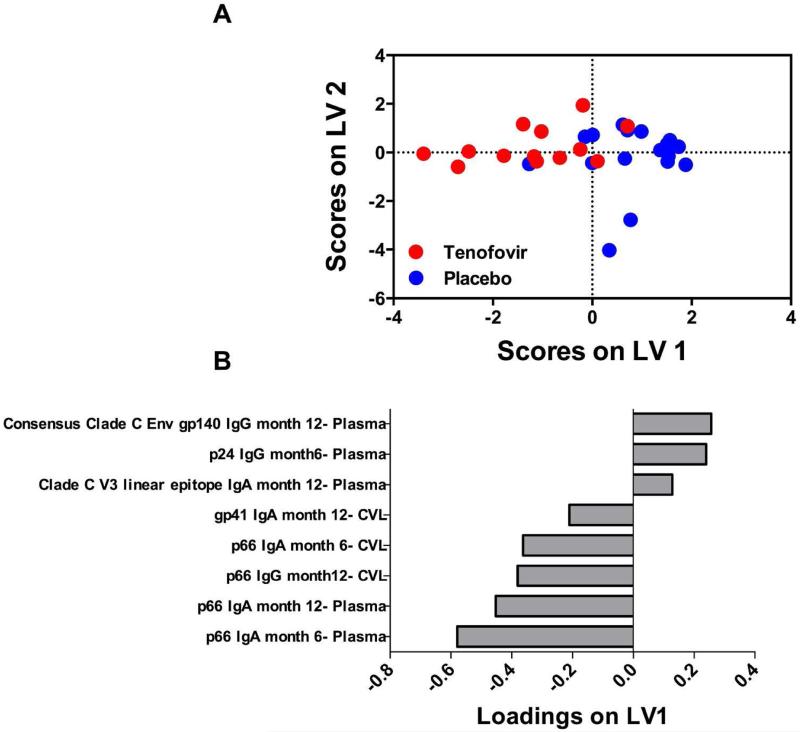
A multivariate approach that integrates antibody measurements over time and takes tissue compartment into account was used to determine if there were any unique antibody signatures associated with tenofovir use. A feature reduction method (LASSO) and PLSDA was used together to identify the minimum linear combination of antibody specificities that differentiated tenofovir and placebo groups, using 6 and 12 month post-infection time-points. This approach has been used previously to identify molecular signatures associated with disease status36 or cellular behaviours37. LASSO identified a subset of 8 HIV-1 antibody specificities that best differentiated tenofovir and placebo groups, with 77% cross-validation accuracy and 87% calibration accuracy (Figs. 4A and B). Five of these 8 specificities were strongly and positively associated with the tenofovir arm (negatively loaded on LV1; including plasma p66 IgA at months 6 and 12, CVL p66 IgA at month 6, plasma p66 IgG at month 12, and CVL gp41 IgA at month 12), while 3/8 were negatively associated with the tenofovir arm (positively loaded on LV1; including plasma Clade C V3 linear epitope IgA at month 12, p24 IgG at month 6 and Consensus Clade C Env gp140 IgG at month 12) (Fig. 4B). Confirmation of these identified signatures was done by generating 1,000 additional PLSDA models, each with 8 different combinations of antibody specificities selected from the remaining (non-LASSO) measurements. This analysis suggested that the LASSO-selected signature was significantly better for differentiating tenofovir and placebo groups (p<0.01), as both calibration accuracy and cross-validation accuracy were in the 99th percentile rank compared to other models (Suppl. Figs. 6A and B). The identified signature suggested spatio-temporal relationships between HIV-specific antibody specificities that differentiated women in the tenofovir from placebo arm. Early and late antibody relationships between p66 in plasma and CVL contributed most notably to this signature, with plasma p66 IgA (month 6 and 12), CVL p66 IgA and IgG (month 6 and 12, respectively) strongly negatively loaded onto LV1, and strongly associated with the tenofovir arm [the more negative the LV1 loading, the more strong the association is for the tenofovir arm] (Fig. 4B). This suggested that women in the tenofovir group were best characterized by elevated p66 IgA responses over time in both plasma and CVL compared to the placebo group. In addition, lower IgA responses to Clade C V3 linear epitope (month 12), IgG to p24 (month 6), and IgG to Consensus Clade C Env gp140 (month 12), all positively loaded on LV1 and detected in plasma, best characterized women in the tenofovir arm from women in the placebo group [the more positive the LV1 loading, the stronger the association is for the placebo arm]. These results support findings from the univariate analysis, and provide complementary insight into possible integrated relationships between different antibody specificities and provide further evidence of a detectable tenofovir effect on humoral immunity in women subsequent to seroconversion.
Figure 4. Six and 12 month antibody signatures associated with participants in the tenofovir and placebo arms.
LASSO identified a signature of 8 antibody specificities that separated tenofovir (red- n=13) and control groups (blue-n=18) in a PLSDA model with 83% calibration accuracy and 77% cross-validation accuracy (scores plot; A). The loadings plot depicts weighted loadings of individual antibody specificities within the distinguishing signature (B). Five of these specificities were positively associated with tenofovir (negatively loaded on LV1; plasma p66 IgA at months 6 and 12, plasma p66 IgG at month 12, CVL p66 IgA at month 6, and CVL gp41 IgA at month 12) and three were negatively associated with tenofovir (positively loaded on LV1; plasma Clade C V3 linear epitope IgA at month 12, p24 IgG at month 6 and Consensus Clade C Env gp140 IgG at month 12. Latent variable 1 accounted for 24.84% and latent variable 2 accounted for 15.38% of data variance. For the analysis, only 6 and 12 month measurements were used for participants where <4% of the total data was missing.
Compartmental associations for Gag p24 IgGs from women in the tenofovir arm
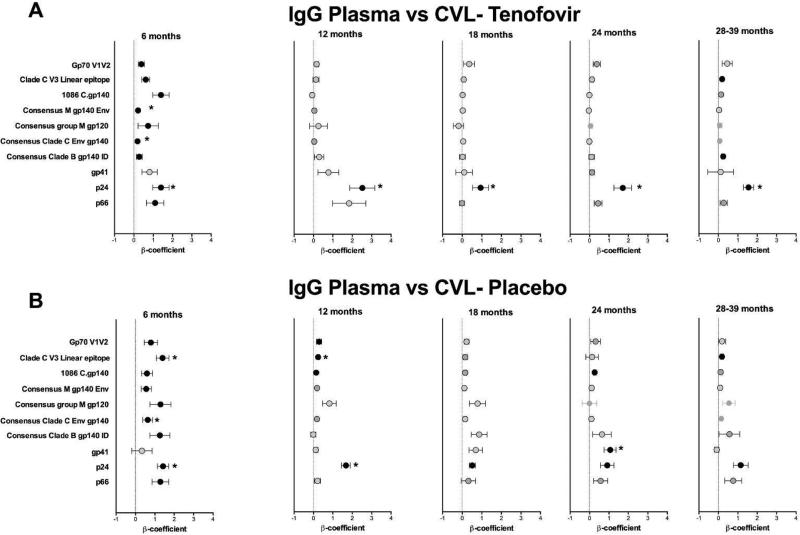
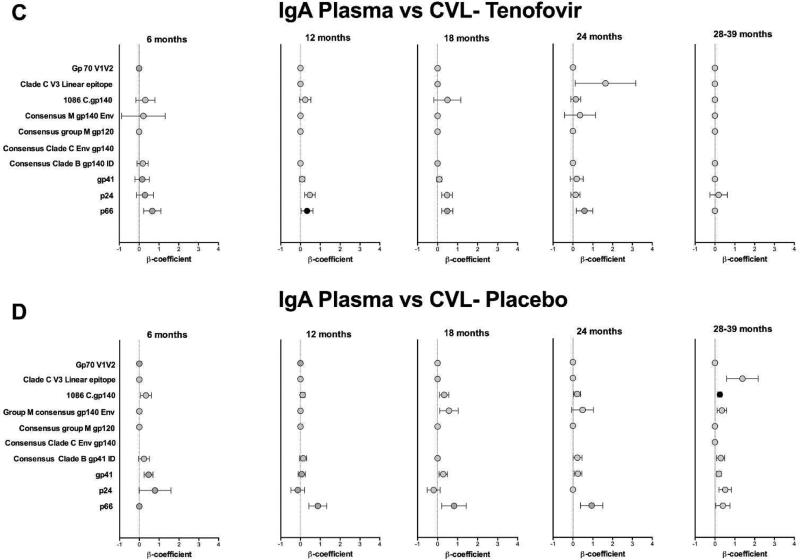
To determine whether HIV-1 specific mucosal antibodies were locally-produced or predominantly transudated from plasma, we compared the titres of HIV-specific IgG and IgA in plasma to those in the CVL. Among women assigned to the tenofovir arm, the plasma p24 IgG responses correlated significantly to those detected in matching CVL, at several time-points post-infection [6 months (β coefficient= 1.4, p<0.0001) until >30 months (β coefficient=1.3, p<0.0001)] (Fig. 5A). In addition, Consensus Clade C Env gp140 and Group M consensus gp140 Env IgG responses at 6 months post-infection in the tenofovir group correlated significantly between the compartments (Fig. 5A). P24 IgG responses in the placebo group showed more restricted and less strong associations between plasma and CVL (Fig. 5B).
Figure 5. p24-specific IgG responses correlate between plasma and CVL in tenofovir-treated women.
Beta (β) coefficients representing correlation between the magnitude of HIV-1-specific responses in plasma and CVL from 3 to 39 months post HIV-1 infection. IgG (a) and IgA (c) shown for the tenofovir arm and IgG (b) and IgA (d) are shown for the placebo arm. The colored circle (●) indicates a statistically significant correlation (P<0.05) (F-test from Linear Mixed Model). An asterisk (*) denotes statistical significance adjusted for false discovery rate. CVL, cervicovaginal lavage.
No correlation was seen between IgA responses in the plasma and CVL at any time-point for either arm, which were detected at lower titres in both compartments (Figs. 5C and D). This suggests that HIV-1 specific IgAs are predominantly locally produced in the female GT although absolute antibody titres may have influenced this poor concordance.
Plasma viral load correlated with HIV-specific IgG and IgA responses
We evaluated whether plasma viral loads were associated with titres of HIV-specific IgGs and IgAs in the plasma. HIV-specific plasma IgG titres correlated significantly with viral load (Table 2). In the tenofovir arm, titres of IgG directed against 1086 C.gp140, Consensus Clade B gp41 ID epitope, and gp41 correlated with plasma viral load, although not after adjusting for multiple comparisons. For HIV-1 specific IgA responses (Table 2), plasma viral load correlated positively with several plasma IgA specificities from women in the placebo arm [clade C V3 linear epitope, Group M consensus gp140 Env, and consensus clade C gp140).
Table 2.
Viral loads correlated with plasma IgG and IgA at 6 months in the tenofovir and placebo arms
| HIV-Antigen | Viral Load versus Plasma IgG (Tenofovir) n=23 | Viral Load versus Plasma IgG (Placebo) n=23 | Viral Load versus Plasma IgA (Tenofovir) n=23 | Viral Load versus Plasma IgA (Placebo) n=23 | ||||
|---|---|---|---|---|---|---|---|---|
| r-value | pvalue | r-value | pvalue | r-value | p-value | r-value | p-value | |
| gp70 V1V2 | 0.004 | NS | 0.39 | 0.06 | 0.26 | NS | 0.17 | NS |
| Clade C V3 Linear Epitope | 0.02 | NS | 0.58 | 0.003* | −0.1 | NS | 0.59 | 0.003* |
| Consensus Group M gp120 | 0.41 | 0.05 | 0.55 | 0.007* | 0.08 | NS | 0.47 | 0.03 |
| 1086 C.gp140 | 0.52 | 0.01 | 0.44 | 0.03* | 0.07 | NS | 0.43 | 0.04 |
| Consensus Group M gp140 | 0.23 | NS | 0.43 | 0.04* | 0.06 | NS | 0.53 | 0.009* |
| Consensus Clade C Env gp140 | 0.35 | 0.09 | 0.44 | 0.03* | 0.19 | NS | 0.53 | 0.009* |
| Consensus Clade B gp41 ID epitope | 0.48 | 0.02 | 0.45 | 0.03* | −0.08 | NS | 0.33 | NS |
| Gp41 | 0.43 | 0.03 | 0.49 | 0.02* | 0.07 | NS | 0.46 | 0.02 |
| p66 | 0.02 | NS | 0.73 | <0.0001* | 0.41 | 0.05 | 0.38 | 0.07 |
| p24 | 0.02 | NS | 0.61 | 0.002* | −0.03 | NS | 0.08 | NS |
P<0.05 indicates statistically significant correlation. NS, not significant.
Remains significant after false discovery rate adjustment.
Similarly, we found broadly negative associations between CD4 cells and HIV-specific IgG responses to several antigens in women in the tenofovir arm at 6 months post-infection, suggesting that HIV clinical status was influencing HIV-specific antibody responses [Table 3; for Consensus Group M gp120 (r=−0.49, p=0.02), 1086 C.gp140 IgG (r=−0.53, p=0.01), Consensus Clade C Env gp140IgG (r=−0.48, p=0.02); although not significant after false discovery rate adjustment]. There were no associations with CD4 cells and IgG responses in the placebo arm.
Table 3.
CD4 cells correlated with plasma IgG and IgA at 6 months in the tenofovir and placebo arms
| HIV-Antigen | CD4 versus Plasma IgG (Tenofovir) n=23 | CD4 versus Plasma IgG (Placebo) n=23 | CD4 versus Plasma IgA (Tenofovir) n=23 | CD4 versus Plasma IgA (Placebo) n=23 | ||||
|---|---|---|---|---|---|---|---|---|
| r-value | p-value | r-value | p-value | r-value | p-value | r-value | p-value | |
| gp70 V1V2 | −0,18 | NS | −0,26 | NS | −0.25 | NS | −0,07 | NS |
| Clade C V3 Linear Epitope | −0,18 | NS | −0,30 | NS | −0,18 | NS | −0,53 | 0.008* |
| Consensus Group M gp120 | −0,49 | 0.02 | −0,28 | NS | −0,48 | 0.02 | −0,47 | 0.02 |
| 1086 C.gp140 | −0.53 | 0.01 | −0.22 | NS | −0.61 | 0.003* | −0.33 | NS |
| Consensus Group M gp140 | −0,25 | NS | −0,25 | NS | −0.61 | 0.002* | −0,51 | 0.01 |
| Consensus Clade C Env gp140 | −0,48 | 0.02 | −0,26 | NS | −0.77 | <0.001* | −0.36 | NS |
| Consensus Clade B gp41 ID epitope | −0,42 | 0.05 | −0,09 | NS | −0,37 | NS | −0,28 | NS |
| Gp41 | −0,40 | 0.06 | −0,05 | NS | 0,42 | 0.05 | −0.42 | 0.05 |
| p66 | −0,03 | NS | −0,24 | NS | −0.43 | 0.05 | −0.50 | 0.02 |
| p24 | −0,005 | NS | −0,10 | NS | 0.02 | NS | −0.05 | NS |
P<0.05 indicates statistically significant correlation. NS, not significant.
Remains significant after false discovery rate adjustment.
For HIV-1 specific IgA responses, viral loads correlated positively in the placebo arm for 8/10 of the IgA antigen specificities [Table 2; Clade C V3 Linear epitope, Group M consensus gp140 Env -IgG, Consensus Clade C Env gp140 remained significant after adjusting for false discovery rate]. Similarly, in the tenofovir arm at 6 months post-infection, we found a significant negative association with CD4 cells and HIV-specific IgA responses [Table 3; including 1086 C.gp140-IgG, Consensus Group M gp140, and Consensus Clade C Env gp140]. These data indicate that both IgG and IgA responses may be driven by viral load which can negatively impact CD4 cells. However, other factors such as characteristics of the transmitted virus, pre-existing immune responses, concurrent infections and host genetics may also contribute.
Conclusions
Women assigned to the tenofovir gel had a distinct p66 IgA signature in both the GT and plasma that differentiated them from those in the placebo arm following HIV-1 infection. The enhanced p66 IgA responses over time in both compartments with a parallel delay or lowering of IgA- specific V3 and IgG-specific Env gp140 responses indicates the impact of prior topical tenofovir on ensuing antibody responses post HIV-1 infection.
An increased frequency and magnitude of HIV-1 specific IgA responses to p66 and p24 in the plasma and GT were seen during the primary infection stage in the tenofovir compared to the placebo arm. Previous studies have shown that IgG rather than IgA are predominant in the type II mucosa of the lower female genital mucosa38,39. However, we previously demonstrated that altered antibody isotypes and specificities were present in CVLs of HESN women participating in the HPTN035 microbicide trial (randomized to either BufferGel or 0.5% PRO2000 or placebo) compared to chronically HIV-infected women21. HIV-exposed-uninfected women in this trial exhibited an increase in different types of Env-specific IgA in the female GT compared to a HIV-1 infected cohort. In this sub-study of the CAPRISA 004 trial, women in the tenofovir arm had a delay of >5 months from enrolment to infection vs women in the placebo arm. The p66 IgA signature in plasma and GT in tenofovir-exposed women may be a consequence of frequent exposure to HIV during sexual intercourse, without productive infection because of the presence of tenofovir, similar to the enhanced IgA responses seen in other HESN cohorts14,21,40. Interestingly, prior studies have suggested that p66 antibodies are associated with the rate of HIV disease progression41, in addition to being associated with HIV-1 controller status42. Thus the role of p66 antibody in our study could also be a surrogate for another immune mechanism that is impacted by tenofovir use.
The significant delay in time to HIV infection in the presence of tenofovir may likely have played an indirect role in modulating specific antibody responses in the absence of HIV infection, as significant differences in p24 and p66 binding antibody response rates and magnitude were seen >6 months post-infection. A limitation of this study is that samples immediately prior to infection or during very early acute infection were not available for investigation. Oral PrEP trials have shown immuno-modulatory effects of tenofovir in those who became infected, with effects ranging from preserved CD4 cells to lower levels of systemic inflammation43. In topical PrEP studies, Gag-specific T cell responses in the absence of infection has been demonstrated7 with use of tenofovir gel. During the acute stage of breakthrough infections in the CAPRISA 004 study, Laeyendecker et al. showed a delay in avidity maturation of specific antibody responses in women assigned to the tenofovir gel9,10. While studies showed decreased antibody titres with ARV-treatment in individuals already infected with HIV-111–13, we found increased antibody response rates and magnitudes for V1-V2 IgG, p66 and p24 specific IgA and IgG during HIV-1 breakthrough infection in individuals exposed to tenofovir before infection after controlling for sexually transmitted diseases. Women with breakthrough infections in the tenofovir arm were reported to have an episodic use of 3-4 weeks of tenofovir gel post-infection4. In addition, high levels (3.1 × 106 ng/ml) of tenofovir-diphopshate (TFV-DP) were achieved in the genital tract with topical application44. The half-life of TFV-DP (active metabolite) is approximately 40 days in the genital tract45 suggesting that the levels of TFV at mucosal sites were maintained for at least 6 weeks or 40 days after the last application of the tenofovir gel and this may explain the delay in antibody responses in the first few months post-infection. Our study is different from Laeyendecker et al.10 in that their study measured antibody avidity at early time-points. Here, we measured the magnitude and breadth of the antibody response, not the avidity, to both envelope and non-envelope HIV-1 antigens. These data suggest that prior tenofovir use may prime the immune system7,8 upon exposure to HIV46, leading to increased p66 and p24 specific IgA and IgG that can distinguish patients with mucosally applied tenofovir during the primary stage of HIV-infection.
In the RV144 vaccine trial, plasma gp70 V1V2 binding IgGs, including V1V2 specific IgG3, correlated with decreased HIV-1 infection risk25,27,47. Whether the same specificity was present in the GT of uninfected vaccinees was not evaluated in the trial. Transudation of HIV-1 specific antibodies from the systemic circulation into secretions in the lower female GT has been described48. Many factors may affect the transudation of antibodies across the mucosa, including vaginal pH49,50, the type of antibody glycosylation51, and heterogeneity among individuals. Our data indicated that in the presence or absence of tenofovir, gp70 V1V2 IgG were detected in the GT in a small proportion of women throughout infection (26% in the tenofovir and 22% in the placebo arm overall at 28-39 months), despite 100% of the women displaying plasma gp70 V1V2 IgG specificity (100% for both tenofovir and placebo at 28-39 months). This indicates that there may be differential transudation of certain IgG specificities which are regulated by mechanisms that are yet to be determined. Whether these antibodies confer protection in the GT and whether tenofovir impacts the IgG subclass profile 25, requires further study. Observations for little to no gp70 V1V2 specific IgA in either the plasma or CVL suggests that certain antigen specificities may be less likely to have a mucosal IgA response. Although we did not measure the secretory component of IgA in this study (another limitation of this study), our data suggests that IgA responses may be generated both locally in the GT and systemically. Additional studies are needed to determine the relative proportion of locally produced versus systemic IgA responses in the female GT, and whether HIV-1 specific IgA in different compartments may indeed protect or increase the risk for HIV-acquisition27,52,53. The menstrual cycle and female hormones may influence the composition of mucosal IgGs and possibly IgAs. We were unable to assess this in the current study as all women were on some form of hormonal contraception, and stage of the menstrual cycle in those cycling was not recorded.
The direct relationship between plasma viral loads and the magnitude of Env-specific IgGs suggested that B cell immune responses may have been driven primarily through frequent antigen stimulation or exposure54. IgG responses were found to be locally produced in the macaque GT17, after SIVmac239Δnef vaccination and in response to live SIV exposure. Another study demonstrated that rectal Env-specific IgG responses were a key correlate of protection following heterologous prime boost vaccination55. In the gut lamina propria of mice, local B cell receptor editing, IgG repertoire and B cell development were shown to be regulated by extracellular signals from commensal microbes56, and the same could be true for the female GT57. The strong and significant cross-compartment correlations for most of the Env-specific IgGs may be reflective of local T cell specificities in the GT. To achieve sterilizing protection against HIV, a combination of potent polyfunctional humoral immune responses that reach the GT and circulating T cell-mediated immunity may be crucial. Whether HIV-specific antibodies in the GT would confer protection through combined direct neutralization and Fc-associated effector functions remains unknown58–64.
In summary, we identified specific alterations in the plasma and mucosal responses to HIV-1 infection following tenofovir gel use in those women who became infected. Our analyses revealed an antibody signature that distinguished women in the tenofovir arm post-infection from those in the placebo arm. Further work is needed to evaluate whether tenofovir has a direct or indirect immunomodulatory role that resulted in these differences in the humoral response to infection. We have corroborated earlier findings that systemic HIV-1 specific IgG responses likely transudate into the GT65,66 and that the IgA responses may be locally produced. These findings underscore the importance of both locally produced or transduced antibody responses in the genital tract to prevent infection52,53,67–69. Information from this study is directly relevant to both vaccine and PrEP strategies, which aim to induce protective antibodies at mucosal surfaces.
Supplementary Material
Acknowledgements
We thank all the CAPRISA 002 Acute Infection Study participants who are continuing to make an important contribution to HIV research. The scientific and supportive role of the whole CAPRISA 002 study and protocol teams are gratefully acknowledged.
Tenofovir was provided by Gilead Sciences and the gel was manufactured and supplied for the CAPRISA 004 trial by CONRAD. We thank Drs. Hua-Xin Liao, M. Anthony Moody and Barton Haynes (Duke Human Vaccine Institute (DHVI) for HIV-1 mAb and antigen reagents and Glenn Overman (DHVI) for expert technical assistance in IgG depletion of plasma and CVL samples. Over the last decade, the CAPRISA 002 study team has received support from the National Institute of Allergy and infectious Disease (NIAID), National Institutes of Health (NIH) (grants # AI51794, #AI104387, #AI115981, #AI116086), from CONRAD (USAID co-operative grant #GP00-08-00005-00, subproject agreement # PPA-09-046), from the National Research Foundation (grant # 67385), the Medical Research Council of South Africa, the Technology Innovation Agency, and the Columbia University-Southern African Fogarty AIDS International Training and Research Programme (AITRP) funded by the Fogarty International Center, NIH (grant # D43TW00231). DA was funded through the Medical Research Council of South Africa Self-Initiated Grant (MRC SIR), and the National Research Foundation (NRF) of South Africa training funds to carry out this study. This work was also supported by NIH NIAID HVTN Laboratory Center UM1AI068618 and NIH NIAID Duke Center for AIDS Research Immunology Core P30 AI 64518.
Footnotes
Conflict of Interest: The authors declare that there is no conflict of interest.
References
- 1.Grant RM, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N. Engl. J. Med. 2010;363:2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeten JM, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N. Engl. J. Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molina JM, et al. On Demand PrEP With Oral TDF-FTC in MSM: Results of the ANRS Ipergay Trial | CROI Conference.. Conf. Retroviruses Opportunistic Infect. (CROI); Seattle, Washington. 23-26 Febr. 2015; Abstract 23LB (Seattle, Washington, 2015) [Google Scholar]
- 4.Abdool Karim Q, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marrazzo JM, et al. Tenofovir-Based Preexposure Prophylaxis for HIV Infection among African Women. N. Engl. J. Med. 2015;372:509–518. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCormack S, et al. Pragmatic Open-Label Randomised Trial of Preexposure Prophylaxis: The PROUD Study | CROI Conference.. Conf. Retroviruses Opportunistic Infect. (CROI); Seattle, Washington. 23-26 Febr. 2015; Seattle, Washington, 2015. [Google Scholar]
- 7.Cranage M, et al. Prevention of SIV Rectal Transmission and Priming of T Cell Responses in Macaques after Local Pre-exposure Application of Tenofovir Gel. PLoS Medicine. 5(8):e157. doi: 10.1371/journal.pmed.0050157. doi:10.1371/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mureithi MW, et al. Preservation HIV-1 specific IFNγ+ CD4+ T cell responses in breakthrough infections following exposure to Tenofovir Gel in the CAPRISA 004 microbicide trial. JAIDS J. Acquir. Immune Defic. Syndr. 2012;1 doi: 10.1097/QAI.0b013e31824f53a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtis KA, et al. Delayed Maturation of Antibody Avidity but Not Seroconversion in Rhesus Macaques Infected With Simian HIV During Oral Pre-Exposure Prophylaxis. J. Acquir. Immune Defic. Syndr. 2011;57:355–362. doi: 10.1097/QAI.0b013e3182234a51. [DOI] [PubMed] [Google Scholar]
- 10.Laeyendecker O, et al. Antibody Maturation in Women who Acquire HIV Infection While Using Antiretroviral Pre-Exposure Prophylaxis. J. Infect. Dis. 2015 doi: 10.1093/infdis/jiv110. doi:10.1093/infdis/jiv110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hare CB, et al. Seroreversion in subjects receiving antiretroviral therapy during acute/early HIV infection. Clin. Infect. Dis. 2006;42:700–8. doi: 10.1086/500215. [DOI] [PubMed] [Google Scholar]
- 12.O'Sullivan CE, et al. Epstein Barr virus and human immunodeficiency virus serological responses and viral burdens in HIV-infected patients treated with HAART. J. Med. Virol. 2002;67:320–6. doi: 10.1002/jmv.10080. [DOI] [PubMed] [Google Scholar]
- 13.Payne H, et al. Reactivity of routine HIV antibody tests in children who initiated antiretroviral therapy in early infancy as part of the Children with HIV Early Antiretroviral Therapy (CHER) trial: a retrospective analysis. Lancet Infect. Dis. doi: 10.1016/S1473-3099(15)00087-0. doi:10.1016/S1473-3099(15)00087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devito C, et al. Mucosal and plasma IgA from HIV-1-exposed uninfected individuals inhibit HIV-1 transcytosis across human epithelial cells. J. Immunol. 2000;165:5170–5176. doi: 10.4049/jimmunol.165.9.5170. [DOI] [PubMed] [Google Scholar]
- 15.Belyakov IM, Ahlers JD, Berzofsky JA. Mucosal AIDS vaccines: current status and future directions. Expert Rev. Vaccines. 2004;3:S65–73. doi: 10.1586/14760584.3.4.s65. [DOI] [PubMed] [Google Scholar]
- 16.Smith AJ, et al. Live simian immunodeficiency virus vaccine correlate of protection: immune complex-inhibitory fc receptor interactions that reduce target cell availability. J. Immunol. 2014;193:3126–33. doi: 10.4049/jimmunol.1400822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Q, et al. Live simian immunodeficiency virus vaccine correlate of protection: local antibody production and concentration on the path of virus entry. J. Immunol. 2014;193:3113–25. doi: 10.4049/jimmunol.1400820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tudor D, et al. HIV-1 gp41-specific monoclonal mucosal IgAs derived from highly exposed but IgG-seronegative individuals block HIV-1 epithelial transcytosis and neutralize CD4(+) cell infection: an IgA gene and functional analysis. Mucosal Immunol. 2009;2:412–26. doi: 10.1038/mi.2009.89. [DOI] [PubMed] [Google Scholar]
- 19.Mazzoli S, et al. HIV specific mucosal and cellular immunity in HIV seronegative partners of HIV-seropositive individuals. Nat. Med. 1997;3:1250–7. doi: 10.1038/nm1197-1250. [DOI] [PubMed] [Google Scholar]
- 20.Kaul R, et al. Mucosal IgA in exposed, uninfected subjects: evidence for a role in protection against HIV infection. AIDS. 2001;15:431–2. doi: 10.1097/00002030-200102160-00026. [DOI] [PubMed] [Google Scholar]
- 21.Seaton KE, et al. HIV-1 Specific IgA Detected in Vaginal Secretions of HIV Uninfected Women Participating in a Microbicide Trial in Southern Africa Are Primarily Directed Toward gp120 and gp140 Specificities. PLoS One. 2014;9:e101863. doi: 10.1371/journal.pone.0101863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yates NL, et al. HIV-1 gp41 envelope IgA is frequently elicited after transmission but has an initial short response half-life. Mucosal Immunol. 2013;6:692–703. doi: 10.1038/mi.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bebell LM, et al. Relationship between levels of inflammatory cytokines in the genital tract and CD4+ cell counts in women with acute HIV-1 infection. J. Infect. Dis. 2008;198:710–4. doi: 10.1086/590503. [DOI] [PubMed] [Google Scholar]
- 24.Tomaras GD, et al. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J. Virol. 2008;82:12449–63. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yates NL, et al. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV 1 infection risk and declines soon after vaccination. Sci. Transl. Med. 2014;6:228ra39. doi: 10.1126/scitranslmed.3007730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yates NL, et al. Multiple HIV-1 specific IgG3 responses decline during acute HIV 1: implications for detection of incident HIV infection. AIDS. 2011;25:2089–97. doi: 10.1097/QAD.0b013e32834b348e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haynes BF, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 2012;366:1275–86. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao H-X, et al. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity. 2013;38:176–86. doi: 10.1016/j.immuni.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao HX, et al. Antigenicity and immunogenicity of transmitted/founder, consensus, and chronic envelope glycoproteins of human immunodeficiency virus type 1. J. Virol. 2013;87:4185–201. doi: 10.1128/JVI.02297-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao F, et al. Antigenicity and immunogenicity of a synthetic human immunodeficiency virus type 1 group m consensus envelope glycoprotein. J. Virol. 2005;79:1154–63. doi: 10.1128/JVI.79.2.1154-1163.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaschen B, et al. Diversity considerations in HIV-1 vaccine selection. Science. 2002;296:2354–60. doi: 10.1126/science.1070441. [DOI] [PubMed] [Google Scholar]
- 32.Liao H-X, et al. A group M consensus envelope glycoprotein induces antibodies that neutralize subsets of subtype B and C HIV-1 primary viruses. Virology. 2006;353:268–82. doi: 10.1016/j.virol.2006.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris L, et al. Isolation of a human anti-HIV gp41 membrane proximal region neutralizing antibody by antigen-specific single B cell sorting. PLoS One. 2011;6:e23532. doi: 10.1371/journal.pone.0023532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tibshirani R. Regression Shrinkage and Selection via the Lasso. J. R. Stat. Soc. Ser. B. 1996;58:267–288. [Google Scholar]
- 35.Lau KS, et al. In vivo systems analysis identifies spatial and temporal aspects of the modulation of TNF α induced apoptosis and proliferation by MAPKs. Sci. Signal. 2011;4:ra16. doi: 10.1126/scisignal.2001338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lau KS, et al. Multi-scale in vivo systems analysis reveals the influence of immune cells on TNF-α induced apoptosis in the intestinal epithelium. PLoS Biol. 2012;10:e1001393. doi: 10.1371/journal.pbio.1001393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janes KA, et al. A systems model of signaling identifies a molecular basis set for cytokine induced apoptosis. Science. 2005;310:1646–53. doi: 10.1126/science.1116598. [DOI] [PubMed] [Google Scholar]
- 38.Woof JM, Mestecky J. Mucosal immunoglobulins. Immunol. Rev. 2005;206:64–82. doi: 10.1111/j.0105-2896.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- 39.Russell MW, Mestecky J. Humoral immune responses to microbial infections in the genital tract. Microbes Infect. 2002;4:667–77. doi: 10.1016/s1286-4579(02)01585-x. [DOI] [PubMed] [Google Scholar]
- 40.Devito C, et al. Cross-clade HIV-1 specific neutralizing IgA in mucosal and systemic compartments of HIV-1 exposed, persistently seronegative subjects. J. Acquir. Immune Defic. Syndr. 2002;30:413–20. doi: 10.1097/00042560-200208010-00007. [DOI] [PubMed] [Google Scholar]
- 41.Moja P, et al. Prognostic value of antibodies to p66 during the course of HIV1 infection. P. Moja, V. Cheynet, T. Bourlet, F. Mallet, F. Lucht, B. Pozzetto, C. Genin. 1997. Progn. value antibodies to p66 Dur. course HIV1 Infect. Immunol. Lett. 56 133. 1997;1-3:133. [Google Scholar]
- 42.French MA, et al. Isotype-switched immunoglobulin G antibodies to HIV Gag proteins may provide alternative or additional immune responses to ‘protective’ human leukocyte antigen-B alleles in HIV controllers. AIDS. 2013;27:519–28. doi: 10.1097/QAD.0b013e32835cb720. [DOI] [PubMed] [Google Scholar]
- 43.Kersh EN, et al. Reduced inflammation and CD4 loss in acute SHIV infection during oral pre-exposure prophylaxis. J. Infect. Dis. 2012;206:770–9. doi: 10.1093/infdis/jis422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hendrix CW, et al. MTN-001: randomized pharmacokinetic cross-over study comparing tenofovir vaginal gel and oral tablets in vaginal tissue and other compartments. PLoS One. 2013;8:e55013. doi: 10.1371/journal.pone.0055013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Louissaint NA, et al. Single dose pharmacokinetics of oral tenofovir in plasma, peripheral blood mononuclear cells, colonic tissue, and vaginal tissue. AIDS Res. Hum. Retroviruses. 2013;29:1443–50. doi: 10.1089/aid.2013.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kersh EN, et al. T cell chemo-vaccination effects after repeated mucosal SHIV exposures and oral pre-exposure prophylaxis. PLoS One. 2011;6:e19295. doi: 10.1371/journal.pone.0019295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zolla-Pazner S, et al. Vaccine-induced IgG Antibodies to V1V2 Regions of Multiple HIV-1 Subtypes Correlate with Decreased Risk of HIV-1 Infection. PloS One. 2014;9:e87572. doi: 10.1371/journal.pone.0087572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johansson M, Lycke NY. Immunology of the human genital tract. Curr. Opin. Infect. Dis. 2003;16:43–9. doi: 10.1097/00001432-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 49.Li Z, et al. Transfer of IgG in the female genital tract by MHC class I-related neonatal Fc receptor (FcRn) confers protective immunity to vaginal infection. Proc. Natl. Acad. Sci. U. S. A. 2011;108:4388–93. doi: 10.1073/pnas.1012861108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gupta S, et al. The Neonatal Fc receptor (FcRn) enhances human immunodeficiency virus type 1 (HIV-1) transcytosis across epithelial cells. PLoS Pathog. 2013;9:e1003776. doi: 10.1371/journal.ppat.1003776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ackerman ME, et al. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J. Clin. Invest. 2013;123:2183–92. doi: 10.1172/JCI65708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou M, Ruprecht RM. Are anti-HIV IgAs good guys or bad guys? Retrovirology. 2014;11:109. doi: 10.1186/s12977-014-0109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sholukh AM, et al. Defense-in-depth by mucosally administered anti-HIV dimeric IgA2 and systemic IgG1 mAbs: Complete protection of rhesus monkeys from mucosal SHIV challenge. Vaccine. 2015;33:2086–95. doi: 10.1016/j.vaccine.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nicholas KJ, et al. B cell responses to HIV antigen are a potent correlate of viremia in HIV-1 infection and improve with PD 1 blockade. PLoS One. 2013;8:e84185. doi: 10.1371/journal.pone.0084185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barouch DH, et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 2012;482:89–93. doi: 10.1038/nature10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wesemann DR, et al. Microbial colonization influences early B-lineage development in the gut lamina propria. Nature. 2013;501:112–5. doi: 10.1038/nature12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anahtar MN, et al. Cervicovaginal Bacteria Are a Major Modulator of Host Inflammatory Responses in the Female Genital Tract. Immunity. 2015;42:965–976. doi: 10.1016/j.immuni.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiao P, et al. Multiple vaccine-elicited nonneutralizing antienvelope antibody activities contribute to protective efficacy by reducing both acute and chronic viremia following simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J. Virol. 2010;84:7161–73. doi: 10.1128/JVI.00410-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Letvin NL, et al. Immune and Genetic Correlates of Vaccine Protection Against Mucosal Infection by SIV in Monkeys. Sci. Transl. Med. 2011;3:81ra36. doi: 10.1126/scitranslmed.3002351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Florese RH, et al. Contribution of nonneutralizing vaccine-elicited antibody activities to improved protective efficacy in rhesus macaques immunized with Tat/Env compared with multigenic vaccines. J. Immunol. 2009;182:3718–27. doi: 10.4049/jimmunol.0803115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gómez-Román VR, et al. Vaccine-elicited antibodies mediate antibody-dependent cellular cytotoxicity correlated with significantly reduced acute viremia in rhesus macaques challenged with SIVmac251. J. Immunol. 2005;174:2185–9. doi: 10.4049/jimmunol.174.4.2185. [DOI] [PubMed] [Google Scholar]
- 62.Hessell AJ, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–4. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 63.Barouch DH, et al. Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys. Cell. 2013;155:531–9. doi: 10.1016/j.cell.2013.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bournazos S, et al. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell. 2014;158:1243–53. doi: 10.1016/j.cell.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bard E, et al. Validation of a high sensitive immunoenzymatic assay to establish the origin of immunoglobulins in female genital secretions. J. Immunoassay Immunochem. 2002;23:145–62. doi: 10.1081/IAS-120003658. [DOI] [PubMed] [Google Scholar]
- 66.Mestecky J, Alexander RC, Wei Q, Moldoveanu Z. Methods for evaluation of humoral immune responses in human genital tract secretions. Am. J. Reprod. Immunol. 2011;65:361–7. doi: 10.1111/j.1600-0897.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat. Rev. Immunol. 2006;6:148–58. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 68.Devito C, et al. Intranasal HIV-1-gp 160-DNA/gp41 Peptide Prime Boost Immunization Regimen in Mice Results in Long-Term HIV-1 Neutralizing Humoral Mucosal and Systemic Immunity. J. Immunol. 2004;173:7078–7089. doi: 10.4049/jimmunol.173.11.7078. [DOI] [PubMed] [Google Scholar]
- 69.Duerr A. Update on mucosal HIV vaccine vectors. Curr. Opin. HIV AIDS. 2010;5:397–403. doi: 10.1097/COH.0b013e32833d2e39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.