Abstract
Isocyanates differ from many other xenobiotics in their ability to form S-linked conjugates with glutathione (GSH) through direct nucleophilic addition reactions (e.g. without enzymatic “preactivation” and/or transferase activity), potentially predisposing them to metabolism via the mercapturic acid pathway. In vivo, mono-isocyanates are metabolized via the mercapturic acid pathway and excreted as N-acetylated cysteine conjugates, however, the metabolism of di-isocyanates remains unclear. We assessed the ability of purified human γ-glutamyl transpeptidase-1 (GGT-1), a primary enzyme of the mercapturic acid pathway, to cleave S-linked GSH conjugates of 4,4’-methylene diphenyl diisocyanate (MDI) and 1,6-hexamethylene diisocyanate (HDI), two widely used industrial chemicals. A combination of liquid chromatography (LC), tandem mass spectrometry (MS/MS) and hydrogen-deuterium exchange studies confirmed GGT-1 mediated formation of the 607.2 and 525.2 m/z (M+H)+ ions corresponding to bis(cys-gly)-MDI and bis(cys-gly)-HDI, the cleavage products expected from the corresponding bis(GSH)-diisocyanate conjugates. Additional intermediate metabolites and mono(cys-gly)-conjugates with partially hydrolyzed diisocyanate were also observed. Consistent with GGT enzyme kinetics, metabolism proceeded more rapidly under conditions that favored transpeptidation vs. hydrolytic mechanisms of cleavage. Together the data demonstrate the capacity of human GGT-1 to cleave GSH conjugates of both aromatic and aliphatic diisocyanates, suggesting a potential role in their metabolism.
Keywords: glutathione, metabolism, mercapturic acid pathway
INTRODUCTION
Chemicals that contain reactive isocyanate (N=C=O) groups possess toxicity related to their capacity to undergo rapid nucleophilic addition reactions with functional groups on “self” molecules (Fuchs and Valade 1951, Gross, Whetzel et al. 1975, Bucher 1987, Wisnewski, Liu et al. 2010). Glutathione, which is present in high concentrations in the lower respiratory tract is postulated to be a major reaction target for inhaled isocyanates, and readily forms S-linked thiocarbamate products (GSH-isocyanate “conjugates”) under physiologic conditions (Cantin, North et al. 1987, Slatter, Rashed et al. 1991, Wisnewski, Liu et al. 2013, Wisnewski, Mhike et al. 2013). The ability of N=C=O to directly conjugate with GSH, without the need for enzymatic “preactivation” or glutathione-S-transferases (required for many other xenobiotics) may predispose isocyanate chemicals to metabolism via the mercapturic acid pathway.
GSH conjugation and metabolism via the mercapturic acid pathway has been established as a major route by which certain mono-isocyanate chemicals are excreted in vivo (Slatter, Rashed et al. 1991). In rats exposed to methyl isocyanate, GSH-methyl isocyanate conjugates have been detected in bile and N-acetylated cysteine-methyl isocyanate conjugates (the predicted end-product of methylisocyanate metabolism via the mercapturic acid pathway) are excreted in the urine (Pearson, Slatter et al. 1990, Slatter, Rashed et al. 1991). Mono-isocyanate breakdown products of some anti-neoplastic drugs are similarly conjugated with GSH and excreted as N-acetylated cysteine derivatives in humans (Davis, Kassahun et al. 1993).
The metabolism of diisocyanates, important industrial chemicals with well-recognized adverse effects on respiratory health, remains less clearly understood (Redlich and Karol 2002, Tarlo 2008). Limited animal studies to date have been hampered by technical difficulties including gastrointestinal (vs. inhalational) ingestion, scrubbing effects of rodent upper airways, and pH-instability of S-linked thiocarbamates (Kennedy, Stock et al. 1989, Kennedy, Singh et al. 1993, Kennedy, Wilson et al. 1994, Morris and Buckpitt 2009, Pauluhn 2014). A single study of MDI exposed rats by Gledhill et al identified an (M+H)+ ion at 388 m/z, consistent with that predicted for the N-acetylated-cysteine conjugate of partially hydrolyzed MDI, but did not characterize this metabolite (Gledhill, Wake et al. 2005). In vitro studies provide clear evidence for rapid formation of S-linked GSH conjugates, however the ability of GSH-diisocyanate conjugates to be metabolized via the mercapturic acid pathway has yet to be assessed (Reisser, Schmidt et al. 2002, Wisnewski, Hettick et al. 2011, Wisnewski, Liu et al. 2013, Wisnewski, Mhike et al. 2013). In this report, we directly evaluate the ability of human GGT-1, the primary enzyme of the mercapturic acid pathway, to cleave GSH-diisocyanate conjugates under well controlled in vitro conditions.
If diisocyanate chemicals are metabolized via the mercapturic acid pathway, then their corresponding mercapturic acid derivatives may serve as biomarkers amenable to occupational exposure surveillance. Indeed, substantial precedence exists for using mercapturic acid derivatives to monitor exposure to other hazardous chemicals present in the workplace (De Rooij, Commandeur et al. 1998, Maestri, Ghittori et al. 1997). In many cases, mercapturic acid derivatives serve as highly specific exposure biomarkers, despite representing only a small portion of the total metabolites derived from a particular exposure (Boogaard and van Sittert 1995, Ghittori, Maestri et al. 1997). The results of the present investigation are discussed in the context of metabolite-based approaches to workplace diisocyanate exposure surveillance.
MATERIALS AND METHODS
Generation of GSH-MDI Conjugates
Reduced glutathione, GSH (CAS # 70-18-8), and 4,4’-methylenebis(phenyl isocyanate) or MDI (CAS # 101-68-8) were from Sigma-Aldrich (St. Louis, MO) and were of ≥ 98.0% purity. GSH was reacted with MDI as previously described (Wisnewski, Liu et al. 2013). Briefly, 50 µl of 10% (w/v) MDI in acetone from JT Baker (Phillipsburg, NJ) was added dropwise with stirring to 25 ml of 10 mM GSH in 200 mM sodium phosphate, pH 7.4 (final acetone concentration 0.2% v/v). The reaction mixture was rotated end-over-end for 2 hours at 37°C, and then centrifuged at 10,000 g, and 0.2 µm filtered before use.
Generation of GSH-HDI Conjugates
GSH-HDI conjugates were made under mixed (vapor-liquid) phase exposure conditions as previously described (Wisnewski, Mhike et al. 2013). Briefly, 1 mM GSH in deionized water was placed in open petri dishes and exposed to HDI vapors obtained by passive diffusion from puriss grade HDI (CAS Number: 822-06-0) (Sigma-Aldrich), ≥99% purity by gas chromatography, with a refractive index (n20/D) = 1.453, and a density of 1.047 g/mL at 20°C. Samples exposed to HDI vapors for 18 hours were centrifuged at 10,000 g and 0.2 µm filtered before use.
GGT-1 Enzyme Treatment of GSH-Diisocyanate Reaction Products
Five hundred µl of 1mM GSH-MDI or GSH-HDI was mixed with 50 µl of human GGT-1 enzyme (1.8 mg/ml, 11.1 U/mg) from SCIPAC (Sittingbourne, Kent; U.K.). Experiments were performed in the absence and in the presence of 50 µl of 200 mM glycylglycine (in 200 mM sodium phosphate buffer pH 8.0) as an acceptor molecule for transpeptidation. Experiments with GSH-HDI were allowed to proceed for 15 min at GGT-1’s optimal temperature, 37°C (Farrance, Krauja et al. 1975). Experiments with GSH-MDI were initially performed for 60 minutes at a temperature lower than optimal (e.g. 22°C) as previously described (Sener and Yardimci 2005), since MDI thiocarbamates are more susceptible than aliphatic thiocarbamates to hydrolysis and/or transcarbamoylation reactions at 37°C (Chipinda, Stetson et al. 2006, Wisnewski, Liu et al. 2013, Wisnewski, Mhike et al. 2013). However, subsequent studies at 37°C (data not shown) yielded identical results. Chemical structures proposed for GGT-1 metabolites of GSH-diisocyanate were drawn using ChemBioDraw Ultra 14.0 (CambridgeSoft Corporation; Cambridge, MA).
LC-MS and hydrogen-deuterium (H-D) exchange LC-MS
LC-MS was performed on an Agilent 6550 Q-TOF system coupled to an Agilent 1290 Infinity LC system, using a rapid resolution HT Zorbax Eclipse Plus C18 column (2.1 × 50 mm, 1.8 µm), also from Agilent Technologies (Santa Clara, CA). Samples were mixed 1:1 in buffer A (water containing 0.1% formic acid) before loading and were eluted with 40% buffer B (acetonitrile containing 0.1% formic acid) over 8 min, increasing to 95% buffer B by 10 min. Positive ion electrospray was performed using the following parameters: gas temp- 280°C, gas flow- 11 l/min, nebulizer-40 psig, sheath gas temp- 350°C, sheath gas flow-11, Vcap-4000 V, nozzle voltage-2000 V, fragmentor voltage– 175 V, skimmer voltage 65 V, octopole RF peak voltage 750 V. The data acquisition range was from 110–1700 m/z. H-D exchange LC-MS was performed on duplicate samples identically assessed using buffer A made with “normal” or heavy (D2O) water and the number of exchangeable hydrogens was based on increase in the mass/charge ratio (m/z) of the selected ions, as previously described (Nassar 2003). Data were acquired and analyzed using Mass Hunter Workstation software from Agilent.
MS/MS analysis
MS/MS analysis was performed on an LTQ Orbitrap XL (Thermo Scientific; Waltham, MA) by the Yale Keck Center as previously described (Stone, DeAngelis et al. 1998, Wisnewski, Liu et al. 2013). The fragmentation patterns of monoisotopic ions corresponding to specific GSH-diisocyanate metabolites were analyzed following collision-induced dissociation (CID). Briefly, the LTQ Orbitrap was equipped with a Waters (Milford, MA) nanoAcquity UPLC system, and used a Waters Symmetry® C18 180µm × 20 mm trap column and a 1.7 µm, BEH130 C18, 75 µm × 250 mm nanoAcquity™ UPLC™ column (37°C). Trapping was done at 5µl/min, 99% Buffer A (100% water, 0.1% formic acid) for 3 min. Sample separation is performed at 300 nl/min with Buffer A: 99.9% water 0.1% formic acid and Buffer B: 99.925% CH3CN, 0.075% formic acid. MS was acquired on the Orbitrap using 1 microscan, and a maximum inject time of 900 followed by 6 data dependent MS/MS acquisitions in the ion trap.
RESULTS
Identification of GGT-1 metabolites of Aromatic MDI-GSH Reaction Products
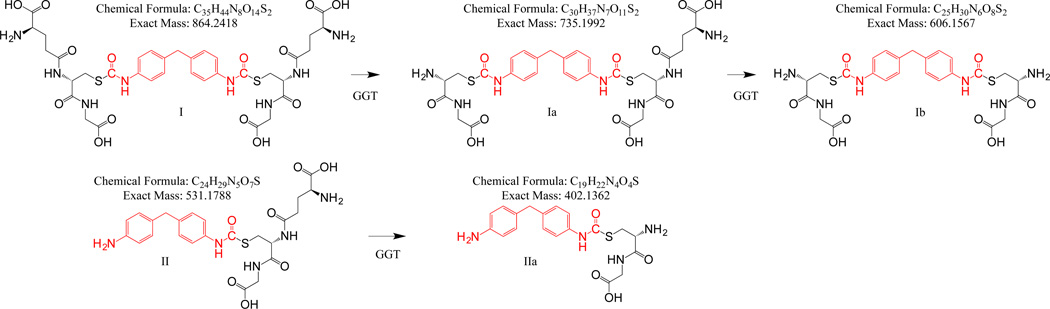
Preliminary HPLC-UV studies (not shown) suggested that GGT-1 could cleave GSH-MDI conjugates, and prompted subsequent LC-MS studies to better characterize these enzyme-dependent products. As shown in Figures 1 and 2 and in prior publications (Reisser, Schmidt et al. 2002, Wisnewski, Liu et al. 2013), S-linked bis(GSH)-MDI, and mono(GSH)-S-MDI* (where * represents one N=C=O hydrolyzed to an NH2), are major products resulting from direct nucleophilic addition of MDI to GSH. Incubation of these GSH-MDI conjugates with GGT-1, resulted in the formation of new (M+H)+ ions, with m/z’s corresponding to the predicted GGT-1 cleavage products of bis(GSH)-diisocyanate, and mono(GSH)-S-diisocyanate*, as depicted in Figure 1. The partially metabolized cys-gly-MDI-GSH (Ia in Figure 1) was the most prominent GGT-1 metabolite observed under reaction conditions that favor γ-glutamate hydrolysis. However, under conditions that favor γ-glutamate transpeptidation (e.g. in the presence of acceptor molecule, gly-gly), incubation with GGT-1 resulted in new molecules with m/z values corresponding to completely processed bis(cys-gly)-MDI and mono(cys-gly)-MDI* metabolites (Ib, and IIa in Figure 1, and supplemental data Fig. S1). Furthermore, when GSH-MDI was metabolized by GGT-1 in the presence of acceptor molecule gly-gly, an ion with the m/z value expected for the transpeptidation product (e.g. glu-gly-gly) was also observed (Supplemental data Fig. S2).
Figure 1.
Major S-linked GSH-MDI reaction products and proposed chemical structures for metabolites resulting from enzymatic cleavage by human GGT-1.
Figure 2.
Extracted ion chromatograms for GSH-MDI and expected metabolites resulting from cleavage by human GGT-1. Panel A shows EIC for the major mono and bis(GSH)-MDI reaction products (starting material) with m/z’s of 532.18 and 865.24**. Panels B and C show EICs for the major GSH-MDI reaction products and the expected (cys-gly)-MDI metabolites resulting from their cleavage by GGT-1, in the presence (thin dashed line) or absence (heavy solid line) of purified human GGT-1. Experiments were performed in the absence (Panel B) or presence (Panel C) of acceptor dipeptide (gly-gly). Metabolites include mono(cys-gly)-MDI*= 403.14 m/z, bis(cys-gly)-MDI= 607.16 m/z, and (cys-gly)-MDI-GSH= 736.20 m/z. See Figure S1 in supplemental materials for corresponding MS data ** double peak of bis(GSH)-MDI is consistently observed and may result from on-column intramolecular rearrangement, in-source fragmentation, or unrecognized causes.
Characterization of GGT-1 Metabolites of MDI-GSH by MS/MS and H-D Exchange
We further characterized the structure of the GGT-1 dependent metabolites of GSH-MDI through MS/MS, hydrogen-deuterium exchange LC-MS, and theoretical analysis, as shown in Table 1 and supplemental data (Figures S3–S6). During MS/MS analysis of the newly described GGT-1 dependent GSH-MDI metabolites (e.g. ions with m/z’s corresponding to Ia, Ib, or IIa), CID of the parent (M+H)+ ions revealed fragmentation patterns consistent with the structures proposed in Figure 1. The number of exchangeable hydrogens on the GGT-1 metabolites of GSH-MDI, based on mass increase when LC-MS analysis was performed with D2O vs. H2O (Table 1 and supplemental data Figure S6), was also consistent with the chemical structures proposed in Figure 1A. Furthermore, the molecular mass data satisfy the nitrogen rule (Table 1) and the ring double bond equivalent (RDBE) rule (not shown) for the novel GSH-MDI metabolite structures (Pellegrin 1983, Badertscher, Bischofberger et al. 2001). Thus, LC-MS, MS/MS, H-D exchange LC-MS, and theoretical calculations support the metabolism of GSH-MDI to cys-gly-MDI-GSH (Ia), bis(cys-gly)-MDI (Ib), and mono(cys-gly)-MDI* (IIa) by human GGT-1.
Table 1.
Characteristics of GGT-1 metabolites of GSH-MDI
| GGT-1 Metabolite | Formula | Mass (M+H) m/z | H-D Exchangea | Nitrogen Ruleb | Major MS/MS Ions (m/z) | |||
|---|---|---|---|---|---|---|---|---|
| Observed | Theoretical | Observed | Expected | Observed | Expected | |||
| cys-gly-MDI-GSH (Ia) |
C30H37N7O11S2 | 736.2082 | 736.2071 | 12 | 12 | odd | odd | 718.22(−H2O) 661.19 cys-MDI-GSH 607.16 bis(cys-gly)-MDI 589.19 bis(cys-gly)-MDI(−H2O) 532.22 mono(GSH)-MDI* |
| bis(cys-gly)-MDI (Ib) |
C25H30N6O8S2 | 607.1643 | 607.1647 | 10 | 10 | even | even | 589.14(−H2O) 532.10 (cys-gly)-MDI-cys 429.09 (cys-gly)-MDI 403.12 (cys-gly)-MDI* |
| mono(cys-gly)-MDI* (IIa) |
C19H22N4O4S | 403.1439 | 403.1405 | 7 | 7 | even | even | 385.18(−H2O) 310.04 cys-MDI*(−H2O) |
H-D Exchange = the number of hydrogen atoms exchangeable with D2O by LC-MS (see supplemental data Fig S6)
Nitrogen Rule = organic compounds (made exclusively of hydrogen, carbon, nitrogen, oxygen, and sulfur) that contain none, or an even number of, nitrogen atoms will have an even mass number; an odd number of nitrogen atoms results in an odd mass number
Identification of GGT-1 Metabolites of Aliphatic HDI-GSH
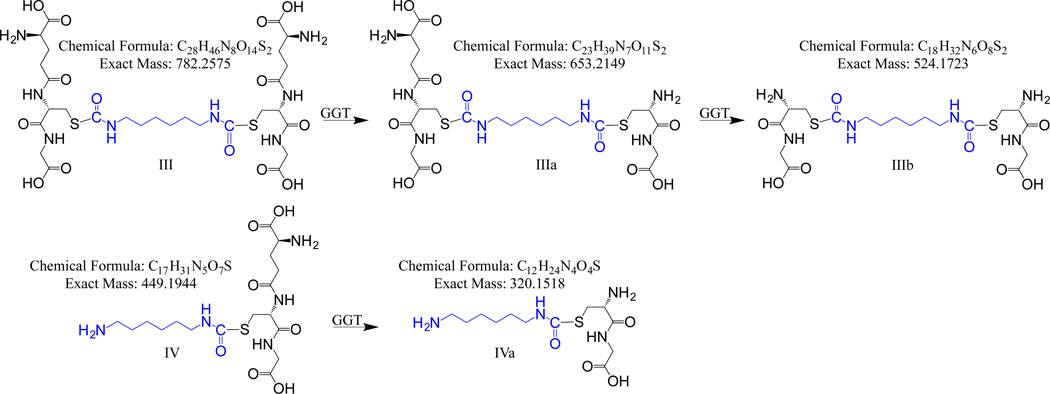
We similarly evaluated the capacity of human GGT-1 to cleave GSH conjugates of the aliphatic diisocyanate, HDI, which include bis(GSH)-S-HDI and mono(GSH)-S-HDI* (where * represents hydrolysis of one N=C=O group to NH2) as shown in Figures 3 and 4, and in prior reports (Wisnewski, Mhike et al. 2013). Incubation of GSH-HDI conjugates with human GGT-1 resulted in the production of new (M+H)+ ions with m/z values that match the predicted GGT-1 metabolites of GSH-HDI (shown in Figure 3), based on LC-MS (Figure 4, and supplemental Figure S7), MS/MS, and H-D exchange studies (Table 2). The partially metabolized cys-gly-HDI-GSH and mono(GSH)-HDI* were most prominent under conditions that favor γ-glutamate hydrolysis. However, under conditions that favor γ-glutamate transpeptidation (e.g. in the presence of gly-gly as an acceptor molecule) greater accumulation of the completely processed bis(cys-gly)-HDI (IIIb in Figure 3) and mono(cys-gly)-HDI* were observed (Figure 4). Thus, LC-MS, MS/MS, and H-D exchange, together with data on MDI-GSH, support the metabolism of GSH-HDI to (cys-gly)-HDI-GSH, bis(cys-gly)-HDI and mono(cys-gly)-HDI* by human GGT-1. The structures proposed for these major GGT-1 metabolites of GSH-HDI are further consistent with the nitrogen and RDBE rules of organic chemistry.
Figure 3.
Major S-linked GSH-HDI reaction products and proposed chemical structures for metabolites resulting from enzymatic cleavage by human GGT-1.
Figure 4.
Extracted ion chromatograms for GSH-HDI and expected metabolites resulting from cleavage by human GGT1. Panel A shows EIC for the major mono and bis(GSH)-HDI reaction products (starting material) with m/z’s of 450.19 and 783.26. Panels B and C show EICs for GSH-HDI and the expected (cys-gly)-HDI metabolites resulting from their cleavage by GGT1, in the presence (thin dashed line) or absence (heavy solid line) of enzyme. Experiments were performed in the absence (Panel B) or presence (Panel C) of acceptor dipeptide (gly-gly). Metabolites include mono(cys-gly)-HDI*= 321.2 m/z, bis(cys-gly)-HDI= 525.2 m/z, and (cys-gly)-HDI-GSH= 654.2.
Table 2.
Characteristics of GGT-1 metabolites of GSH-HDI
| GGT-1 Metabolite | Formula | Mass (M+H) m/z | H-D Exchangea | Nitrogen Ruleb | Major MS/MS Ions (m/z) | |||
|---|---|---|---|---|---|---|---|---|
| Observed | Theoretical | Observed | Expected | Observed | Expected | |||
| cys-gly-HDI-GSH (IIIa) |
C23H39N7O11S2 | 654.2209 | 654.2284 | 12 | 12 | odd | odd | 636.25(−H2O) 579.24cys-HDI-GSH 525.18bis(cys-gly)-HDI 507.21 bis(cys-gly)-HDI(−H2O) 450.20 mono(GSH)-HDI* |
| bis(cys-gly)-HDI (IIIb) |
C18H32N6O8S2 | 525.1798 | 525.1802 | 10 | 10 | even | even | 507.21(−H2O) 450.15 (cys-gly)-HDI-cys 347.17 (cys-gly)-HDI 321.19 (cys-gly)-HDI* |
| mono(cys-gly)-HDI* (IVa) |
C12H24N4O4S | 321.1609 | 321.1597 | 7 | 7 | even | even | 303.16(−H2O) 228.10cys-HDI*(−H2O) |
H-D Exchange = the number of hydrogen atoms exchangeable with D2O by LC-MS
Nitrogen Rule = organic compounds (made exclusively of hydrogen, carbon, nitrogen, oxygen, and sulfur) that contain none, or an even number of, nitrogen atoms will have an even mass number; an odd number of nitrogen atoms results in an odd mass number
DISCUSSION
The present study demonstrates that GSH conjugates of MDI and HDI, important industrial chemicals with well-recognized adverse health effects, can be cleaved by human GGT-1. Cys(gly)-diisocyanate reaction products resulting from GGT-1 enzymatic cleavage of GSH-diisocyanate conjugates were readily identified using a combination of LC-MS, MS/MS, and H-D exchange LC-MS analyses. The findings suggest the potential for diisocyanate metabolism via the mercapturic acid pathway, as previously documented for mono-isocyanates (Slatter, Rashed et al. 1991).
An important consideration regarding diisocyanate metabolism via the mercapturic acid pathway is the potential reversibility of S-linked isocyanate conjugates, and their ability to carbamoylate other molecules (Pearson, Slatter et al. 1990, Pearson, Slatter et al. 1991, Slatter, Rashed et al. 1991, Wisnewski, Liu et al. 2013). Thus, a GSH-mediated pathway is unlikely to fulfill a conventional detoxification role for diisocyanates, however, it may provide an elimination pathway for at least a portion of internalized chemical. Precedence for this hypothesis is provided from studies with (mono) methylisocyanate, whose mercapturic acid derivative can be found in the urine of exposed animals, despite similar instability of its S-linked thiocarbamate conjugates (Slatter, Rashed et al. 1991).
The present findings may be relevant to future efforts of preventing diisocyanate-induced disease. In this regard, excreted diisocyanate metabolites may serve as biomarkers for monitoring workplace exposure levels and ensuring the efficacy of industrial hygiene efforts. The present findings, together with prior work on mono-isocyanate (Slatter, Rashed et al. 1991), suggest N-acetylated cysteine-diisocyanate conjugates may represent urinary metabolites, whose levels could serve as the basis for such exposure biomonitoring. Mercapturic acid derivatives of other potentially hazardous chemicals have proven useful for biomonitoring occupational as well as environmental exposures (De Rooij, Commandeur et al. 1998, Maestri, Ghittori et al. 1997). Although some mercapturic acid derivatives are “non-specific” biomarkers (e.g. may result from exposure to different chemicals), many are highly specific and provide an accurate approach to exposure biomonitoring, even if they represent only a minor fraction of the total metabolites resulting from a given exposure (e.g. styrene, benzene) (Sanduja, Ansari et al. 1989, Boogaard and van Sittert 1995, Ghittori, Maestri et al. 1997). Recent advances in LC/MS and related metabolomic technology facilitate the identification and quantification of such potentially useful urinary metabolites (Kotapati, Esades et al 2015, Sterz, Scherer et al. 2012, Manini, Andreoli et al. 2000).
The strengths and weaknesses of the present study are important to recognize in interpreting the present findings. The major strength of the investigation is the use of highly precise methods (e.g. high performance liquid chromatography, tandem mass spectrometry and H-D exchange) for characterizing GGT-1 mediated metabolites under well-defined conditions. The major weakness is that experiments were performed entirely in vitro and define only the primary step along the mercapturic acid pathway of metabolism. Further studies, including in vivo investigations in animal models or exposed human subjects will be necessary to fully assess the biological relevance of the present findings.
CONCLUSIONS
In summary, human GGT-1 was shown to metabolize GSH-diisocyanate conjugates in vitro. Chemical structures for the newly described (cys-gly)-diisocyanate metabolites are supported by LC-MS, MS/MS, and H-D LC-MS exchange data. Given the propensity for diisocyanates to form S-linked GSH conjugates and the well-described role of GGT-1 in the metabolism and elimination of other GSH-xenobiotic conjugates (including monoisocyanates), the data suggest GGT-1 could participate in the metabolism of the industrially important diisocyanates, MDI and HDI.
Supplementary Material
ACKNOWLEDGMENTS
We would like to acknowledge Dr. Terrence Wu from the Yale West Campus, Director of the Mass Spectrometry/Proteomics section of the Molecular Institute for Discovery, for his direction and assistance with LC-MS and LC-MS/MS. We are also indebted to personnel from the Yale Keck Center, especially Ted Voss, Mary LoPresti, Jean Kanyo, and Drs. Stone, Lam, and Colangelo, for their expert assistance with sample analysis.
ABBREVIATIONS USED IN THE PAPER
- EIC
extracted ion chromatogram
- CID
collision-induced dissociation
- GGT
gamma glutamyl transpeptidase
- GSH
reduced glutathione
- HDI
hexamethylene diisocyanate
- H-D
hydrogen-deuterium
- MDI
methylene diphenyl diisocyanate
- m/z
mass-to-charge ratio
- RDBE
ring-double bond equivalent
- *
N=C=O group hydrolyzed to primary amine.
Footnotes
DECLARATION OF INTERESTS
The authors report no declaration of interest.
REFERENCES
- Badertscher M, Bischofberger K, Munk ME, Pretsch E. A novel formalism to characterize the degree of unsaturation of organic molecules. J Chem Inf Comput Sci. 2001;41(4):889–893. doi: 10.1021/ci000135o. [DOI] [PubMed] [Google Scholar]
- Boogaard PJ, van Sittert NJ. Biological monitoring of exposure to benzene: a comparison between S-phenylmercapturic acid, trans,trans-muconic acid, and phenol. Occup Environ Med. 1995;52:611–620. doi: 10.1136/oem.52.9.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher JR. The toxicity of methyl isocyanate: Where do we stand? Environ Health Perspect. 1987;72:197–198. doi: 10.1289/ehp.8772197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantin AM, North SL, Hubbard RC, Crystal RG. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol (1985) 1987;63(1):152–157. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- Chipinda I, Stetson SJ, Depree GJ, Simoyi RH, Siegel PD. Kinetics and mechanistic studies of the hydrolysis of diisocyanate-derived bis-thiocarbamates of cysteine methyl ester. Chem Res Toxicol. 2006;19(3):341–350. doi: 10.1021/tx050311t. [DOI] [PubMed] [Google Scholar]
- Davis MR, Kassahun K, Jochheim CM, Brandt KM, Baillie TA. Glutathione and N-acetylcysteine conjugates of 2-chloroethyl isocyanate. Identification as metabolites of N,N'-bis(2-chloroethyl)-N-nitrosourea in the rat and inhibitory properties toward glutathione reductase in vitro. Chem Res Toxicol. 1993;6(3):376–383. doi: 10.1021/tx00033a020. [DOI] [PubMed] [Google Scholar]
- De Rooij BM, Commandeur JNM, Vermeulen NPE. Mercapturic acids as biomarkers of exposure to electrophilic chemicals: applications to environmental and industrial chemicals. Biomarkers: biochemical indicators of exposure, response, and susceptibility to chemicals. 1998;3:239–303. doi: 10.1080/135475098231101. [DOI] [PubMed] [Google Scholar]
- Farrance I, Krauja VW, Dennis PM. The determination of gamma-glutamyl transpeptidase by reaction rate assay at 37 degrees C. Pathology. 1975;7(3):237–243. doi: 10.3109/00313027509094413. [DOI] [PubMed] [Google Scholar]
- Fuchs S, Valade P. [Clinical and experimental study of some cases of poisoning by desmodur T (1-2-4 and 1-2-6 di-isocyanates of toluene)] Arch Mal Prof. 1951;12(2):191–196. [PubMed] [Google Scholar]
- Ghittori S, Maestri L, Imbriani M, Capodaglio E, Cavalleri A. Urinary excretion of specific mercapturic acids in workers exposed to styrene. Am J Ind Med. 1997;31:636–644. doi: 10.1002/(sici)1097-0274(199705)31:5<636::aid-ajim20>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Gledhill A, Wake A, Hext P, Leibold E, Shiotsuka R. Absorption, distribution, metabolism and excretion of an inhalation dose of [14C] 4,4'-methylenediphenyl diisocyanate in the male rat. Xenobiotica. 2005;35(3):273–292. doi: 10.1080/00498250500057591. [DOI] [PubMed] [Google Scholar]
- Gross M, Whetzel NK, Folk JE. Alkyl isocyanates as active site-directed inactivators of guinea pig liver transglutaminase. J Biol Chem. 1975;250(19):7693–7699. [PubMed] [Google Scholar]
- Kennedy AL, Singh G, Alarie Y, Brown WE. Autoradiographic analyses of guinea pig airway tissues following inhalation exposure to 14C-labeled methyl isocyanate. Fundam Appl Toxicol. 1993;20(1):57–67. doi: 10.1006/faat.1993.1007. [DOI] [PubMed] [Google Scholar]
- Kennedy AL, Stock MF, Alarie Y, Brown WE. Uptake and distribution of 14C during and following inhalation exposure to radioactive toluene diisocyanate. Toxicol Appl Pharmacol. 1989;100(2):280–292. doi: 10.1016/0041-008x(89)90314-1. [DOI] [PubMed] [Google Scholar]
- Kennedy AL, Wilson TR, Stock MF, Alarie Y, Brown WE. Distribution and reactivity of inhaled 14C-labeled toluene diisocyanate (TDI) in rats. Arch Toxicol. 1994;68(7):434–443. doi: 10.1007/s002040050094. [DOI] [PubMed] [Google Scholar]
- Kotapati S, Esades A, Matter B, Le C, Tretyakova N. High throughput HPLC-ESI-MS/MS methodology for mercapturic acid metabolites of 1,3-butadiene: Biomarkers of exposure and bioactivation. Chem Biol Interact. 2015;241:23–31. doi: 10.1016/j.cbi.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestri L, Ghittori S, Imbriani M. Determination of specific mercapturic acids as an index of exposure to environmental benzene, toluene, and styrene. Ind Health. 1997;35:489–501. doi: 10.2486/indhealth.35.489. [DOI] [PubMed] [Google Scholar]
- Manini P, Andreoli R, Bergamaschi E, De Palma G, Mutti A, Niessen WM. A new method for the analysis of styrene mercapturic acids by liquid chromatography/electrospray tandem mass spectrometry. Rapid Commun Mass Spectrom. 2000;14:2055–2060. doi: 10.1002/1097-0231(20001115)14:21<2055::AID-RCM134>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Morris JB, Buckpitt AR. Upper respiratory tract uptake of naphthalene. Toxicol Sci. 2009;111(2):383–391. doi: 10.1093/toxsci/kfp138. [DOI] [PubMed] [Google Scholar]
- Nassar AE. Online hydrogen-deuterium exchange and a tandem-quadrupole time-of-flight mass spectrometer coupled with liquid chromatography for metabolite identification in drug metabolism. J Chromatogr Sci. 2003;41(8):398–404. doi: 10.1093/chromsci/41.8.398. [DOI] [PubMed] [Google Scholar]
- Pauluhn J. Development of a respiratory sensitization/elicitation protocol of toluene diisocyanate (TDI) in Brown Norway rats to derive an elicitation-based occupational exposure level. Toxicology. 2014;319:10–22. doi: 10.1016/j.tox.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Pearson PG, Slatter JG, Rashed MS, Han DH, Baillie TA. Carbamoylation of peptides and proteins in vitro by S-(N-methylcarbamoyl)glutathione and S-(N-methylcarbamoyl)cysteine, two electrophilic S-linked conjugates of methyl isocyanate. Chem Res Toxicol. 1991;4(4):436–444. doi: 10.1021/tx00022a007. [DOI] [PubMed] [Google Scholar]
- Pearson PG, Slatter JG, Rashed MS, Han DH, Grillo MP, Baillie TA. S-(N-methylcarbamoyl)glutathione: a reactive S-linked metabolite of methyl isocyanate. Biochem Biophys Res Commun. 1990;166(1):245–250. doi: 10.1016/0006-291x(90)91937-n. [DOI] [PubMed] [Google Scholar]
- Pellegrin V. Molecular formulas of organic compounds: the nitrogen rule and degree of unsaturation. Journal of Chemical Education. 1983;60(8):626. [Google Scholar]
- Redlich CA, Karol MH. Diisocyanate asthma: clinical aspects and immunopathogenesis. Int Immunopharmacol. 2002;2(2–3):213–224. doi: 10.1016/s1567-5769(01)00174-6. [DOI] [PubMed] [Google Scholar]
- Reisser M, Schmidt BF, Brown WE. Synthesis, characterization, and solvolysis of mono- and bis-S-(glutathionyl) adducts of methylene-bis-(phenylisocyanate) (MDI) Chem Res Toxicol. 2002;15(10):1235–1241. doi: 10.1021/tx0255020. [DOI] [PubMed] [Google Scholar]
- Sanduja R, Ansari GA, Boor PJ. 3-Hydroxypropylmercapturic acid: a biologic marker of exposure to allylic and related compounds. J Appl Toxicol. 1989;9:235–238. doi: 10.1002/jat.2550090406. [DOI] [PubMed] [Google Scholar]
- Sener A, Yardimci T. Activity determination, kinetic analyses and isoenzyme identification of gamma glutamyltransferase in human neutrophils. J Biochem Mol Biol. 2005;38:343–349. doi: 10.5483/bmbrep.2005.38.3.343. [DOI] [PubMed] [Google Scholar]
- Slatter JG, Rashed MS, Pearson PG, Han DH, Baillie TA. Biotransformation of methyl isocyanate in the rat. Evidence for glutathione conjugation as a major pathway of metabolism and implications for isocyanate-mediated toxicities. Chem Res Toxicol. 1991;4(2):157–161. doi: 10.1021/tx00020a006. [DOI] [PubMed] [Google Scholar]
- Sterz K, Scherer G, Krumsiek J, Theis FJ, Ecker J. Identification and quantification of 1-hydroxybutene-2-yl mercapturic acid in human urine by UPLC- HILIC-MS/MS as a novel biomarker for 1,3-butadiene exposure. Chem Res Toxicol. 2012;25:1565–1567. doi: 10.1021/tx3002862. [DOI] [PubMed] [Google Scholar]
- Stone KL, DeAngelis R, LoPresti M, Jones J, Papov VV, Williams KR. Use of liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS) for routine identification of enzymatically digested proteins separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Electrophoresis. 1998;19(6):1046–1052. doi: 10.1002/elps.1150190620. [DOI] [PubMed] [Google Scholar]
- Tarlo SM. Occupational exposures and adult asthma. Immunol Allergy Clin North Am. 2008;28(3):563–576. viii. doi: 10.1016/j.iac.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Wisnewski AV, Hettick JM, Siegel PD. Toluene diisocyanate reactivity with glutathione across a vapor/liquid interface and subsequent transcarbamoylation of human albumin. Chem Res Toxicol. 2011;24(10):1686–1693. doi: 10.1021/tx2002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnewski AV, Liu J, Redlich CA. Antigenic changes in human albumin caused by reactivity with the occupational allergen diphenylmethane diisocyanate. Anal Biochem. 2010;400(2):251–258. doi: 10.1016/j.ab.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnewski AV, Liu J, Redlich CA. Connecting glutathione with immune responses to occupational methylene diphenyl diisocyanate exposure. Chem Biol Interact. 2013;205(1):38–45. doi: 10.1016/j.cbi.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnewski AV, Mhike M, Hettick JM, Liu J, Siegel PD. Hexamethylene diisocyanate (HDI) vapor reactivity with glutathione and subsequent transfer to human albumin. Toxicol In Vitro. 2013;27(2):662–671. doi: 10.1016/j.tiv.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.