Abstract
Background
5-Methoxy-N,N-dimethyltryptamine (5-MeO-DMT) and harmaline are indolealkylamine (IAA) drugs often abused together. Our recent studies have revealed the significant effects of co-administered harmaline, a monoamine oxidase inhibitor (MAOI), on 5-MeO-DMT pharmacokinetics and thermoregulation. This study was to delineate the impact of harmaline and 5-MeO-DMT on home-cage activity in mouse models, as well as the contribution of serotonin (5-HT) receptors.
Methods
Home-cage activities of individual animals were monitored automatically in the home cages following implantation of telemetry transmitters and administration of various doses of IAA drugs and 5-HT receptor antagonists. Area under the effect curve (AUEC) of mouse activity values were calculated by trapezoidal rule.
Results
High dose of harmaline (15 mg/kg, ip) alone caused an early-phase (0–45 min) hypoactivity in mice that was fully attenuated by 5-HT1A receptor antagonist WAY-100635, whereas a late-phase (45–180 min) hyperactivity that was reduced by 5-HT2A receptor antagonist MDL-100907. 5-MeO-DMT (10 and 20 mg/kg, ip) alone induced biphasic effects, an early-phase (0–45 min) hypoactivity that was completely attenuated by WAY-100635, and a late-phase (45–180 min) hyperactivity that was fully suppressed by MDL-100907. Interestingly, co-administration of MAOI harmaline (2–15 mg/kg) with a subthreshold dose of 5-MeO-DMT (2 mg/kg) induced excessive hyperactivities at late phase (45–180 min) that could be abolished by either WAY-100635 or MDL-100907.
Conclusions
Co-administration of MAOI with 5-MeO-DMT provokes excessive late-phase hyperactivity, which involves the activation of both 5-HT1A and 5-HT2A receptors.
Introduction
Indolealkylamine (IAA) drugs are 5-hydroxytryptamine (5-HT or serotonin) analogs that are able to modulate various physiological and psychological functions including body temperature, attention and behavior [1–3]. Many IAAs are found as psychoactive ingredients of a variety of plant and animal preparations used for medicine, religion and recreation purposes [4–8]. IAAs are also recognized as a major class of drugs of abuse, among which 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT or by the street name “5-MEO”) and 5-methoxy-N,N-diisopropyltryptamine (5-MeO-DiPT or “Foxy” and “Foxy methoxy”) were placed into Schedule I under the Controlled Substances Act in the United States by Drug Enforcement Administration (DEA) in 2011 and 2004, respectively [9, 10]. There are also many reports on IAA intoxications in recent years which include several fatal cases related to the abuse of 5-MeO-DMT or 5-MeO-DiPT [11–19].
5-MeO-DMT is metabolically inactivated by monoamine oxidase A (MAO-A), and thus it is often abused with MAO-A inhibitor (MAOI), e.g., harmaline, to achieve an improved hallucinogenic effect [20]. The pharmacokinetic drug-drug interactions among IAA compounds have been nicely demonstrated using 5-MeO-DMT and MAOI harmaline as model drugs [21–24]. In particular, co-administration of MAOI harmaline leads to a remarkably elevated and prolonged systemic and cerebral exposure to 5-MeO-DMT and an active metabolite bufotenine. In addition, MAOI harmaline greatly increases brain 5-HT levels in mice through the inhibition of 5-HT deamination metabolism [25]. Consequently, 5-MeO-DMT pharmacological effects may be significantly altered by co-administered MAOI harmaline [26–29]. Mechanistically, 5-MeO-DMT is rather a relatively less selective 5-HT receptor agonist and it is able to bind to 5-HT1A, 5-HT2A and 5-HT2C receptors with modest to high affinities [5, 30–34]. Due to the presence of consistent pharmacokinetic and pharmacodynamic interactions [2, 24], co-administration of 5-MeO-DMT and MAOI may cause hyperserotonergic tone or even serotonin toxicity/syndrome, which exhibits a number of characteristic features in patients and animal models (e.g., neuromuscular excitation such as shivering and tremor, autonomic stimulation such as hyperthermia and tachycardia, and altered mental/behavioral status such as confusion, anxiety, and activity), and has become a more prevalent clinical issue [35–37].
Indeed our recent study has revealed the potentiation of 5-MeO-DMT-induced hyperthermia by MAOI harmaline, and defined the contribution of 5-HT1A and 5-HT2A receptors [28]. In this study, we aimed to delineate the effects of co-administered MAOI harmaline with 5-MeO-DMT on home-cage activities in mice maintained in home cages using an automated telemetry system. In addition, 5-HT1A receptor antagonist WAY-100635 and 5-HT2A receptor antagonists MDL-100907 were utilized to define the serotonergic mechanisms underlying home-cage activities altered by harmaline and 5-MeO-DMT. These results would advance the mechanistic understanding of IAA pharmacological effects on behaviors and the risks of interactions between IAA drugs of abuse.
Material and Methods
Chemicals and Materials
Harmaline hydrochloride dihydrate, 5-MeO-DMT oxalate, and N-N-(2-pyridinyl) cyclohexanecarboxamide trihydrochloride (WAY-100635) were bought from Sigma-Aldrich (St. Louis, MO). (R)-(+)-(2,3-dimethoxyphenyl)-[1-[2-(4-fluorophenyl)ethyl]-4-piperidyl]methanol (MDL-100907) was a generous gift from Sanofi-Aventis (Paris, France). Carprofen (Rimadyl) was purchased from Pfizer Inc. (New York, NY). Isoflurane (AErrane) was bought from Baxter Healthcare (Deerfield, IL). All drugs for injection were dissolved in saline and were administered as their free base weights. An injection volume of 10 ml/kg was used for both intraperitoneal (ip) and subcutaneous (sc) injections.
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee at University at Buffalo, The State University of New York. Age-matched male FVB/N mice (25–35 g; The Jackson Laboratory, Bar Harbor, ME, USA) were housed in an animal care facility maintained at 20 ± 2.0°C on a 12-h light/dark cycle (lights on from 6 AM to 6 PM) with ad libitum food and water.
Surgical Preparations
All the surgery procedures were performed under aseptic conditions as described earlier [28]. A sterile Physiotel TA10TA-F20 telemetry transmitter (Data Sciences International, St. Paul, MN) was implanted into the peritoneal cavity. Mice were anaesthetized with isoflurane in oxygen (4%, reduced as necessary). Carprofen (5 mg/kg) as an analgesic was injected sc immediately after surgery and oral (po) dosed for 2 more days. After surgery, animals were individually housed for recovery and conditioned for 2 weeks before being used for activity studies in home cages (overall dimensions: mm 365×207×140 ht).
Experimental Procedures
All animals were tested in their home cages in an isolated and quiet room between 10:30 A.M. and 4:30 P.M.. On the afternoon before an experimental day, mice were weighted and returned to their home cages, which were placed on individual configured receivers (Data Sciences International, St. Paul, MN). The telemetry transmitter was activated for overnight stabilization and acquisition of baseline activities before experiments. Harmaline (0, 2, 5 or 15 mg/kg; N = 14 mice in each group), 5-MeO-DMT (0, 2, 10 or 20 mg/kg; N = 14 mice in each group) or their combination (0, 2, 5 or 15 mg/kg harmaline plus 2 mg/kg 5-MeO-DMT; N = 11 mice per group) were ip administered to the mice. In the combination studies, harmaline was injected 15 min before 5-MeO-DMT treatment. To define the role of 5-HT1A and 5-HT2A receptors in harmaline, 5-MeO-DMT and their combination elicited behavior changes, mice were pretreated sc with either 5-HT1A receptor antagonist WAY-100635 (1 mg/kg; N = 7 mice per group) or 5-HT2A receptor antagonist MDL-100907 (1 mg/kg; N = 7 mice in each group). For harmaline (15 mg/kg) treatment, antagonists were dosed 15 min before the administration of harmaline. For 5-MeO-DMT (20 mg/kg) alone and harmaline (2 m/kg) plus 5-MeO-DMT (2 mg/kg) treatments, antagonists were given 15 min before the administration of 5-MeO-DMT. Control animals were given drug vehicle (saline) by following the same injection protocols. After drug administration, the locomotor activities (counts) of individual animals were continuously recorded during the procedures using the same receivers (Data Sciences International, St. Paul, MN), which were controlled by the Ponemah software (Data Sciences International).
Data Acquisition and Analysis
Mouse home-cage activities (counts) were recorded every 10 seconds and the average values within a 15 min period were calculated and used for data analysis. Area under the effect curve (AUEC) of home-cage activity values were calculated by trapezoidal method (GraphPad Prism 5, GraphPad Software Inc., San Diego, CA). Because drug-induced changes of animal activities were biphasic, AUEC values were calculated for two periods, an early phase at 0–45 min and late-phase at 45–180 min which better indicated biphasic effects. Depending on the number of groups and variances, data were compared with one-way or two-way ANOVA followed by Bonferroni’s post-hoc tests (GraphPad Prism 5). Difference was considered statistically significant when p < 0.05.
Results
High dose of harmaline is able to alter the home-cage activities of mice
Administration of vehicle (0 mg/kg harmaline) introduced some stress to the mice and led to a transient (0–45 min) increase in home-cage activities, which completely returned to the baseline levels later (45–180 min) (Figure 1A). Compared to the vehicle control treatment, lower doses of harmaline (2 and 5 mg/kg) had no significant effects on mouse home-cage activity (Figure 1A), which is also indicated by the lack of change in AUEC values (Figure 1B and 1C). Interestingly, a higher dose of harmaline (15 mg/kg) significantly reduced the activities of mice at early times (0–45 min) and slightly enhanced the home-cage activities at late phase (45–180 min) (Figure 1A). The impact of high dose of harmaline on mouse activities is also evidenced by a significant change of AUEC0–45 min (Figure 1B) and AUEC45–180min values (Figure 1C).
Figure 1.
Higher dose (15 mg/kg) of harmaline (HAR) significantly reduced the early-phase (0–45 min) home-cage activity and elevated late-phase (45 – 180 min) activity in mice (Two-way ANOVA with Bonferroni’s post-hoc test: p < 0.0001 for drug treatment, time and interaction; ***p < 0.001 at indicated time points, compared to vehicle control), whereas lower doses (2 or 5 mg/kg) of harmaline showed no effect on home-cage activity (A). This is clearly indicated by the AUEC values (B and C). Values are mean ± SD (N = 14 in each group). Baseline represents mouse home-cage activity without any interference. Harmaline or vehicle was injected ip at 0 min. AUEC values were calculated by trapezoidal rule. One-way ANOVA with Bonferroni’s post-hoc Multiple Comparison Test for AUEC values: F = 4.462, R2 = 0.2711, and p = 0.0092 for the four groups of AUEC0–45min values (B), and F = 3.134, R2 = 0.1942, and p = 0.0363 for AUEC45–180min values (C); *p < 0.05 as compared with the specified groups.
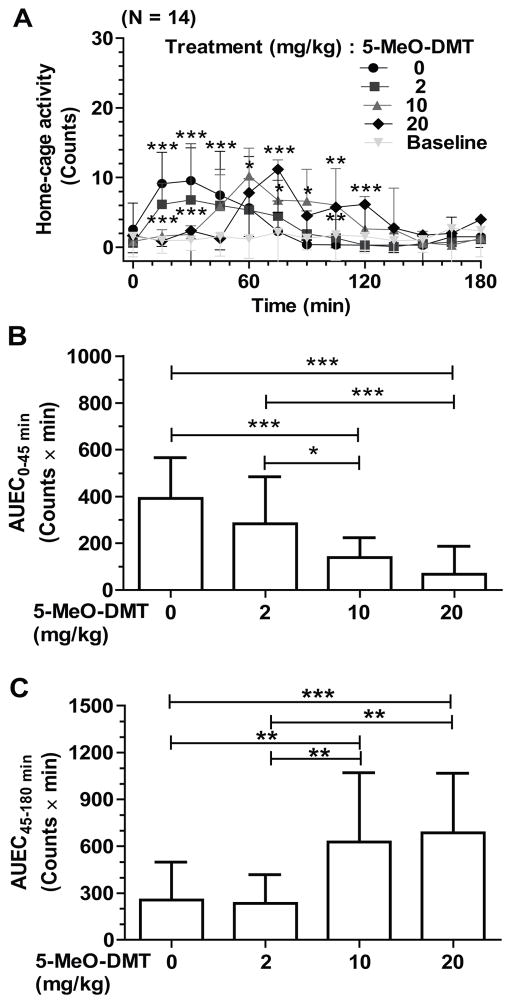
5-MeO-DMT causes biphasic effects on mouse home-cage activities, a hypoactivity at early phase and hyperactivity at late phase
Compared with vehicle control treatment, administration of 5-MeO-DMT consistently induced biphasic effects on mouse home-cage activities (Figure 2). 5-MeO-DMT suppressed mouse activities at early phase (0–45 min) in a dose dependent manner, which became more obvious at 10 and 20 mg/kg dose levels (Figure 2A and 2B). Furthermore, 10 and 20 mg/kg of 5-MeO-DMT caused a significant increase in home-cage activity at the late phase (45–180 min), while 2 mg/kg of 5-MeO-DMT had no significant influence (Figure 2A and 2C). The biphasic effects of 5-MeO-DMT on home-cage activity are nicely demonstrated by a significantly decreased AUEC0–45 min value (Figure 2B) and an increased AUEC45–180min value (Figure 2C).
Figure 2.
5-MeO-DMT caused biphasic effects on mouse home-cage activity (A) in a dose dependent manner. As indicated by the AUEC values (B and C), higher doses (10 and 20 mg/kg) of 5-MeO-DMT led to hypoactivity at early phase (0–45 min) and hyperactivity at late phase (45–180 min). Values are mean ± SD (N = 14 in each group). Baseline represents the activity without any interference. 5-MeO-DMT or vehicle was injected ip at 0 min. AUEC values were calculated by trapezoidal rule. Two-way ANOVA with Bonferroni’s post-hoc test for the activity data (A): p < 0.0001 for drug treatment, time and interaction; *p < 0.05, **p < 0.01, and ***p < 0.001 at indicated time points, when the 10 and 20 mg/kg dose was compared to vehicle control. One-way ANOVA with Bonferroni’s post-hoc Multiple Comparison Test for AUEC values: F = 15.82, R2 = 0.5490, and p < 0.0001 for the four groups of AUEC0–45min values (B), and F = 10.31, R2 = 0.4423, and p < 0.001 for AUEC45–180min values (C); *p < 0.05, **p < 0.01, and ***p < 0.001 as compared with the indicated groups.
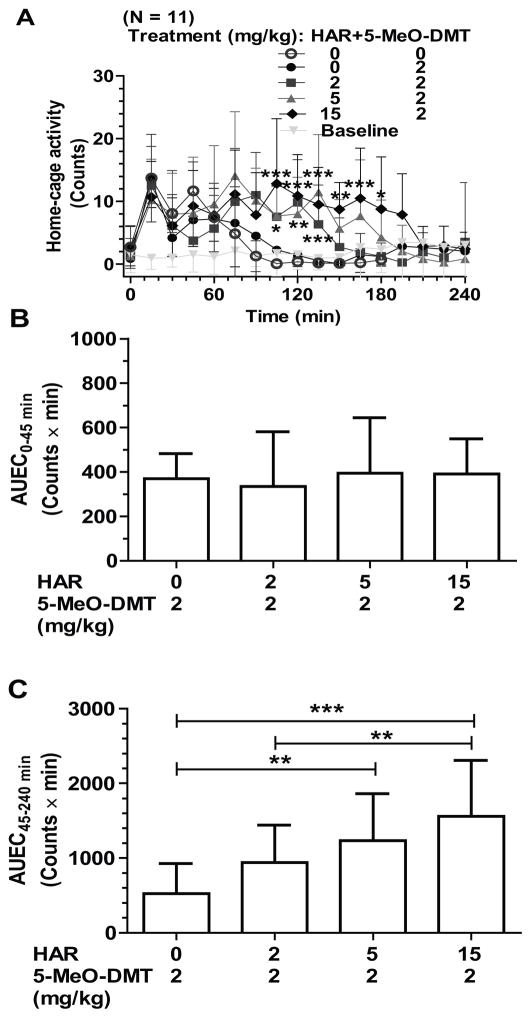
Co-administration of MAOI harmaline with a small dose of 5-MeO-DMT produces excessive hyperactivities in mice at late phase
We then chose the 2 mg/kg 5-MeO-DMT dose to examine whether MAOI harmaline alters 5-MeO-DMT pharmacological effects, given the above observation that this dose of 5-MeO-DMT had no or minimal effects on mouse home-cage activities (Figure 2) and our recent finding that the two drugs interact significantly at the pharmacokinetic level [21, 23]. Indeed the vehicle plus 2 mg/kg 5-MeO-DMT treatment did not cause any significant change in mouse activities, as compared with vehicle plus vehicle treatments (data not shown). However, co-administration of MAOI harmaline with 5-MeO-DMT led to a dose-dependent increase in late-phase hyperactivity in mice although there was no significant effect on early-phase home-cage activity (Figure 3). As indicated by the AUEC45–180min values, 2–15 mg/kg harmaline increased the home-cage activities by 2- to 3-fold, as compared with the 2 mg/kg of 5-MeO-DMT treatment alone (Figure 3C). The results demonstrate a prominent impact on animal home-cage activities when MAOI harmaline is co-administered with a small dose of 5-MeO-DMT. On the other hand, the small dose (2 mg/kg) of 5-MeO-DMT attenuated the early-phase hypoactivity and enhanced the late-phase hyperactivity that were induced by 15 mg/kg of harmaline alone (Figure 1), indicating the influence of 5-MeO-DMT on harmaline drug effects.
Figure 3.
Co-administration of harmaline (HAR) with a small dose of 5-MeO-DMT (2 mg/kg) induced excessive hyperactivity at late phase (45–180 min) in a dose dependent manner (A). This is clearly manifested by the calculated AUEC values (B and C). 5-MeO-DMT was administered ip 15 min after the ip treatment with harmaline (at 0 min). Values are mean ± SD (N = 11 in each group). Baseline represents the home-cage activity without any interference. AUEC values were calculated by trapezoidal rule. Two-way ANOVA with Bonferroni’s post-hoc test for the activity data (A): p < 0.0001 for drug treatment, time and interaction; *p < 0.05, **p < 0.01, and ***p < 0.001 for the specified time points, when 5 or 10 mg/kg harmaline plus 5-MeO-DMT treatment was compared to vehicle plus 5-MeO-DMT treatment. One-way ANOVA with Bonferroni’s post-hoc Multiple Comparison Test for the AUEC values: F = 0.5779, R2 = 0.05463, and p = 0.6340 for the four groups of AUEC0–45min values (B), and F = 22.58, R2 = 0.6724, and p < 0.0001 for AUEC45–180min values (C); **p < 0.01 and ***p < 0.001 as compared with the specified groups.
Harmaline-induced early-phase hypoactivity is mediated by the activation of 5-HT1A receptor
To define the serotonergic mechanisms behind the change of animal activities by harmaline, we examined the impact of pretreatment of selective 5-HT1A receptor antagonist WAY-100635 and 5-HT2A receptor antagonist MDL-100907, respectively (Figure 4). Our data showed that WAY-100635 completely attenuated the early-phase hypoactivity elicited by 15 mg/kg of harmaline, whereas it had no effect on the late-phase hyperactivity (Figure 4A–4C). On the other hand, MDL-100907 exhibited no significant effects on harmaline-induced changes in home-cage activity (Figure 4D–4F), although MDL-100907 itself inhibited the home-cage activities in mice at early stage. These results point to an important role of 5-HT1A receptor in harmaline-induced early-phase hypoactivity.
Figure 4.
WAY-100635 (WAY, a selective 5-HT1A receptor antagonist) fully attenuated 15 mg/kg of harmaline (HAR)-induced early-phase (0–45 min) hypoactivity, whereas it had no impact on late-phase (45–180 min) hyperactivity (A–C). In contrast, MDL-100907 (MDL, a selective 5-HT2A receptor antagonist) showed no effect on harmaline-induced changes in home-cage activity (D–F). WAY or MDL (1 mg/kg) or vehicle was injected sc at 0 min, and harmaline (15 mg/kg) or vehicle was injected ip at 15 min. Values are mean ± SD (N = 7 per group). AUEC values were calculated by trapezoidal rule. Two-way ANOVA with Bonferroni’s post-hoc test for the activity data (A & D): p < 0.0001 for drug treatment, time and interaction; *p < 0.05 for the specified time points, when WAY or MDL plus harmaline treatment was compared to vehicle plus harmaline treatment. One-way ANOVA with Bonferroni’s post-hoc Multiple Comparison Test for AUEC values: F = 2.113, R2 = 0.2605, and p = 0.1342 for the four groups of WAY-treated AUEC0–45min values (B), and F = 3.936, R2 = 0.3599, and p = 0.0225 for WAY-treated AUEC45–180min values (C); F = 4.085, R2 = 0.4051, and p = 0.0224 for the four groups of MDL-treated AUEC0–45min values (E), and F = 2.079, R2 = 0.2290, and p = 0.1336 for MDL-treated AUEC45–180min values (F); *p < 0.05 as compared with the indicated groups.
5-MeO-DMT-elicited early-phase hypoactivity is mainly mediated by the activation of 5-HT1A receptor, whereas late-phase hyperactivity is determined by the activation of 5-HT2A receptor
Likewise we employed the 5-HT1A and 5-HT2A receptor antagonists to evaluate serotonergic mechanisms underlying the alternation of mouse home-cage activities by a large dose (20 mg/kg) of 5-MeO-DMT (Figure 5). As indicated by the home-cage activity profiles (Figure 5A and 5D) and AUEC values (Figure 5B and 5F), pretreatment of WAY-100635 completely attenuated the early-phase hypoactivity induced by 20 mg/kg of 5-MeO-DMT, whereas MDL-100907 fully blocked the late-phase hyperactivity. In contrast, WAY-100635 had no impact on the late-phase hyperactivity and MDO-100907 showed only a minor effect on early-phase hypoactivity (Figure 5C and 5E). These results suggest that 5-MeO-DMT-induced biphasic effects on home-cage activity are mediated by 5-HT1A and 5-HT2A receptor at early and late phase, respectively.
Figure 5.
WAY-100635 (WAY) completely attenuated 5-MeO-DMT-induced early-phase (0–45 min) hypoactivity (A–C), while MDL-100907 (MDL) fully suppressed 5-MeO-DMT-elicited late-phase (45–180 min) hyperactivity (D–F). WAY or MDL (1 mg/kg) or drug vehicle was injected sc at 0 min, and 5-MeO-DMT (20 mg/kg) or vehicle was injected ip at 15 min. Values are mean ± SD (N = 7 per group). AUEC values were calculated according to trapezoidal rule. Two-way ANOVA with Bonferroni’s post-hoc test for the activity data (A & D): p≤ 0.001 for drug treatment, time and interaction; *p < 0.05, **p < 0.01, and ***p < 0.001 for the specified time points, when WAY or MDL plus 5-MeO-DMT treatment was compared to vehicle plus 5-MeO-DMT treatment. One-way ANOVA with Bonferroni’s post-hoc Multiple Comparison Test for the AUEC values: F = 4.490, R2 = 0.4280, and p = 0.0161 for the four groups of WAY-treated AUEC0–45min values (B), and F = 4.599, R2 = 0.4339, and P = 0.0147 for WAY-treated AUEC45–180min values (C); F = 15.37, R2 = 0.7192, and p < 0.0001 for the four groups of MDL-treated AUEC0–45min values (E), and F = 6.745, R2 = 0.5292, and p = 0.0030 for MDL-treated AUEC45–180min values (F); *p < 0.05, **p < 0.01, and ***p < 0.001 as compared with the specified groups.
Both 5-HT1A and 5-HT2A receptors contribute to the excessive, late-phase hyperactivity induced by co-administered harmaline and 5-MeO-DMT
The early-phase home-cage activities in mice treated with 2 mg/kg harmaline plus 2 mg/kg 5-MeO-DMT were not affected by pretreatment of WAY-100635 (Figure 6A–6B) but sharply reduced by MDL-100907 (Figure 6D–6E) as MDL-100907 itself suppressed mouse home-cage activities. Interestingly, both WAY-100635 and MDL-100907 was able to attenuate the excessive, late-phase (45–180 min) hyperactivity induced by 2 mg/kg of harmaline plus 2 mg/kg of 5-MeO-DMT treatment, as indicated by the home-cage activity profiles (Figures 6A and 6D) and AUEC values (Figure 6C and 6F). Together these results indicate that the late-phase hyperactivity induced by harmaline-5-MeO-DMT combination involves the actions of both 5-HT1A and 5-HT2A receptors, which is different from the hyperactivity induced by 5-MeO-DMT alone.
Figure 6.
Both WAY-100635 (WAY) (A–C) and MDL-100907 (MDL) (D–F) completely attenuated the excessive, late-phase (45–180 min) hyperactivity induced by co-administered harmaline and 5-MeO-DMT. In addition, MDL suppressed the early-phase (0–45 min) hyperactivity. WAY or MDL (1 mg/kg) or vehicle was injected sc at 0 min, and harmaline (2 mg/kg) and 5-MeO-DMT (2 mg/kg) or corresponding vehicles were injected ip at 0 and 15min, respectively. Values are mean ± SD (N = 7 per group). Baseline represents the home-cage activity without any intervention. AUEC values were calculated according to trapezoidal rule. Two-way ANOVA with Bonferroni’s post-hoc test for the activity data (A & D): p < 0.0001 for drug treatment, time and interaction; *p < 0.05, **p < 0.01, and ***p < 0.001 for the specified time points, when WAY or MDL plus harmaline-5-MeO-DMT treatment was compared to vehicle plus harmaline-5-MeO-DMT treatment. One-way ANOVA with Bonferroni’s post-hoc Multiple Comparison Test for the AUEC values: F = 0.2957, R2 = 0.04696, and p = 0.8280 for the four groups of WAY-treated AUEC0–45min values (B), and F = 15.10, R2 = 0.7156, and p < 0.0001 for WAY-treated AUEC45–180min values (C); F = 14.41, R2 = 0.6070, and p < 0.0001 for the four groups of MDL-treated AUEC0–45min values (E), and F = 9.276, R2 = 0.6072, and P = 0.0006 for MDL-treated AUEC45–180min values (F); **p < 0.01, and ***p < 0.001 as compared with the specified groups.
Discussion
Our recent studies have demonstrated the remarkable effects of MAOI harmaline on 5-MeO-DMT pharmacokinetics [21, 23] besides the elevation of cerebral 5-HT levels in animal models [25]. As a result, co-administration of MAOI harmaline with 5-MeO-DMT may lead to an increased risk of hyperthermia in mice [28]. The present study revealed biphasic effects of 5-MeO-DMT on mouse home-cage activities, in which the early-phase hypoactivity was mediated by 5-HT1A receptor and late-phase hyperactivity was determined by 5-HT2A receptor. In addition, co-administration of MAOI harmaline with 5-MeO-DMT was able to dose-dependently induce excessive hyperactivity at late phase (45–180 min) that could be completely attenuated by either 5-HT1A or 5-HT2A receptor antagonist.
The biphasic effects of a higher dose of harmaline (15 mg/kg) on home-cage or locomotor activity revealed in mice (Figures 1 and 4) using the automated Telemetry system [38–40] is consistent with the very recent findings on biphasic effects of harmaline on mobility (locomotor activity) obtained from rats using fully automated Force Plate Actimeters [41]. However, different from a partial attenuation of the late-phase hyperactivity by Lu AF21934, a positive allosteric modulator of metabotropic glutamate receptor 4 (mGlu4) [41], the present study showed a complete attenuation of the early-phase hypoactivity by 5-HT1A receptor antagonist WAY-100635 (Figure 4). While harmaline interacts with various receptors [41–43], the contribution of 5-HT1A receptor to mouse home-cage activity may be explained by the facts that harmaline acts as a 5-HT1A receptor agonist [28, 42, 44], and harmaline-mediated inhibition of MAO-A leads to a remarkable increase of 5-HT level in central nervous system where 5-HT itself exhibits a higher affinity to 5-HT1A receptor than other 5-HT receptors [25, 45, 46]. In addition, the critical role of 5-HT1A receptor in hypoactivity, different from the transient hyperactivity caused by handling and injection stresses (Figure 1), is in agreement with previous findings on the importance of 5-HT1A receptor in the control of locomotor activities in rats [47].
The present study also revealed a clear biphasic effect for 5-MeO-DMT in the modulation of mouse home-cage activity, an early-phase hypoactivity (0–45 min) followed by hyperactivity (45–180 min) (Figures 2 and 5). This observation is consistent with previous findings on the biphasic effects (hypoactivity at 0–30 min and hyperactivity at 30–60 min) in rodents using the Behavior Pattern Monitor (BPM) method [26, 33, 47], supporting the characteristic effects of hallucinogens in the modulation of locomotor and exploratory behavior. Further investigation for the serotonergic mechanisms behind home-cage activity revealed that not 5-HT2A receptor antagonist MDL-100907 but 5-HT1A receptor antagonist WAY-100635 blocked 5-MeO-DMT-induced initial phase hypoactivity (Figure 5), which agrees with the role of 5-HT1A receptor [33, 47]. However, our study also showed that, as time progressed to the late stage (e.g. 45–180 min post-treatment), 5-HT2A receptor became a determinant factor because the late-phase hyperactivity was fully suppressed by MDL-100907 whereas the impact of WAY-100635 was minimal (Figure 5). Therefore, the mechanistic actions of 5-MeO-DMT are similar as nonselective 5-HT agonist hallucinogens lysergic acid diethylamide (LSD) and 3,4,5-trimethoxyphenethylamine (mescaline), which both exert an initial attenuation of crossings that is abolishable by 5-HT1 antagonist propranolol, and a late-phase elevation in crossings that may be attenuated by 5-HT2 antagonist ritanserin [48, 49].
When MAOI harmaline (2, 5 and 15 mg/kg) was co-administered with the small dose of 5-MeO-DMT (2 mg/kg), mice showed excessive late-phase hyperactivities but no change of early-phase activities (Figures 3 and 6), although higher dose of harmaline (15 mg/kg) itself suppressed mouse home-cage activities (Figure 1). This observation may indicate an antagonism between 5-MeO-DMT and harmaline at these dose combinations, and thus it is different from the biphasic effects on rodent locomotor activities (initial decrease at 10–20 min, and an increase at 40–70 min) induced by distinct dose combinations of MAOI plus 5-MeO-DMT [26, 29]. In addition, the present study revealed that the late-phase hyperactivity induced by harmaline-5-MeO-DMT combination was completely attenuated not only by 5-HT1A receptor antagonist but also 5-HT2A receptor antagonist (Figure 6), which is different from previous findings on the attenuation of late-phase hyperlocomotion in rodents only by 5-HT2A receptor antagonist [26, 29]. This could be attributed to the differences in experimental settings, doses of drugs, animal models, and methods to monitor animal activities.
In vitro studies have revealed that cytochrome P450 2D6 (CYP2D6), one of the most important polymorphic Phase I drug-metabolizing enzymes, metabolizes harmaline and 5-MeO-DMT [50, 51]. In vivo studies with wild-type and CYP2D6-humanized (Tg-CYP2D6) mice have also demonstrated significant effects of CYP2D6 status on harmaline pharmacokinetics and the production of active metabolite from 5-MeO-DMT [21, 23, 28, 52], which might consequently influence drug response or pharmacodynamics. In our studies we found that, at current dose levels, harmaline plus 5-MeO-DMT treatments caused the same degrees of change in home-cage activities among wild-type and Tg-CYP2D6 mice (data not shown). This observation agrees with our recent findings using stimulus control assay [27] and it may be associated with relatively high variability in animal behavioral studies that could mask rather a limited impact of CYP2D6 status on the behavioral effects of IAA drugs.
In summary, the present study demonstrated that 5-MeO-DMT followed the trait of many hallucinogenic drugs to induce biphasic effects on mouse home-cage activities, among which the early-phase hypoactivity was mediated by 5-HT1A receptor and the late-phase hyperactivity was determined by 5-HT2A receptor. Furthermore, co-administration of MAOI harmaline with 5-MeO-DMT caused excessive activities at late phase, which could be fully attenuated by either 5-HT1A or 5-HT2A receptor antagonist. These findings may improve the understanding of hazards of combined use of IAA agents and provide insights into developing effective means to treat IAA intoxications.
Highlights.
High dose of MAOI harmaline reduces mouse home-cage activity at early phase that is mediated by 5-HT1A receptor, and increases mouse mobility at late-phase that is controlled by 5-HT2A receptor.
5-MeO-DMT has biphasic effects on mouse home-cage activities. The early-phase hypoactivity is mediated by 5-HT1A receptor, and late-phase hyperactivity is determined by 5-HT2A receptor.
Co-administration of MAOI harmaline with 5-MeO-DMT provokes excessive late-phase hyperactivity, which may be attenuated by either 5-HT1A or 5-HT2A receptor antagonist.
Acknowledgments
This project was supported by Award Number R01DA021172 from the National Institute on Drug Abuse, National Institutes of Health (NIH). We also thank Dr. Donald E. Mager for helpful discussions and language editing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Araujo AM, Carvalho F, de Bastos ML, Guedes de Pinho P, Carvalho M. The hallucinogenic world of tryptamines: an updated review. Arch Toxicol. 2015;89:1151–73. doi: 10.1007/s00204-015-1513-x. [DOI] [PubMed] [Google Scholar]
- 2.Yu AM. Indolealkylamines: biotransformations and potential drug-drug interactions. The AAPS journal. 2008;10:242–53. doi: 10.1208/s12248-008-9028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halberstadt AL. Recent advances in the neuropsychopharmacology of serotonergic hallucinogens. Behavioural brain research. 2015;277:99–120. doi: 10.1016/j.bbr.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruno R, Matthews AJ, Dunn M, Alati R, McIlwraith F, Hickey S, et al. Emerging psychoactive substance use among regular ecstasy users in Australia. Drug and alcohol dependence. 2012;124:19–25. doi: 10.1016/j.drugalcdep.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 5.Halberstadt AL, Geyer MA. Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology. 2011;61:364–81. doi: 10.1016/j.neuropharm.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKenna DJ. Clinical investigations of the therapeutic potential of ayahuasca: rationale and regulatory challenges. Pharmacology & therapeutics. 2004;102:111–29. doi: 10.1016/j.pharmthera.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Winstock AR, Kaar S, Borschmann R. Dimethyltryptamine (DMT): prevalence, user characteristics and abuse liability in a large global sample. Journal of psychopharmacology. 2014;28:49–54. doi: 10.1177/0269881113513852. [DOI] [PubMed] [Google Scholar]
- 8.McIlhenny EH, Riba J, Barbanoj MJ, Strassman R, Barker SA. Methodology for and the determination of the major constituents and metabolites of the Amazonian botanical medicine ayahuasca in human urine. Biomed Chromatogr. 2011;25:970–84. doi: 10.1002/bmc.1551. [DOI] [PubMed] [Google Scholar]
- 9.DEA DoJ. Schedules of controlled substances: placement of 5-methoxy-N,N-dimethyltryptamine into Schedule I of the Controlled Substances Act. Final rule. Fed Regist. 2010;75:79296–300. [PubMed] [Google Scholar]
- 10.DEA DoJ. Schedules of controlled substances: placement of alpha-methyltryptamine and 5-methoxy-N,N-diisopropyltryptamine into schedule I of the Controlled Substances Act. Final rule. Fed Regist. 2004;69:58950–3. [PubMed] [Google Scholar]
- 11.Brush DE, Bird SB, Boyer EW. Monoamine oxidase inhibitor poisoning resulting from Internet misinformation on illicit substances. J Toxicol Clin Toxicol. 2004;42:191–5. doi: 10.1081/clt-120030949. [DOI] [PubMed] [Google Scholar]
- 12.Long H, Nelson LS, Hoffman RS. Alpha-methyltryptamine revisited via easy Internet access. Vet Hum Toxicol. 2003;45:149. [PubMed] [Google Scholar]
- 13.Muller AA. New drugs of abuse update: Foxy Methoxy. J Emerg Nurs. 2004;30:507–8. doi: 10.1016/j.jen.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 14.Sklerov J, Levine B, Moore KA, King T, Fowler D. A fatal intoxication following the ingestion of 5-methoxy-N,N-dimethyltryptamine in an ayahuasca preparation. Journal of analytical toxicology. 2005;29:838–41. doi: 10.1093/jat/29.8.838. [DOI] [PubMed] [Google Scholar]
- 15.Smolinske SC, Rastogi R, Schenkel S. Foxy methoxy: a new drug of abuse. Journal of medical toxicology : official journal of the American College of Medical Toxicology. 2005;1:22–5. [PubMed] [Google Scholar]
- 16.Tanaka E, Kamata T, Katagi M, Tsuchihashi H, Honda K. A fatal poisoning with 5-methoxy-N,N-diisopropyltryptamine, Foxy. Forensic Sci Int. 2006;163:152–4. doi: 10.1016/j.forsciint.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 17.Hill SL, Thomas SH. Clinical toxicology of newer recreational drugs. Clin Toxicol (Phila) 2011;49:705–19. doi: 10.3109/15563650.2011.615318. [DOI] [PubMed] [Google Scholar]
- 18.Bjornstad K, Hulten P, Beck O, Helander A. Bioanalytical and clinical evaluation of 103 suspected cases of intoxications with psychoactive plant materials. Clin Toxicol (Phila) 2009;47:566–72. doi: 10.1080/15563650903037181. [DOI] [PubMed] [Google Scholar]
- 19.Fuse-Nagase Y, Nishikawa T. Prolonged delusional state triggered by repeated ingestion of aromatic liquid in a past 5-methoxy-N, N-diisopropyltryptamine abuser. Addiction science & clinical practice. 2013;8:9. doi: 10.1186/1940-0640-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ott J. Pharmepena-Psychonautics: Human intranasal, sublingual and oral pharmacology of 5-methoxy-N,N-dimethyl-tryptamine. J Psychoactive Drugs. 2001;33:403–7. doi: 10.1080/02791072.2001.10399925. [DOI] [PubMed] [Google Scholar]
- 21.Jiang XL, Shen HW, Mager DE, Yu AM. Pharmacokinetic interactions between monoamine oxidase A inhibitor harmaline and 5-methoxy-N,N-dimethyltryptamine, and the impact of CYP2D6 status. Drug Metab Dispos. 2013;41:975–86. doi: 10.1124/dmd.112.050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen HW, Jiang XL, Yu AM. Development of a LC-MS/MS method to analyze 5-methoxy-N,N-dimethyltryptamine and bufotenine, and application to pharmacokinetic study. Bioanalysis. 2009;1:87–95. doi: 10.4155/bio.09.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen HW, Wu C, Jiang XL, Yu AM. Effects of monoamine oxidase inhibitor and cytochrome P450 2D6 status on 5-methoxy-N,N-dimethyltryptamine metabolism and pharmacokinetics. Biochemical pharmacology. 2010;80:122–8. doi: 10.1016/j.bcp.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen HW, Jiang XL, Winter JC, Yu AM. Psychedelic 5-methoxy-N,N-dimethyltryptamine: metabolism, pharmacokinetics, drug interactions, and pharmacological actions. Curr Drug Metab. 2010;11:659–66. doi: 10.2174/138920010794233495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng J, Zhen Y, Miksys S, Beyoglu D, Krausz KW, Tyndale RF, et al. Potential role of CYP2D6 in the central nervous system. Xenobiotica; the fate of foreign compounds in biological systems. 2013;43:973–84. doi: 10.3109/00498254.2013.791410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halberstadt AL, Buell MR, Masten VL, Risbrough VB, Geyer MA. Modification of the effects of 5-methoxy-N,N-dimethyltryptamine on exploratory behavior in rats by monoamine oxidase inhibitors. Psychopharmacology. 2008;201:55–66. doi: 10.1007/s00213-008-1247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winter JC, Amorosi DJ, Rice KC, Cheng K, Yu AM. Stimulus control by 5-methoxy-N,N-dimethyltryptamine in wild-type and CYP2D6-humanized mice. Pharmacol Biochem Behav. 2011;99:311–5. doi: 10.1016/j.pbb.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang XL, Shen HW, Yu AM. Potentiation of 5-methoxy-N,N-dimethyltryptamine-induced hyperthermia by harmaline and the involvement of activation of 5-HT1A and 5-HT2A receptors. Neuropharmacology. 2015;89:342–51. doi: 10.1016/j.neuropharm.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halberstadt AL, Nichols DE, Geyer MA. Behavioral effects of alpha,alpha,beta,beta-tetradeutero-5-MeO-DMT in rats: comparison with 5-MeO-DMT administered in combination with a monoamine oxidase inhibitor. Psychopharmacology. 2012;221:709–18. doi: 10.1007/s00213-011-2616-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winter JC, Filipink RA, Timineri D, Helsley SE, Rabin RA. The paradox of 5-methoxy-N,N-dimethyltryptamine: an indoleamine hallucinogen that induces stimulus control via 5-HT1A receptors. Pharmacol Biochem Behav. 2000;65:75–82. doi: 10.1016/s0091-3057(99)00178-1. [DOI] [PubMed] [Google Scholar]
- 31.Riga MS, Soria G, Tudela R, Artigas F, Celada P. The natural hallucinogen 5-MeO-DMT, component of Ayahuasca, disrupts cortical function in rats: reversal by antipsychotic drugs. The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2014;17:1269–82. doi: 10.1017/S1461145714000261. [DOI] [PubMed] [Google Scholar]
- 32.van den Buuse M, Ruimschotel E, Martin S, Risbrough VB, Halberstadt AL. Enhanced effects of amphetamine but reduced effects of the hallucinogen, 5-MeO-DMT, on locomotor activity in 5-HT(1A) receptor knockout mice: implications for schizophrenia. Neuropharmacology. 2011;61:209–16. doi: 10.1016/j.neuropharm.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halberstadt AL, Koedood L, Powell SB, Geyer MA. Differential contributions of serotonin receptors to the behavioral effects of indoleamine hallucinogens in mice. Journal of psychopharmacology. 2011;25:1548–61. doi: 10.1177/0269881110388326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roth BL, Choudhary MS, Khan N, Uluer AZ. High-affinity agonist binding is not sufficient for agonist efficacy at 5-hydroxytryptamine2A receptors: evidence in favor of a modified ternary complex model. J Pharmacol Exp Ther. 1997;280:576–83. [PubMed] [Google Scholar]
- 35.Haberzettl R, Bert B, Fink H, Fox MA. Animal models of the serotonin syndrome: A systematic review. Behavioural brain research. 2013;256C:328–45. doi: 10.1016/j.bbr.2013.08.045. [DOI] [PubMed] [Google Scholar]
- 36.Boyer EW, Shannon M. The serotonin syndrome. The New England journal of medicine. 2005;352:1112–20. doi: 10.1056/NEJMra041867. [DOI] [PubMed] [Google Scholar]
- 37.Kant S, Liebelt E. Recognizing serotonin toxicity in the pediatric emergency department. Pediatr Emerg Care. 2012;28:817–21. doi: 10.1097/PEC.0b013e31826289d9. quiz 22–4. [DOI] [PubMed] [Google Scholar]
- 38.Kassai F, Kedves R, Gyertyan I, Tuka B, Fulop F, Toldi J, et al. Effect of a kynurenic acid analog on home-cage activity and body temperature in rats. Pharmacol Rep. 2015;67:1188–92. doi: 10.1016/j.pharep.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 39.Gelzer AR, Ball HA. Validation of a telemetry system for measurement of blood pressure, electrocardiogram and locomotor activity in beagle dogs. Clin Exp Hypertens. 1997;19:1135–60. doi: 10.3109/10641969709083209. [DOI] [PubMed] [Google Scholar]
- 40.Vivanco P, Studholme KM, Morin LP. Drugs that prevent mouse sleep also block light-induced locomotor suppression, circadian rhythm phase shifts and the drop in core temperature. Neuroscience. 2013;254:98–109. doi: 10.1016/j.neuroscience.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ossowska K, Wardas J, Berghauzen-Maciejewska K, Glowacka U, Kuter K, Pilc A, et al. Lu AF21934, a positive allosteric modulator of mGlu4 receptors, reduces the harmaline-induced hyperactivity but not tremor in rats. Neuropharmacology. 2014;83:28–35. doi: 10.1016/j.neuropharm.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 42.Nasehi M, Jamshidi-Mehr M, Khakpai F, Zarrindast MR. Possible involvement of CA1 5-HT1B/1D and 5-HT2A/2B/2C receptors in harmaline-induced amnesia. Pharmacol Biochem Behav. 2014;125:70–7. doi: 10.1016/j.pbb.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 43.Ossowska K, Glowacka U, Kosmowska B, Wardas J. Apomorphine enhances harmaline-induced tremor in rats. Pharmacol Rep. 2015;67:435–41. doi: 10.1016/j.pharep.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 44.Abdel-Fattah AF, Matsumoto K, Gammaz HA, Watanabe H. Hypothermic effect of harmala alkaloid in rats: involvement of serotonergic mechanism. Pharmacol Biochem Behav. 1995;52:421–6. doi: 10.1016/0091-3057(95)00131-f. [DOI] [PubMed] [Google Scholar]
- 45.Spencer DG, Jr, Glaser T, Traber J. Serotonin receptor subtype mediation of the interoceptive discriminative stimuli induced by 5-methoxy-N,N-dimethyltryptamine. Psychopharmacology. 1987;93:158–66. doi: 10.1007/BF00179927. [DOI] [PubMed] [Google Scholar]
- 46.Abdel-Fattah AF, Matsumoto K, Murakami Y, Adel-Khalek Gammaz H, Mohamed MF, Watanabe H. Central serotonin level-dependent changes in body temperature following administration of tryptophan to pargyline- and harmaline-pretreated rats. Gen Pharmacol. 1997;28:405–9. doi: 10.1016/s0306-3623(96)00300-x. [DOI] [PubMed] [Google Scholar]
- 47.Krebs-Thomson K, Ruiz EM, Masten V, Buell M, Geyer MA. The roles of 5-HT1A and 5-HT2 receptors in the effects of 5-MeO-DMT on locomotor activity and prepulse inhibition in rats. Psychopharmacology. 2006;189:319–29. doi: 10.1007/s00213-006-0566-1. [DOI] [PubMed] [Google Scholar]
- 48.Mittman SM, Geyer MA. Dissociation of multiple effects of acute LSD on exploratory behavior in rats by ritanserin and propranolol. Psychopharmacology. 1991;105:69–76. doi: 10.1007/BF02316866. [DOI] [PubMed] [Google Scholar]
- 49.Palenicek T, Balikova M, Bubenikova-Valesova V, Horacek J. Mescaline effects on rat behavior and its time profile in serum and brain tissue after a single subcutaneous dose. Psychopharmacology. 2008;196:51–62. doi: 10.1007/s00213-007-0926-5. [DOI] [PubMed] [Google Scholar]
- 50.Yu AM, Idle JR, Herraiz T, Kupfer A, Gonzalez FJ. Screening for endogenous substrates reveals that CYP2D6 is a 5-methoxyindolethylamine O-demethylase. Pharmacogenetics. 2003;13:307–19. doi: 10.1097/01.fpc.0000054094.48725.b7. [DOI] [PubMed] [Google Scholar]
- 51.Yu AM, Idle JR, Krausz KW, Kupfer A, Gonzalez FJ. Contribution of individual cytochrome P450 isozymes to the O-demethylation of the psychotropic beta-carboline alkaloids harmaline and harmine. J Pharmacol Exp Ther. 2003;305:315–22. doi: 10.1124/jpet.102.047050. [DOI] [PubMed] [Google Scholar]
- 52.Wu C, Jiang XL, Shen HW, Yu AM. Effects of CYP2D6 status on harmaline metabolism, pharmacokinetics and pharmacodynamics, and a pharmacogenetics-based pharmacokinetic model. Biochemical pharmacology. 2009;78:617–24. doi: 10.1016/j.bcp.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]