Abstract
The growing field of evo-devo is increasingly demonstrating the complexity of steps involved in genetic, intracellular regulatory, and extracellular environmental control of the development of phenotypes. A key result of such work is an account for the remarkable plasticity of organismal form in many species based on relatively minor changes in regulation of highly conserved genes and genetic processes. Accounting for behavioral plasticity is of similar potential interest but has received far less attention. Of particular interest is plasticity in communication systems, where human language represents an ultimate target for research. The present paper considers plasticity of language capabilities in a comparative framework, focusing attention on examples of a remarkable fact: Whereas there exist design features of mature human language that have never been observed to occur in nonhumans in the wild, many of these features can be developed to notable extents when nonhumans are enculturated through human training (especially with intensive social interaction). These examples of enculturated developmental plasticity across extremely diverse taxa suggest, consistent with the evo-devo theme of highly conserved processes in evolution, that human language is founded in part on cognitive capabilities that are indeed ancient and that even modern humans show self-organized emergence of many language capabilities in the context of rich enculturation, built on the special social/ecological history of the hominin line. Human culture can thus be seen as a regulatory system encouraging language development in the context of a cognitive background with many highly conserved features.
Evo-devo as a framework for new perspectives on language evolution and development
Evolutionary-developmental biology or evo-devo is not a new field, but rather the elaboration of a long-term trend, increasingly emphasizing that natural selection tends to target developmental processes and that evolutionary change tends to proceed by adjusting intracellular regulatory mechanisms (Carroll, 2005). Another feature of evo-devo is emphasis on “conserved” systems that produce widely different organismal forms through minor regulatory changes (Kirschner & Gerhart, 2005). Thus point mutations, duplications or deletions may change the timing or scope of expression of conserved “toolkit genes”, yielding vastly different phenotypes, often different species. Similarly, organismal form can be affected dramatically by environment, a fact known for over 100 years—e.g., Bonellia viridis (green spoonworm) larvae are initially undifferentiated sexually, floating in ocean water. Falling on or near a female spoonworm, they develop into 1–3 mm-long males. Falling on the ocean floor, they develop into ~50-times larger females (Hertwig, 1894). Thus environmental conditions can yield dramatically different organismal forms from one genotype (Newman, 1989).
The evo-devo agenda predicts that environmental conditions can radically modify behavioral phenotypes as well, in relatively short time frames, sometimes with concomitant changes in form. Darwin’s finches provide a good example: both beak type and feeding behavior were modified from a highly conserved background through regulatory changes naturally selected under differing environmental pressures on different islands.
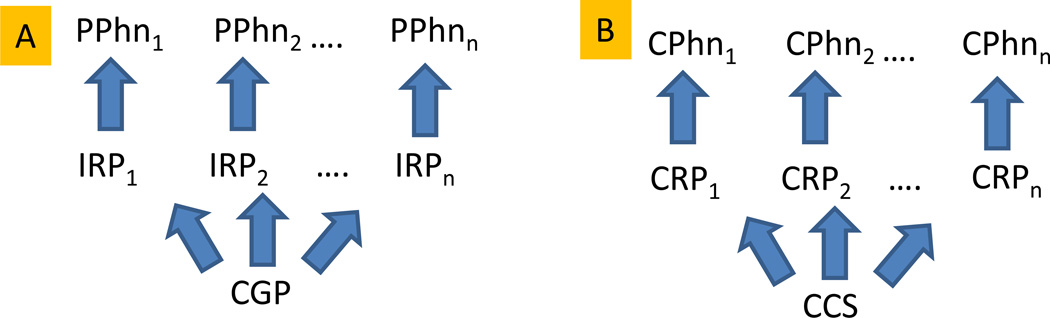
Language also presents an important case of developmental plasticity. Whereas specific human languages differ, humans around the world can be thought to share a phenotype at the level of “infrastructural capabilities” (Oller, 2000) or “design features” (Hockett & Altmann, 1968) of language. In the context of the evo-devo agenda we ask: 1) What design features are essentially unique to humans? And 2) what role might environment, especially human enculturation, play in regulating language-approximating phenotypes across species? We review evidence to illustrate that the evo-devo theme (Figure 1A) can be applied by analogy to language-approximating phenotypes in many human-trained animals (Figure 1B). In both cases conserved processes regulated by environmental conditions produce significantly modified phenotypes.
Figure 1. Conserved evolutionary processes and developmental plasticity.
A. Organismal form (PPhn=Physical Phenotypes) can vary dramatically based on minor adjustments in regulatory processes (IRP=Intracellular Regulatory Processes) that determine the expression of protein-coding genes, which are often themselves highly conserved (CGP=Conserved Genetic Processes) as toolkits shared across widely different taxa. Evolution can thus produce vast differences in species in relatively few generations, maintaining a core of conserved genetic processes across all of them.
B. Similarly, we argue, communicative capabilities (CPhn=Communicative Phenotypes) in a variety of non-humans can vary substantially within generation based on exposure to differing human training (CRP=Cultural Regulatory Processes), which appears to exploit cognitive systems that are highly conserved across many species (CCS=Conserved Cognitive Systems) to produce a variety of potential “language-approximating” phenotypes. Different modern human languages can also be thought of as different phenotypes, determined by cultural regulation. However, mature languages share a wide variety of “design features” around the world, although these features are not generally shared with nonhumans in the wild. Fig. 2B portrays the phenotypic plasticity seen across many species in response to human enculturation.
Of course, many have argued that human language also depends upon enculturation (e.g., Tomasello, 1996) and that language evolution has involved ratcheting interactions of growth in culture and in inherent capabilities necessary to learn language (Christiansen & Kirby, 2003; Elman et al., 1996). We argue that evidence from nonhumans learning to recognize and use language-like structures offers a unique perspective on conserved cognitive systems shared across many taxa, systems that form a basis for at least minimal command of many language-design features if human training and interaction are brought to bear. The arguments are that 1) many nonhumans share critical foundations with humans in language-relevant cognitive systems, and 2) even for humans, enculturation may play a critical role in command of infrastructural features of language. The design features discussed represent a small set that can be addressed with current empirical evidence (for rationale on selection see Supplementary Material 1, SM1). The species to be discussed have been selected primarily on the basis of available data (see SM2) compiled predominantly from peer-reviewed studies (see SM3).
Design features of language and enculturation of non-human learners
Symbolism/semanticity
Whereas animals in the wild use various signals to transmit illocutionary functions (Austin, 1962; Griebel & Oller, 2008), no indisputable report exists of any case of fully referential symbolism or semanticity in natural animal communication (see SM1). In language, words can refer to anything conceivable (entity, event, being, quality, state…), but animal signals appear predominantly to express states of senders (fearful, angry, solicitous…) and to induce states and action tendencies in receivers rather than to transmit referential/semantic content about the external world. The possibility that animals ever transmit external-world information in their natural signals is in dispute (Stegmann, 2013) in part because animal receivers seem capable of inferring information not actually encoded in signals from correlations between signal occurrence and the external world (Owren & Rendall, 2001) (and see SM1).
In dramatic contrast, nonhumans from a wide variety of taxa have learned to understand and often produce symbols with semantic content if they experience intensive human training. As examples, a border collie is reported to have learned to retrieve over 1000 objects on voice command (Pilley & Reid, 2011), chimpanzees and bonobos have been trained to comprehend and produce scores to hundreds of signs or lexigrams (Gardner, Gardner, & Van Cantfort, 1989; Premack, 1971; Savage-Rumbaugh et al., 1993), and Grey parrots have learned to talk, using scores of spoken words and phrases with referential content (Pepperberg, 2010).
Animal referential word learning seems to be dependent on intensive, long-term training, and seems to be facilitated by direct human interaction and/or observation of human interaction. The claim that a border collie could learn words by fast mapping (Kaminski, Call, & Fischer, 2004) has been called into question empirically (Griebel & Oller, 2012), but it is generally agreed now that semanticity, with production/comprehension of hundreds of labels, compared to many thousands for humans, can be instilled in many animals with persistent human enculturation.
Displacement
Humans use referential terms in such a way that they are utterly free of the here-and-now, referring to things in the past, future, and in any location, real or imaginary, a capability called displacement (see SM1). Such reference has never been observed in nonhumans in the wild although evidence of displacement in communication has been suggested, for example, in honeybees (von Frisch, 1967; Riley, Greggers, Smith, Reynolds, & R. Menzel, 2005) and chimpanzees (E. Menzel, 1988). These indications, however, fall far short of referential displacement as in human words, if for no other reason, because there is no “lexicon” of semantic items in these animal communications; the system may operate according to simpler routines where receivers derive information from correlations between signaler actions and situations, even without lexically-coded information in signals themselves.
But again, human training can enable such abilities. For example, C. Menzel and colleagues (1999) showed that, when an experimenter hid one of more than 30 objects in a nearby woods, a language-trained chimpanzee could, from her enclosure hours later, touch the appropriate lexigrams to indicate, with extremely high reliability, the identity and location of the object to an uninformed keeper. This game proved the chimpanzee labeled items using the design feature of displacement, as the objects labeled were always displaced in both space and time.
Functional flexibility
Any referential word or sentence in a human language can be used to serve a wide variety of illocutionary functions (Austin, 1962). Thus, we can use the word “pig” to serve an aggressive function (“You pig!”), as a question (“Is this a pig?”), as a statement (“This is a pig.”), as a warning (“Watch out, a pig!”), as an endearment (“My sweet little pig!”), as an example (which we are doing here), etc. In all these cases “pig” refers to a type of mammal with certain characteristics (the semantic content), but the illocutionary force can vary dramatically, with emotional valences ranging from positive, to neutral, to negative (see SM1).
Active investigation exists about the extent to which animal communication in the wild may show flexibility of the relation between signal and function (Crockford & Boesch, 2005; Laporte & Zuberbühler, 2011), a pursuit that is helping to moderate the claim of classical ethology that animal signals have one-to-one mappings between signal and function (Lorenz, 1951). A substantial difference, however, between the extent of functional flexibility in animal and human communication is not in dispute—for example, no reports demonstrate that any animal signal in the wild is used with a full range of illocutionary valences from positive (e.g., exultation) to negative (e.g., aggressive). But all normal adult humans can use words these ways, and even three-month-old human infants use several prespeech vocalizations with illocutionary forces ranging from positive, to neutral, to negative (Oller et al., 2013).
The picture can change, however, after intensive human training of animals to use referential labels. The type of training appears important, because one chimpanzee trained in a strict reward/reinforcement paradigm used his acquired vocabulary almost exclusively as requests for food, hugs, or tickling, a single illocutionary function (Terrace, 1979). Other experimental animals raised in human-like social conditions with only intermittent reinforcement have shown much more diverse illocutions. In addition to requests, they could also query What, Who, and Where: e.g., the Grey parrot Alex asked: “What color?” to his reflection. He had been trained to respond to that question, but learned to produce it via observation only (Pepperberg, 1999). Human-trained animals could inform their trainers about novel things, e.g., the chimpanzee Washoe would climb a tree from which she could see who was arriving by car and would sign names of arriving people to trainers and/or other chimps on the ground, thus not just naming, but informing (an additional illocution). Some animals also commented on objects or events (e.g., Washoe, on hearing barking in the neighborhood, signed “dog”, Gardner et al. 1989)
Washoe was reported to use “swear words” appended to her utterances to express the illocution of insult, signing “dirty” before the name of a person with whom she was displeased, although she also could use the term merely as a description. Another insult, or perhaps a dare, was creative use of the signs “you bird”, meaning “you’re chicken”. Washoe interfered in a fight between her adopted son and another juvenile male, whom she slapped, and then she produced the sign “go”, which in other cases she used as a description, but here as a command. The chimpanzee Tatu signed “black” as a description but, for reasons unknown, also to indicate she thought something to be beautiful or “cool” (Gardner et al. 1989). Alex similarly used “wanna go (back, chair, shoulder, etc.)” to request movement or that a trainer leave (“go away”), and also as a descriptive comment (“I’m gonna go away”) as he broke contact with a trainer (Pepperberg, 1999).
Such reports provide evidence for notable diversification of functional usage by nonhumans of human-trained labels. The reports at least demonstrate multiple illocutionary uses of the same human-trained label, and at least (in Washoe’s “dirty”) both negative (insult) and neutral (descriptive) types and (in Tatu’s “black”), both positive (adulation) and neutral (descriptive)—a clear step toward functional flexibility of the sort found in language. These cases provide much more convincing demonstrations of functional flexibility than in cases reported for in-the-wild communication by nonhumans.
Serial ordering/recombination
Human language involves systematic recombination of words and morphemes, forming indefinitely long sentences of semantic material (see SM1). Only weak evidence exists for even minimal “syntax” for in-the-wild animal communication, although some have argued that combinations of calls or of calls and gestures such as drumming have effects on receivers that suggest modifications of function by the combinations (e.g., Clay & Zuberbühler, 2011).
Some human-trained animals in contrast—including parrots, dogs, primates, dolphins and pinnipeds—comprehend differences in meaning for at least short human-generated sequences of words or lexical-like symbols presented in different orders (e.g., Gisiner & Schusterman, 1992; Herman, Richards, & Wolz, 1984; Pepperberg, 1999; Savage-Rumbaugh et al., 1993; Pilley, 2013).
Production of serially-ordered lexemes in human-trained animals has been questioned (Terrace, Petitto, Sanders, & Bever, 1979), even though sequences, mostly of 2–4 lexemes, have been reported in at least human-trained parrots and great apes (Gardner et al., 1989; Pepperberg, 2004). Still it is not clear that ordering is itself a systematic indicator of meaning in such cases. In reported cases where lexemes were not used in consistent sequences to indicate meaning, trainers could often interpret by context (e.g. “give orange me” or “me give orange”) and/or accompanying gestures, e.g., a begging hand. Once again human-trained animals, although far from producing language per se, appear to have produced much more language-like behavior after intensive human communicative interaction and training. Alex the parrot even engaged in phonemic or phonetic recombination (Pepperberg, 1999, 2010), creating novel vocalizations (e.g., “banerry” for apple”) out of parts of existent labels (banana, cherry) or sounds and labels (e.g., “s-none” as a precursor to “seven”) (see SM4).
Cultural transmission
Language is inherently cultural, with semantic elements transmitted across generations. Data on birds suggest cultural transmission of signals, though not semantic elements (SM1). A few reports exist of cultural transmission of behaviors from one generation to another in great apes (Boesch, 1991; Hannah & McGrew, 1987), but no convincing examples of learned communicative signals thus transmitted. The case of sign-language trained chimpanzees offers, however, one intriguing view of possible cultural transmission of learned lexemes in primates. When Washoe was living with a group of sign-trained chimpanzees, who often signed to each other, she was given an adoptive son, Loulis. Human trainers were not allowed to sign in Loulis’ presence. After seven years in the group, Loulis acquired ~70 signs (Fouts et al. 1989). Researchers also observed that Washoe and the other chimpanzees had acquired a few new signs from each other over the same period. A few documented observations also existed of Washoe actively trying to teach Loulis a new sign.
An observational opportunity such as the one Fouts et al. developed is unique. Few language-trained apes have lived together in a socially nurturant environment, and Loulis represents the only case we know of where another ape lacking prior human-training has been allowed to grow up in such an environment. The result suggests that cultural transmission of language-approximating lexemes to a second generation individual is possible for chimpanzees trained by humans (SM3 for issues regarding peer-review of this work). We wonder if there will ever be another opportunity to confirm this result.
Why not in the wild?
At this point, we must ask how it is possible for animals in captivity to learn lexemes while not developing such elements in the wild. Clearly, considerable cognitive foundations of minimal lexical learning are in place across many taxa, and given the variety of species capable of learning human labels, we might suspect that with intensive training, similar capacities might be demonstrated in many other mammals and birds, perhaps even in reptiles or fish. Clearly, neither evolutionary distance from humans nor absolute brain size is a major factor here (relative brain size or brain organization could of course be more important predictors) given that a parrot is one of the champions of all nonhuman learners of language-approximating communication skills, even demonstrating capacities such as phonological or phonetic awareness (Pepperberg, 2010, and SM4). Some researchers suggest that label learning is based, at least in part at the very earliest of stages, on very basic associative-learning mechanisms that are shared across species ranging at least from mammals to birds (see SM5).
The simple answer to “why not in the wild” seems to be that no animal society appears to have developed to the point of providing the cultural support necessary to initiate the chain of events that would bring such features as strong functional flexibility or semanticity into the communicative repertoire. Much speculation exists that the hominin line profited in communicative evolution from increases in social group size and complexity (Dunbar, 1996) and from intensified parental attention to their altricial infants and their signaling behavior (Locke, 2006; Oller & Griebel, 2006, and SM6).
One conclusion from animal language studies is that method is crucial. Best results on all fronts, including spontaneity of communication, number of labels, and usage complexity have been achieved with total immersion into human culture with nonhumans raised as much as possible like human children, with maximal social interaction. This approach has worked with parrots, dogs, and great apes. For example, the Gardners did not use operant conditioning; signs were learned during daily interactions in the home. Trainers often molded chimpanzees’ hands for correct configuration, but reinforcement was merely social attention and the acquisition of a relevant object or pursuance of the relevant action. Trainers prompted chimpanzees in ways teachers commonly prompt human children, and like children, chimpanzees were often denied their requests, and were more likely to get what they wanted if they made themselves understood. Parrots have learned best when trained with interactive social modeling demonstrating the connection between vocal labels and related objects or attributes; they have failed when exposed merely to audio or videotapes of human speech (Pepperberg, 1999). Interactive teaching also evoked spontaneous signing by chimpanzees and vocalizations by parrots, just as seems to occur with human children (and see SM6 on birdsong learning).
The results suggest many animals categorize things and events in ways not unlike those of humans and these shared conceptions allow many nonhumans to associate arbitrary labels with things and events if they experience appropriate interactions with humans. Perhaps given our evolutionary past, this should not be surprising. But it does take us aback to recognize that in a single generation, an enculturated cross-fostered ape learned to communicate with language-approximating behavior at a level a lot like that of a 2-year-old human. We have no test yet of how far this kind of language-approximating behavior could go across multiple generations supported by human training and conspecific interaction, but the Loulis result intriguingly suggests there may be considerable room for growth.
The findings reviewed here also support the evo-devo compatible idea that human language may be substantially self-organized under the influence of human culture (see Christiansen & Dale, 2004). Ancient hominins may have experienced multiple rounds of communicative growth, including at each round, expansion in language-learning capabilities through natural selection, as well as advancement in hominin communicative culture that would have placed further selective pressure on learning capabilities (e.g., via Baldwin Effects). If the reasoning is correct, hominins would have progressively distanced themselves from their primate background in communication, because at each step, culture would have provided a mechanism of selection for more powerful communication both within and across generations.
Supplementary Material
Acknowledgments
This research was supported in part by NIDCD R01 DC011027, the Plough Foundation and The Alex Foundation.
References
- Austin JL. How to do things with words. London: Oxford University. Press; 1962. [Google Scholar]
- Boesch C. Teaching among wild chimpanzees. Animal Behavior. 1991;41:530–532. [Google Scholar]
- Carroll SB. Endless forms most beautiful: The new science of evo devo and the making of the animal kingdom. New York: W. W. Norton; 2005. [Google Scholar]
- Christiansen MH, Dale R. The role of learning and development in language evolution: A connectionist perspective. In: Oller DK, Griebel U, editors. The evolution of communication systems: A comparative approach. MIT Press; 2004. pp. 91–110-yy. [Google Scholar]
- Christiansen MH, Kirby S. Language evolution: Consensus and controversies. Trends in Cognitive Sciences. 2003;7(7):300–307. doi: 10.1016/s1364-6613(03)00136-0. [DOI] [PubMed] [Google Scholar]
- Clay Z, Zuberbühler K. Bonobos extract meaning from call sequences. PLoS One. 2011;6(4):1–10. doi: 10.1371/journal.pone.0018786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockford C, Boesch C. Call combinations in wild chimpanzees. Behaviour. 2005;142(4):397–421. doi: [Google Scholar]
- Dunbar RIM. Gossiping, grooming and the evolution of language. Cambridge, MA: Harvard University Press; 1996. [Google Scholar]
- Elman JL, Bates E, Johnson MH, Karmiloff-Smith A, Parisi D, Plunkett K. Rethinking innateness: A connectionist perspective on development. Cambridge, MA: MIT Press; 1996. [Google Scholar]
- Fouts R, Fouts DH, Van Cantfort TE. The infant Loulis learns signs from cross-fostered chimpanzees. In: Gardner RA, Gardner BT, Van Cantfort TE, editors. Teaching sign language to chimpanzees. Albany, New York: State University of New York Press; 1989. pp. 280–292. [Google Scholar]
- von Frisch K. The dance language and orientation of bees. Cambridge, MA: Belknap Press of Harvard University Press; 1967. [Google Scholar]
- Gardner RA, Gardner BT, Van Cantfort TE, editors. Teaching sign language to chimpanzees. Suny Press; 1989. [Google Scholar]
- Gisiner R, Schusterman RJ. Sequence, syntax, and semantics: Responses of a language-trained sea lion (Zalophus californianus) to novel sign combinations. Journal of Comparative Psychology. 1992;106:78–91. [Google Scholar]
- Griebel U, Oller DK. Evolutionary forces favoring contextual flexibiliity. In: Oller DK, Griebel U, editors. Evolution of communicative flexibility: Complexity, creativity and adaptability in human and animal communication. Cambridge, MA: MIT Press; 2008. pp. 9–40. [Google Scholar]
- Griebel U, Oller DK. Vocabulary learning in a Yorkshire terrier: Slow mapping of spoken words. PLoS ONE. 2012;7(2) doi: 10.1371/journal.pone.0030182. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah AC, McGrew WC. Chimpanzees using stones to crack open oil palm nuts in Liberia. Primates. 1987;28:31–46. [Google Scholar]
- Herman LM, Richards DG, Wolz JP. Comprehension of sentences by bottlenosed dolphins. Cognition. 1984;16:129–219. doi: 10.1016/0010-0277(84)90003-9. [DOI] [PubMed] [Google Scholar]
- Hertwig O. Grundzüge einer Entwicklungstheorie der Organismen. Jena: Gustav Fischer; 1894. Zeit- und Streitfragen der Biologie. Band 1. Präformation oder Epigenese? [Google Scholar]
- Hockett CF, Altmann SA. A note on design features. In: Sebeok TA, editor. Animal communication: techniques of study and results of research. Bloomington: Indiana University Press; 1968. pp. 61–72. [Google Scholar]
- Kaminski J, Call J, Fischer J. Word learning in a domestic dog: Evidence for "fast mapping". Science. 2004;304(5677):1682–1683. doi: 10.1126/science.1097859. [DOI] [PubMed] [Google Scholar]
- Kirschner MW, Gerhart JC. The plausibillity of life: Resolving Darwin's dilemma. New Haven, CT: Yale University Press; 2005. [Google Scholar]
- Laporte MNC, Zuberbühler K. The development of a greeting signal in wild chimpanzees. Developmental Science. 2011;14(5):1220–1234. doi: 10.1111/j.1467-7687.2011.01069.x. [DOI] [PubMed] [Google Scholar]
- Locke JL. Parental selection of vocal behavior: Crying, cooing, babbling, and the evolution of language. Human Nature. 2006;17:155–168. doi: 10.1007/s12110-006-1015-x. [DOI] [PubMed] [Google Scholar]
- Lorenz KZ. Ausdrucksbewegungen höherer Tiere. Naturwissenschaften. 1951;38:113–116. [Google Scholar]
- Menzel CR. Unprompted recall and reporting of hidden objects by a chimpanzee (Pan troglodytes) after extended delays. Journal of Comparative Psychology. 1999;113(4):426–434. doi: 10.1037/0735-7036.113.4.426. [DOI] [PubMed] [Google Scholar]
- Menzel E. A group of young chimpanzees in a 1-acre field: leadership and communicaiton. In: Byrne RW, Whiten A, editors. Machiavellian intelligence. Oxford, England: Clarendon Press; 1988. pp. 155–159. [Google Scholar]
- Newman RA. Developmental plasticity of Scaphiopus couchii tadpoles in an unpredictable environment. Ecology. 1989;70:1775–1787. [Google Scholar]
- Oller DK. The emergence of the speech capacity. Mahwah, NJ: Lawrence Erlbaum Associates; 2000. [Google Scholar]
- Oller DK, Buder EH, Ramsdell HL, Warlaumont AS, Chorna L, Bakeman R. Functional flexibility of infant vocalization and the emergence of language. Proceedings of the National Academy of Sciences. 2013;110(16):6318–6332. doi: 10.1073/pnas.1300337110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oller DK, Griebel U. How the language capacity was naturally selected: Altriciality and long immaturity. Commentary on Locke, J. Bogin, B: Language and life history: A new perspective on the evolution and development of linguistic communication. Behavioral and Brain Sciences. 2006;29:293–294. [Google Scholar]
- Owren MJ, Rendall D. Sound on the rebound: Bringing form and function back to the forefront in understanding nonhuman primate vocal signaling. Evolutionary Anthropology. 2001;10:58–71. [Google Scholar]
- Pepperberg IM. The Alex studies. Harvard University Press; 1999. [Google Scholar]
- Pepperberg IM. The evolution of communication from an avian perspective. In: Oller DK, Griebel U, editors. The evolution of communication systems: A comparative approach. MIT Press; 2004. pp. 171–192. [Google Scholar]
- Pepperberg IM. Vocal learning in Grey parrots: A brief review of perception, production, and cross-species comparisons. Brain & Language. 2010;115:81–91. doi: 10.1016/j.bandl.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Pilley JW. Border collie comprehends sentences containing a prepositional object, verb, and direct object. Learning and Motivation. 2013;44(4):229–240. [Google Scholar]
- Pilley JW, Reid AK. Border collie comprehends object names as verbal referents. Behavioural Processes. 2011;86(2):184–195. doi: 10.1016/j.beproc.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Premack D. Language in chimpanzee? Science. 1971;172:808–822. doi: 10.1126/science.172.3985.808. [DOI] [PubMed] [Google Scholar]
- Riley JR, Greggers U, Smith AD, Reynolds DR, Menzel R. The flight paths of honeybees recruited by the waggle dance. Nature. 2005;435(7039):205–207. doi: 10.1038/nature03526. [DOI] [PubMed] [Google Scholar]
- Savage-Rumbaugh ES, Murphy J, Sevcik RA, Brakke KE, Williams SL, Rumbaugh DM. Language comprehension in ape and child. Monographs of the Society for Research in Child Development. 1993;233:1–258. [PubMed] [Google Scholar]
- Seyfarth RM, Cheney DL, Marler P. Vervet monkey alarm calls: Semantic communication in a free-ranging primate. Animal Behaviour. 1980;28:1070–1094. [Google Scholar]
- Stegmann UE. Animal communication theory: Information and influence. Cambridge, UK: Cambridge University Press; 2013. [Google Scholar]
- Terrace HS. Nim: A chimpanzee who learned sign language. New York: Columbia University Press; 1979. [Google Scholar]
- Terrace HS, Petitto LA, Sanders RJ, Bever TG. Can an ape create a sentence? Science. 1979;206(4421):891–902. doi: 10.1126/science.504995. [DOI] [PubMed] [Google Scholar]
- Tomasello M. The cultural roots of language. In: Velikovsky B, Rumbaugh D, editors. Communicating meaning: The evolution and development of language. Mahwah, NJ: Lawrence Erlbaum Associates, Inc; 1996. pp. 275–308. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.